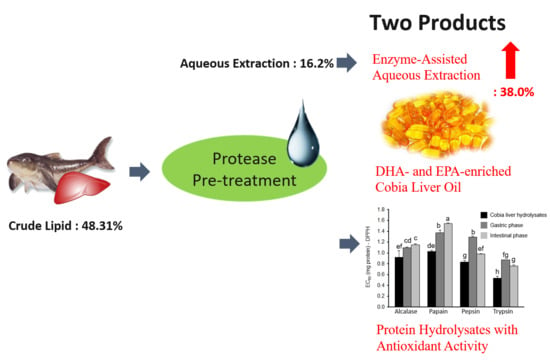
Enzyme-Assisted Aqueous Extraction of Cobia Liver Oil and Protein Hydrolysates with Antioxidant Activity
Abstract
1. Introduction
2. Results and Discussions
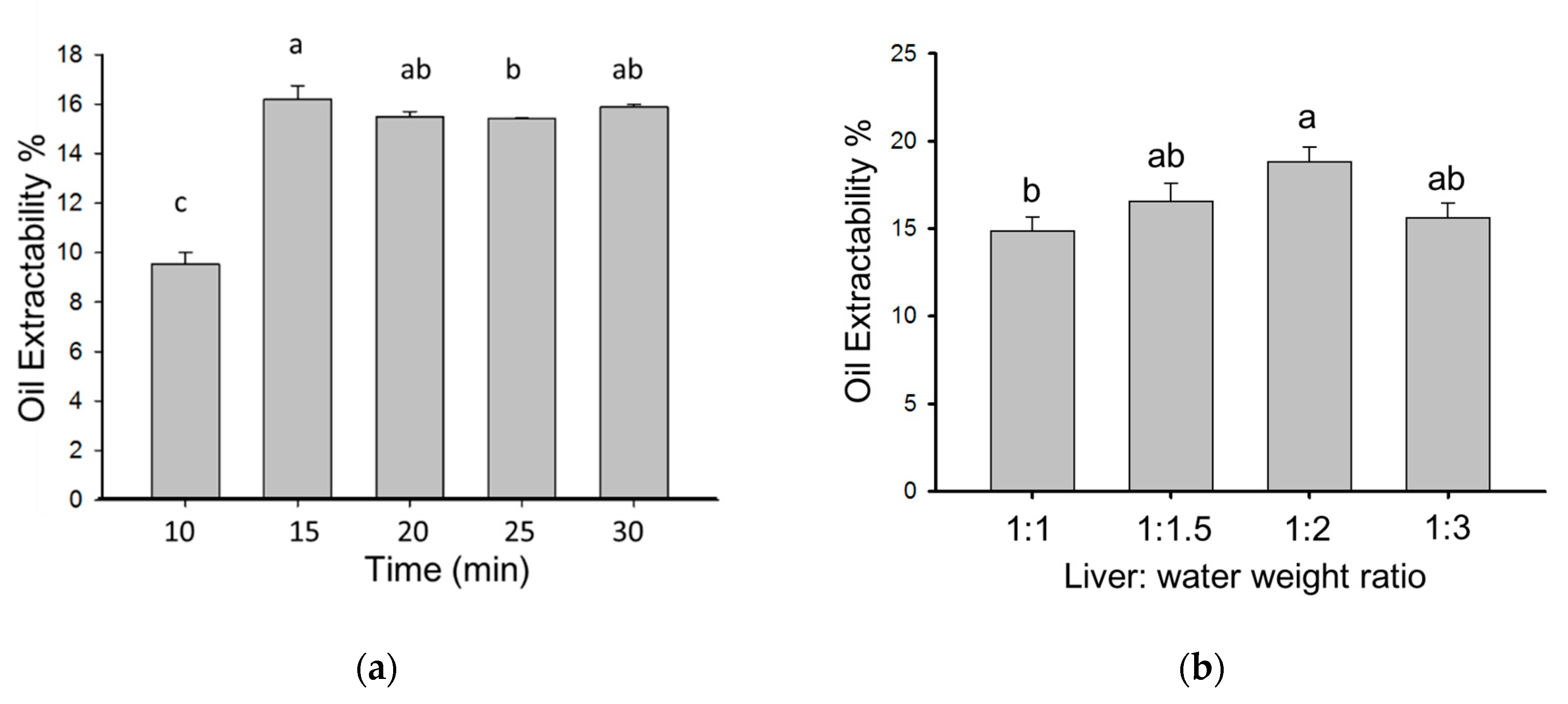
2.1. Optimal Conditions of Aqueous Extraction
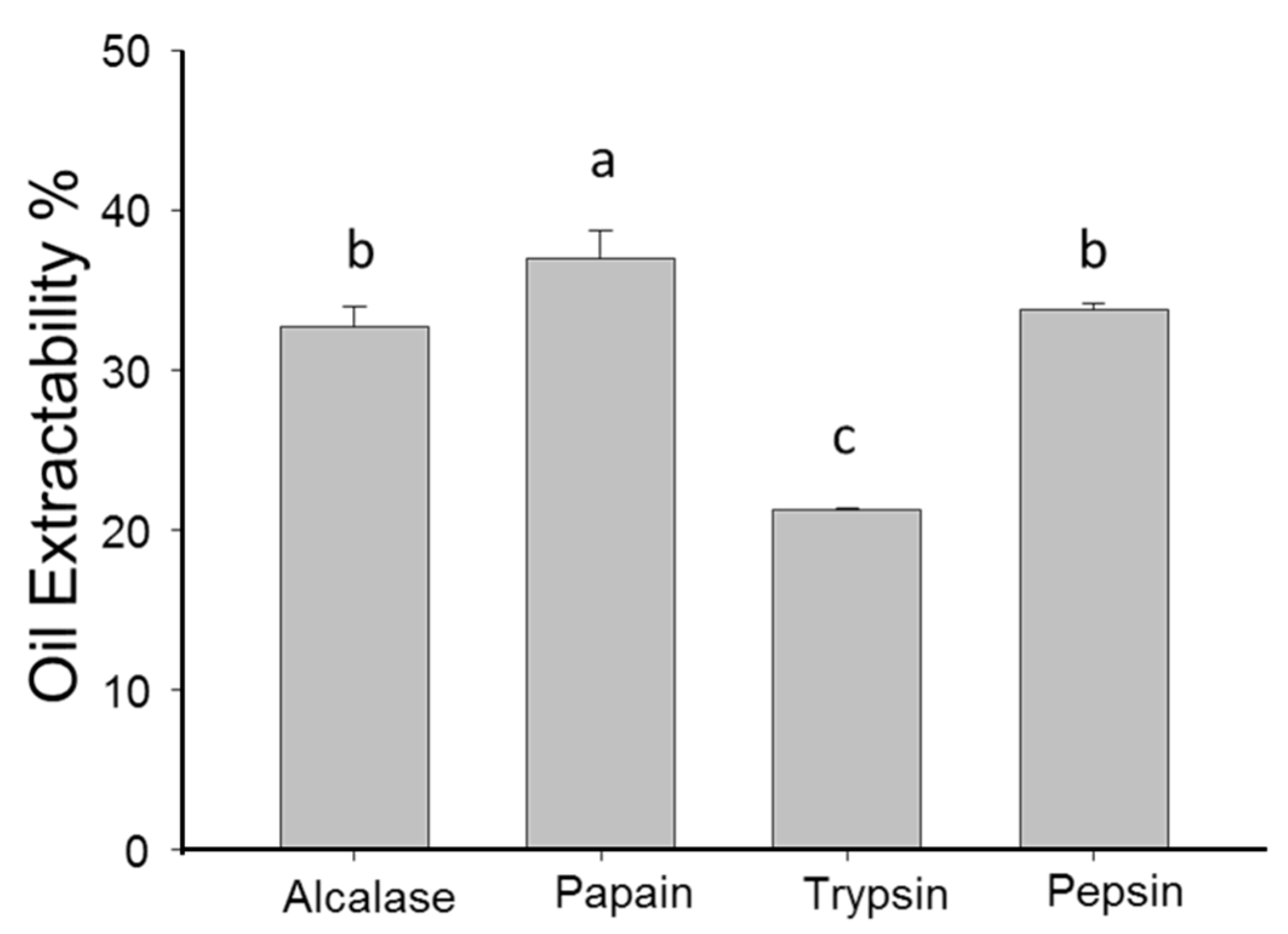
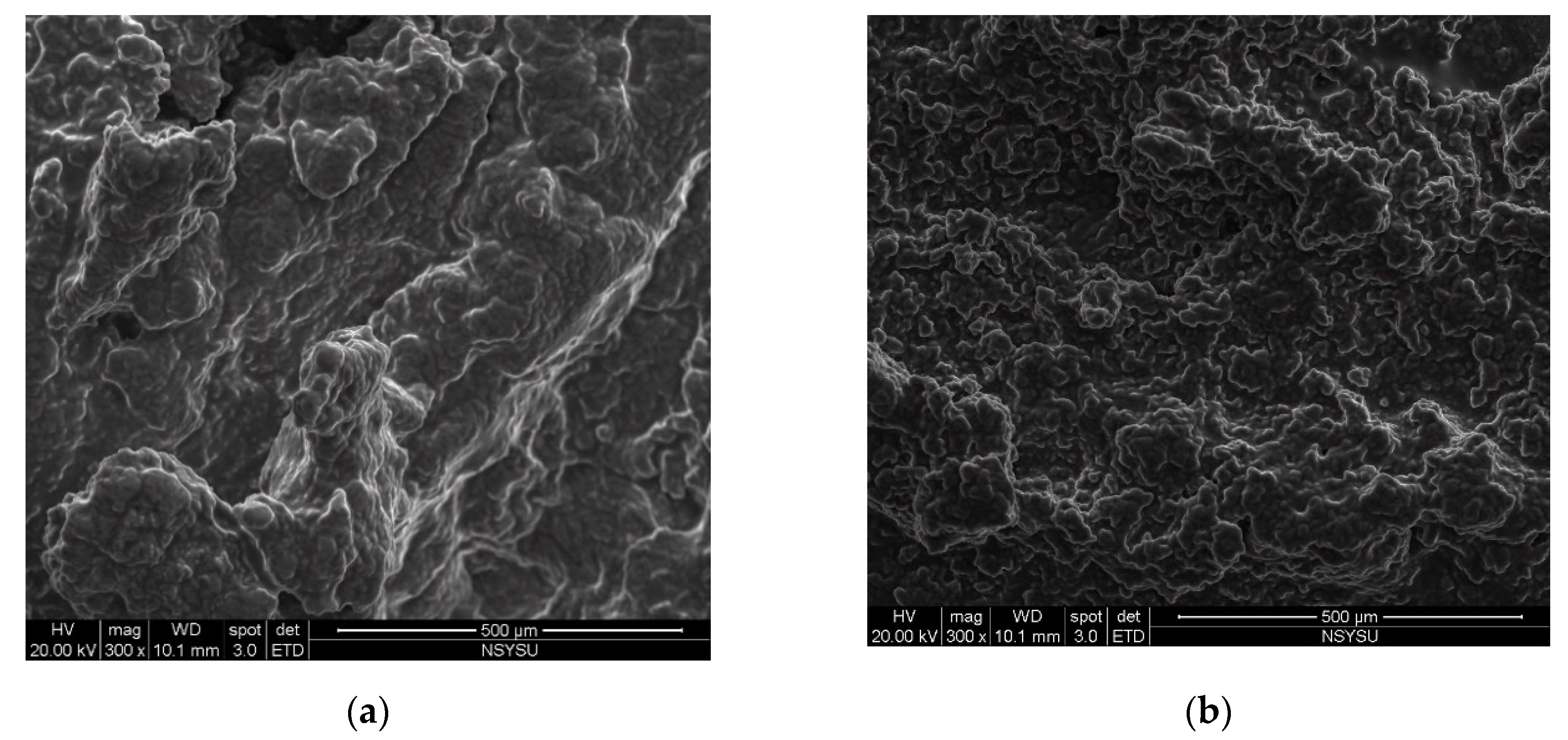
2.2. Effects of Different Proteases Pretreatment on Oil Extractability
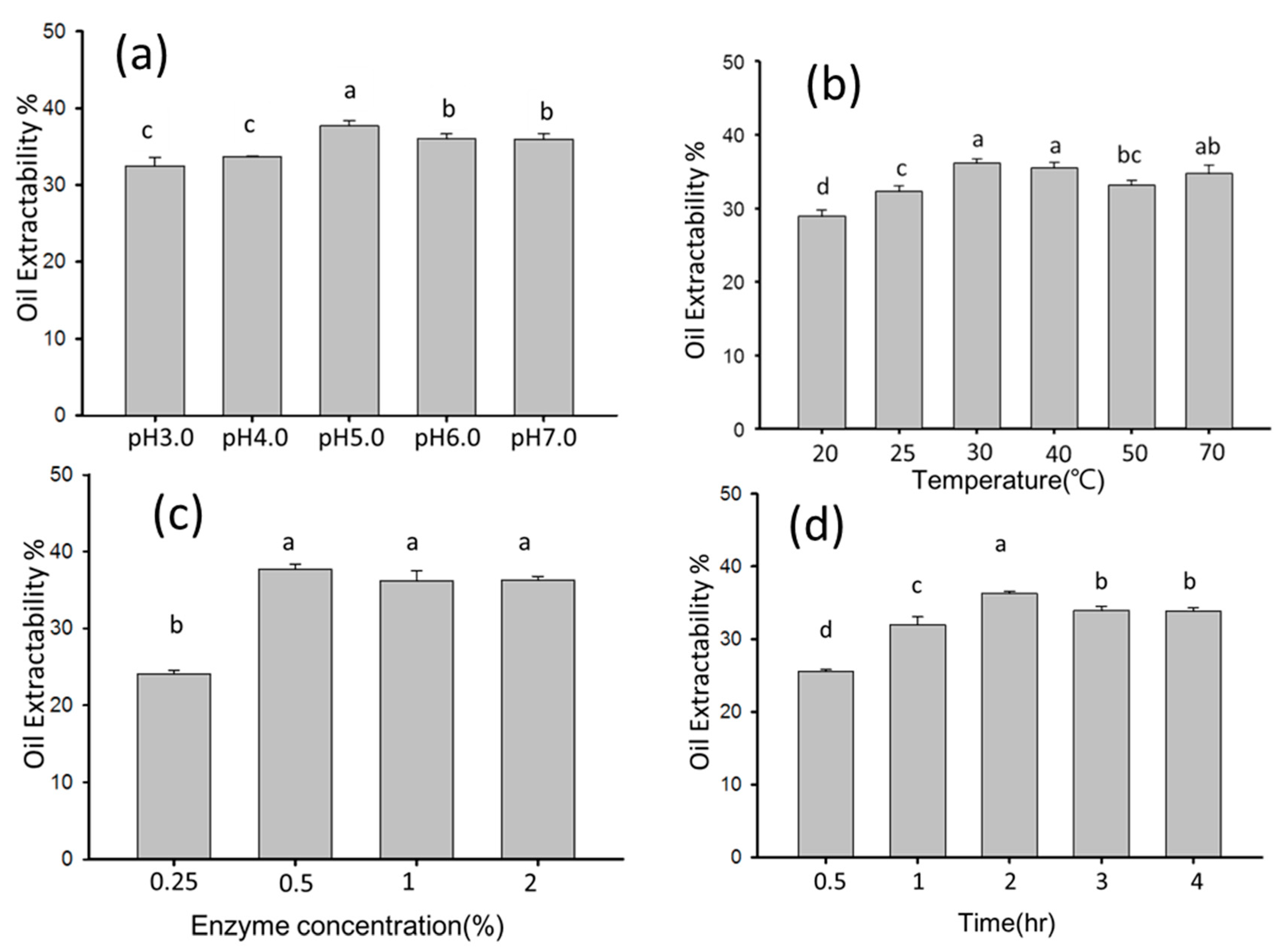
2.3. Optimal Conditions of Enzyme-Assisted Aqueous Extraction
2.4. Fatty Acid Composition
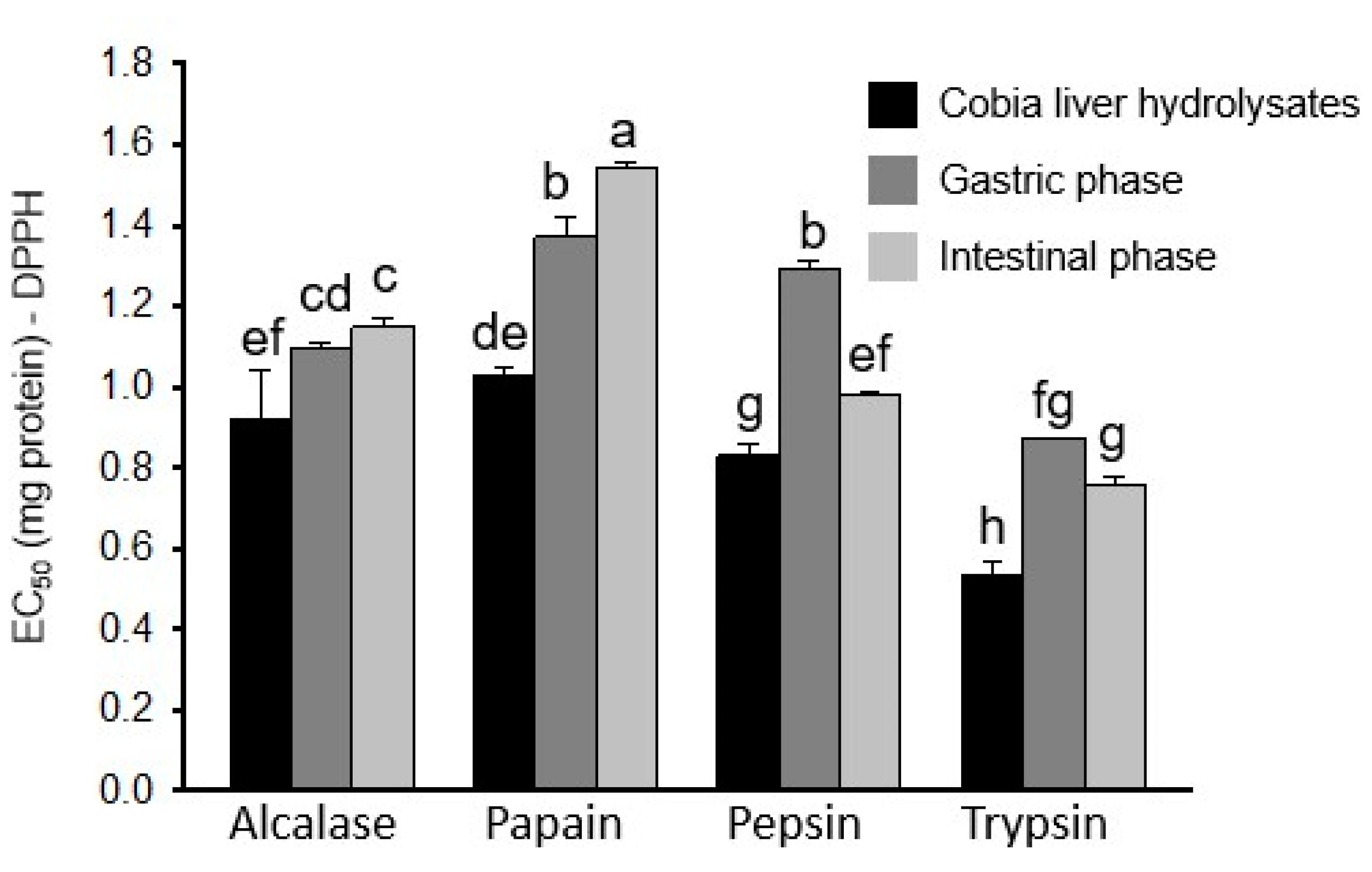
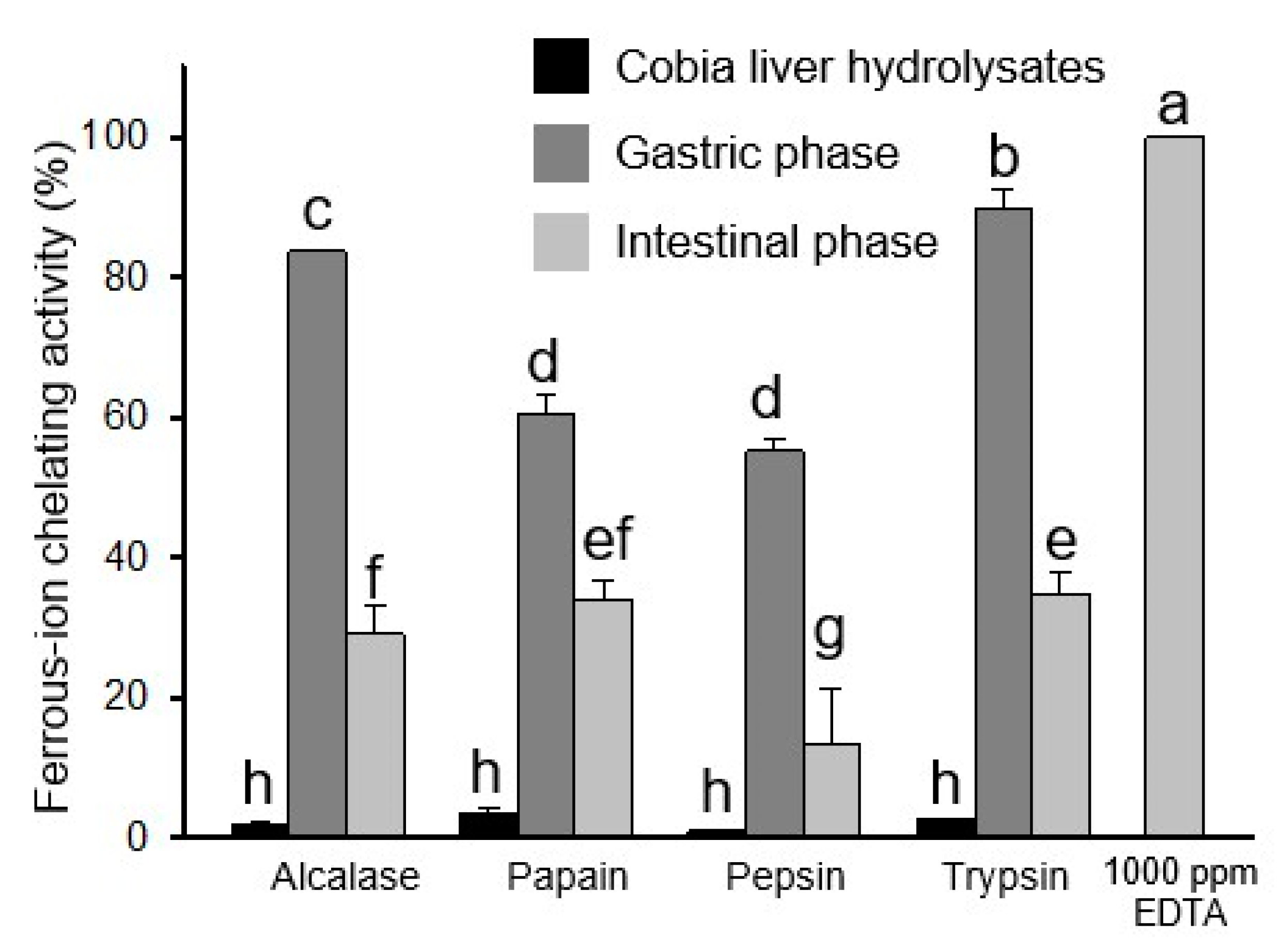
2.5. Antioxidant Activities of Protein Hydrolysates After In Vitro Simulated Gastro-Intestinal Digestion
3. Materials and Methods
3.1. Materials
3.2. Aqueous Extraction
3.3. Enzyme-Assisted Aqueous Extraction
3.4. In Vitro Simulated Gastro-Intestinal Digestion
3.5. Antioxidant Activity Analysis
3.6. Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, C.-T.; Miao, S.; Nan, F.-H.; Jung, S.-M. Study on regional production and economy of cobia Rachycentron canadum commercial cage culture. Aquac. Int. 2011, 19, 649–664. [Google Scholar] [CrossRef]
- Wu, Y.-S.; Chen, Y.-Y.; Ueng, P.-S.; Nan, F.-H. Effects of medicinal herbs “Plantago asiatica”, “Houttuynia cordata” and “Mentha haplocalyx” on non-specific immune responses of cobia (Rachycentron canadum). Fish Shellfish Immunol. 2016, 58, 406–414. [Google Scholar] [CrossRef] [PubMed]
- FAO. Cultured Aquatic Species Information Programme Rachycentron canadum (Linnaeus, 1766). Available online: http://www.fao.org/fishery/culturedspecies/Rachycentron_canadum/en (accessed on 22 October 2020).
- Kuo, C.H.; Liao, H.Z.; Wang, Y.H.; Wang, H.M.D.; Shieh, C.J.; Tseng, C.Y. Highly efficient extraction of EPA/DHA-enriched oil from cobia liver using homogenization plus sonication. Eur. J. Lipid Sci. Technol. 2017, 119, 1600466. [Google Scholar] [CrossRef]
- Šimat, V.; Vlahović, J.; Soldo, B.; Skroza, D.; Ljubenkov, I.; Generalić Mekinić, I. Production and refinement of omega-3 rich oils from processing by-products of farmed fish species. Foods 2019, 8, 125. [Google Scholar] [CrossRef] [PubMed]
- Manuelli, M.; Della Guardia, L.; Cena, H. Enriching diet with n-3 PUFAs to help prevent cardiovascular diseases in healthy adults: Results from clinical trials. Int. J. Mol. Sci. 2017, 18, 1552. [Google Scholar] [CrossRef]
- Méndez, L.; Dasilva, G.; Taltavull, N.; Romeu, M.; Medina, I. Marine lipids on cardiovascular diseases and other chronic diseases induced by diet: An insight provided by proteomics and lipidomics. Mar. Drugs 2017, 15, 258. [Google Scholar] [CrossRef]
- De Castro, G.S.; Deminice, R.; Simões-Ambrosio, L.M.C.; Calder, P.C.; Júnior, A.A.J.; Vannucchi, H. Dietary docosahexaenoic acid and eicosapentaenoic acid influence liver triacylglycerol and insulin resistance in rats fed a high-fructose diet. Mar. Drugs 2015, 13, 1864–1881. [Google Scholar] [CrossRef]
- Yang, M.; Gong, S.; Ye, S.Q.; Lyman, B.; Geng, L.; Chen, P.; Li, D.-Y. Non-alcoholic fatty liver disease in children: Focus on nutritional interventions. Nutrients 2014, 6, 4691–4705. [Google Scholar] [CrossRef]
- Duttaroy, A.K. Docosahexaenoic acid supports feto-placental growth and protects cardiovascular and cognitive function: A mini review. Eur. J. Lipid Sci. Technol. 2016, 118, 1439–1449. [Google Scholar] [CrossRef]
- Dawczynski, C.; Dittrich, M.; Neumann, T.; Goetze, K.; Welzel, A.; Oelzner, P.; Völker, S.; Schaible, A.; Troisi, F.; Thomas, L. Docosahexaenoic acid in the treatment of rheumatoid arthritis: A double-blind, placebo-controlled, randomized cross-over study with microalgae vs. sunflower oil. Clin. Nutr. 2018, 37, 494–504. [Google Scholar] [CrossRef]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Kay, A.; Mills-Lamptey, B. Effect of solvent extraction parameters on the recovery of oil from spent coffee grounds for biofuel production. Waste Biomass Valorization 2019, 10, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Soto, C.; Chamy, R.; Zuniga, M. Enzymatic hydrolysis and pressing conditions effect on borage oil extraction by cold pressing. Food Chem. 2007, 102, 834–840. [Google Scholar] [CrossRef]
- Samaram, S.; Mirhosseini, H.; Tan, C.P.; Ghazali, H.M. Ultrasound-assisted extraction (UAE) and solvent extraction of papaya seed oil: Yield, fatty acid composition and triacylglycerol profile. Molecules 2013, 18, 12474–12487. [Google Scholar] [CrossRef] [PubMed]
- Khoei, M.; Chekin, F. The ultrasound-assisted aqueous extraction of rice bran oil. Food Chem. 2016, 194, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chu, K.; Li, H.; Chen, L.; Zhang, Y.; Tang, X. Extraction of Lepidium apetalum seed oil using supercritical carbon dioxide and anti-oxidant activity of the extracted oil. Molecules 2011, 16, 10029–10045. [Google Scholar] [CrossRef]
- Hu, B.; Li, C.; Zhang, Z.; Zhao, Q.; Zhu, Y.; Su, Z.; Chen, Y. Microwave-assisted extraction of silkworm pupal oil and evaluation of its fatty acid composition, physicochemical properties and antioxidant activities. Food Chem. 2017, 231, 348–355. [Google Scholar] [CrossRef]
- Rhein-Knudsen, N.; Ale, M.T.; Meyer, A.S. Seaweed hydrocolloid production: An update on enzyme assisted extraction and modification technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Chen, B.-Y.; Liu, Y.-C.; Chen, J.-H.; Shieh, C.-J. Production of resveratrol by piceid deglycosylation using cellulase. Catalysts 2016, 6, 32. [Google Scholar] [CrossRef]
- Lin, J.-A.; Kuo, C.-H.; Chen, B.-Y.; Li, Y.; Liu, Y.-C.; Chen, J.-H.; Shieh, C.-J. A novel enzyme-assisted ultrasonic approach for highly efficient extraction of resveratrol from Polygonum cuspidatum. Ultrason. Sonochem. 2016, 32, 258–264. [Google Scholar] [CrossRef]
- Huo, S.; Wang, Z.; Cui, F.; Zou, B.; Zhao, P.; Yuan, Z. Enzyme-assisted extraction of oil from wet microalgae Scenedesmus sp. G4. Energies 2015, 8, 8165–8174. [Google Scholar] [CrossRef]
- Encalada, A.M.I.; Pérez, C.D.; Flores, S.K.; Rossetti, L.; Fissore, E.N.; Rojas, A.M. Antioxidant pectin enriched fractions obtained from discarded carrots (Daucus carota L.) by ultrasound-enzyme assisted extraction. Food Chem. 2019, 289, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Ihua, M.W.; Guihéneuf, F.; Mohammed, H.; Margassery, L.M.; Jackson, S.A.; Stengel, D.B.; Clarke, D.J.; Dobson, A.D. Microbial population changes in decaying Ascophyllum nodosum result in macroalgal-polysaccharide-degrading bacteria with potential applicability in enzyme-assisted extraction technologies. Mar. Drugs 2019, 17, 200. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Anwar, F. Aqueous enzymatic sesame oil and protein extraction. Food Chem. 2011, 125, 679–684. [Google Scholar] [CrossRef]
- Souza, T.S.P.; Dias, F.F.; Koblitz, M.G.B.; Bell, J.M.L.N.D.M. Aqueous and enzymatic extraction of oil and protein from almond cake: A comparative study. Processes 2019, 7, 472. [Google Scholar] [CrossRef]
- Han, L.; Zhang, S.; Qi, B.-K.; Li, H.; Xie, F.-Y.; Li, Y. Molecular distillation-induced deacidification of soybean oil isolated by enzyme-assisted aqueous extraction: Effect of distillation parameters. Appl. Sci. 2019, 9, 2123. [Google Scholar] [CrossRef]
- Deng, B.X.; Li, B.; Li, X.D.; Zaaboul, F.; Jiang, J.; Li, J.W.; Li, Q.; Cao, P.R.; Liu, Y.F. Using short-wave infrared radiation to improve aqueous enzymatic extraction of peanut oil: Evaluation of peanut cotyledon microstructure and oil quality. Eur. J. Lipid Sci. Technol. 2018, 120, 1700285. [Google Scholar] [CrossRef]
- Karamać, M.; Kosińska-Cagnazzo, A.; Kulczyk, A. Use of different proteases to obtain flaxseed protein hydrolysates with antioxidant activity. Int. J. Mol. Sci. 2016, 17, 1027. [Google Scholar] [CrossRef]
- Chow, C.J.; Yang, J.I. The effect of process variables for production of cobia (Rachycentron canadum) skin gelatin hydrolysates with antioxidant properties. J. Food Biochem. 2011, 35, 715–734. [Google Scholar] [CrossRef]
- Jia, J.; Zhou, Y.; Lu, J.; Chen, A.; Li, Y.; Zheng, G. Enzymatic hydrolysis of Alaska pollack (Theragra chalcogramma) skin and antioxidant activity of the resulting hydrolysate. J. Sci. Food Agric. 2010, 90, 635–640. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y.; Regenstein, J.; Su, X. In vitro and in vivo anti-oxidation and anti-fatigue effect of monkfish liver hydrolysate. Food Biosci. 2017, 18, 9–14. [Google Scholar] [CrossRef]
- Ahn, C.B.; Lee, K.H.; Je, J.Y. Enzymatic production of bioactive protein hydrolysates from tuna liver: Effects of enzymes and molecular weight on bioactivity. Int. J. Food Sci. Technol. 2010, 45, 562–568. [Google Scholar] [CrossRef]
- Je, J.-Y.; Lee, K.-H.; Lee, M.H.; Ahn, C.-B. Antioxidant and antihypertensive protein hydrolysates produced from tuna liver by enzymatic hydrolysis. Food Res. Int. 2009, 42, 1266–1272. [Google Scholar] [CrossRef]
- Fan, J.; He, J.; Zhuang, Y.; Sun, L. Purification and identification of antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) frame protein. Molecules 2012, 17, 12836–12850. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Li, T.; Yu, R.; Yan, C.; Ren, S.; Zhao, Y. Antioxidant activities of hydrolysates of Arca subcrenata prepared with three proteases. Mar. Drugs 2008, 6, 607–619. [Google Scholar] [CrossRef]
- Xu, S.; Shen, Y.; Li, Y. Antioxidant activities of sorghum kafirin alcalase hydrolysates and membrane/gel filtrated fractions. Antioxidants 2019, 8, 131. [Google Scholar] [CrossRef]
- Yang, J.-I.; Ho, H.-Y.; Chu, Y.-J.; Chow, C.-J. Characteristic and antioxidant activity of retorted gelatin hydrolysates from cobia (Rachycentron canadum) skin. Food Chem. 2008, 110, 128–136. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Attkan, A.K.; Panghal, A.; Singh, V.K.; Garg, M.K. Novel oil extraction technologies: Process conditions, quality parameters, and optimization. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3–20. [Google Scholar] [CrossRef]
- Zhu, B.W.; Qin, L.; Zhou, D.Y.; Wu, H.T.; Wu, J.; Yang, J.F.; Li, D.M.; Dong, X.P.; Murata, Y. Extraction of lipid from sea urchin (Strongylocentrotus nudus) gonad by enzyme-assisted aqueous and supercritical carbon dioxide methods. Eur. Food Res. Technol. 2010, 230, 737–743. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, S.; Liu, Y.; Wu, S.; Ran, J. Optimization of enzyme-assisted extraction of the Lycium barbarum polysaccharides using response surface methodology. Carbohydr. Polym. 2011, 86, 1089–1092. [Google Scholar] [CrossRef]
- Wu, S.; Gong, G.; Wang, Y.; Li, F.; Jia, S.; Qin, F.; Ren, H.; Liu, Y. Response surface optimization of enzyme-assisted extraction polysaccharides from Dictyophora indusiata. Int. J. Biol. Macromol. 2013, 61, 63–68. [Google Scholar] [CrossRef]
- Rajalakshmi, N.; Sundaram, P.V. Stability of native and covalently modified papain. Protein Eng. Des. Sel. 2015, 8, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yuan, Q.; Zhang, Y.; Li, J.; Zhu, X.; Zhao, L.; Wen, J.; Liu, J.; Zhao, L.; Zhao, J. Enzyme-assisted extraction optimization, characterization and antioxidant activity of polysaccharides from sea cucumber Phyllophorus proteus. Molecules 2018, 23, 590. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, F.; Li, T.; Liu, D.; Xu, Y.; Yang, Y. Enzyme assisted extraction, purification and structure analysis of the polysaccharides from naked pumpkin seeds. Appl. Sci. 2018, 8, 1866. [Google Scholar] [CrossRef]
- Sharma, A.; Khare, S.K.; Gupta, M.N. Enzyme-assisted aqueous extraction of rice bran oil. J. Am. Oil Chem. Soc. 2001, 78, 949–951. [Google Scholar] [CrossRef]
- Huang, Y.; Yin, Z.; Guo, J.; Wang, F.; Zhang, J. Oil extraction and evaluation from yellow horn using a microwave-assisted aqueous saline process. Molecules 2019, 24, 2598. [Google Scholar] [CrossRef]
- Sarker, M.Z.I.; Selamat, J.; Habib, A.S.M.; Ferdosh, S.; Akanda, M.J.H.; Jaffri, J.M. Optimization of supercritical CO2 extraction of fish oil from viscera of African catfish (Clarias gariepinus). Int. J. Mol. Sci. 2012, 13, 11312–11322. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, C.-Y.; Chen, J.-W.; Wang, H.-M.D.; Shieh, C.-J. Concentration of docosahexaenoic and eicosapentaenoic acid from cobia liver oil by acetone fractionation of fatty acid salts. Appl. Biochem. Biotechnol. 2020, 192, 517–529. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Huang, C.-Y.; Lee, C.-L.; Kuo, W.-C.; Hsieh, S.-L.; Shieh, C.-J. Synthesis of DHA/EPA ethyl esters via lipase-catalyzed acidolysis using Novozym® 435: A kinetic study. Catalysts 2020, 10, 565. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Q.; Qu, Y.; Ye, F. Antioxidant activity and anti-exercise-fatigue effect of highly denatured soybean meal hydrolysate prepared using neutrase. J. Food Sci. Technol. 2015, 52, 1982–1992. [Google Scholar] [CrossRef]
- Rajapakse, N.; Mendis, E.; Jung, W.-K.; Je, J.-Y.; Kim, S.-K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem. 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Wattanasiritham, L.; Theerakulkait, C.; Wickramasekara, S.; Maier, C.S.; Stevens, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhao, M.; Shi, J.; Wang, J.; Jiang, Y.; Cui, C.; Kakuda, Y.; Xue, S.J. Purification and identification of antioxidant peptides from grass carp muscle hydrolysates by consecutive chromatography and electrospray ionization-mass spectrometry. Food Chem. 2008, 108, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Samaranayaka, A.G.; Li-Chan, E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regenstein, J.M.; Ren, J. Changes in the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chem. 2010, 120, 810–816. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Cheng, S.; Han, F. Aqueous enzymatic extraction of oil and protein hydrolysates from peanut. Food Sci. Technol. Res. 2008, 14, 533. [Google Scholar] [CrossRef]
- Hathwar, S.C.; Bijinu, B.; Rai, A.K.; Narayan, B. Simultaneous recovery of lipids and proteins by enzymatic hydrolysis of fish industry waste using different commercial proteases. Appl. Biochem. Biotechnol. 2011, 164, 115–124. [Google Scholar] [CrossRef]
- Chi, C.-F.; Hu, F.-Y.; Wang, B.; Li, Z.-R.; Luo, H.-Y. Influence of amino acid compositions and peptide profiles on antioxidant capacities of two protein hydrolysates from skipjack tuna (Katsuwonus pelamis) dark muscle. Mar. Drugs 2015, 13, 2580–2601. [Google Scholar] [CrossRef]
- Farvin, K.S.; Andersen, L.L.; Nielsen, H.H.; Jacobsen, C.; Jakobsen, G.; Johansson, I.; Jessen, F. Antioxidant activity of Cod (Gadus morhua) protein hydrolysates: In vitro assays and evaluation in 5% fish oil-in-water emulsion. Food Chem. 2014, 149, 326–334. [Google Scholar] [CrossRef]
- Wu, H.-C.; Chen, H.-M.; Shiau, C.-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, S.; Li, L.; He, J.; Cheng, W.; Ren, G. In vitro antioxidant activity of peptides from simulated gastro-intestinal digestion products of Cyprinus carpio haematopterus scale gelatin. Foods 2019, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Kuo, C.-H.; Lee, C.-H. Antibacterial and antioxidant capacities and attenuation of lipid accumulation in 3T3-L1 adipocytes by low-molecular-weight fucoidans prepared from compressional-puffing-pretreated sargassum crassifolium. Mar. Drugs 2018, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.-R.; Yu, H.-C.; Huang, C.-Y.; Kuo, J.-M.; Chang, C.; Shieh, C.-J.; Kuo, C.-H. Bioprocessed Production of Resveratrol-Enriched Rice Wine: Simultaneous Rice Wine Fermentation, Extraction, and Transformation of Piceid to Resveratrol from Polygonum cuspidatum Roots. Foods 2019, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Gnanou, J.; Srinivas, S.; Kurpad, A. Automated derivatization with o-phthalaldehyde for the estimation of amino acids in plasma using reversed-phase high performance liquid chromatography. Indian J. Biochem. Biophys. 2004, 41, 322–325. [Google Scholar] [PubMed]
- Sultana, H.; Onodera, R.; Or-Rashid, M.M.; Wadud, S. Convenient method for the determination of arginine and its related compounds in rumen fluid by reversed-phase high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 2001, 755, 321–329. [Google Scholar] [CrossRef]
| Fatty Acid (%) | Alcalase | Papain | Pepsin | Trypsin | Aqueous | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | 1.11 | ± | 0.13 | 1.03 | ± | 0.04 | 1.07 | ± | 0.08 | 1.00 | ± | 0.05 | 1.57 | ± | 0.22 |
| C16:0 | 29.12 | ± | 0.30 | 28.34 | ± | 0.18 | 29.00 | ± | 0.13 | 28.63 | ± | 0.26 | 27.96 | ± | 1.36 |
| C16:1 | 4.46 | ± | 0.09 | 4.35 | ± | 0.04 | 4.49 | ± | 0.20 | 4.31 | ± | 0.00 | 6.69 | ± | 0.19 |
| C18:0 | 11.38 | ± | 0.12 | 11.17 | ± | 0.01 | 11.25 | ± | 0.43 | 11.43 | ± | 0.15 | 8.05 | ± | 0.27 |
| C18:1n9 | 33.51 | ± | 0.49 | 33.93 | ± | 0.01 | 33.46 | ± | 0.42 | 33.76 | ± | 0.08 | 32.92 | ± | 1.04 |
| C18:2n6 | 0.56 | ± | 0.02 | 0.56 | ± | 0.02 | 0.54 | ± | 0.01 | 0.55 | ± | 0.03 | 0.84 | ± | 0.15 |
| C18:3n3 | 0.57 | ± | 0.04 | 0.58 | ± | 0.00 | 0.57 | ± | 0.02 | 0.57 | ± | 0.02 | 0.68 | ± | 0.12 |
| C20:0 | 0.33 | ± | 0.02 | 0.33 | ± | 0.01 | 0.34 | ± | 0.00 | 0.34 | ± | 0.01 | 0.24 | ± | 0.02 |
| C20:1 | 1.23 | ± | 0.06 | 1.25 | ± | 0.01 | 1.26 | ± | 0.01 | 1.25 | ± | 0.04 | 1.22 | ± | 0.06 |
| C22:0 | 0.18 | ± | 0.01 | 0.15 | ± | 0.03 | 0.16 | ± | 0.00 | 0.18 | ± | 0.04 | 0.11 | ± | 0.03 |
| C20:5n3 (EPA) | 3.80 | ± | 0.08 | 3.93 | ± | 0.03 | 3.81 | ± | 0.07 | 3.86 | ± | 0.15 | 4.15 | ± | 0.65 |
| C22:6n3 (DHA) | 13.75 | ± | 0.20 | 14.37 | ± | 0.21 | 14.05 | ± | 0.18 | 14.13 | ± | 0.29 | 15.59 | ± | 1.52 |
| SFA 2 | 42.12 | ± | 0.43 1a | 41.01 | ± | 0.21 a | 41.82 | ± | 0.33 a | 41.58 | ± | 0.38 a | 37.92 | ± | 1.44 b |
| MUFA | 39.20 | ± | 0.47 b | 39.54 | ± | 0.02 b | 39.21 | ± | 0.50 b | 39.32 | ± | 0.07 b | 40.82 | ± | 1.17 a |
| PUFA | 18.68 | ± | 0.29 a,b | 19.45 | ± | 0.23 a,b | 18.97 | ± | 0.28 a,b | 19.10 | ± | 0.45 a,b | 21.26 | ± | 2.42 a |
| EPA + DHA | 17.55 | ± | 0.24 a,b | 18.30 | ± | 0.24 a,b | 17.86 | ± | 0.25 a,b | 17.99 | ± | 0.41 a,b | 19.73 | ± | 2.16 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-H.; Kuo, C.-H.; Lee, C.-L.; Kuo, W.-C.; Tsai, M.-L.; Sun, P.-P. Enzyme-Assisted Aqueous Extraction of Cobia Liver Oil and Protein Hydrolysates with Antioxidant Activity. Catalysts 2020, 10, 1323. https://doi.org/10.3390/catal10111323
Wang Y-H, Kuo C-H, Lee C-L, Kuo W-C, Tsai M-L, Sun P-P. Enzyme-Assisted Aqueous Extraction of Cobia Liver Oil and Protein Hydrolysates with Antioxidant Activity. Catalysts. 2020; 10(11):1323. https://doi.org/10.3390/catal10111323
Chicago/Turabian StyleWang, Yu-Hsiang, Chia-Hung Kuo, Chien-Liang Lee, Wen-Cheng Kuo, Mei-Ling Tsai, and Pei-Pei Sun. 2020. "Enzyme-Assisted Aqueous Extraction of Cobia Liver Oil and Protein Hydrolysates with Antioxidant Activity" Catalysts 10, no. 11: 1323. https://doi.org/10.3390/catal10111323
APA StyleWang, Y.-H., Kuo, C.-H., Lee, C.-L., Kuo, W.-C., Tsai, M.-L., & Sun, P.-P. (2020). Enzyme-Assisted Aqueous Extraction of Cobia Liver Oil and Protein Hydrolysates with Antioxidant Activity. Catalysts, 10(11), 1323. https://doi.org/10.3390/catal10111323