Entrapping Immobilisation of Lipase on Biocomposite Hydrogels toward for Biodiesel Production from Waste Frying Acid Oil
Abstract
1. Introduction
2. Results
2.1. Characterisation of Hydrogels
2.1.1. Water Content of Hydrogels
2.1.2. Swelling Behaviour
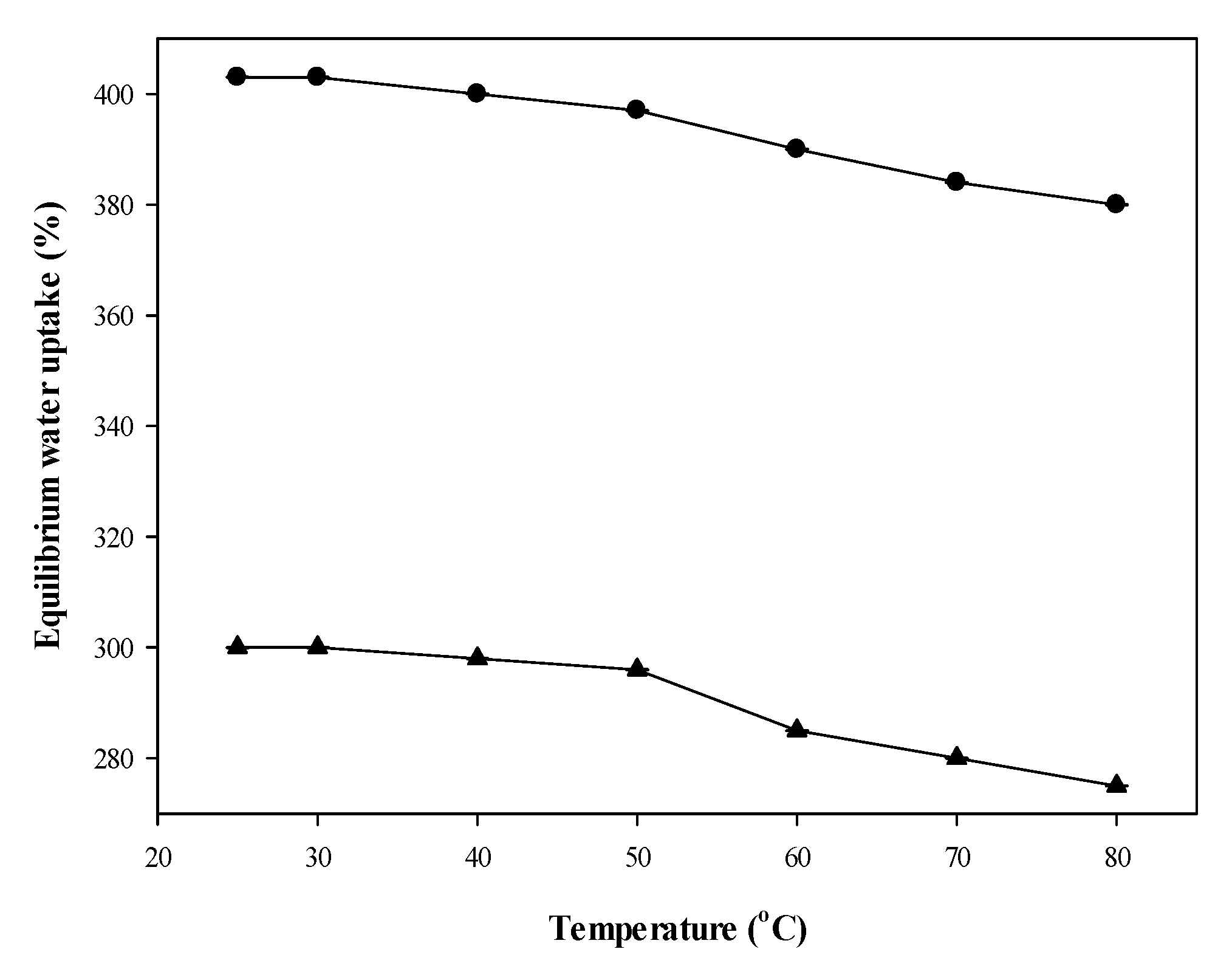
2.1.3. Test of Temperature Sensitivity
2.2. Lipase Activity and Immobilisation Yield
2.3. Effect of Time on Biodiesel Production
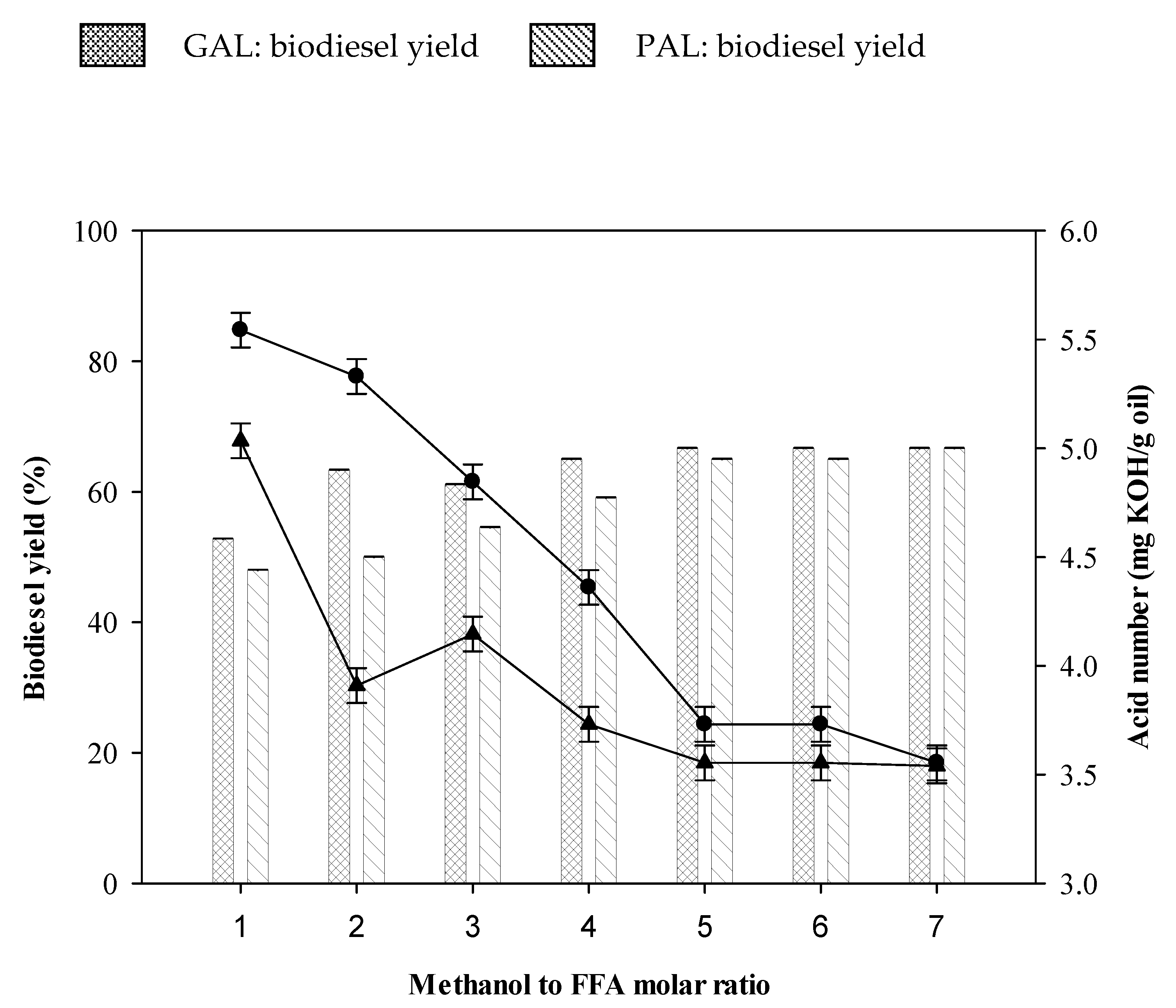
2.4. Effect of Methanol to Fatty Acids Molar Ratio on Biodiesel Production
2.5. Effect of Temperature on Biodiesel Production
2.6. Effect of Agitation Rate on Biodiesel Production
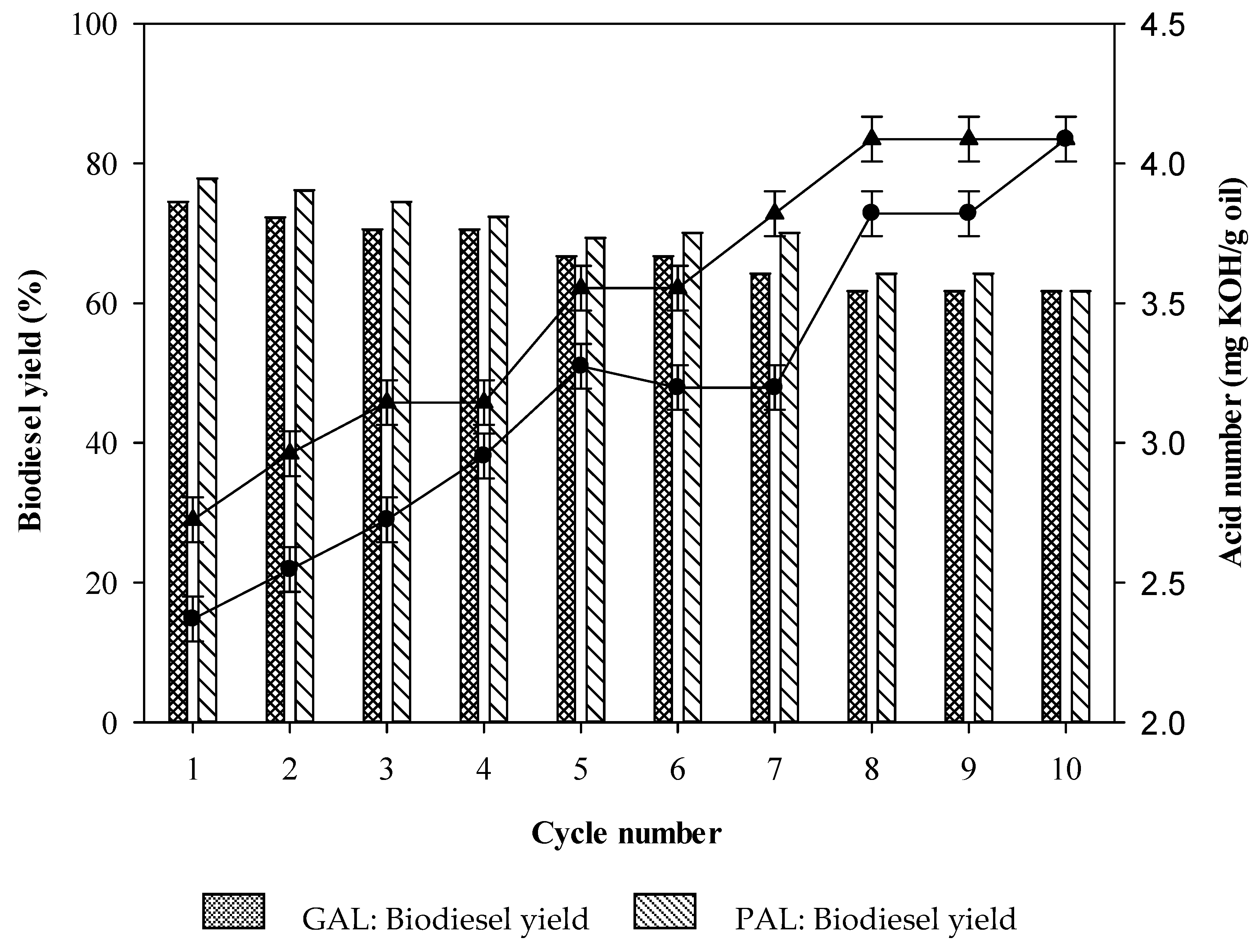
2.7. Reusability of GAL/PAL Beads during Biodiesel Production
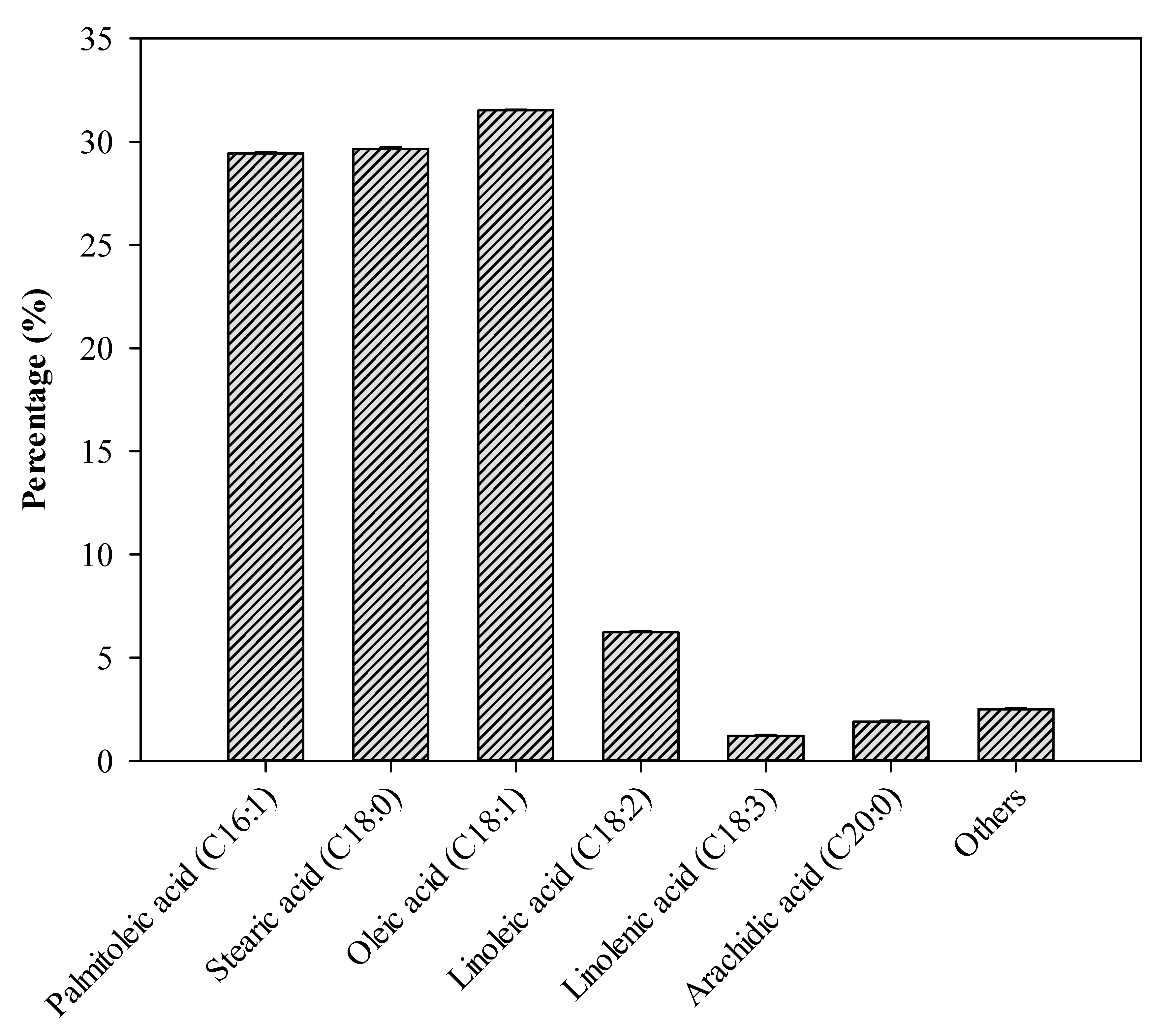
2.8. Biodiesel Composition
2.9. Prediction of Biodiesel Properties
3. Materials and Methods
3.1. Raw Materials
3.2. Preparation of Hydrogels
3.3. Characterisation of Hydrogels
3.3.1. Water Content of Hydrogels
3.3.2. Swelling Behaviour of Hydrogels
3.3.3. Temperature Sensitivity Test
3.4. Immobilisation of Rhizopus Oryzae Lipase (rROL) on Hydrogels
3.5. Esterification of WFAO for Biodiesel Production
3.6. Analytical Techniques
3.6.1. Lipase Activity
3.6.2. Percentage of Immobilisation Yield
3.6.3. Biodiesel Analysis
3.6.4. Reusability Test
3.6.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shokri, A.; Daraei, P.; Zereshki, S. Water decolorization using waste cooking oil: An optimized green emulsion liquid membrane by RSM. J. Water Process Eng. 2020, 33, 101021. [Google Scholar] [CrossRef]
- Xiong, Y.; Miao, W.F.; Wang, N.N.; Chen, H.M.; Wang, X.R.; Wang, J.Y.; Tan, Q.L.; Chen, S.P. Solid alcohol based on waste cooking oil: Synthesis, properties, micromorphology and simultaneous synthesis of biodiesel. Waste Manag. 2019, 85, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Chung, Z.L.; Tan, Y.H.; Chan, Y.S.; Kansedo, J.; Mubarak, N.M.; Ghasemi, M.; Abdullah, M.O. Life cycle assessment of waste cooking oil for biodiesel production using waste chicken eggshell derived CaO as catalyst via transesterification. Biocatal. Agric. Biotechnol. 2019, 21, 101317. [Google Scholar] [CrossRef]
- Dwiarti, L.; Ali, E.; Park, E.Y. Enhancement of lipase catalyzed-fatty acid methyl esters production from waste activated bleaching earth by nullification of lipase inhibitors. Bioresour. Technol. 2010, 101, 14–20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, G.; Liu, J.; Yao, J.; Qi, Y.; Yan, B. Biodiesel production from waste cooking oil in a magnetically fluidized bed reactor using whole-cell biocatalysts. Energy Convers. Manag. 2017, 138, 556–564. [Google Scholar] [CrossRef]
- de Haro, J.C.; Garrido, M.D.P.; Pérez, Á.; Carmona, M.; Rodríguez, J.F. Full conversion of oleic acid to estolides esters, biodiesel and choline carboxylates in three easy steps. J. Clean. Prod. 2018, 184, 579–584. [Google Scholar] [CrossRef]
- Kumar, D.; Das, T.; Giri, B.S.; Verma, B. Optimization of biodiesel synthesis from nonedible oil using immobilized bio-support catalysts in jacketed packed bed bioreactor by response surface methodology. J. Clean. Prod. 2020, 224, 118700. [Google Scholar] [CrossRef]
- Urrutia, P.; Arrieta, R.; Alvarez, L.; Cardenas, C.; Mesa, M.; Wilson, L. Immobilization of lipases in hydrophobic chitosan for selective hydrolysis of fish oil: The impact of support functionalization on lipase activity, selectivity and stability. Int. J. Biol. Macromol. 2018, 108, 674–686. [Google Scholar] [CrossRef]
- Oliveira, A.F.; Bastos, R.G.; de la Torre, L.G. Bacillus subtilis immobilization in alginate microfluidic-based microparticles aiming to improve lipase productivity. Biochem. Eng. J. 2019, 143, 110–120. [Google Scholar] [CrossRef]
- Fernandez-Arrojo, L.; Rodriguez-Colinas, B.; Gutierrez-Alonso, P.; Fernandez-Lobato, M.; Alcalde, M.; Ballesteros, A.O.; Plou, F.J. Dried alginate-entrapped enzymes (DALGEEs) and their application to the production of fructooligosaccharides. Process Biochem. 2013, 48, 677–682. [Google Scholar] [CrossRef]
- Zhao, X.; Qi, F.; Yuan, C.; Du, W.; Liu, D. Lipase-catalyzed process for biodiesel production: Enzyme immobilization, process simulation and optimization. Renew. Sustain. Energy Rev. 2015, 44, 182–197. [Google Scholar] [CrossRef]
- İnal, M.; Işıklan, N.; Yiğitoğlu, M. Preparation and characterization of pH-sensitive alginate- g-poly (N-vinyl-2-pyrrolidone)/gelatin blend beads. J. Ind. Eng. Chem. 2017, 52, 128–137. [Google Scholar] [CrossRef]
- Castillo López, B.; Esteban Cerdán, L.; Robles Medina, A.; Navarro López, E.; Martín Valverde, L.; Hita Peña, E.; González Moreno, P.A.; Molina Grima, E. Production of biodiesel from vegetable oil and microalgae by fatty acid extraction and enzymatic esterification. J. Biosci. Bioeng. 2015, 119, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Pilipenko, N.; Gonçalves, O.H.; Bona, E.; Fernandes, I.P.; Pinto, J.A.; Sorita, G.D.; Leimann, F.V.; Barreiro, M.F. Tailoring swelling of alginate-gelatin hydrogel microspheres by crosslinking with calcium chloride combined with transglutaminase. Carbohydr. Polym. 2019, 223, 115035. [Google Scholar] [CrossRef] [PubMed]
- Takei, T.; Yoshihara, R.; Danjo, S.; Fukuhara, Y.; Evans, C.; Tomimatsu, R.; Ohzuno, Y.; Yoshida, M. Hydrophobically-modified gelatin hydrogel as a carrier for charged hydrophilic drugs and hydrophobic drugs. Int. J. Biol. Macromol. 2020, 149, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Ahmad, T.; Aadil, R.M.; Spotti, M.J.; Bakry, A.M.; Khan, I.M.; Zhao, L.; Riaz, T.; Tong, Q. Pectin polymers as wall materials for the nano-encapsulation of bioactive compounds. Trends Food Sci. Technol. 2019, 90, 35–46. [Google Scholar] [CrossRef]
- Shahrampour, D.; Khomeiri, M.; Razavi, S.M.A.; Kashiri, M. Development and characterization of alginate/pectin edible films containing Lactobacillus plantarum KMC 45. LWT 2020, 118, 108758. [Google Scholar] [CrossRef]
- Vaziri, A.S.; Alemzadeh, I.; Vossoughi, M.; Khorasani, A.C. Co-microencapsulation of Lactobacillus plantarum and DHA fatty acid in alginate-pectin-gelatin biocomposites. Carbohydr. Polym. 2018, 199, 266–275. [Google Scholar] [CrossRef]
- Pulat, M.; Akalin, G.O. Preparation and characterization of gelatin hydrogel support for immobilization of Candida Rugosa lipase. Artif. Cells Nanomed. Biotechnol. 2013, 41, 145–151. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Development and characterization of composite edible films based on sodium alginate and pectin. J. Food Eng. 2013, 115, 459–465. [Google Scholar] [CrossRef]
- Satar, R.; Husain, Q. Catalyzed degradation of disperse dyes by calcium alginate-pectin entrapped bitter gourd (Momordica charantia) peroxidase. J. Environ. Sci. 2011, 23, 1135–1142. [Google Scholar] [CrossRef]
- Batista, K.A.; Purcena, L.L.A.; Alves, G.L.; Fernandes, K.F. A pectin-lipase derivative as alternative copolymer for lipase assay. J. Mol. Catal. B Enzym. 2014, 102, 25–32. [Google Scholar] [CrossRef]
- Chambi, H.N.M.; Grosso, C.R.F. Mechanical and water vapor permeability properties of biodegradables films based on methylcellulose, glucomannan, pectin and gelatin. Ciência e Tecnol. Aliment. 2011, 31, 739–746. [Google Scholar] [CrossRef]
- Feki, A.; Hamdi, M.; Jaballi, I.; Zghal, S.; Nasri, M.; Ben Amara, I. Conception and characterization of a multi-sensitive composite chitosan-red marine alga-polysaccharide hydrogels for insulin controlled-release. Carbohydr. Polym. 2020, 236, 116046. [Google Scholar] [CrossRef] [PubMed]
- Mellinas, C.; Ramos, M.; Jiménez, A.; Garrigós, M.C. Recent trends in the use of pectin from agro-waste residues as a natural-based biopolymer for food packaging applications. Materials 2020, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Di Giuseppe, M.; Law, N.; Webb, B.A.; Macrae, R.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef]
- Cejudo-Sanchesa, J.; Orrego, A.H.; Jaime-Mendozaab, A.; Ghobadi, R.; Moreno-Perezd, S.; Fernandez-Lorente, G.; Rocha-Martina, J.; Guisan, J.M. High stabilization of immobilized Rhizomucor miehei lipase by additional coating with hydrophilic crosslinked polymers: Poly-allylamine/Aldehyde–dextran. Process Biochem. 2020, 92, 156–163. [Google Scholar] [CrossRef]
- Rodrigues, J.; Canet, A.; Rivera, I.; Osório, N.M.; Sandoval, G.; Valero, F.; Ferreira-Dias, S. Biodiesel production from crude Jatropha oil catalyzed by non-commercial immobilized heterologous Rhizopus oryzae and Carica papaya lipases. Bioresour. Technol. 2016, 213, 88–95. [Google Scholar] [CrossRef]
- Nady, D.; Zaki, A.H.; Raslan, M.; Hozayen, W. Enhancement of microbial lipase activity via immobilization over sodium titanate nanotubes for fatty acid methyl esters production. Int. J. Biol. Macromol. 2020, 146, 1169–1179. [Google Scholar] [CrossRef]
- Adnan, M.; Li, K.; Xu, L.; Yan, Y. X-shaped zif-8 for immobilization rhizomucor miehei lipase via encapsulation and its application toward biodiesel production. Catalysts 2018, 8, 96. [Google Scholar] [CrossRef]
- Nobakht, N.; Faramarzi, M.A.; Shafiee, A.; Khoobi, M.; Rafiee, E. Polyoxometalate-metal organic framework-lipase: An efficient green catalyst for synthesis of benzyl cinnamate by enzymatic esterification of cinnamic acid. Int. J. Biol. Macromol. 2018, 113, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Mehrasbi, M.R.; Mohammadi, J.; Peyda, M.; Mohammadi, M. Covalent immobilization of Candida antarctica lipase on core-shell magnetic nanoparticles for production of biodiesel from waste cooking oil. Renew. Energy 2017, 101, 593–602. [Google Scholar] [CrossRef]
- Jambulingam, R.; Shalma, M.; Shankar, V. Biodiesel production using lipase immobilised functionalized magnetic nanocatalyst from oleaginous fungal lipid. J. Clean. Prod. 2019, 215, 245–258. [Google Scholar] [CrossRef]
- Chen, G.; Ying, M.; Li, W. Enzymatic conversion of waste cooking oils into alternative fuel—Biodiesel. Appl. Biochem. Biotechnol. 2006, 132, 911–921. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, Z.; Liu, C.; Liu, C.; Tan, T. Biodiesel production using lipase immobilized on epoxychloropropane-modified Fe3O4 sub-microspheres. Coll. Surf. B Biointerfaces 2016, 140, 446–451. [Google Scholar] [CrossRef]
- Selvakumar, P.; Sivashanmugam, P. Ultrasound assisted oleaginous yeast lipid extraction and garbage lipase catalyzed transesterification for enhanced biodiesel production. Energy Convers. Manag. 2019, 179, 141–151. [Google Scholar] [CrossRef]
- Ban, K.; Hama, S.; Nishizuka, K.; Kaieda, M.; Matsumoto, T.; Kondo, A.; Noda, H.; Fukuda, H. Repeated use of whole-cell biocatalysts immobilized within biomass support particles for biodiesel fuel production. J. Mol. Catal. B Enzym. 2002, 17, 157–165. [Google Scholar] [CrossRef]
- Vescovi, V.; Rojas, M.J.; Baraldo, A.; Botta, D.C.; Santana, F.A.M.; Costa, J.P.; Machado, M.S.; Honda, V.K.; de Lima Camargo Giordano, R.; Tardioli, P.W. Lipase-catalyzed production of biodiesel by hydrolysis of waste cooking oil followed by esterification of free fatty acids. J. Am. Oil Chem. Soc. 2016, 93, 1615–1624. [Google Scholar] [CrossRef]
- Wang, M.; Nie, K.; Cao, H.; Deng, L.; Wang, F.; Tan, T. Biodiesel production by combined fatty acids separation and subsequently enzymatic esterification to improve the low temperature properties. Bioresour. Technol. 2014, 174, 302–305. [Google Scholar] [CrossRef]
- Karimi, M. Immobilization of lipase onto mesoporous magnetic nanoparticles for enzymatic synthesis of biodiesel. Biocatal. Agric. Biotechnol. 2016, 8, 182–188. [Google Scholar] [CrossRef]
- Kumar, D.; Das, T.; Giri, B.S.; Rene, E.R.; Verma, B. Biodiesel production from hybrid non-edible oil using bio-support beads immobilized with lipase from Pseudomonas cepacia. Fuel 2019, 255, 115801. [Google Scholar] [CrossRef]
- Bi, P.; Wang, J.; Zhang, Y.; Jiang, P.; Wu, X.; Liu, J.; Xue, H.; Wang, T.; Li, Q. From lignin to cycloparaffins and aromatics: Directional synthesis of jet and diesel fuel range biofuels using biomass. Bioresour. Technol. 2015, 183, 10–17. [Google Scholar] [CrossRef]
- Charpe, T.W.; Rathod, V.K. Biodiesel production using waste frying oil. Waste Manag. 2011, 31, 85–90. [Google Scholar] [CrossRef]
- Ashjari, M.; Garmroodi, M.; Amiri Asl, F.; Emampour, M.; Yousefi, M.; Pourmohammadi Lish, M.; Habibi, Z.; Mohammadi, M. Application of multi-component reaction for covalent immobilization of two lipases on aldehyde-functionalized magnetic nanoparticles; production of biodiesel from waste cooking oil. Process Biochem. 2020, 90, 156–167. [Google Scholar] [CrossRef]
- Moazeni, F.; Chen, Y.C.; Zhang, G. Enzymatic transesterification for biodiesel production from used cooking oil, a review. J. Clean. Prod. 2019, 216, 117–128. [Google Scholar] [CrossRef]
- Lopresto, C.G.; Naccarato, S.; Albo, L.; de Paola, M.G.; Chakraborty, S.; Curcio, S.; Calabrò, V. Enzymatic transesterification of waste vegetable oil to produce biodiesel. Ecotoxicol. Environ. Saf. 2015, 121, 229–235. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Virgen-Ortíz, J.J.; Jiménez-Pérez, M.; Yates, M.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Evaluation of different lipase biocatalysts in the production of biodiesel from used cooking oil: Critical role of the immobilization support. Fuel 2017, 200, 1–10. [Google Scholar] [CrossRef]
- Xiang, Y.; Xiang, Y.; Wang, L. Microwave radiation improves biodiesel yields from waste cooking oil in the presence of modified coal fly ash. J. Taibah Univ. Sci. 2017, 11, 1019–1029. [Google Scholar] [CrossRef][Green Version]
- Jung, J.M.; Oh, J.I.; Baek, K.; Lee, J.; Kwon, E.E. Biodiesel production from waste cooking oil using biochar derived from chicken manure as a porous media and catalyst. Energy Convers. Manag. 2018, 165, 628–633. [Google Scholar] [CrossRef]
- Anahas, A.M.P.; Muralitharan, G. Characterization of heterocystous cyanobacterial strains for biodiesel production based on fatty acid content analysis and hydrocarbon production. Energy Convers. Manag. 2018, 157, 423–437. [Google Scholar] [CrossRef]
- Muanruksa, P.; Kaewkannetra, P. Combination of fatty acids extraction and enzymatic esterification for biodiesel production using sludge palm oil as a low-cost substrate. Renew. Energy 2020, 146, 901–906. [Google Scholar] [CrossRef]
- Alviso, D.; Artanaa, G.; Duriezac, T. Prediction of biodiesel physico-chemical properties from its fatty acid composition using genetic programming. Fuel 2020, 264, 116844. [Google Scholar] [CrossRef]
- Khaskheli, A.A.; Talpur, F.N.; Ashraf, M.A.; Cebeci, A.; Jawaid, S.; Afridi, H.I. Monitoring the Rhizopus oryzae lipase catalyzed hydrolysis of castor oil by ATR-FTIR spectroscopy. J. Mol. Catal. B Enzym. 2015, 113, 56–61. [Google Scholar] [CrossRef]
- Li, D.; Qin, X.; Wang, J.; Yang, B.; Wang, W.; Huang, W.; Wang, Y. Hydrolysis of soybean oil to produce diacylglycerol by a lipase from Rhizopus oryzae. J. Mol. Catal. B Enzym. 2015, 115, 43–50. [Google Scholar] [CrossRef]
- Muanruksa, P.; Winterburn, J.; Kaewkannetra, P. A novel process for biodiesel production from sludge palm oil. MethodsX 2019, 6, 2838–2844. [Google Scholar] [CrossRef] [PubMed]

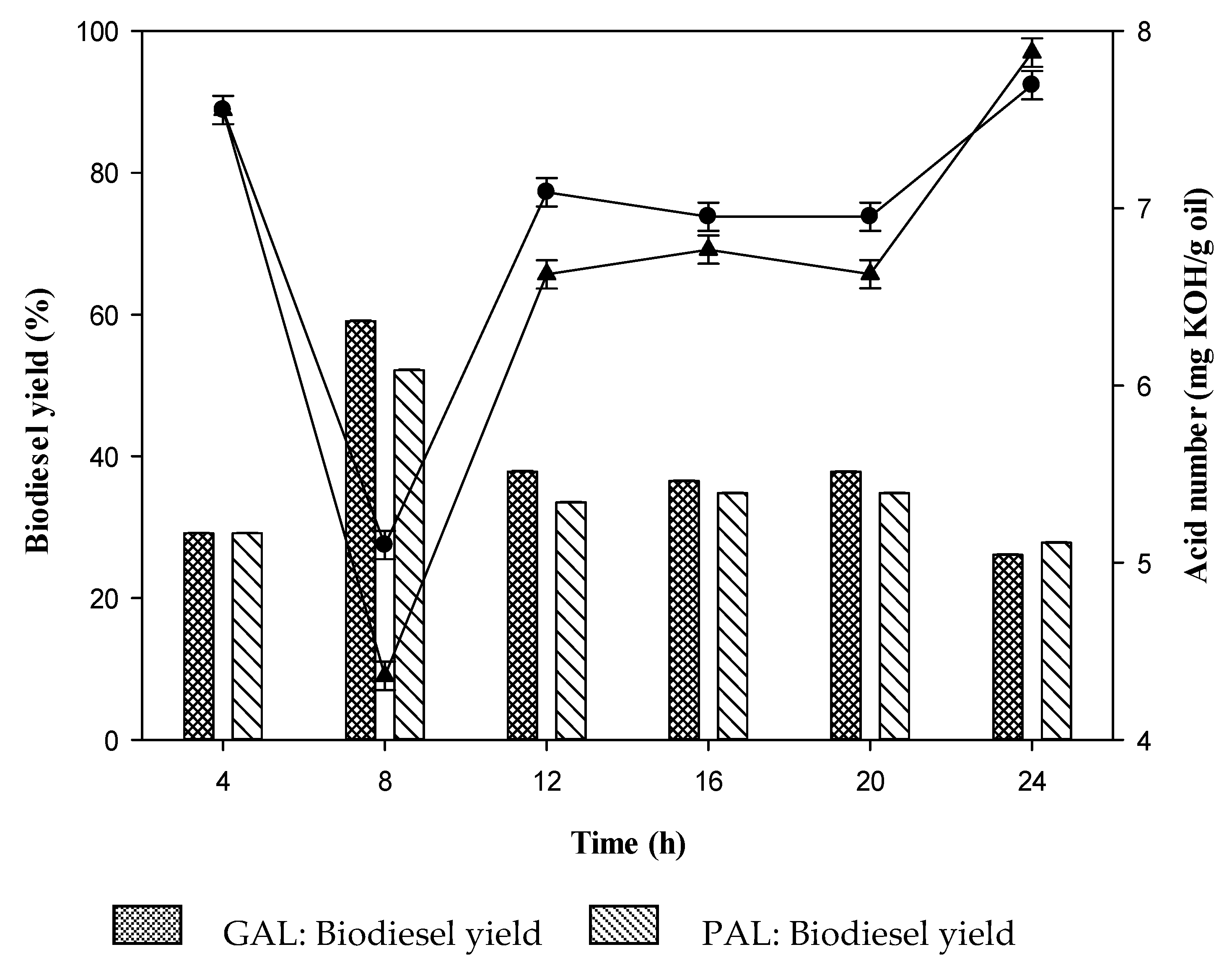

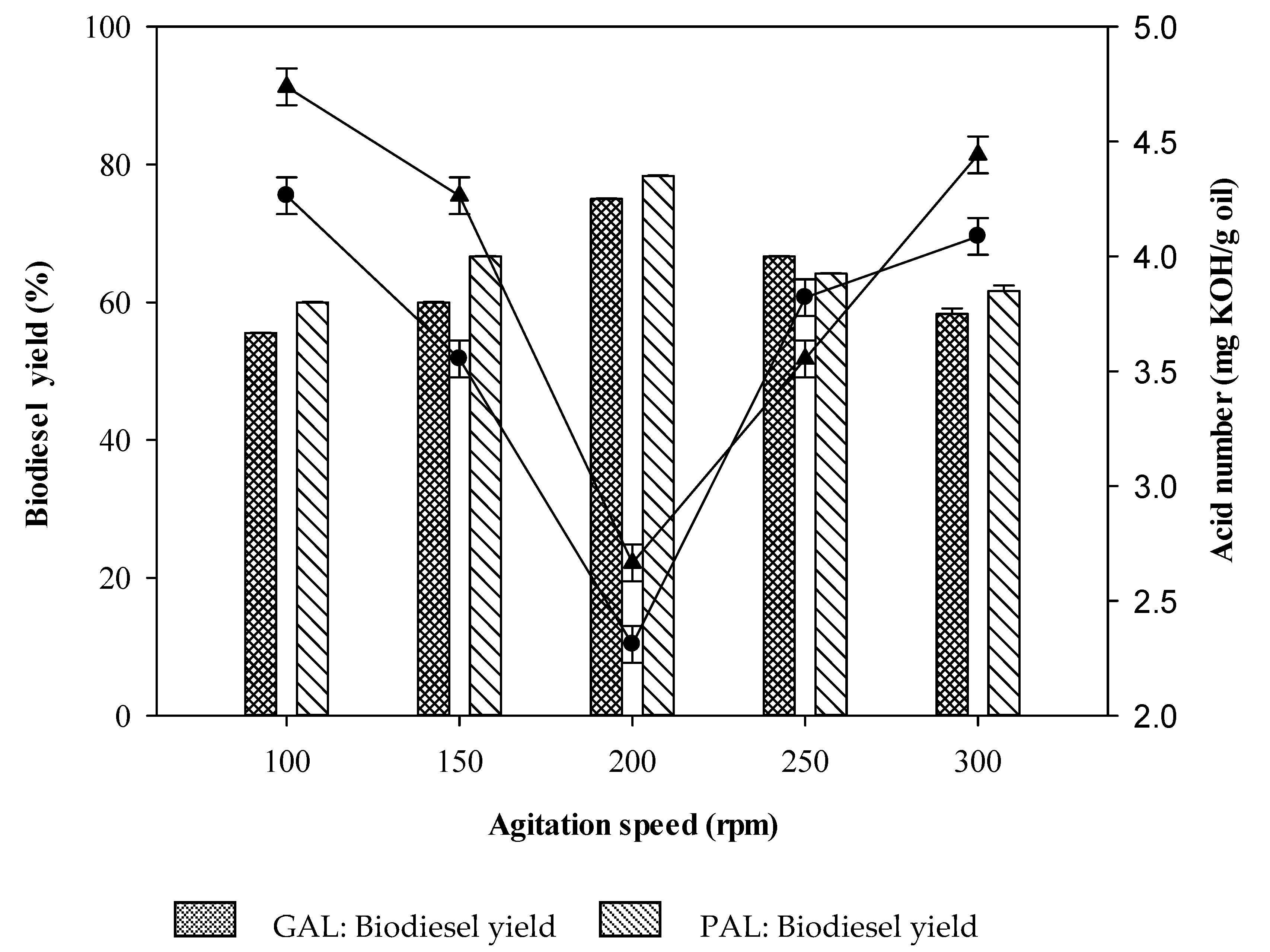
 GAL bead,
GAL bead,  PAL bead. Reaction conditions: acid waste frying oil (AWFO), 50 mL; GAL or PAL beads, 2% w/v; time, 8 h; methanol to fatty acids molar ratio, 4:1 for GAL and 5:1 for PAL; temperature, 50 °C; agitation rate, 200 rpm. Figure 8 indicates the average corresponding to at least three independent experiments. Error bars represent the SD and differences were considered significant at p < 0.05.
PAL bead. Reaction conditions: acid waste frying oil (AWFO), 50 mL; GAL or PAL beads, 2% w/v; time, 8 h; methanol to fatty acids molar ratio, 4:1 for GAL and 5:1 for PAL; temperature, 50 °C; agitation rate, 200 rpm. Figure 8 indicates the average corresponding to at least three independent experiments. Error bars represent the SD and differences were considered significant at p < 0.05.
 GAL bead,
GAL bead,  PAL bead. Reaction conditions: acid waste frying oil (AWFO), 50 mL; GAL or PAL beads, 2% w/v; time, 8 h; methanol to fatty acids molar ratio, 4:1 for GAL and 5:1 for PAL; temperature, 50 °C; agitation rate, 200 rpm. Figure 8 indicates the average corresponding to at least three independent experiments. Error bars represent the SD and differences were considered significant at p < 0.05.
PAL bead. Reaction conditions: acid waste frying oil (AWFO), 50 mL; GAL or PAL beads, 2% w/v; time, 8 h; methanol to fatty acids molar ratio, 4:1 for GAL and 5:1 for PAL; temperature, 50 °C; agitation rate, 200 rpm. Figure 8 indicates the average corresponding to at least three independent experiments. Error bars represent the SD and differences were considered significant at p < 0.05.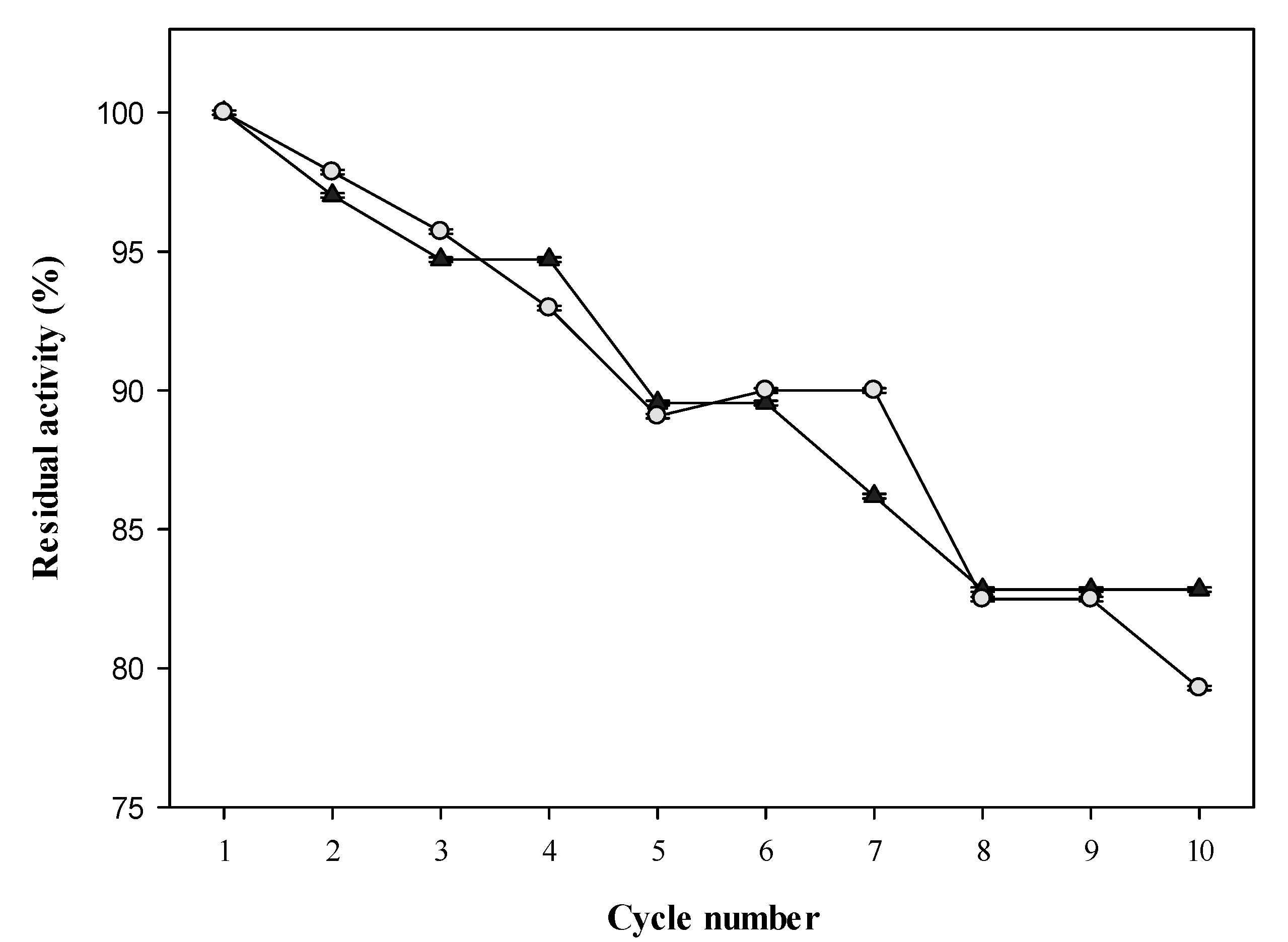
| Enzymes | Carriers | Immobilisation Techniques | Immobilisation Yields * | References |
|---|---|---|---|---|
| Lipase from R. oryzae (rROL) | Gelatin-alginate | Entrapment | 97.61 | This work |
| Lipase from R. oryzae (rROL) | Pectin-alginate | Entrapment | 98.30 | This work |
| Lipase from R. oryzae (rROL) | Lewatit VPOC 1600 | Adsorption | 77.40 | [28] |
| Lipase from R. oryzae (rROL) | Amberlite IRA-96 | Covalent binding | 58.80 | [28] |
| Lipase from R. oryzae (rROL) | Lifetech ECR1030M | Adsorption | 79.50 | [28] |
| Lipase from R. oryzae (rROL) | Lifetech ECR8285M | Covalent binding | 78.40 | [28] |
| Lipase from R. oryzae (rROL) | Lifetech AP1090M | Adsorption | 70.10 | [28] |
| Lipase from Aspergillus niger | Sodium titanate nanotubes | Adsorption | 80.00 | [29] |
| Lipase from R. miehei | X-shaped zeolitic imidazolate frameworks (ZIF-8) | Encapsulation | 90.00 | [30] |
| Porcine pancreatic lipase | PW@MIL-100(Fe) | Encapsulation | 91.00 | [31] |
| Lipase from Candida antarctica | Fe3O4@SiO2 core-shell magnetic nanoparticles | Covalent binding | 84.00 | [32] |
| References | Processes and Conditions | Catalysts | Biodiesel Yield (%) | Reusability (Cycles) |
|---|---|---|---|---|
| This study | Esterification: 2% w/v biocatalyst, 50 °C, 4:1 methanol to fatty acids molar ratio and agitation speed 200 rpm for 8 h. Esterification: 2% w/v biocatalyst, 50 °C, 5:1 methanol to fatty acids molar ratio and agitation speed 200 rpm for 8 h. | GAL bead PAL bead | 75.00 78.33 | 7 7 |
| [43] | Transesterification: 5% biocatalyst, 45 °C, 3:1 methanol to oil ratio for 24 h. | Lipase from P. fluorescens | 63.84 | - |
| [46] | Transesterification: 3% w/w biocatalyst, 37 °C, 3:1 methanol to oil ratio and agitation speed 200 rpm for 24 h. | Immobilised P. cepacia lipase on epoxy-acrylic resin | 46.32 | 6 |
| [32] | Transesterification: 5% w/w biocatalyst, 37 °C, 3:1 methanol to oil ratio for 96 h. | Immobilised C. antractica lipase on core-shellmagnetic nanoparticles | 100 | 6 |
| [47] | Transesterification: 10% w/w biocatalyst, 30 °C, 3:1 methanol to oil ratio, 0.25% w/w water and agitation speed 350 rpm for 24 h. | Immobilised T. lanuginosus lipase on Octadecyl methacylate | 80 | - |
| [44] | Transesterification: 15.2% w/w biocatalyst, 40 °C, 29.2% w/w t-butanol to oil ratio and 40.5% w/w water adsorbent for 48 h. | Immobilised lipase from R. miehei on silica core-shell magnetic nanoparticles (Fe3O4@SiO2) | 57.5 | 5 |
| Parameters | Test Methods | Predicted Values * | European Standard |
|---|---|---|---|
| Kinematic viscosity (mm2/s) | ASTM D445 | 5.09 | 3.5–5.0 |
| Flash point (°C) | ASTM D93 | 132.89 | ˃101 |
| Cloud point (K) | ASTM D2500 | 286.52 | No specification |
| Pour point (K) | ASTM D97 | 280.35 | No specification |
| Cold filter plugging point (K) | ASTM D6371 | 273.13 | No specification |
| Cetane number | ASTM D613 | 54.80 | ˃51 |
| Iodine number | EN14111 | 72.47 | <120 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muanruksa, P.; Dujjanutat, P.; Kaewkannetra, P. Entrapping Immobilisation of Lipase on Biocomposite Hydrogels toward for Biodiesel Production from Waste Frying Acid Oil. Catalysts 2020, 10, 834. https://doi.org/10.3390/catal10080834
Muanruksa P, Dujjanutat P, Kaewkannetra P. Entrapping Immobilisation of Lipase on Biocomposite Hydrogels toward for Biodiesel Production from Waste Frying Acid Oil. Catalysts. 2020; 10(8):834. https://doi.org/10.3390/catal10080834
Chicago/Turabian StyleMuanruksa, Papasanee, Praepilas Dujjanutat, and Pakawadee Kaewkannetra. 2020. "Entrapping Immobilisation of Lipase on Biocomposite Hydrogels toward for Biodiesel Production from Waste Frying Acid Oil" Catalysts 10, no. 8: 834. https://doi.org/10.3390/catal10080834
APA StyleMuanruksa, P., Dujjanutat, P., & Kaewkannetra, P. (2020). Entrapping Immobilisation of Lipase on Biocomposite Hydrogels toward for Biodiesel Production from Waste Frying Acid Oil. Catalysts, 10(8), 834. https://doi.org/10.3390/catal10080834