Development of FTIR Spectroscopy Methodology for Characterization of Boron Species in FCC Catalysts
Abstract
:1. Introduction
2. Results and Discussion
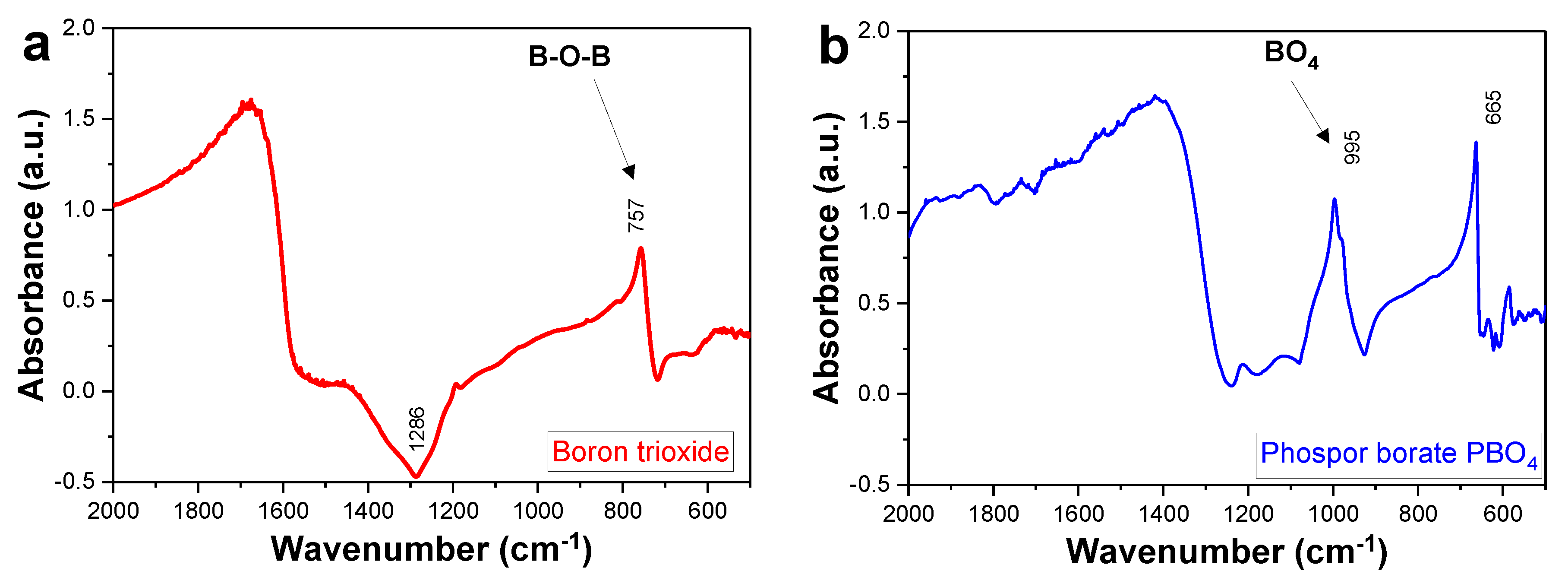
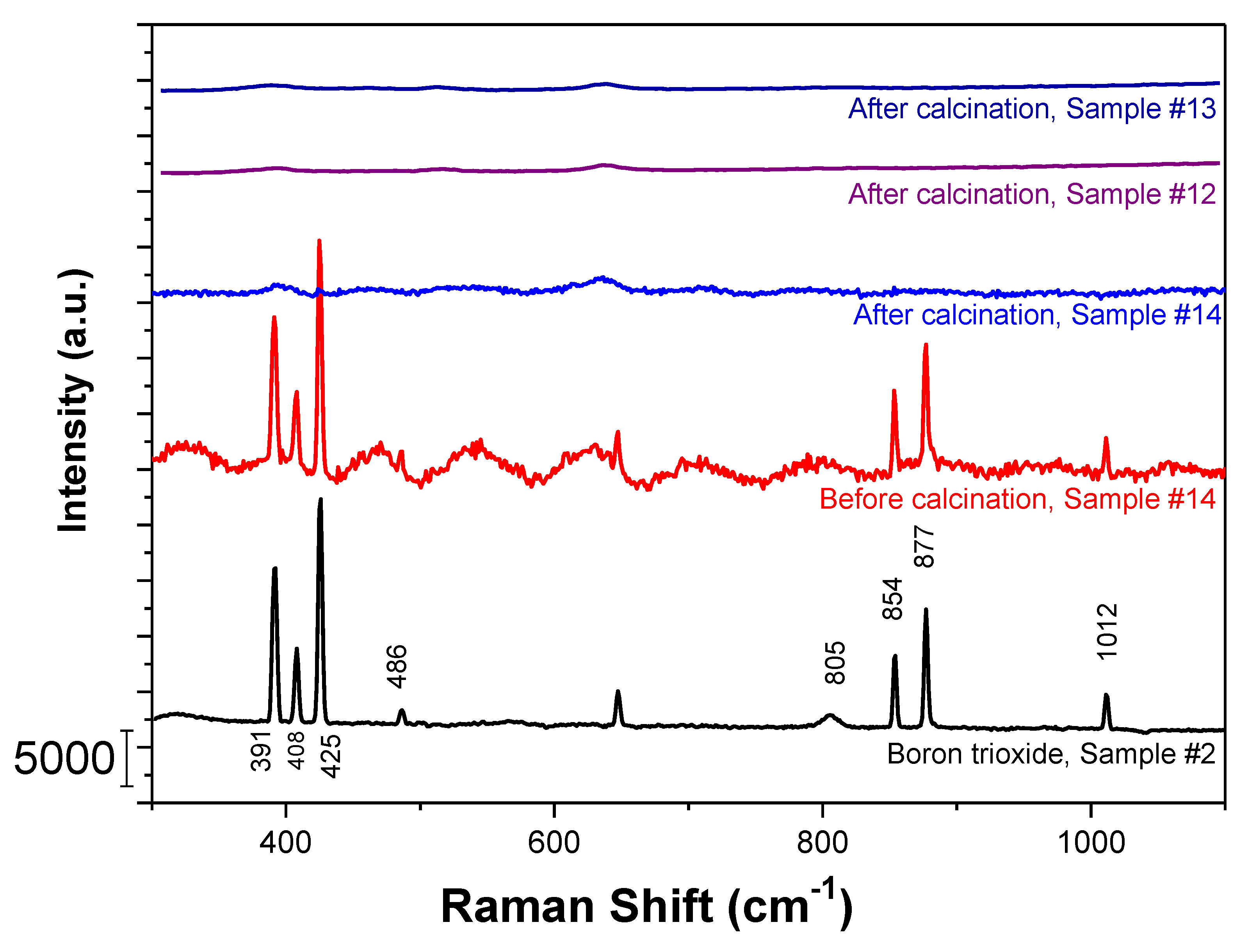
2.1. Boron Trioxide Characterization via FTIR
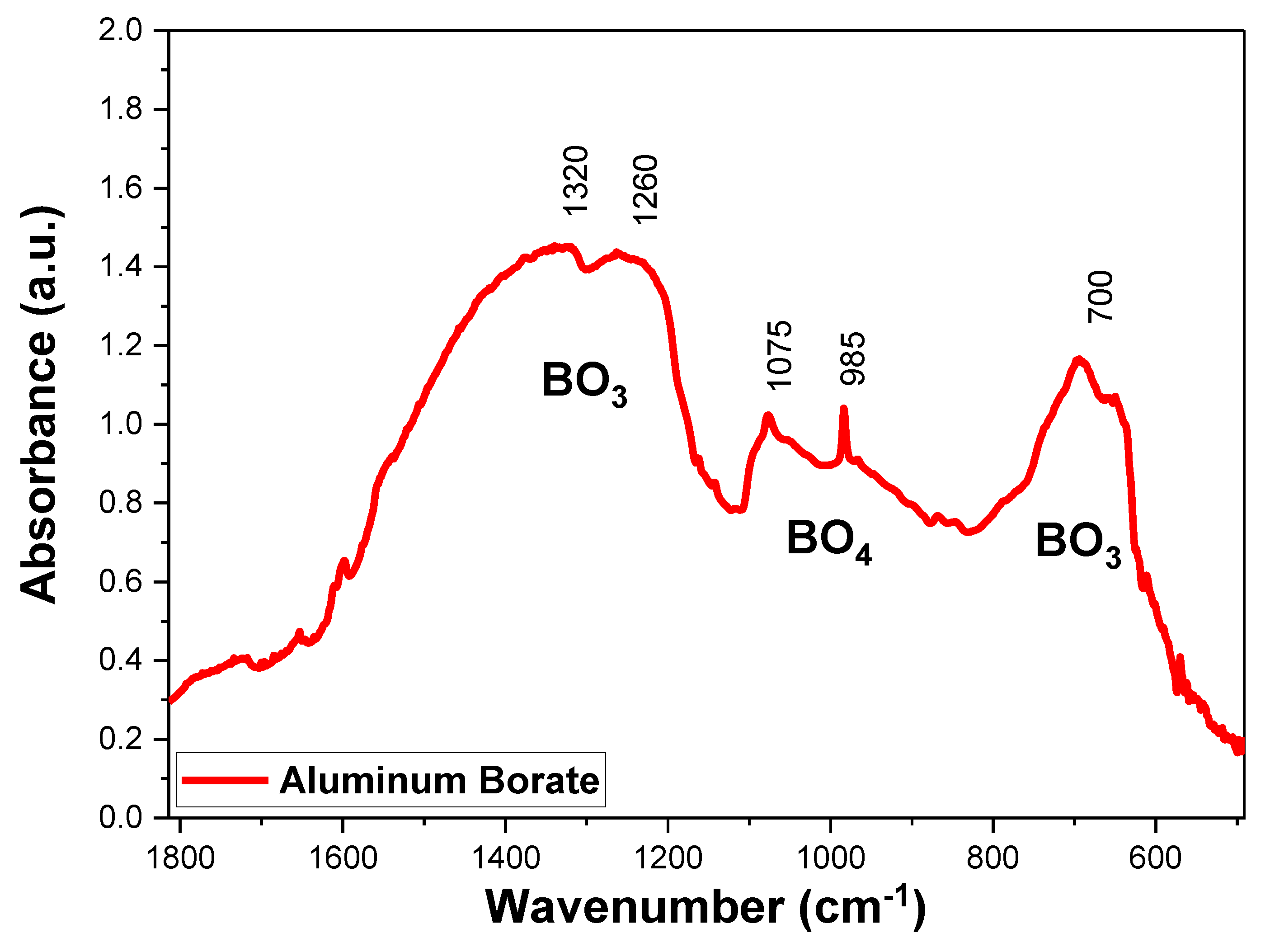
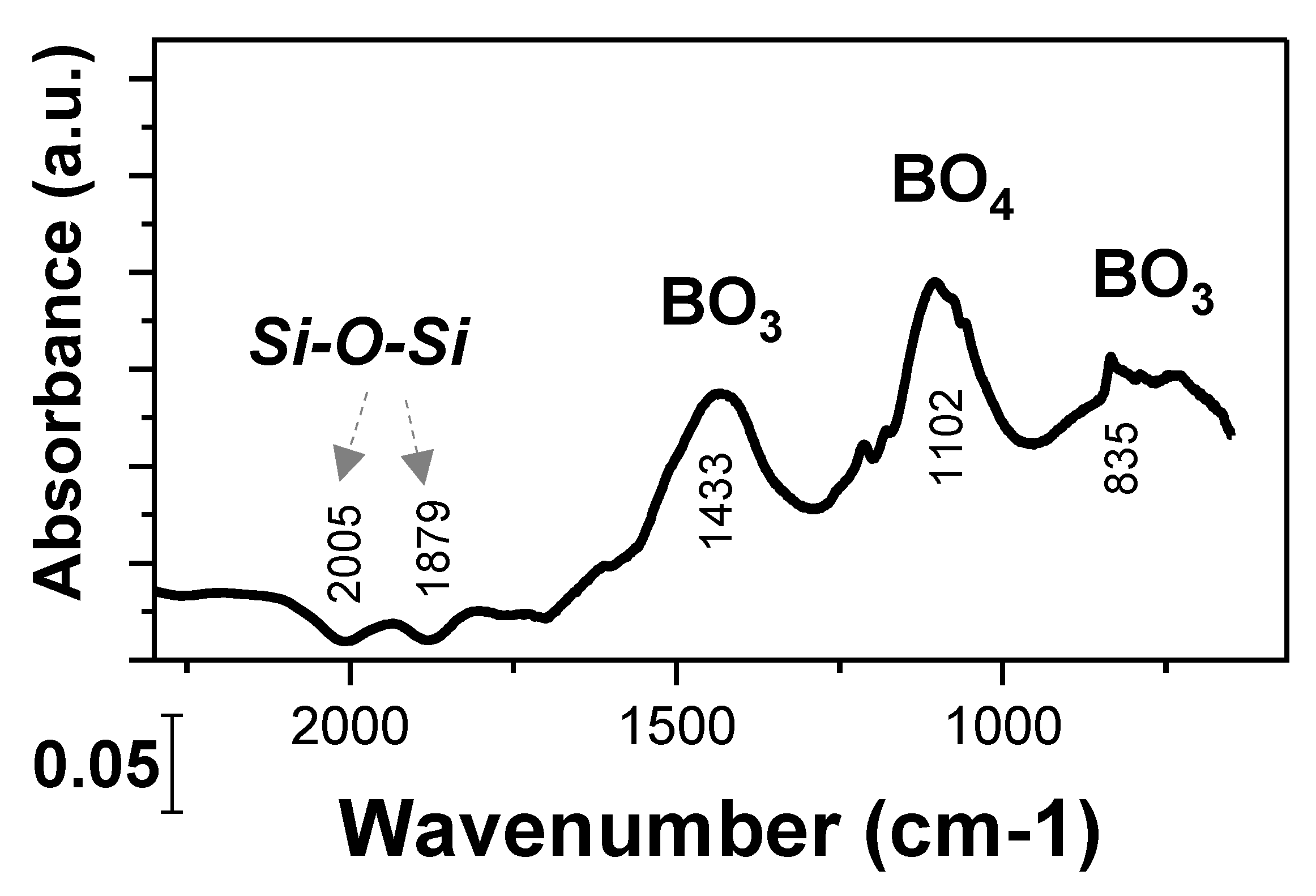
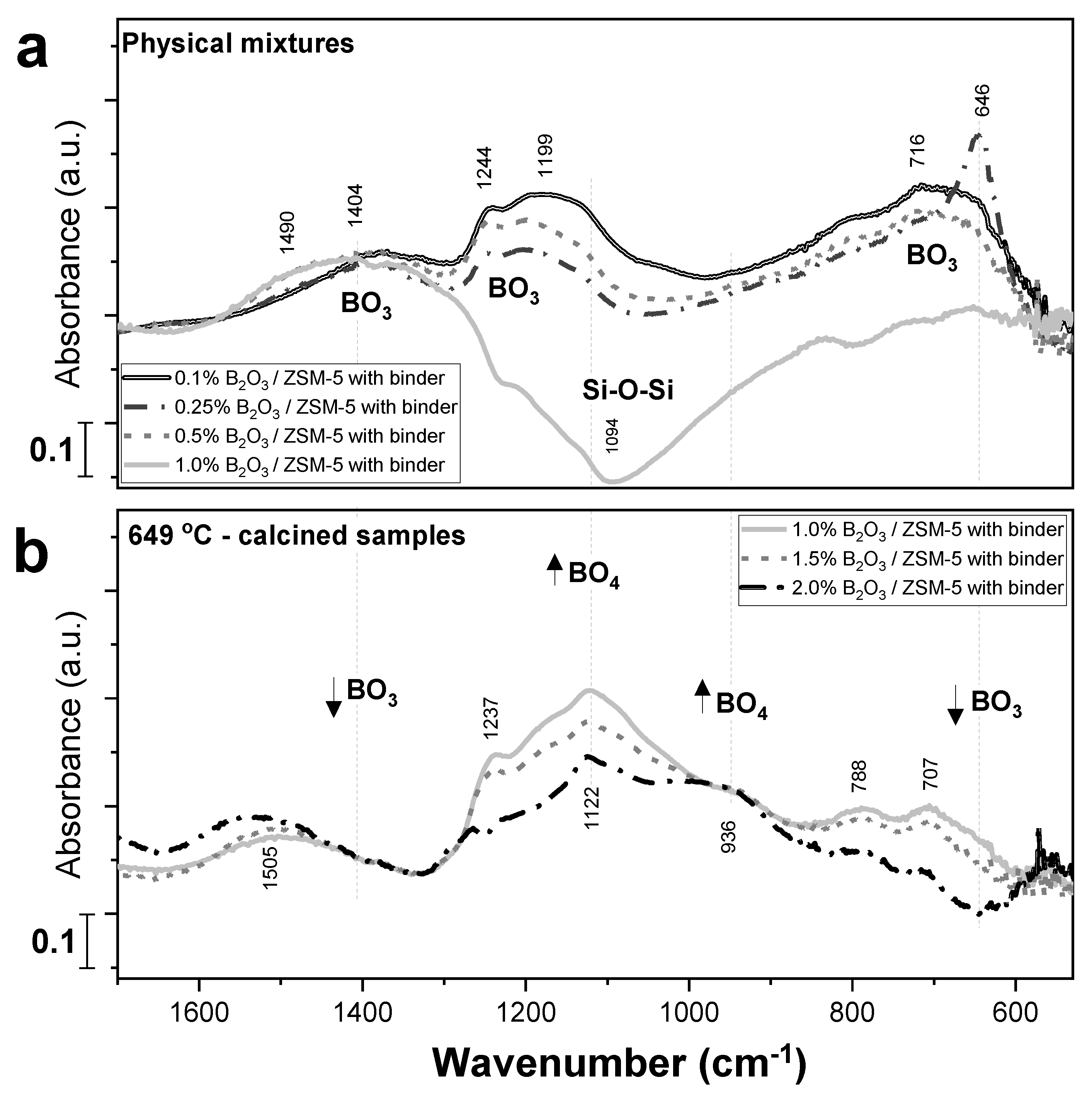
2.2. Borate Structure Characterization via FTIR
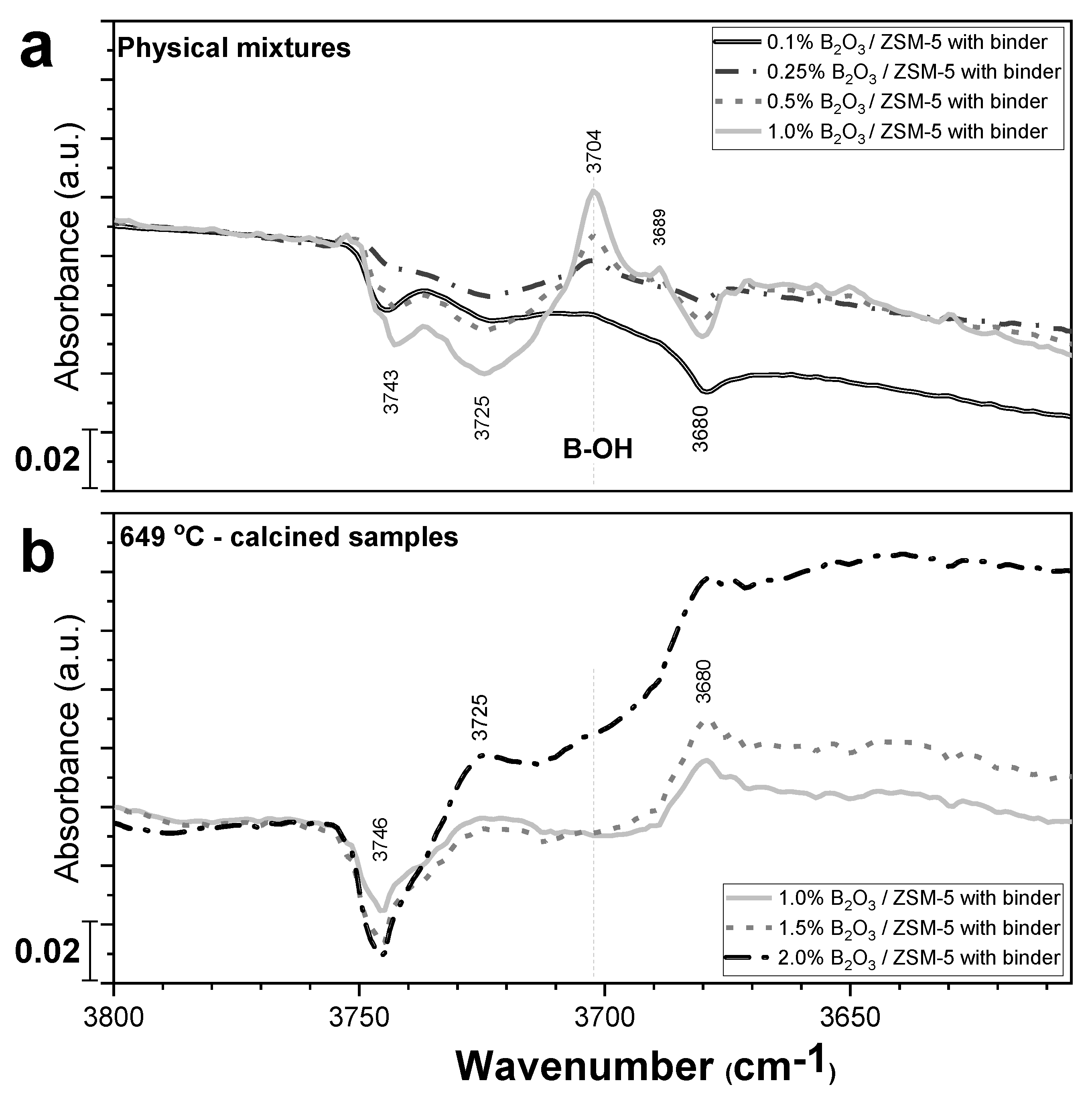
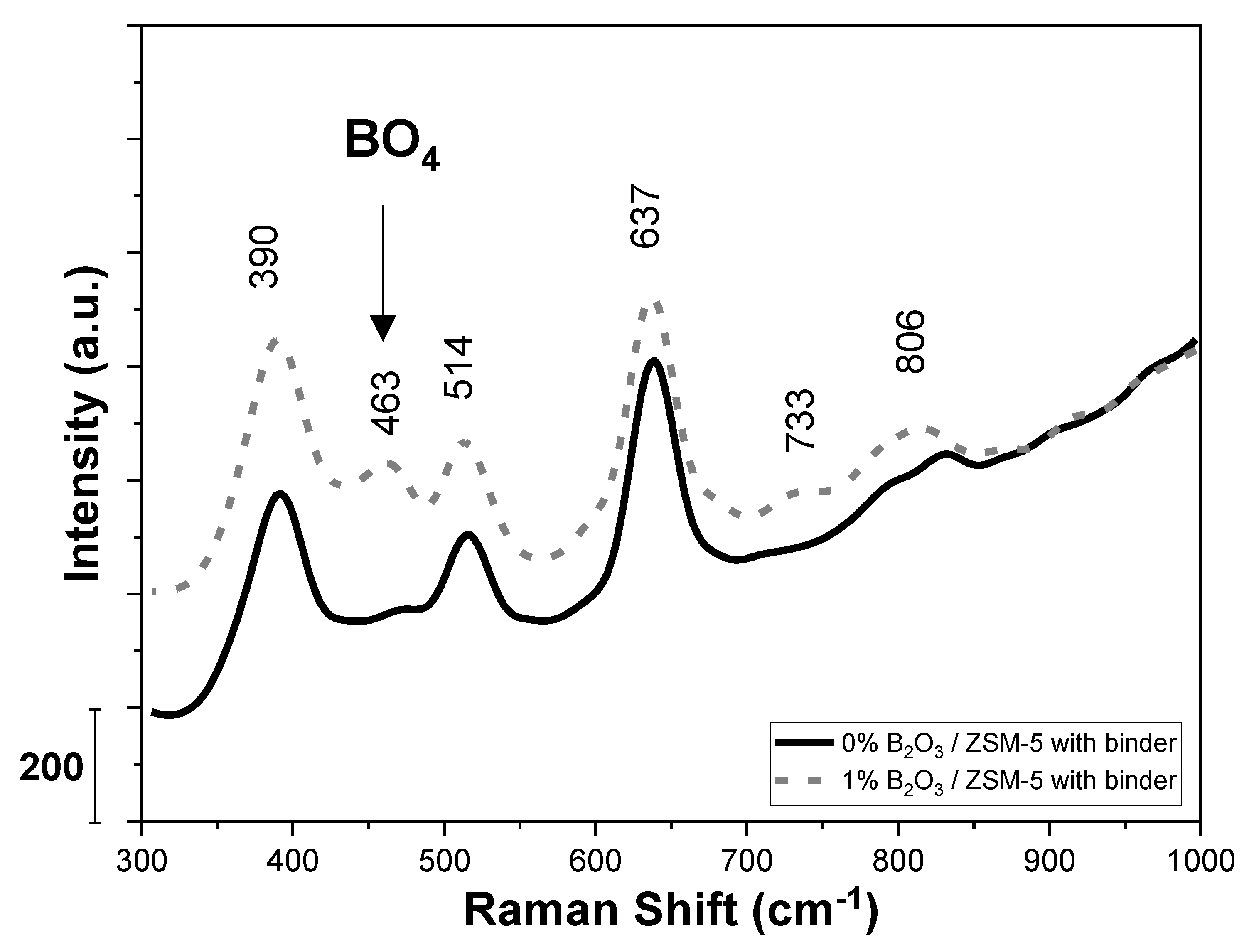
2.3. Characterization of Boron via Other Spectroscopic Techniques
3. Materials and Methods
3.1. Sample Information
3.2. Raman Spectroscopy
3.3. Infrared Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, S.; Shackleford, A.; McGuire, R., Jr.; Smith, G.M.; Yilmaz, B. Creative catalysis. Hydrocarb. Eng. 2015, 20, 46–52. [Google Scholar] [CrossRef] [Green Version]
- European Chemicals Agency. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/6449/1 (accessed on 15 March 2019).
- European Chemicals Agency. Available online: https://echa.europa.eu/documents/10162/6b35228c-45c4-4ed8-88b0-823fafb0d795 (accessed on 15 March 2019).
- Duydu, Y.; Başaran, N.; Ustundag, A.; Aydın, S.; Ündeger, Ü.; Ataman, O.Y.; Aydos, K.; Düker, Y.; Ickstadt, K.; Waltrup, B.S.; et al. Assessment of DNA integrity (COMET assay) in sperm cells of boron-exposed workers. Arch. Toxicol. 2012, 86, 27–35. [Google Scholar] [CrossRef]
- Duydu, Y.; Başaran, N.; Ustundag, A.; Aydın, S.; Ündeger, Ü.; Ataman, O.Y.; Aydos, K.; Düker, Y.; Ickstadt, K.; Waltrup, B.S.; et al. Reproductive toxicity parameters and biological monitoring in occupationally and environmentally boron-exposed persons in Bandırma, Turkey. Arch. Toxicol. 2011, 85, 589–600. [Google Scholar] [CrossRef]
- Glass Alliance Europe. Available online: https://www.glassallianceeurope.eu/images/cont/gae-reach-position-paper-on-boron-trioxide_1_file.pdf (accessed on 15 March 2019).
- IPC—Association Connecting Electronics Industries. Available online: http://www.ipc.org/4.0_Knowledge/4.1_Standards/Position-Statement-3-12d-TG_REACH-SVHC_E-Glass-B2O3.pdf (accessed on 15 March 2019).
- Intel. Available online: https://www.intel.com/content/dam/www/programmable/us/en/pdfs/support/reliability/environmental/reach/intel-dcl-product-content-reach-oct2017.pdf (accessed on 15 March 2019).
- Berlin, J. Analysis of boron with energy dispersive X-ray spectrometry. Imaging Microsc. 2011, 13, 19–21. [Google Scholar]
- Ong, C.W.; Huang, H.; Zheng, B.; Kwok, R.W.M.; Hui, Y.Y.; Lau, W.M. X-ray photoemission spectroscopy of nonmetallic materials: Electronic structures of boron and BxOy. J. Appl. Phys. 2004, 95, 3527. [Google Scholar] [CrossRef] [Green Version]
- Sen, S.; Xu, Z.; Stebbins, J.F. Temperature dependent structural changes in borate, borosilicate and boroaluminate liquids: High-resolution 11B, 29Si and 27Al NMR studies. J. Non-Cryst. Solids 1998, 226, 29–40. [Google Scholar] [CrossRef]
- Galeener, F.L.; Lucovsky, G.; Mikkelsen, J.C., Jr. Vibrational spectra and the structure of pure vitreous B2O3. Phys. Rev. B 1980, 22, 3983–3990. [Google Scholar] [CrossRef]
- Gautam, C.; Yadav, A.K.; Singh, A.K. A review on infrared spectroscopy of borate glasses with effects of different additives. ISRN Ceram. 2012, 2012, 17. [Google Scholar] [CrossRef] [Green Version]
- Kmeel, P.; Bukovee, P. Boron phosphate: Its synthesis, gradual crystallisation and characterisation of bulk properties. Acta Chim. Slov. 1999, 46, 161–171. [Google Scholar]
- Baykal, A.; Kizilyalli, M.; Toprak, M.; Kniep, R. Hydrothermal and microwave synthesis of boron phosphate, BPO4. Turk. J. Chem. 2001, 25, 425–432. [Google Scholar]
- Kamitsos, E.; Patsis, A.; Karakassides, M.; Chryssikos, G. Infrared reflectance spectra of lithium borate glasses. J. Non-Cryst. Solids 1990, 126, 52–67. [Google Scholar] [CrossRef]
- Stoch, L.; Środa, M. Infrared spectroscopy in the investigation of oxide glasses structure. J. Mol. Struct. 1999, 511–512, 77–84. [Google Scholar] [CrossRef]
- Ram, S. Infrared study of the dynamics of boroxol rings in the crystallization of BaFe12O19 microcrystals in borate glasses. Phys. Rev. B 1995, 51, 6280. [Google Scholar] [CrossRef] [PubMed]
- Pisarski, W.A.; Pisarska, J.; Ryba-Romanowski, W. Structural role of rare earth ions in lead borate glasses evidenced by infrared spectroscopy: BO3 <-> BO4 conversion. J. Mol. Struct. 2005, 744–747, 515–520. [Google Scholar] [CrossRef]
- Gielisse, P.J.M.; Foster, W.R. The system Al2O3–B2O3. Nature 1962, 195, 69. [Google Scholar] [CrossRef]
- Gutsev, G.L.; Weatherford, C.A.; Johnson, L.E.; Jena, P. Structure and properties of the aluminum borates Al(BO2)n and Al(BO2)n−, (n = 1–4). J. Comput. Chem. 2011, 33, 416–424. [Google Scholar] [CrossRef]
- Blay, V.; Louis, B.; Miravalles, R.; Yokoi, T.; Peccatiello, K.A.; Clough, M.; Yilmaz, B. Engineering zeolites for catalytic cracking to light olefins. ACS Catal. 2017, 7, 6542–6566. [Google Scholar] [CrossRef]
- Clough, M.; Pope, J.C.; Lin, L.T.X.; Komvokis, V.; Pan, S.S.; Yilmaz, B. Nanoporous materials forge a path forward to enable sustainable growth: Technology advancements in fluid catalytic cracking. Microporous Mesoporous Mater. 2017, 254, 45–58. [Google Scholar] [CrossRef]
- Rulmont, A.; Almou, M. Vibrational spectra of metaborates with infinite chain structure: LiBO2, CaB2O4, SrB2O4. Spectrochim. Acta Part A Mol. Spectrosc. 1989, 45, 603–610. [Google Scholar] [CrossRef]
- Umari, P.; Pasquarello, A. Fraction of boroxol rings in vitreous boron oxide from a first-principles analysis of raman and NMR spectra. Phys. Rev. Lett. 2005, 95, 137401. [Google Scholar] [CrossRef]
- Simon, G.; Hehlen, B.; Vacher, R.; Courtens, E. Courtens, Hyper-Raman scattering analysis of the vibrations in vitreous boron oxide. Phys. Rev. B 2007, 76, 054210. [Google Scholar] [CrossRef] [Green Version]
- Voronko, Y.K.; Sobol, A.A.; Shukshin, V.E. Structure of boron-oxygen groups in crystalline, molten, and glassy alkali-metal and alkaline-earth metaborates. Inorg. Mater. 2012, 48, 732–737. [Google Scholar] [CrossRef]
- Barrio, R.A.; Castillo-Alvarado, F.L.; Galeener, F.L. Structural and vibrational model for vitreous boron oxide. Phys. Rev. B 1991, 44, 7313. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.S.; Lin, L.T.X.; Komvokis, V.; Spann, A.; Clough, M.; Yilmaz, B. Nanomaterials fueling the world. In Nanomaterials for Sustainable Energy; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2015. [Google Scholar]
- Komvokis, V.; Tan, L.X.; Clough, M.; Yilmaz, B. Zeolites in fluid catalytic cracking (FCC). In Zeolites in Sustainable Chemistry; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
| Sample ID | Description | Sample Contents and Treatment |
|---|---|---|
| 1 | Phosphor borate, PBO4, Alfa Aesar | commercial material |
| 2 | Boron trioxide | commercial material |
| 3 | aluminum borate, Al18B4O33, BOC Sciences | commercial material |
| 4 | 0.5% B2O3/USY zeolite, (silico)aluminate matrices | Cyclic Propylene Steaming (CPS) deactivated |
| 5 | 0.1% B2O3/ZSM-5 with alumina binder | physical mixture |
| 6 | 0.25% B2O3/ZSM-5 with alumina binder | physical mixture |
| 7 | 0.5% B2O3/ZSM-5 with alumina binder | physical mixture |
| 8 | 1% B2O3/ZSM-5 with alumina binder | physical mixture |
| 9 | 1% B2O3/ZSM-5 with alumina binder | 649 °C-calcination |
| 10 | 1.5% B2O3/ZSM-5 with alumina binder | 649 °C-calcination |
| 11 | 2% B2O3/ZSM-5 with alumina binder | 649 °C-calcination |
| 12 | 0% B2O3 ZSM-5 with alumina binder | Fluidizable microspheres |
| 13 | 1% B2O3/ZSM-5 with alumina binder | Fluidizable microspheres |
| 14 | 2% B2O3/Silicoaluminate matrix | Fluidizable microspheres |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.C.; Gao, X.; Yilmaz, B. Development of FTIR Spectroscopy Methodology for Characterization of Boron Species in FCC Catalysts. Catalysts 2020, 10, 1327. https://doi.org/10.3390/catal10111327
Zhang CC, Gao X, Yilmaz B. Development of FTIR Spectroscopy Methodology for Characterization of Boron Species in FCC Catalysts. Catalysts. 2020; 10(11):1327. https://doi.org/10.3390/catal10111327
Chicago/Turabian StyleZhang, Claire Chunjuan, Xingtao Gao, and Bilge Yilmaz. 2020. "Development of FTIR Spectroscopy Methodology for Characterization of Boron Species in FCC Catalysts" Catalysts 10, no. 11: 1327. https://doi.org/10.3390/catal10111327
APA StyleZhang, C. C., Gao, X., & Yilmaz, B. (2020). Development of FTIR Spectroscopy Methodology for Characterization of Boron Species in FCC Catalysts. Catalysts, 10(11), 1327. https://doi.org/10.3390/catal10111327







