Soot Combustion over Niobium-Doped Cryptomelane (K-OMS-2) Nanorods—Redox State of Manganese and the Lattice Strain Control the Catalysts Performance
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural and Morphological Characterization
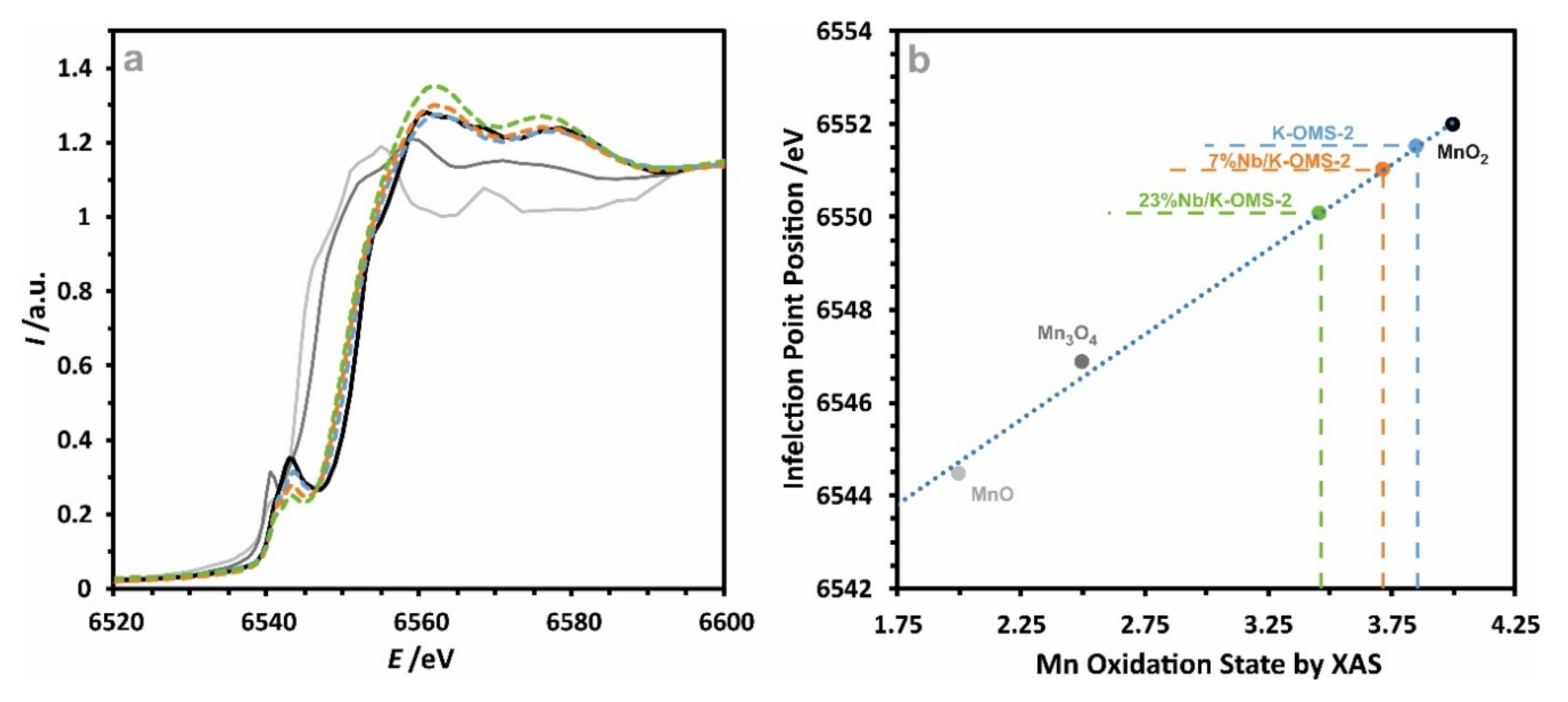
2.2. Chemical Composition and Manganese Redox State Analysis
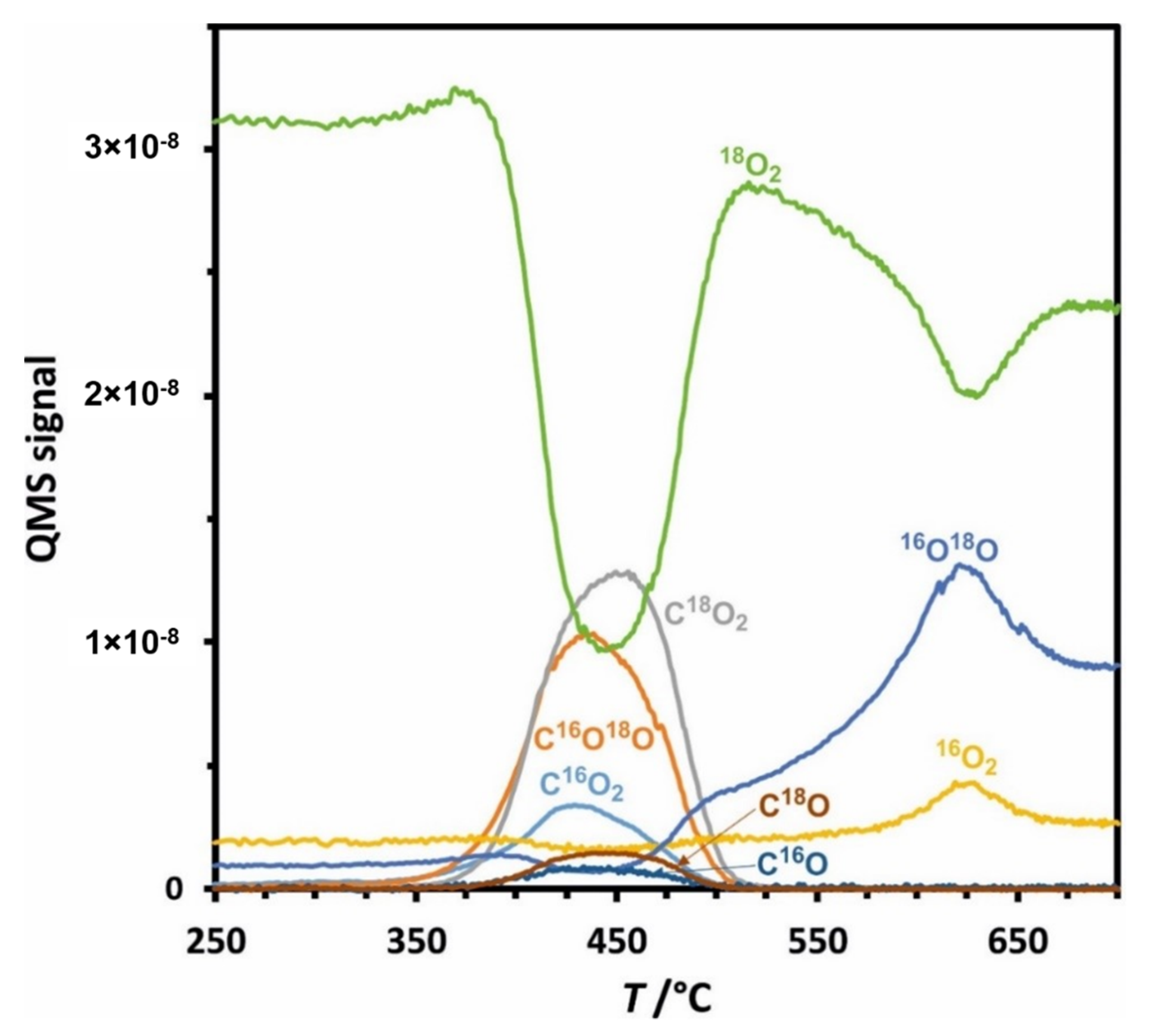
2.3. Catalytic Activity
3. Materials and Methods
3.1. Catalyst Synthesis
3.2. Catalyst Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Setten, B.A.A.L.; Makkee, M.; Moulijn, J.A. Science and technology of catalytic diesel particulate filters. Catal. Rev. Sci. Eng. 2001, 43, 489–564. [Google Scholar] [CrossRef]
- Fino, D. Diesel emission control: Catalytic filters for particulate removal. Sci. Technol. Adv. Mater. 2007, 8, 93–100. [Google Scholar] [CrossRef]
- Wei, E.T.; Shu, H.P. Nitroaromatic carcinogens in diesel soot: A review of laboratory findings. Am. J. Public Health 1983, 73, 1085–1088. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Legutko, P.; Jakubek, T.; Kaspera, W.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Strong Enhancement of deSoot Activity of Transition Metal Oxides by Alkali Doping: Additive Effects of Potassium and Nitric Oxide. Top. Catal. 2017, 60, 162–170. [Google Scholar] [CrossRef]
- Johnson, T.V. Diesel emission control in review. SAE Int. J. Fuels Lubr. 2009, 2, 1–12. [Google Scholar] [CrossRef]
- Caroca, J.; Villata, G.; Fino, D.; Russo, N. Comparison of different diesel particulate filters. Top. Catal. 2009, 52, 2076–2082. [Google Scholar] [CrossRef]
- Cui, B.; Zhou, L.; Li, K.; Liu, Y.Q.; Wang, D.; Ye, Y.; Li, S. Holey Co-Ce oxide nanosheets as a highly efficient catalyst for diesel soot combustion. Appl. Catal. B Environ. 2020, 267, 118670. [Google Scholar] [CrossRef]
- Gardini, D.; Christensen, J.M.; Damsgaard, C.D.; Jensen, A.D.; Wagner, J.B. Visualizing the mobility of silver during catalytic soot oxidation. Appl. Catal. B Environ. 2016, 183, 28–36. [Google Scholar] [CrossRef]
- Hernández-Giménez, A.M.; Castelló, D.L.; Bueno-López, A. Diesel soot combustion catalysts: Review of active phases. Chem. Pap. 2014, 68, 1154–1168. [Google Scholar] [CrossRef]
- Álvarez-Docio, C.M.; Portela, R.; Reinosa, J.J.; Rubio-Marcos, F.; Granados-Miralles, C.; Pascual, L.; Fernández, J.F. Pt-free CoAl2O4 catalyst for soot combustion with NOx/O2. Appl. Catal. A Gen. 2020, 591, 117404. [Google Scholar] [CrossRef]
- Jakubek, T.; Hudy, C.; Gryboś, J.; Manyar, H.; Kotarba, A. Thermal Transformation of Birnessite (OL) Towards Highly Active Cryptomelane (OMS-2) Catalyst for Soot Oxidation. Catal. Lett. 2019, 149, 2218–2225. [Google Scholar] [CrossRef]
- Chen, X.; Shen, Y.F.; Suib, S.L.; O’Young, C.L. Characterization of manganese oxide octahedral molecular sieve (M-OMS-2) materials with different metal cation dopants. Chem. Mater. 2002, 14, 940–948. [Google Scholar] [CrossRef]
- Kaspera, W.; Indyka, P.; Sojka, Z.; Kotarba, A. Bridging the gap between tight and loose contacts for soot oxidation by vanadium doping of cryptomelane nanorods catalyst using NO2 as an oxygen carrier. Catal. Sci. Technol. 2018, 8, 3183–3192. [Google Scholar] [CrossRef]
- Jiménez, R.; García, X.; López, T.; Gordon, A.L. Catalytic combustion of soot. Effects of added alkali metals on CaO-MgO physical mixtures. Fuel Process. Technol. 2008, 89, 1160–1168. [Google Scholar] [CrossRef]
- Jakubek, T.; Kaspera, W.; Legutko, P.; Stelmachowski, P.; Kotarba, A. How to Efficiently Promote Transition Metal Oxides by Alkali Towards Catalytic Soot Oxidation. Top. Catal. 2016, 59, 1083–1089. [Google Scholar] [CrossRef]
- Jakubek, T.; Kaspera, W.; Legutko, P.; Stelmachowski, P.; Kotarba, A. Surface versus bulk alkali promotion of cobalt-oxide catalyst in soot oxidation. Catal. Commun. 2015, 71, 37–41. [Google Scholar] [CrossRef]
- Pecchi, G.; Cabrera, B.; Delgado, E.J.; García, X.; Jimenez, R. Activity of KNbO3 as catalyst for soot combustion: Effect of the preparation method. Appl. Catal. A Gen. 2013, 453, 341–348. [Google Scholar] [CrossRef]
- Neyertz, C.A.; Banús, E.D.; Miró, E.E.; Querini, C.A. Potassium-promoted Ce0.65Zr0.35O2 monolithic catalysts for diesel soot combustion. Chem. Eng. J. 2014, 248, 394–405. [Google Scholar] [CrossRef]
- Wagloehner, S.; Kureti, S. Study on the mechanism of the oxidation of soot on Fe2O3 catalyst. Appl. Catal. B Environ. 2012, 125, 158–165. [Google Scholar] [CrossRef]
- Wasalathanthri, N.D.; Guild, C.; Nizami, Q.A.; Dissanayake, S.L.; He, J.; Kerns, P.; Fee, J.; Achola, L.; Rathnayake, D.; Weerakkody, C.; et al. Niobium-substituted octahedral molecular sieve (OMS-2) materials in selective oxidation of methanol to dimethoxymethane. RSC Adv. 2019, 9, 32665–32673. [Google Scholar] [CrossRef]
- Chen, C.H.; Njagi, E.C.; Chen, S.Y.; Horvath, D.T.; Xu, L.; Morey, A.; Mackin, C.; Joesten, R.; Suib, S.L. Structural Distortion of Molybdenum-Doped Manganese Oxide Octahedral Molecular Sieves for Enhanced Catalytic Performance. Inorg. Chem. 2015, 54, 10163–10171. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Wang, J.; Casey, W.H. Structure, stability and photocatalytic H2 production by Cr-, Mn-, Fe-, Co-, and Ni-substituted decaniobate clusters. Dalt. Trans. 2014, 43, 17928–17933. [Google Scholar] [CrossRef] [PubMed]
- Becerra, M.-E.; Arias, N.-P.; Giraldo, O.-H.; López-Suárez, F.-E.; Illán-Gómez, M.-J.; Bueno-López, A. Alumina-Supported Manganese Catalysts for Soot Combustion Prepared by Thermal Decomposition of KMnO4. Catalysts 2012, 2, 352–367. [Google Scholar] [CrossRef]
- Becerra, M.E.; Arias, N.P.; Giraldo, O.H.; López Suárez, F.E.; Illán Gómez, M.J.; Bueno López, A. Soot combustion manganese catalysts prepared by thermal decomposition of KMnO4. Appl. Catal. B Environ. 2011, 102, 260–266. [Google Scholar] [CrossRef]
- Sultana, S.; Ye, Z.; Veerapandian, S.K.P.; Löfberg, A.; De Geyter, N.; Morent, R.; Giraudon, J.M.; Lamonier, J.F. Synthesis and catalytic performances of K-OMS-2, Fe/K-OMS-2 and Fe-K-OMS-2 in post plasma-catalysis for dilute TCE abatement. Catal. Today 2018, 307, 20–28. [Google Scholar] [CrossRef]
- Legutko, P.; Pęza, J.; Villar Rossi, A.; Marzec, M.; Jakubek, T.; Kozieł, M.; Adamski, A. Elucidation of Unexpectedly Weak Catalytic Effect of Doping with Cobalt of the Cryptomelane and Birnessite Systems Active in Soot Combustion. Top. Catal. 2019, 62, 599–610. [Google Scholar] [CrossRef]
- Ding, Y.S.; Shen, X.F.; Sithambaram, S.; Gomez, S.; Kumar, R.; Crisostomo, V.M.B.; Suib, S.L.; Aindow, M. Synthesis and catalytic activity of cryptomelane-type manganese dioxide nanomaterials produced by a novel solvent-free method. Chem. Mater. 2005, 17, 5382–5389. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Legutko, P.; Jakubek, T.; Indyka, P.; Sojka, Z.; Holmlid, L.; Kotarba, A. Emission of highly excited electronic states of potassium from cryptomelane nanorods. Phys. Chem. Chem. Phys. 2015, 17, 26289–26294. [Google Scholar] [CrossRef]
- Ortiz, A.L.; Shaw, L. X-ray diffraction analysis of a severely plastically deformed aluminum alloy. Acta Mater. 2004, 52, 2185–2197. [Google Scholar] [CrossRef]
- Cheney, M.A.; Birkner, N.R.; Ma, L.; Hartmann, T.; Bhowmik, P.K.; Hodge, V.F.; Steinberg, S.M. Synthesis and characterization of inorganic double helices of cryptomelane nanomaterials. Colloids Surf. A Physicochem. Eng. Asp. 2006, 289, 185–192. [Google Scholar] [CrossRef]
- Stelmachowski, P.; Legutko, P.; Jakubek, T.; Kotarba, A. Phase evolution and electronic properties of cryptomelane nanorods. J. Alloys Compd. 2018, 767, 592–599. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Chandra Bose, A.; Rameshbabu, N. X-ray peak broadening studies of nanocrystalline hydroxyapatite by WilliamsonHall analysis. Phys. B Condens. Matter 2010, 405, 4256–4261. [Google Scholar] [CrossRef]
- Englman, R.; Dow, J.D. The Jahn–Teller Effect in Molecules and Crystals. Phys. Today 1974, 27, 57. [Google Scholar] [CrossRef]
- Gao, T.; Glerup, M.; Krumeich, F.; Nesper, R.; Fjellvâg, H.; Norby, P. Microstructures and spectroscopic properties of cryptomelane-type manganese dioxide nanofibers. J. Phys. Chem. C 2008, 112, 13134–13140. [Google Scholar] [CrossRef]
- Campbell, I.H. The Effects of Microcrystal Size and Shape on the One Phonon Raman Spectra of crystalline semiconductors. Semiconductors 1986, 58, 739–741. [Google Scholar] [CrossRef]
- Wang, J.B.; Zhong, H.M.; Li, Z.F.; Lu, W. Raman study for E 2 phonon of ZnO in Zn1−xMnxO nanoparticles. J. Appl. Phys. 2005, 97, 086105. [Google Scholar] [CrossRef]
- Xing, S.; Chun, H.U.; Jiuhui, Q.U.; Hong, H.E.; Yang, M.I.N. Characterization and Reactivity of MnOx Supported on Mesoporous Zirconia for Herbicide 2,4-D Mineralization with Ozone. Environ. Sci. Technol. 2008, 42, 3363–3368. [Google Scholar] [CrossRef]
- Ye, Y.; Shen, F.; Wang, H.; Chen, R. SSZ-13-supported manganese oxide catalysts for low temperature selective catalytic reduction of NOx by NH3. J. Chem. Sci. 2017, 129, 765–774. [Google Scholar] [CrossRef]
- Fernández López, E.; Sánchez Escribano, V.; Gallardo-Amores, J.M.; Resini, C.; Busca, G. Structural and morphological characterization of Mn-Zr mixed oxides prepared by a sol-gel method. Solid State Sci. 2002, 4, 951–961. [Google Scholar] [CrossRef]
- Velu, S.; Shah, N.; Jyothi, T.M.; Sivasanker, S. Effect of manganese substitution on the physicochemical properties and catalytic toluene oxidation activities of Mg-Al layered double hydroxides. Microporous Mesoporous Mater. 1999, 33, 61–75. [Google Scholar] [CrossRef]
- Liu, W.X.; Zhu, X.L.; Liu, S.Q.; Gu, Q.Q.; Meng, Z. Da Near-Infrared-Driven Selective Photocatalytic Removal of Ammonia Based on Valence Band Recognition of an α-MnO2/N-Doped Graphene Hybrid Catalyst. ACS Omega 2018, 3, 5537–5546. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.A.; Adán, C.; Araujo, A.S.; Guedes, A.P.M.A.; Marugán, J. Synthesis, Characterization, and Photonic Efficiency of Novel Photocatalytic Niobium Oxide Materials. Glob. Chall. 2017, 1, 1700066. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wu, G.; Guan, N.; Li, L. Nb2O5/TiO2 heterojunctions: Synthesis strategy and photocatalytic activity. Appl. Catal. B Environ. 2014, 152–153, 280–288. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef]
- Nelson, A.J.; Reynolds, J.G.; Roos, J.W. Core-level satellites and outer core-level multiplet splitting in Mn model compounds. J. Vac. Sci. Technol. Vac. Surf. Films 2000, 18, 1072–1076. [Google Scholar] [CrossRef]
- Nelson, A.J.; Reynolds, J.G.; Christou, G. Spin-state effects on the outer core-level multiplet structures for high-spin Mn molecular clusters. J. Appl. Phys. 2003, 93, 2536–2539. [Google Scholar] [CrossRef]
- Junta, J.L.; Hochella, M.F. Manganese (II) oxidation at mineral surfaces: A microscopic and spectroscopic study. Geochim. Cosmochim. Acta 1994, 58, 4985–4999. [Google Scholar] [CrossRef]
- Galakhov, V.R.; Demeter, M.; Bartkowski, S.; Neumann, M.; Ovechkina, N.A.; Kurmaev, E.Z.; Lobachevskaya, N.I.; Mukovskii, Y.M.; Mitchell, J.; Ederer, D.L. Mn (formula presented) exchange splitting in mixed-valence manganites. Phys. Rev. B Condens. Matter Mater. Phys. 2002, 65, 1–4. [Google Scholar] [CrossRef]
- Li, X.; Zou, Q.; Wei, Y.; Zhou, X.; Wang, Z.; Xu, A.; Ruan, X. Graphite assisted room-temperature synthesis of structurally defected OMS-2 nanorods for peroxymonosulfate activation. Appl. Surf. Sci. 2019, 497, 143770. [Google Scholar] [CrossRef]
- Zhang, T.; Lei, W.; Liu, P.; Rodriguez, J.A.; Yu, J.; Qi, Y.; Liu, G.; Liu, M. Organic Pollutant Photodecomposition by Ag/KNbO3 Nanocomposites: A Combined Experimental and Theoretical Study. J. Phys. Chem. C 2016, 120, 2777–2786. [Google Scholar] [CrossRef]
- Deng, H.; Kang, S.; Ma, J.; Wang, L.; Zhang, C.; He, H. Role of Structural Defects in MnOx Promoted by Ag Doping in the Catalytic Combustion of Volatile Organic Compounds and Ambient Decomposition of O3. Environ. Sci. Technol. 2019, 53, 10871–10879. [Google Scholar] [CrossRef]
- Piszora, P. Temperature dependence of the order and distribution of Mn3+ and Mn4+ cations in orthorhombic LiMn2O4. J. Alloys Compd. 2004, 382, 112–118. [Google Scholar] [CrossRef]
- Chu, Q.; Wang, X.; Zhang, X.; Li, Q.; Liu, X. Buckled layers in K0.66Mn2O4·0.28H2O and K0.99Mn3O6·1.25H2O synthesized at high pressure: Implication for the mechanism of layer-to-tunnel transformation in manganese oxides. Inorg. Chem. 2011, 50, 2049–2051. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Su, D.; Liu, L.; Chisnell, R.; Ong, S.P.; Chen, H.; Toumar, A.; Idrobo, J.C.; Lei, Y.; et al. Direct visualization of the Jahn-Teller effect coupled to Na ordering in Na5/8MnO2. Nat. Mater. 2014, 13, 586–592. [Google Scholar] [CrossRef]
- Legutko, P.; Stelmachowski, P.; Trȩbala, M.; Sojka, Z.; Kotarba, A. Role of electronic factor in soot oxidation process over tunnelled and layered potassium iron oxide catalysts. Top. Catal. 2013, 56, 489–492. [Google Scholar] [CrossRef]
- Legutko, P.; Kaspera, W.; Jakubek, T.; Stelmachowski, P.; Kotarba, A. Influence of potassium and NO addition on catalytic activity in soot combustion and surface properties of iron and manganese spinels. Top. Catal. 2013, 56, 745–749. [Google Scholar] [CrossRef][Green Version]
- Legutko, P.; Kaspera, W.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Boosting the catalytic activity of magnetite in soot oxidation by surface alkali promotion. Catal. Commun. 2014, 56, 139–142. [Google Scholar] [CrossRef]
- Benedek, R. Role of Disproportionation in the Dissolution of Mn from Lithium Manganate Spinel. J. Phys. Chem. C 2017, 121, 22049–22053. [Google Scholar] [CrossRef]
- Muzykantov, V.S.; Panov, G.I.; Boreskov, G.K. Determination of the Types of Homo-Molecular Oxygen Exchange on Oxides. Kinet. Katal. 1973, 14, 948–955. [Google Scholar]
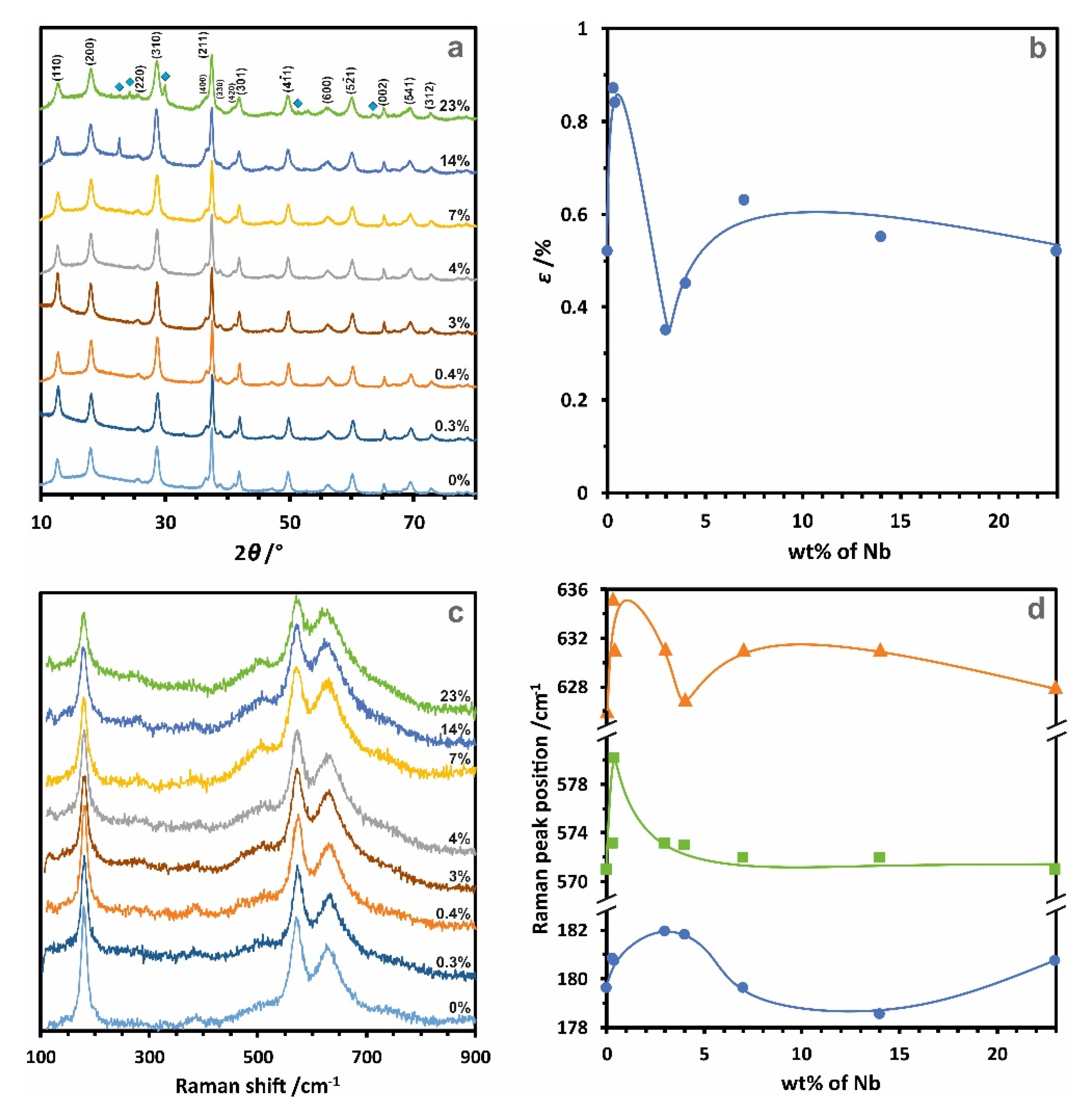
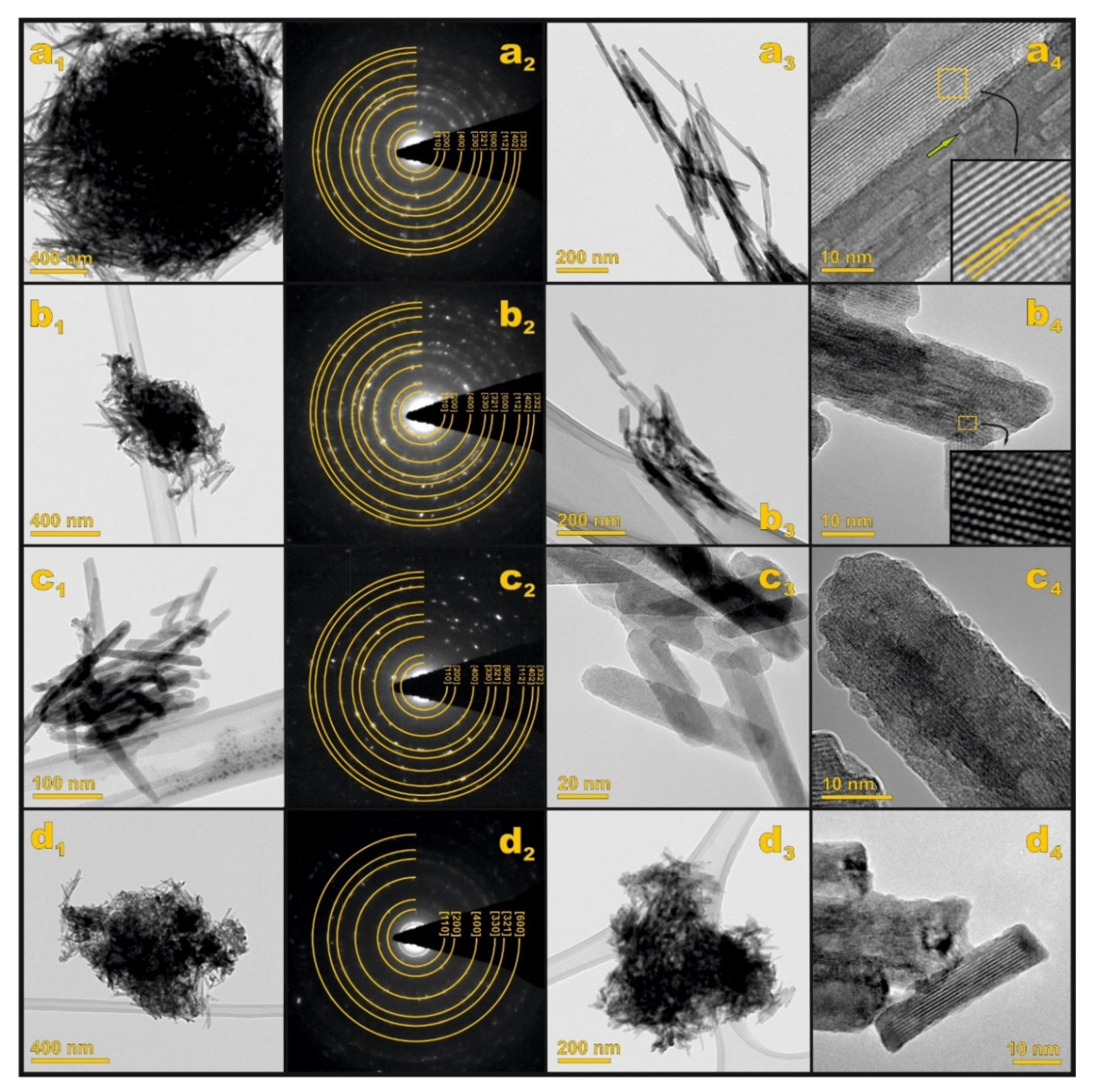
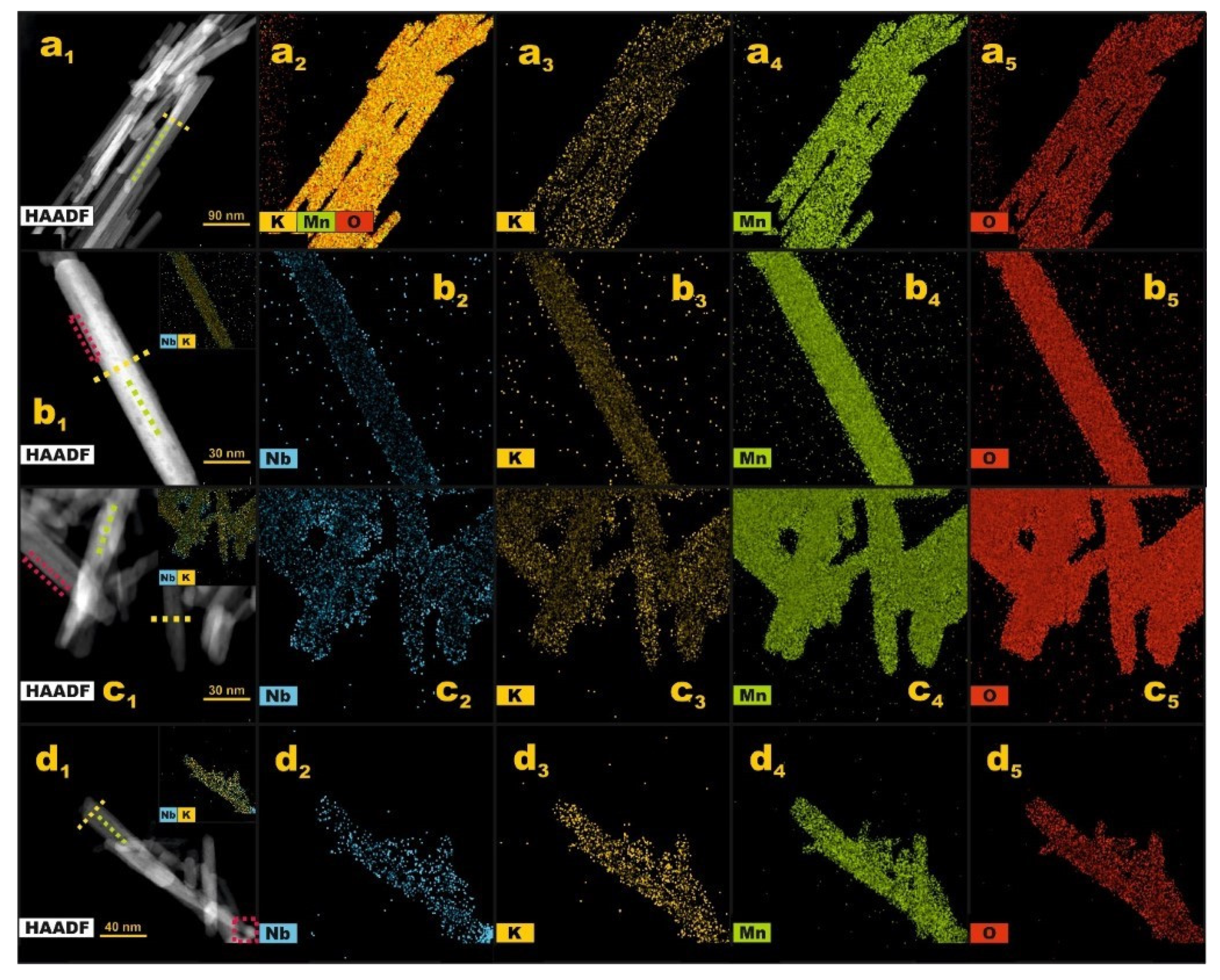



| Nb Loading/wt% | dW-H/nm * | ε/% ** | SSA/m2·g−1 *** |
|---|---|---|---|
| 0 | 30 | 0.52 | 57 |
| 0.3 | 32 | 0.87 | 57 |
| 0.4 | 37 | 0.84 | 57 |
| 3 | 35 | 0.35 | 63 |
| 4 | 41 | 0.45 | 64 |
| 7 | 36 | 0.63 | 72 |
| 14 | 22 | 0.55 | 75 |
| 23 | 21 | 0.52 | 75 |
| Nb Loading/wt% | Average Length/nm | <AR> | K/Mn | Mn/O |
|---|---|---|---|---|
| 0 | 350 | 20.7 | 0.12 | 0.63 |
| 3 | 87 | 5.9 | 0.14 | 0.61 |
| 7 | 123 | 8.2 | 0.09 | 0.68 |
| 23 | 47 | 3.8 | 0.11 | 0.59 |
| Nb Loading/wt% * | XRF/Norm% ** | XPS/Norm% | NbXPS/NbXRF | Mn/O | |||||
|---|---|---|---|---|---|---|---|---|---|
| K | Mn | Nb | K | Mn | Nb | O *** | |||
| 0 | 0.84 | 8.00 | - | 0.95 | 8.00 | - | 11.8 | - | 0.68 |
| 0.3 | 0.94 | 7.99 | 0.01 | 0.95 | 7.97 | 0.03 | 12.3 | 3.00 | 0.65 |
| 0.4 | 0.77 | 7.98 | 0.02 | 0.77 | 7.96 | 0.04 | 11.5 | 2.00 | 0.69 |
| 3 | 0.78 | 7.90 | 0.10 | 0.73 | 7.80 | 0.20 | 12.0 | 2.00 | 0.65 |
| 4 | 0.72 | 7.80 | 0.20 | 0.78 | 7.80 | 0.20 | 12.2 | 1.00 | 0.64 |
| 7 | 0.77 | 7.70 | 0.30 | 0.78 | 7.80 | 0.20 | 12.1 | 0.67 | 0.65 |
| 14 | 0.78 | 7.30 | 0.70 | 0.78 | 7.70 | 0.30 | 12.6 | 0.42 | 0.61 |
| 23 | 0.82 | 6.80 | 1.20 | 1.10 | 7.30 | 0.70 | 13.0 | 0.42 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legutko, P.; Gryboś, J.; Fedyna, M.; Janas, J.; Wach, A.; Szlachetko, J.; Adamski, A.; Yu, X.; Zhao, Z.; Kotarba, A.; et al. Soot Combustion over Niobium-Doped Cryptomelane (K-OMS-2) Nanorods—Redox State of Manganese and the Lattice Strain Control the Catalysts Performance. Catalysts 2020, 10, 1390. https://doi.org/10.3390/catal10121390
Legutko P, Gryboś J, Fedyna M, Janas J, Wach A, Szlachetko J, Adamski A, Yu X, Zhao Z, Kotarba A, et al. Soot Combustion over Niobium-Doped Cryptomelane (K-OMS-2) Nanorods—Redox State of Manganese and the Lattice Strain Control the Catalysts Performance. Catalysts. 2020; 10(12):1390. https://doi.org/10.3390/catal10121390
Chicago/Turabian StyleLegutko, Piotr, Joanna Gryboś, Monika Fedyna, Janusz Janas, Anna Wach, Jakub Szlachetko, Andrzej Adamski, Xuehua Yu, Zhen Zhao, Andrzej Kotarba, and et al. 2020. "Soot Combustion over Niobium-Doped Cryptomelane (K-OMS-2) Nanorods—Redox State of Manganese and the Lattice Strain Control the Catalysts Performance" Catalysts 10, no. 12: 1390. https://doi.org/10.3390/catal10121390
APA StyleLegutko, P., Gryboś, J., Fedyna, M., Janas, J., Wach, A., Szlachetko, J., Adamski, A., Yu, X., Zhao, Z., Kotarba, A., & Sojka, Z. (2020). Soot Combustion over Niobium-Doped Cryptomelane (K-OMS-2) Nanorods—Redox State of Manganese and the Lattice Strain Control the Catalysts Performance. Catalysts, 10(12), 1390. https://doi.org/10.3390/catal10121390