Fe-Exchanged Small-Pore Zeolites as Ammonia Selective Catalytic Reduction (NH3-SCR) Catalysts
Abstract
1. Introduction
2. Synthesis of Small-Pore Fe/Zeolites
3. Fe Speciation of Small-Pore Fe/Zeolites
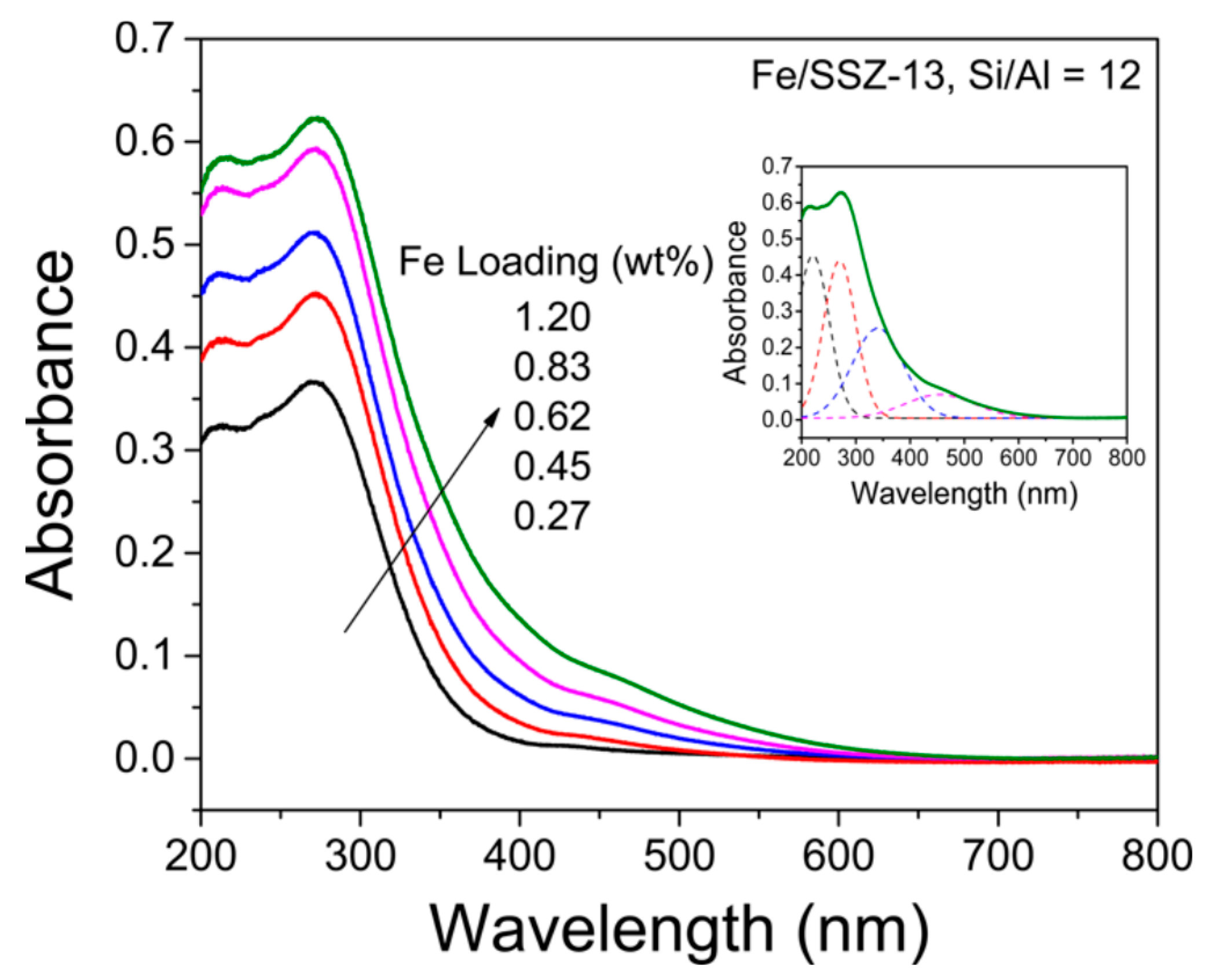
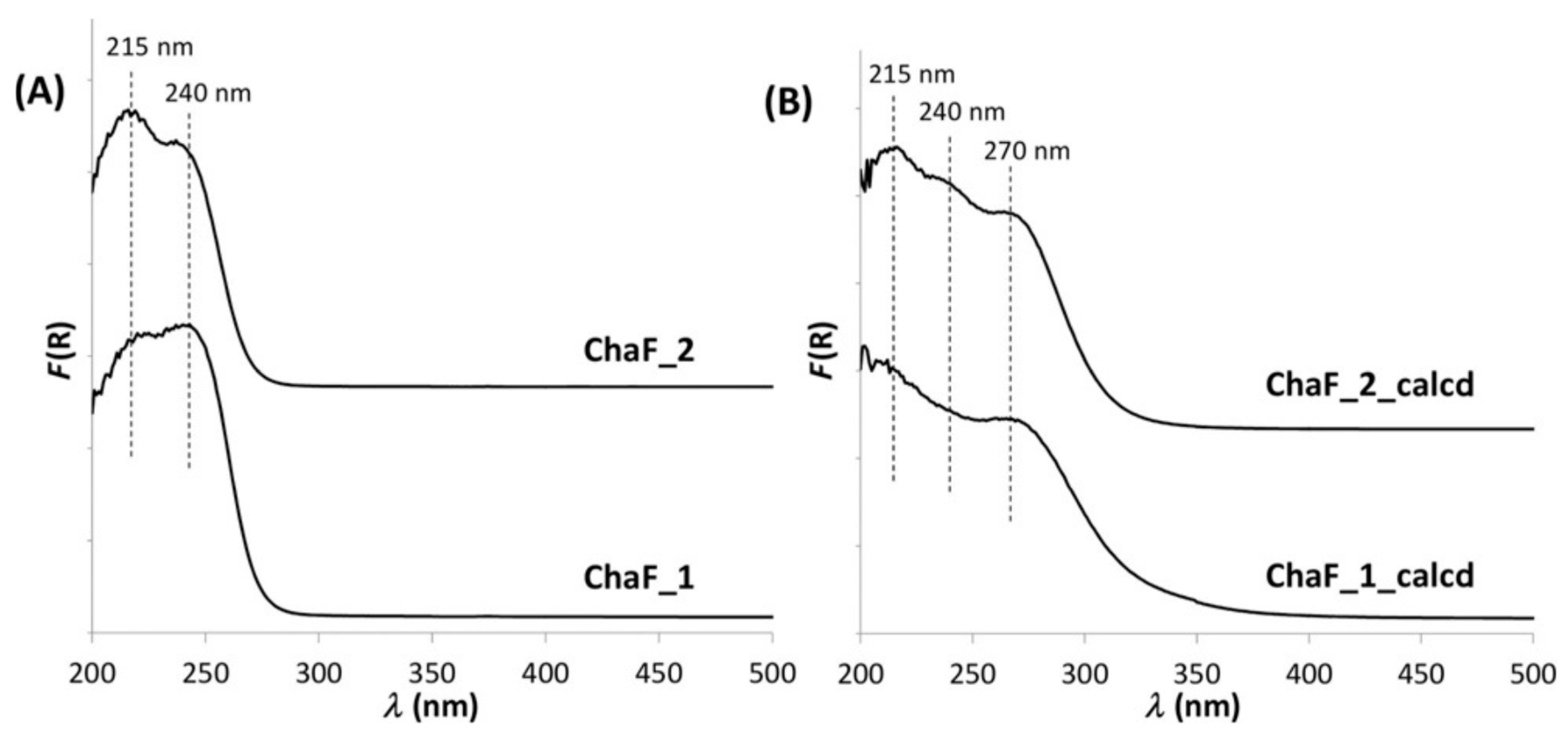
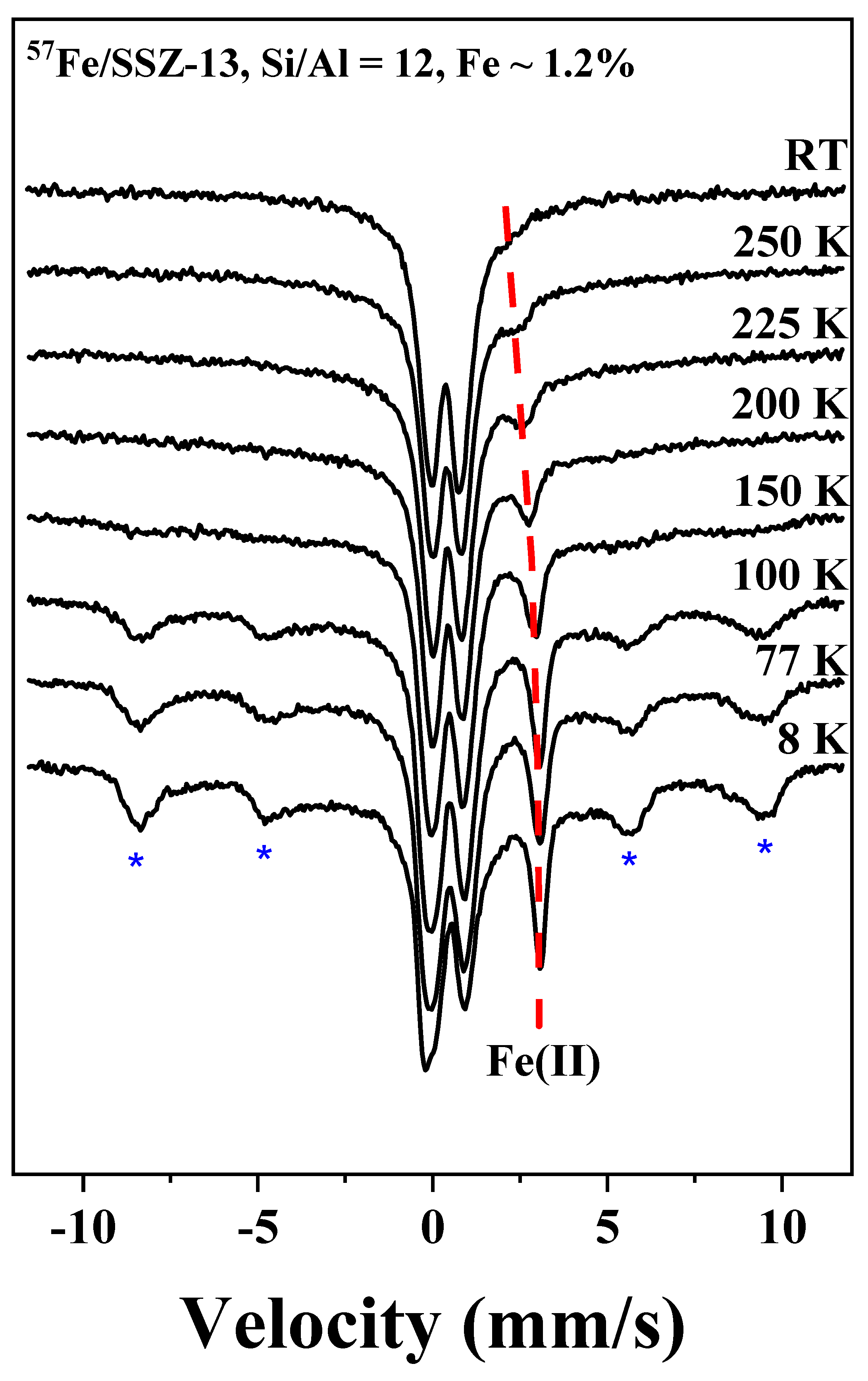
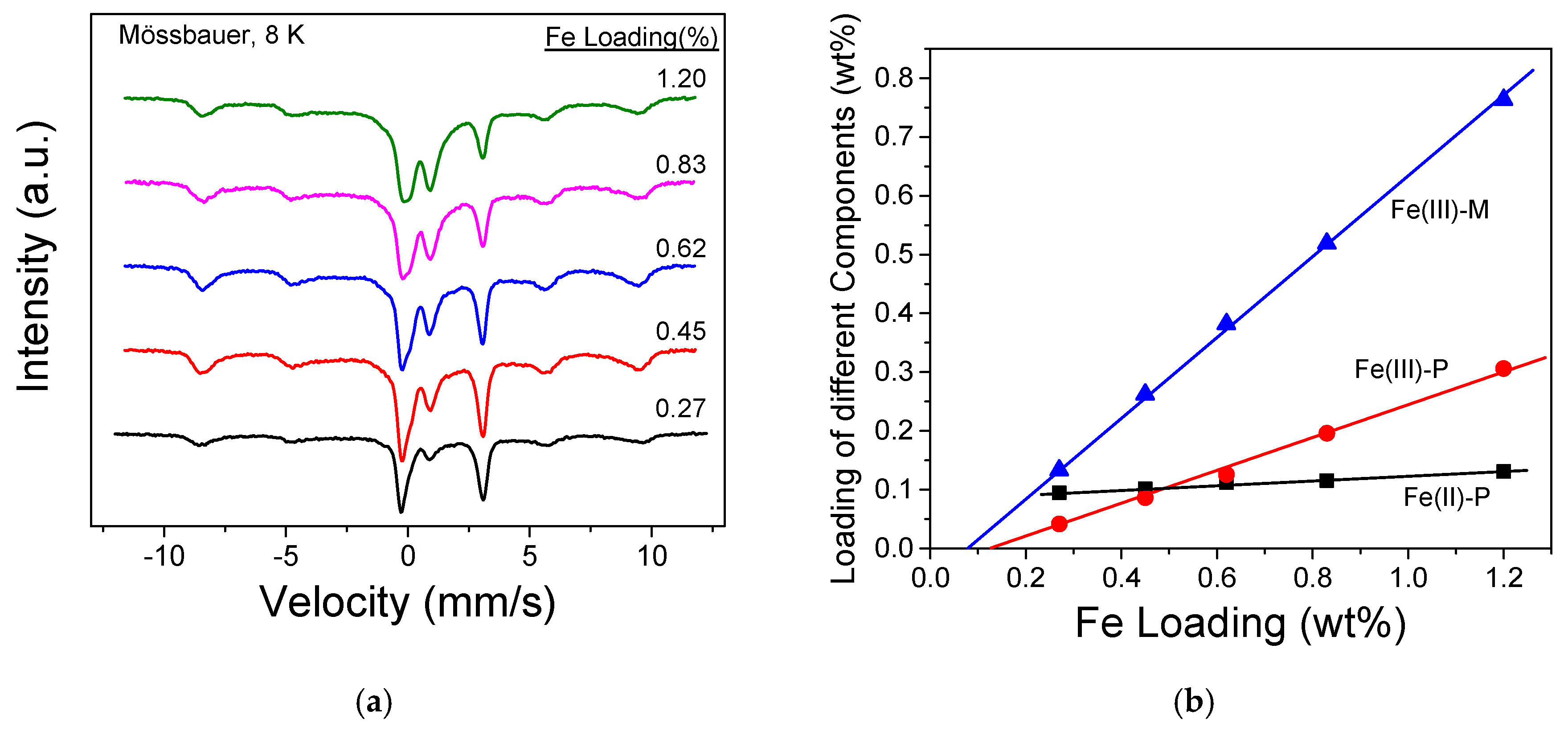
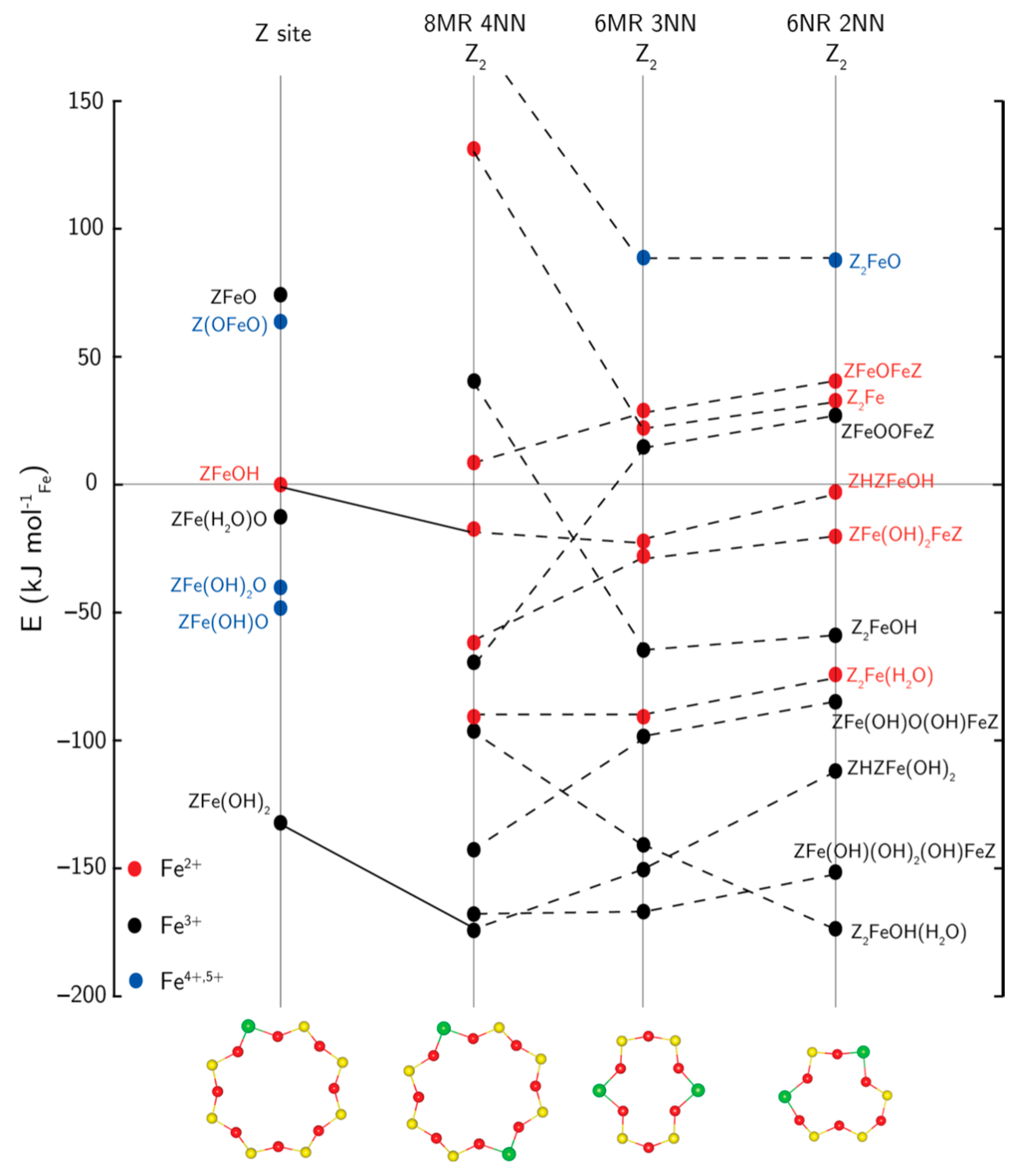
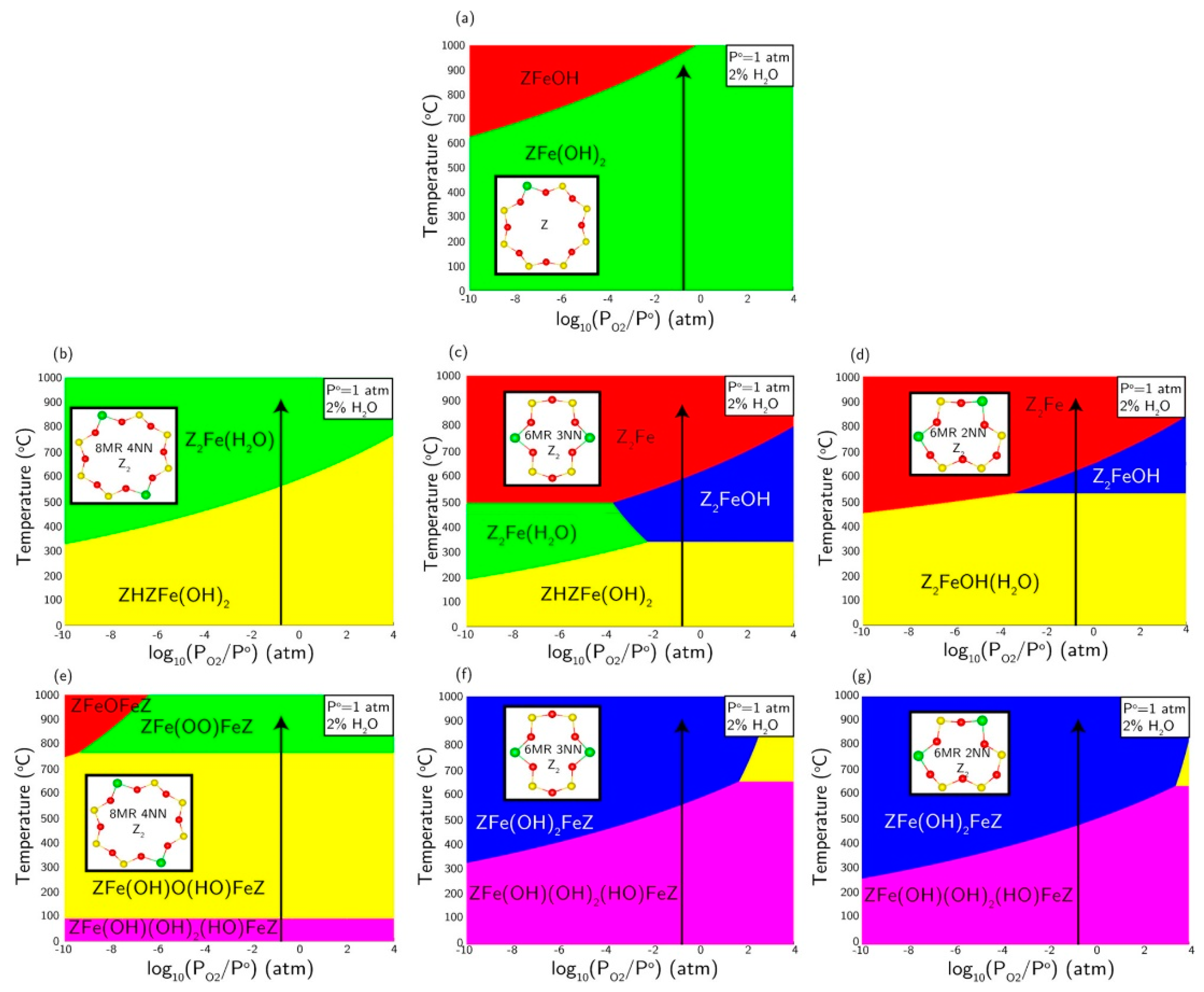
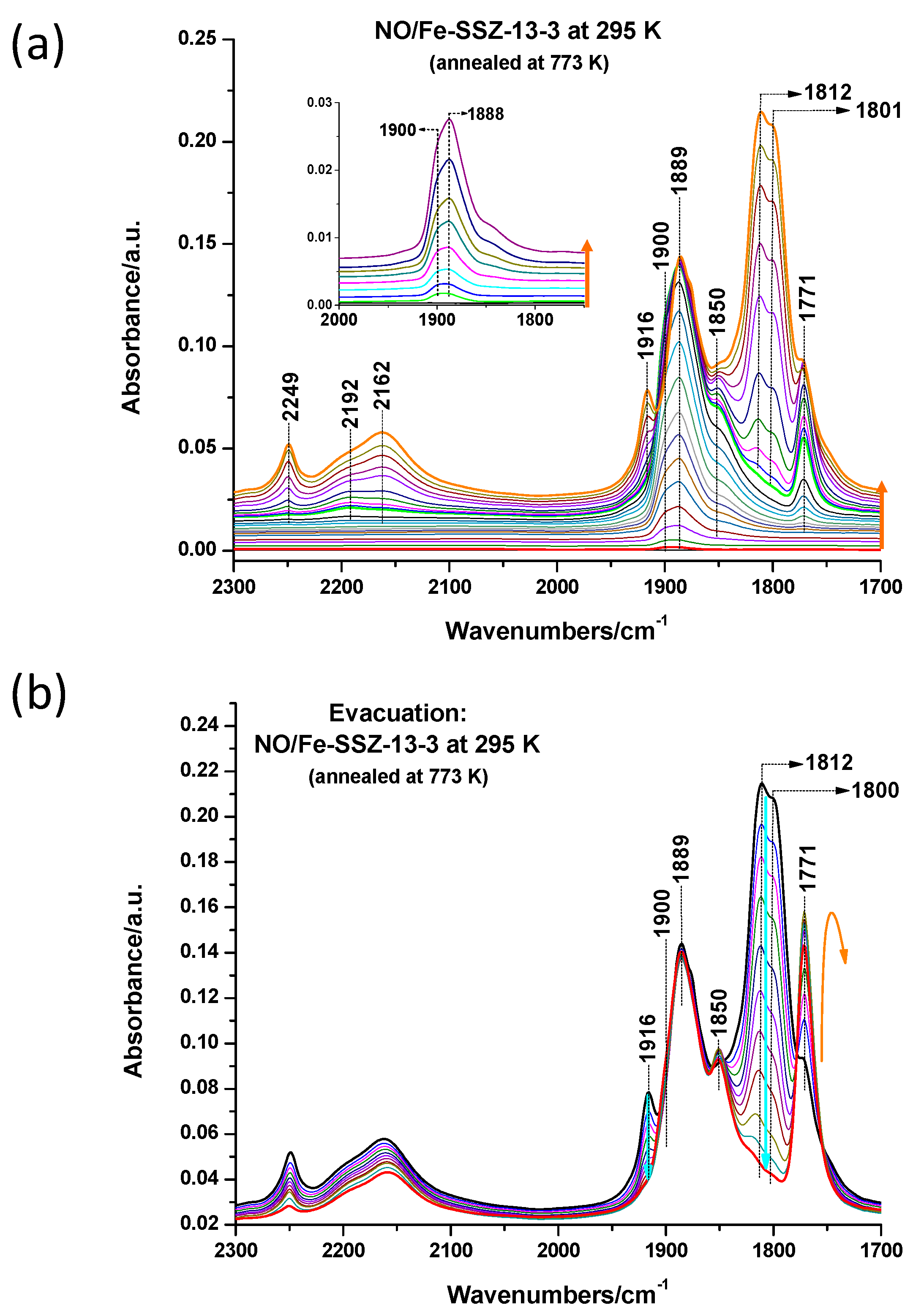
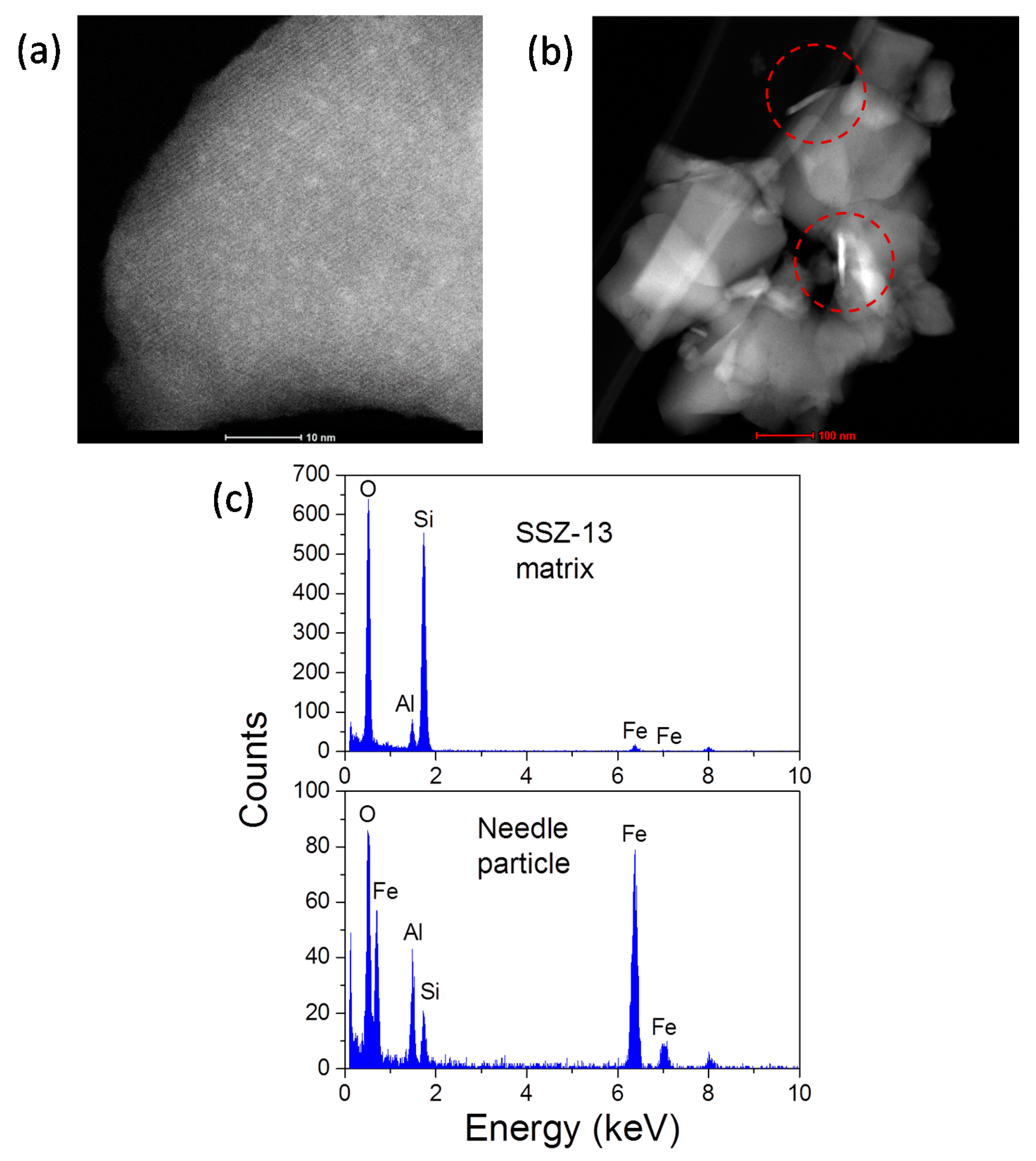
3.1. Fe Species in Freshly Prepared Small-Pore Fe/Zeolites
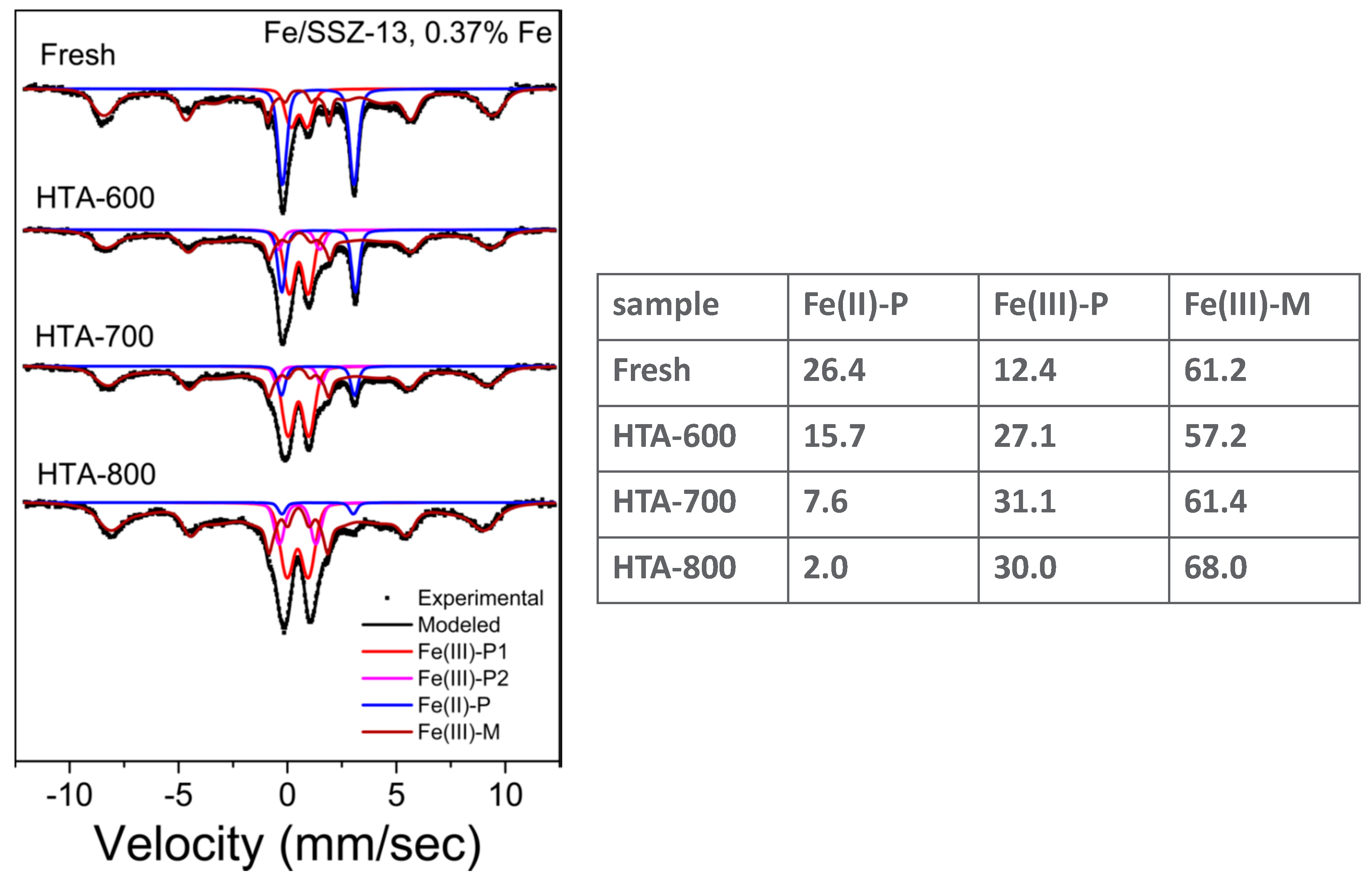
3.2. Fe-Ion Transformations during Hydrothermal Aging
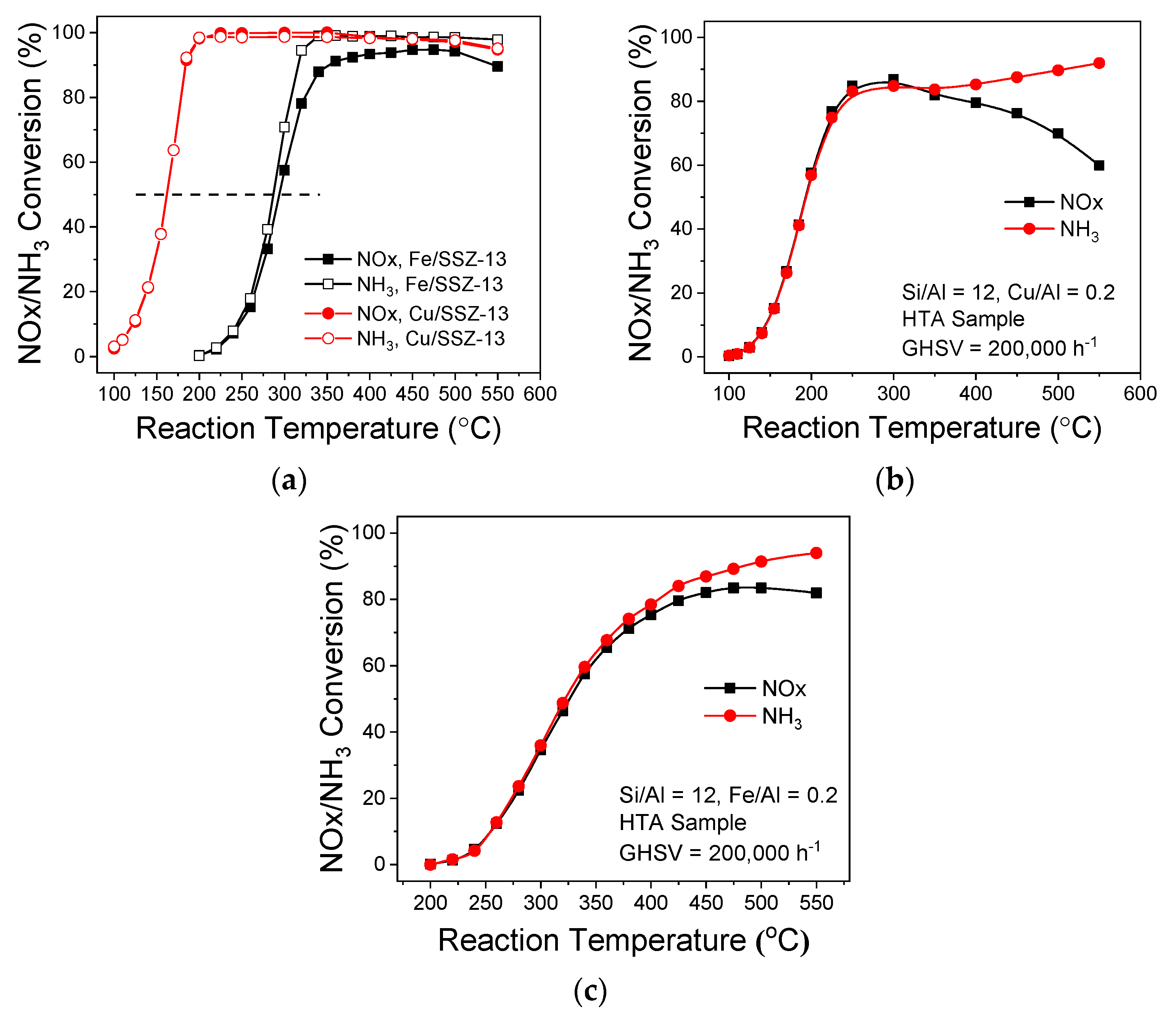
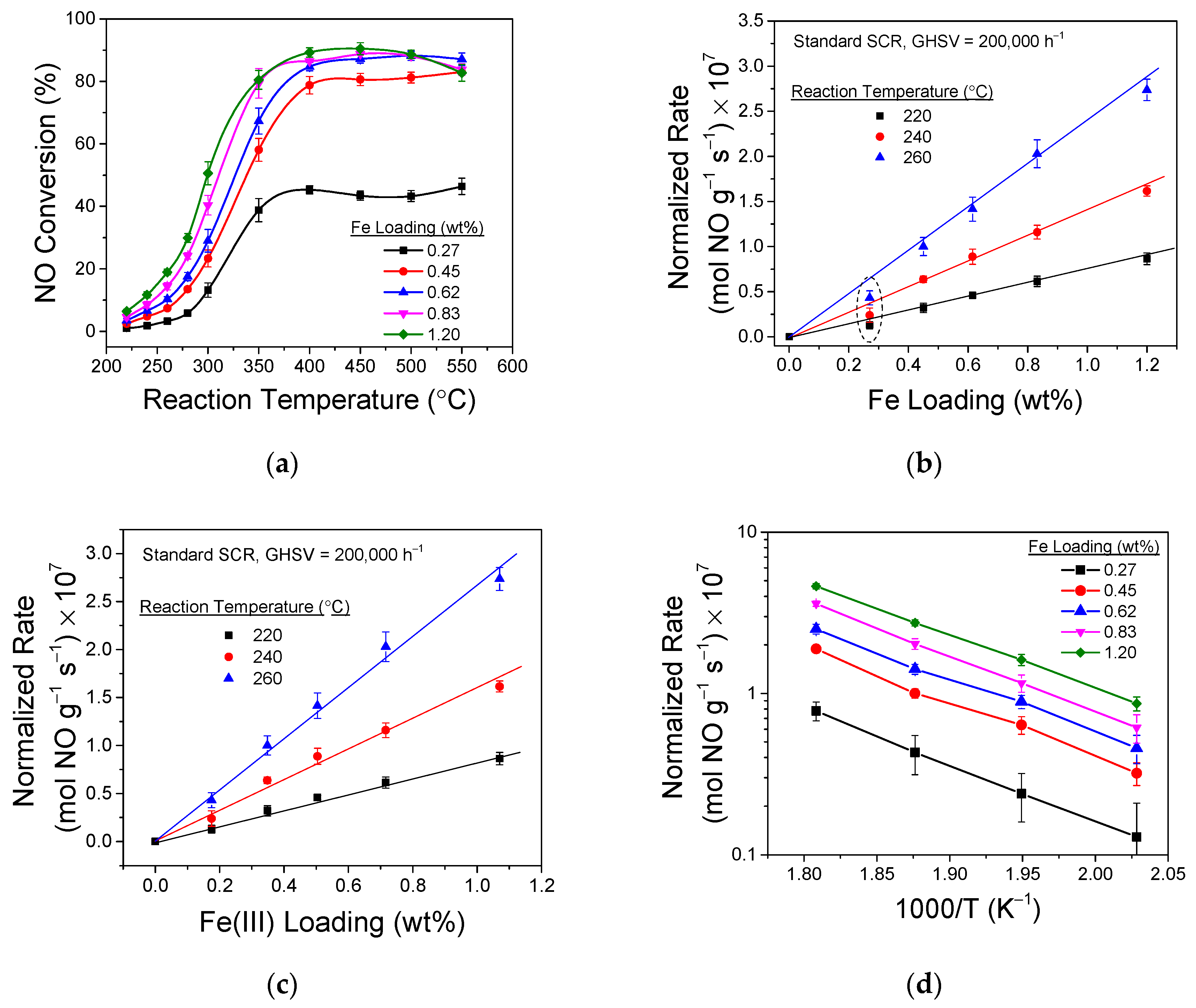
4. Small-Pore Fe/Zeolites as SCR Catalysts: Structure-Function Correlations
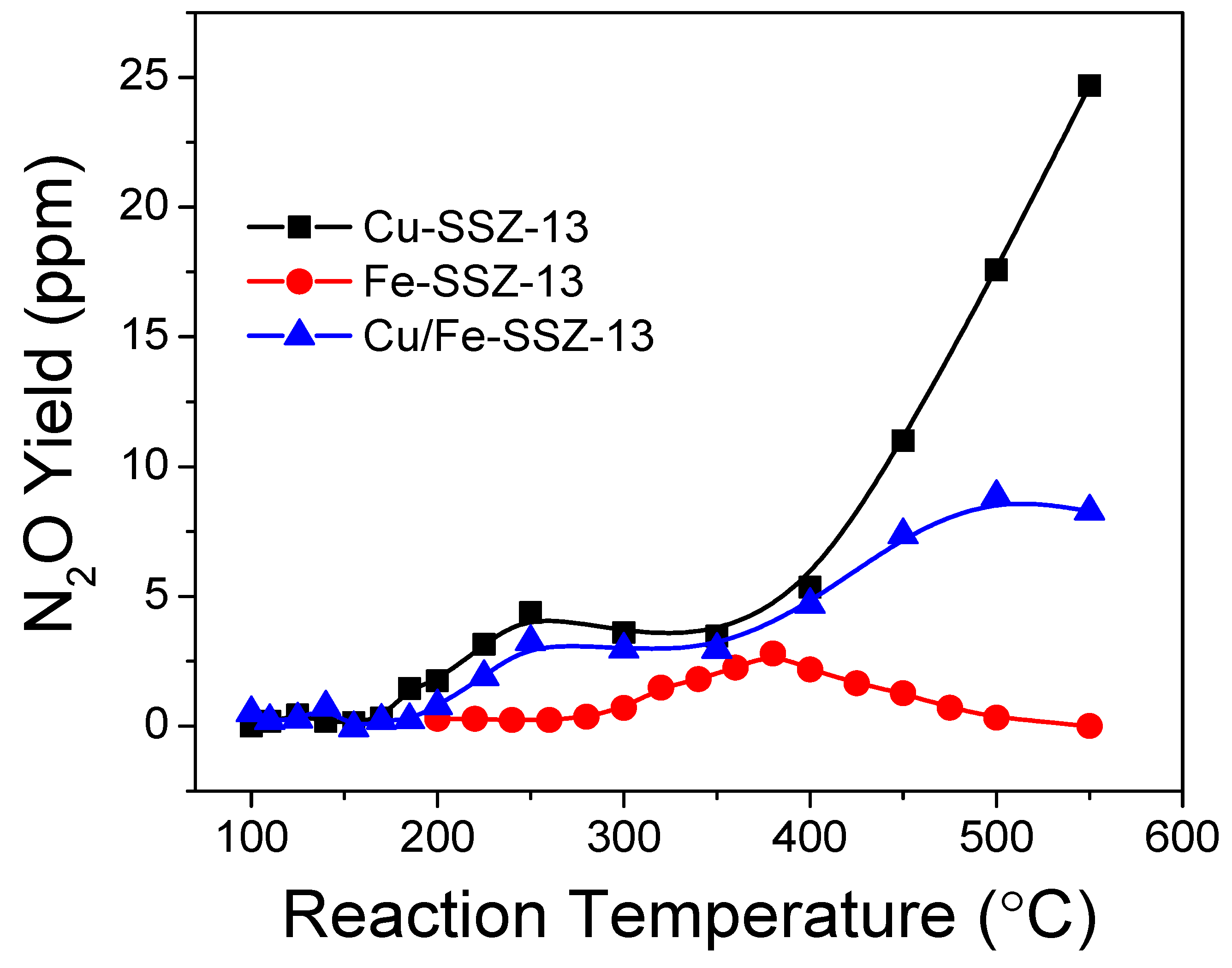
5. Small-Pore Fe/Zeolites as SCR Catalysts: Application Aspects
6. Future Perspectives
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Andersen, J.; Bailie, J.E.; Casci, J.L.; Chen, H.Y.; Fedeyko, J.M.; Foo, R.K.S.; Rajaram, R.R. Transion Metal/Zeolite SCR Catalysts. International Patent WO/2008/132452, 6 November 2008. [Google Scholar]
- Bull, I.; Xue, W.M.; Burk, P.; Boorse, R.S.; Jaglowski, W.M.; Koermer, G.S.; Moini, A.; Patchett, J.A.; Dettling, J.C.; Caudle, T.M. Copper CHA Zeolite Catalysts. U.S. Patent 7,601,662 B2, 13 October 2009. [Google Scholar]
- Bull, I.; Mioni, A.; Koermer, G.S.; Patchett, J.A.; Jaglowski, W.M.; Roth, S. Zeolite Catalyst With Imporved NOx Reduction in SCR. U.S. Patent 7,704,475 B2, 27 April 2010. [Google Scholar]
- Andersen, P.J.; Chen, H.Y.; Fedeyko, J.M.; Weigert, E. Small pore molecular sieve supported copper catalysts durable against lean/rich aging for the reduction of nitrogen oxides. U.S. Patent 7,998,443 B2, 16 August 2011. [Google Scholar]
- Gao, F.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Current Understanding of Cu-Exchanged Chabazite Molecular Sieves for Use as Commercial Diesel Engine DeNO(x) Catalysts. Top Catal. 2013, 56, 1441–1459. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 14, 7371–7405. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Peden, C.H.F. Recent Progress in Atomic-Level Understanding of Cu/SSZ-13 Selective Catalytic Reduction Catalysts. Catalysts 2018, 8, 140. [Google Scholar]
- Peden, C.H.F. Cu/Chabazite catalysts for ‘Lean-Burn’ vehicle emission control. J. Catal. 2019, 373, 384–389. [Google Scholar] [CrossRef]
- Paolucci, C.; di Iorio, J.R.; Ribeiro, F.H.; Gounder, R.; Schneider, W.F. Catalysis Science of NOx Selective Catalytic Reduction With Ammonia Over Cu-SSZ-13 and Cu-SAPO-34. Adv. Catal. 2016, 59, 1–107. [Google Scholar]
- Guan, B.; Zhan, R.; Lin, H.; Huang, Z. Review of state of the art technologies of selective catalytic reduction of NOx from diesel engine exhaust. Appl. Therm. Eng. 2014, 66, 395–414. [Google Scholar] [CrossRef]
- Moliner, M.; Franch, C.; Palomares, E.; Grill, M.; Corma, A. Cu-SSZ-39, an active and hydrothermally stable catalyst for the selective catalytic reduction of NOx. Chem. Commun. 2012, 48, 8264–8266. [Google Scholar] [CrossRef]
- Martin, N.; Boruntea, C.R.; Moliner, M.; Corma, A. Efficient synthesis of the Cu-SSZ-39 catalyst for DeNOx applications. Chem. Commun. 2015, 51, 11030–11033. [Google Scholar] [CrossRef]
- Shan, Y.L.; Shan, W.P.; Shi, X.Y.; Du, J.P.; Yu, Y.B.; He, H. A comparative study of the activity and hydrothermal stability of Al-rich Cu-SSZ-39 and Cu-SSZ-13. Appl. Catal. B Environ. 2020, 264, 118511. [Google Scholar] [CrossRef]
- Jo, D.; Ryu, T.; Park, G.T.; Kim, P.S.; Kim, C.H.; Nam, I.S.; Hong, S.B. Synthesis of High-Silica LTA and UFI Zeolites and NH3-SCR Performance of Their Copper-Exchanged Form. ACS Catal. 2016, 6, 2443–2447. [Google Scholar] [CrossRef]
- Ahn, N.H.; Ryu, T.; Kang, Y.; Kim, H.; Shin, J.; Nam, I.S.; Hong, S.B. The Origin of an Unexpected Increase in NH3-SCR Activity of Aged Cu-LTA Catalysts. ACS Catal. 2017, 7, 6781–6785. [Google Scholar] [CrossRef]
- Ryu, T.; Ahn, N.H.; Seo, S.; Cho, J.; Kim, H.; Jo, D.; Park, G.T.; Kim, P.S.; Kim, C.H.; Bruce, E.L.; et al. Fully Copper-Exchanged High-Silica LTA Zeolites as Unrivaled Hydrothermally Stable NH3-SCR Catalysts. Angew. Chem. Int. Ed. 2017, 56, 3256–3260. [Google Scholar] [CrossRef] [PubMed]
- Brandenberger, S.; Krocher, O.; Tissler, A.; Althoff, R. The State of the Art in Selective Catalytic Reduction of NOx by Ammonia Using Metal-Exchanged Zeolite Catalysts. Catal. Rev. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Li, J.H.; Chang, H.Z.; Ma, L.; Hao, J.M.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts—A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Fritz, A.; Pitchon, V. The current state of research on automotive lean NOx catalysis. Appl. Catal. B Environ. 1997, 13, 1–25. [Google Scholar] [CrossRef]
- Feng, X.B.; Hall, W.K. FeZSM-5: A durable SCR catalyst for NOx removal from combustion streams. J. Catal. 1997, 166, 368–376. [Google Scholar] [CrossRef]
- Chen, H.Y.; Sachtler, W.M.H. Activity and durability of Fe/ZSM-5 catalysts for lean burn NOx reduction in the presence of water vapor. Catal. Today 1998, 42, 73–83. [Google Scholar] [CrossRef]
- Chen, H.Y.; Sachtler, W.M.H. Promoted Fe/ZSM-5 catalysts prepared by sublimation: De-NOx activity and durability in H2O-rich streams. Catal. Lett. 1998, 50, 125–130. [Google Scholar] [CrossRef]
- He, C.H.; Wang, Y.H.; Cheng, Y.S.; Lambert, C.K.; Yang, R.T. Activity stability and hydrocarbon deactivation of Fe/Beta catalyst for SCR of NO with ammonia. Appl. Catal. A Gen. 2009, 368, 121–126. [Google Scholar] [CrossRef]
- Kumar, M.S.; Schwidder, M.; Grunert, W.; Bruckner, A. On the nature of different iron sites and their catalytic role in Fe-ZSM-5 DeNO(x) catalysts: New insights by a combined EPR and UV/VIS spectroscopic approach. J. Catal. 2004, 227, 384–397. [Google Scholar] [CrossRef]
- Brandenberger, S.; Krocher, O.; Tissler, A.; Althoff, R. The determination of the activities of different iron species in Fe-ZSM-5 for SCR of NO by NH3. Appl. Catal. B Environ. 2010, 95, 348–357. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, L.F.; Yang, R.T. Activity, propene poisoning resistance and hydrothermal stability of copper exchanged chabazite-like zeolite catalysts for SCR of NO with ammonia in comparison to Cu/ZSM-5. Appl. Catal. A Gen. 2012, 427, 24–34. [Google Scholar] [CrossRef]
- Yang, X.F.; Wu, Z.L.; Moses-Debusk, M.; Mullins, D.R.; Mahurin, S.M.; Geiger, R.A.; Kidder, M.; Narula, C.K. Heterometal Incorporation in Metal-Exchanged Zeolites Enables Low-Temperature Catalytic Activity of NOx Reduction. J. Phys. Chem. C 2012, 116, 23322–23331. [Google Scholar] [CrossRef]
- Gao, F.; Zheng, Y.; Kukkadapu, R.K.; Wang, Y.L.; Walter, E.D.; Schwenzer, B.; Szanyi, J.; Peden, C.H.F. Iron Loading Effects in Fe/SSZ-13 NH3-SCR Catalysts: Nature of the Fe Ions and Structure-Function Relationships. ACS Catal. 2016, 6, 2939–2954. [Google Scholar] [CrossRef]
- Szanyi, J.; Gao, F.; Kwak, J.H.; Kollar, M.; Wang, Y.L.; Peden, C.H.F. Characterization of Fe2+ ions in Fe,H/SSZ-13 zeolites: FTIR spectroscopy of CO and NO probe molecules. Phys. Chem. Chem. Phys. 2016, 18, 10473–10485. [Google Scholar] [CrossRef]
- Gao, F.; Kollar, M.; Kukkadapu, R.K.; Washton, N.M.; Wang, Y.L.; Szanyi, J.; Peden, C.H.F. Fe/SSZ-13 as an NH3-SCR catalyst: A reaction kinetics and FTIR/Mossbauer spectroscopic study. Appl. Catal. B Environ. 2015, 164, 407–419. [Google Scholar] [CrossRef]
- Gao, F.; Szanyi, J.; Wang, Y.L.; Schwenzer, B.; Kollar, M.; Peden, C.H.F. Hydrothermal Aging Effects on Fe/SSZ-13 and Fe/Beta NH3-SCR Catalysts. Top. Catal. 2016, 59, 882–886. [Google Scholar] [CrossRef]
- Kovarik, L.; Washton, N.M.; Kukadapu, R.; Devaraj, A.; Wang, A.Y.; Wang, Y.L.; Szanyi, J.; Peden, C.H.F.; Gao, F. Transformation of Active Sites in Fe/SSZ-13 SCR Catalysts during Hydrothermal Aging: A Spectroscopic, Microscopic, and Kinetics Study. ACS Catal. 2017, 7, 2458–2470. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.L.; Kollar, M.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. A comparative kinetics study between Cu/SSZ-13 and Fe/SSZ-13 SCR catalysts. Catal. Today 2015, 258, 347–358. [Google Scholar] [CrossRef]
- Wang, A.Y.; Wang, Y.L.; Walter, E.D.; Washton, N.M.; Guo, Y.L.; Lu, G.Z.; Peden, C.H.F.; Gao, F. NH3-SCR on Cu, Fe and Cu plus Fe exchanged beta and SSZ-13 catalysts: Hydrothermal aging and propylene poisoning effects. Catal. Today 2019, 320, 91–99. [Google Scholar] [CrossRef]
- Li, S.C.; Wang, Y.J.; Wu, T.; Schneider, W.F. First-Principles Analysis of Site- and Condition-Dependent Fe Speciation in SSZ-13 and Implications for Catalyst Optimization. ACS Catal. 2018, 8, 10119–10130. [Google Scholar] [CrossRef]
- Zhang, R.Q.; Anderst, E.; Groden, K.; McEwen, J.S. Modeling the Adsorption of NO and NH3 on Fe-SSZ-13 from First-Principles: A DFT Study. Ind. Eng. Chem. Res. 2018, 57, 13396–13405. [Google Scholar] [CrossRef]
- Andonova, S.; Tamm, S.; Montreuil, C.; Lambert, C.; Olsson, L. The effect of iron loading and hydrothermal aging on one-pot synthesized Fe/SAPO-34 for ammonia SCR. Appl. Catal. B Environ. 2016, 180, 775–787. [Google Scholar] [CrossRef]
- Turrina, A.; Dugulan, A.I.; Collier, J.E.; Walton, R.I.; Casci, J.L.; Wright, P.A. Synthesis and activation for catalysis of Fe-SAPO-34 prepared using iron polyamine complexes as structure directing agents. Catal. Sci. Technol. 2017, 7, 4366–4374. [Google Scholar] [CrossRef]
- Martin, N.; Vennestrom, P.N.R.; Thogersen, J.R.; Moliner, M.; Corma, A. Iron-Containing SSZ-39 (AEI) Zeolite: An Active and Stable High-Temperature NH3-SCR Catalyst. Chemcatchem 2017, 9, 1754–1757. [Google Scholar] [CrossRef]
- Martin, N.; Vennestrom, P.N.R.; Thogersen, J.R.; Moliner, M.; Corma, A. Fe-Containing Zeolites for NH3-SCR of NOx: Effect of Structure, Synthesis Procedure, and Chemical Composition on Catalytic Performance and Stability. Chem. Eur. J. 2017, 23, 13404–13414. [Google Scholar] [CrossRef]
- Martin, N.; Paris, C.; Vennestrom, P.N.R.; Thogersen, J.R.; Moliner, M.; Corma, A. Cage-based small-pore catalysts for NH3-SCR prepared by combining bulky organic structure directing agents with modified zeolites as reagents. Appl. Catal. B Environ. 2017, 217, 125–136. [Google Scholar] [CrossRef]
- Ryu, T.; Kang, Y.; Nam, I.S.; Hong, S.B. Iron-exchanged high-silica LTA zeolites as hydrothermally stable NH3-SCR catalysts. React. Chem. Eng. 2019, 4, 1050–1058. [Google Scholar] [CrossRef]
- Brandenberger, S.; Krocher, O.; Tissler, A.; Althoff, R. Estimation of the fractions of different nuclear iron species in uniformly metal-exchanged Fe-ZSM-5 samples based on a Poisson distribution. Appl. Catal. A Gen. 2010, 373, 168–175. [Google Scholar] [CrossRef]
- Hall, W.K.; Feng, X.B.; Dumesic, J.; Watwe, R. Problems in preparation of FeZSM-5 catalysts. Catal. Lett. 1998, 52, 13–19. [Google Scholar] [CrossRef]
- Pieterse, J.A.Z.; Booneveld, S.; van den Brink, R.W. Evaluation of Fe-zeolite catalysts prepared by different methods for the decomposition of N2O. Appl. Catal. B Environ. 2004, 51, 215–228. [Google Scholar] [CrossRef]
- Dusselier, M.; Davis, M.E. Small-Pore Zeolites: Synthesis and Catalysis. Chem. Rev. 2018, 118, 5265–5329. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jentys, A.; Maier, S.M.; Lercher, J.A. Characterization of Fe-Exchanged BEA Zeolite Under NH3 Selective Catalytic Reduction Conditions. J. Phys. Chem. C 2013, 117, 986–993. [Google Scholar] [CrossRef]
- Bordiga, S.; Buzzoni, R.; Geobaldo, F.; Lamberti, C.; Giamello, E.; Zecchina, A.; Leofanti, G.; Petrini, G.; Tozzola, G.; Vlaic, G. Structure and reactivity of framework and extraframework iron in Fe-silicalite as investigated by spectroscopic and physicochemical methods. J. Catal. 1996, 158, 486–501. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. Synthesis and Evaluation of Cu-SAPO-34 Catalysts for Ammonia Selective Catalytic Reduction. 1. Aqueous Solution Ion Exchange. ACS Catal. 2013, 3, 2083–2093. [Google Scholar] [CrossRef]
- Woo, J.; Leistner, K.; Bernin, D.; Ahari, H.; Shost, M.; Zammit, M.; Olsson, L. Effect of various structure directing agents (SDAs) on low-temperature deactivation of Cu/SAPO-34 during NH3-SCR reaction. Catal. Sci. Technol. 2018, 8, 3090–3106. [Google Scholar] [CrossRef]
- Ren, L.M.; Zhu, L.F.; Yang, C.G.; Chen, Y.M.; Sun, Q.; Zhang, H.Y.; Li, C.J.; Nawaz, F.; Meng, X.J.; Xiao, F.S. Designed copper-amine complex as an efficient template for one-pot synthesis of Cu-SSZ-13 zeolite with excellent activity for selective catalytic reduction of NOx by NH3. Chem. Commun. 2011, 47, 9789–9791. [Google Scholar] [CrossRef]
- Turrina, A.; Eschenroeder, E.C.V.; Bode, B.E.; Collier, J.E.; Apperley, D.C.; Cox, P.A.; Casci, J.L.; Wright, P.A. Understanding the structure directing action of copper-polyamine complexes in the direct synthesis of Cu-SAPO-34 and Cu-SAPO-18 catalysts for the selective catalytic reduction of NO with NH3. Micropor. Mesopor. Mat. 2015, 215, 54–167. [Google Scholar] [CrossRef]
- Yin, C.Y.; Cheng, P.F.; Li, X.; Yang, R.T. Selective catalytic reduction of nitric oxide with ammonia over high-activity Fe/SSZ-13 and Fe/one-pot-synthesized Cu-SSZ-13 catalysts. Catal. Sci. Technol. 2016, 6, 7561–7568. [Google Scholar] [CrossRef]
- Wang, A.Y.; Wang, Y.L.; Walter, E.D.; Kukkadapu, R.K.; Guo, Y.L.; Lu, G.Z.; Weber, R.S.; Wang, Y.; Peden, C.H.F.; Gao, F. Catalytic N2O decomposition and reduction by NH3 over Fe/Beta and Fe/SSZ-13 catalysts. J. Catal. 2018, 358, 199–210. [Google Scholar] [CrossRef]
- Borfecchia, E.; Beato, P.; Svelle, S.; Olsbye, U.; Lamberti, C.; Bordiga, S. Cu-CHA—A model system for applied selective redox catalysis. Chem. Soc. Rev. 2018, 47, 8097–8133. [Google Scholar] [CrossRef] [PubMed]
- Bols, M.L.; Hallaert, S.D.; Snyder, B.E.R.; Devos, J.; Plessers, D.; Rhoda, H.M.; Dusselier, M.; Schoonheydt, R.A.; Pierloot, K.; Solomon, E.I.; et al. Spectroscopic Identification of the alpha-Fe/alpha-O Active Site in Fe-CHA Zeolite for the Low-Temperature Activation of the Methane C-H Bond. J. Am. Chem. Soc. 2018, 140, 12021–12032. [Google Scholar] [CrossRef] [PubMed]
- Fickel, D.W.; Lobo, R.F. Copper Coordination in Cu-SSZ-13 and Cu-SSZ-16 Investigated by Variable-Temperature XRD. J. Phys. Chem. C 2010, 114, 1633–1640. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tran, D.; Szanyi, J.; Peden, C.H.F.; Lee, J.H. The Effect of Copper Loading on the Selective Catalytic Reduction of Nitric Oxide by Ammonia Over Cu-SSZ-13. Catal. Lett. 2012, 142, 295–301. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.K.; Min, K.M.; Hong, S.B.; Nam, I.S.; Cho, B.K. Hydrothermal stability of CuSSZ13 for reducing NOx by NH3. J. Catal. 2014, 311, 447–457. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.L.; Walter, E.D.; Washton, N.M.; Mei, D.H.; Kovarik, L.; Engelhard, M.H.; Prodinger, S.; Wang, Y.; Peden, C.H.F.; et al. Toward Rational Design of Cu/SSZ-13 Selective Catalytic Reduction Catalysts: Implications from Atomic-Level Understanding of Hydrothermal Stability. ACS Catal. 2017, 7, 8214–8227. [Google Scholar] [CrossRef]
- Gao, F.; Szanyi, J. On the hydrothermal stability of Cu/SSZ-13 SCR catalysts. Appl. Catal. A Gen. 2018, 560, 185–194. [Google Scholar] [CrossRef]
- Prodinger, S.; Derewinski, M.A.; Wang, Y.L.; Washton, N.M.; Walter, E.D.; Szanyi, J.; Gao, F.; Wang, Y.; Peden, C.H.F. Sub-micron Cu/SSZ-13: Synthesis and application as selective catalytic reduction (SCR) catalysts. Appl. Catal. B Environ. 2017, 201, 461–469. [Google Scholar] [CrossRef]
- Cui, Y.R.; Wang, Y.L.; Mei, D.H.; Walter, E.D.; Washton, N.M.; Holladay, J.D.; Wang, Y.; Szanyi, J.; Peden, C.H.F.; Gao, F. Revisiting effects of alkali metal and alkaline earth co-cation additives to Cu/SSZ-13 selective catalytic reduction catalysts. J. Catal. 2019, 378, 363–375. [Google Scholar] [CrossRef]
- Klinowski, J. Solid-State Nmr-Studies of Molecular-Sieve Catalysts. Chem. Rev. 1991, 91, 1459–1479. [Google Scholar] [CrossRef]
- Schmieg, S.J.; Oh, S.H.; Kim, C.H.; Brown, D.B.; Lee, J.H.; Peden, C.H.F.; Kim, D.H. Thermal durability of Cu-CHA NH3-SCR catalysts for diesel NOx reduction. Catal. Today 2012, 184, 252–261. [Google Scholar] [CrossRef]
- Rahkamaa-Tolonen, K.; Maunula, T.; Lomma, M.; Huuhtanen, M.; Keiski, R.L. The effect of NO2 on the activity of fresh and aged zeolite catalysts in the NH3-SCR reaction. Catal. Today 2005, 100, 217–222. [Google Scholar] [CrossRef]
- Leistner, K.; Olsson, L. Deactivation of Cu/SAPO-34 during low-temperature NH3-SCR. Appl. Catal. B Environ. 2015, 165, 192–199. [Google Scholar] [CrossRef]
- Wang, J.; Fan, D.Q.; Yu, T.; Wang, J.Q.; Hao, T.; Hu, X.Q.; Shen, M.Q.; Li, W. Improvement of low-temperature hydrothermal stability of Cu/SAPO-34 catalysts by Cu2+ species. J. Catal. 2015, 322, 84–90. [Google Scholar] [CrossRef]
- Wang, A.Y.; Chen, Y.; Walter, E.D.; Washton, N.M.; Mei, D.H.; Varga, T.; Wang, Y.L.; Szanyi, J.; Wang, Y.; Peden, C.H.F.; et al. Unraveling the mysterious failure of Cu/SAPO-34 selective catalytic reduction catalysts. Nat. Commun. 2019, 10, 1137. [Google Scholar] [CrossRef]
- Chen, H.Y.; Wei, Z.H.; Kollar, M.; Gao, F.; Wang, Y.L.; Szanyi, J.; Peden, C.H.F. A comparative study of N2O formation during the selective catalytic reduction of NOx with NH3 on zeolite supported Cu catalysts. J. Catal. 2015, 329, 490–498. [Google Scholar] [CrossRef]
- Metkar, P.S.; Salazar, N.; Muncrief, R.; Balakotaiah, V.; Harold, M.P. Selective catalytic reduction of NO with NH3 on iron zeolite monolithic catalysts: Steady-state and transient kinetics. Appl. Catal. B Environ. 2011, 104, 110–126. [Google Scholar] [CrossRef]
- Grossale, A.; Nova, I.; Tronconi, E.; Chatterjee, D.; Weibel, M. The chemistry of the NO/NO2-NH3 “fast” SCR reaction over Fe-ZSM5 investigated by transient reaction analysis. J. Catal. 2008, 256, 312–322. [Google Scholar] [CrossRef]
- Kamasamudram, K.; Currier, N.W.; Chen, X.; Yezerets, A. Overview of the practically important behaviors of zeolite-based urea-SCR catalysts, using compact experimental protocol. Catal. Today 2010, 151, 212–222. [Google Scholar] [CrossRef]
- Nedyalkova, R.; Kamasamudram, K.; Currier, N.W.; Li, J.H.; Yezerets, A.; Olsson, L. Experimental evidence of the mechanism behind NH3 overconsumption during SCR over Fe-zeolites. J. Catal. 2013, 299, 101–108. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Karp, E.M.; Luo, J.Y.; Tonkyn, R.G.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Structure-activity relationships in NH3-SCR over Cu-SSZ-13 as probed by reaction kinetics and EPR studies. J. Catal. 2013, 300, 20–29. [Google Scholar] [CrossRef]
- Bates, S.A.; Verma, A.A.; Paolucci, C.; Parekh, A.A.; Anggara, T.; Yezerets, A.; Schneider, W.F.; Miller, J.T.; Delgass, W.N.; Ribeiro, F.H. Identification of the active Cu site in standard selective catalytic reduction with ammonia on Cu-SSZ-13. J. Catal. 2014, 312, 87–97. [Google Scholar] [CrossRef]
- Li, J.; Li, S.H. A DFT Study toward Understanding the High Activity of Fe-Exchanged Zeolites for the “Fast” Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. J. Phys. Chem. C 2008, 112, 16938–16944. [Google Scholar] [CrossRef]
- Verma, A.A.; Bates, S.A.; Anggara, T.; Paolucci, C.; Parekh, A.A.; Kamasamudram, K.; Yezerets, A.; Miller, J.T.; Delgass, W.N.; Schneider, W.F.; et al. NO oxidation: A probe reaction on Cu-SSZ-13. J. Catal. 2014, 312, 179–190. [Google Scholar] [CrossRef]
- Gao, F.; Mei, D.H.; Wang, Y.L.; Szanyi, J.; Peden, C.H.F. Selective Catalytic Reduction over Cu/SSZ-13: Linking Homo- and Heterogeneous Catalysis. J. Am. Chem. Soc. 2017, 139, 4935–4942. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, C.; Khurana, I.; Parekh, A.A.; Li, S.C.; Shih, A.J.; Li, H.; di Iorio, J.R.; Albarracin-Caballero, J.D.; Yezerets, A.; Miller, J.T.; et al. Dynamic multinuclear sites formed by mobilized copper ions in NOx selective catalytic reduction. Science 2017, 357, 898–903. [Google Scholar] [CrossRef]
- Marberger, A.; Petrov, A.W.; Steiger, P.; Elsener, M.; Krocher, O.; Nachtegaal, M.; Ferri, D. Time-resolved copper speciation during selective catalytic reduction of NO on Cu-SSZ-13. Nat. Catal. 2018, 1, 221–227. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Peng, Y.; Li, K.Z.; Liu, S.; Chen, J.J.; Li, J.H.; Gao, F.; Peden, C.H.F. Using Transient FTIR Spectroscopy to Probe Active Sites and Reaction Intermediates for Selective Catalytic Reduction of NO on Cu/SSZ-13 Catalysts. ACS Catal. 2019, 9, 6137–6145. [Google Scholar] [CrossRef]
- Lomachenko, K.A.; Borfecchia, E.; Negri, C.; Berlier, G.; Lamberti, C.; Beato, P.; Falsig, H.; Bordiga, S. The Cu-CHA deNO(x) Catalyst in Action: Temperature-Dependent NH3-Assisted Selective Catalytic Reduction Monitored by Operando XAS and XES. J. Am. Chem. Soc. 2016, 138, 12025–12028. [Google Scholar] [CrossRef]
- Delahay, G.; Valade, D.; Guzman-Vargas, A.; Coq, B. Selective catalytic reduction of nitric oxide with ammonia on Fe-ZSM-5 catalysts prepared by different methods. Appl. Catal. B Environ. 2005, 55, 149–155. [Google Scholar] [CrossRef]
- Janssens, T.V.W.; Falsig, H.; Lundegaard, L.F.; Vennestrom, P.N.R.; Rasmussen, S.B.; Moses, P.G.; Giordanino, F.; Borfecchia, E.; Lomachenko, K.A.; Lamberti, C.; et al. A Consistent Reaction Scheme for the Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. ACS Catal. 2015, 5, 2832–2845. [Google Scholar] [CrossRef]
- Krocher, O.; Devadas, M.; Elsener, M.; Wokaun, A.; Soger, N.; Pfeifer, M.; Demel, Y.; Mussmann, L. Investigation of the selective catalytic reduction of NO by NH3 on Fe-ZSM5 monolith catalysts. Appl. Catal. B Environ. 2006, 66, 208–216. [Google Scholar] [CrossRef]
- Iwasaki, M.; Shinjoh, H. A comparative study of “standard”, “fast” and “NO2” SCR reactions over Fe/zeolite catalyst. Appl. Catal. A Gen. 2010, 390, 71–77. [Google Scholar] [CrossRef]
- Kumar, A.; Smith, M.A.; Kamasamudram, K.; Currier, N.W.; Yezerets, A. Chemical deSOx: An effective way to recover Cu-zeolite SCR catalysts from sulfur poisoning. Catal. Today 2016, 267, 10–16. [Google Scholar] [CrossRef]
- Wijayanti, K.; Andonova, S.; Kumar, A.; Li, J.H.; Kamasamudram, K.; Currier, N.W.; Yezerets, A.; Olsson, L. Impact of sulfur oxide on NH3-SCR over Cu-SAPO-34. Appl. Catal. B Environ. 2015, 166, 568–579. [Google Scholar] [CrossRef]
- Kumar, A.; Smith, M.A.; Kamasamudram, K.; Currier, N.W.; An, H.; Yezerets, A. Impact of different forms of feed sulfur on small-pore Cu-zeolite SCR catalyst. Catal. Today 2014, 231, 5–82. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Liu, Y.; Kamasamudram, K.; Li, J.H.; Epling, W. SO2 poisoning impact on the NH3-SCR reaction over a commercial Cu-SAPO-34 SCR catalyst. Appl. Catal. B Environ. 2014, 156, 371–377. [Google Scholar] [CrossRef]
- Jangjou, Y.; Do, Q.; Gu, Y.T.; Lim, L.G.; Sun, H.; Wang, D.; Kumar, A.; Li, J.H.; Grabow, L.C.; Epling, W.S. Nature of Cu Active Centers in Cu-SSZ-13 and Their Responses to SO2 Exposure. ACS Catal. 2018, 8, 1325–1337. [Google Scholar] [CrossRef]
- Metkar, P.S.; Harold, M.P.; Balakotaiah, V. Selective catalytic reduction of NOx on combined Fe- and Cu-zeolite monolithic catalysts: Sequential and dual layer configurations. Appl. Catal. B Environ. 2010, 111, 67–80. [Google Scholar] [CrossRef]
- Metkar, P.S.; Harold, M.P.; Balakotaiah, V. Experimental and kinetic modeling study of NH3-SCR of NOx on Fe-ZSM-5, Cu-chabazite and combined Fe- and Cu-zeolite monolithic catalysts. Chem. Eng. Sci. 2013, 87, 51–66. [Google Scholar] [CrossRef]
- Shakya, B.M.; Harold, M.P.; Balakotaiah, V. Simulations and optimization of combined Fe- and Cu-zeolite SCR monolith catalysts. Chem. Eng. J. 2015, 278, 374–384. [Google Scholar] [CrossRef]
- Zhang, T.; Qin, X.; Peng, Y.; Wang, C.Z.; Chang, H.Z.; Chen, J.J.; Li, J.H. Effect of Fe precursors on the catalytic activity of Fe/SAPO-34 catalysts for N2O decomposition. Catal. Commun. 2019, 128, 105706. [Google Scholar] [CrossRef]





| Catalyst | SCR Test Condition | NOx Conversion at 300 °C | Reference |
|---|---|---|---|
| One-pot Fe/SAPO-34, Fe loading 0.27 wt%, washcoated | Catalyst: monolith with length = 20 mm, diameter = 22 mm and cell density of 400 cpsi, contains ~700 mg of washcoaated Fe/SAPO-34. Reaction feed contains 350 ppm NO, 350 ppm NH3, 14% O2, 5% H2O and 5% CO2. The total gas flow is held constant at 3500 mL min−1. | ~70% | [37] |
| Solution exchanged Fe/SSZ-13, Fe loading 1.2 wt% | The reactant feed contains 350 ppm of NO, 350 ppm of NH3, 14% O2, and 2.5% H2O balanced with N2 at a GHSV of 200,000 h−1. | ~50% | [28] |
| Impregnated Fe/SSZ-13, Fe loading 2.5 wt% | Reaction conditions: 400 ppm NO, 400 ppm NH3, 14% O2, 2% H2O, balance helium; 40 mg of catalyst; total flow rate 300 mL min−1; GHSV = 3.45 ×105 h−1. | ~65% | [53] |
| One-pot Fe/SSZ-13, sodium free, Fe loading 1.7 wt% | The reactant feed contains 50 ppm NO, 60 ppm NH3, 10% O2, and 10% H2O; gas flow at 300 mL min−1; 40 mg catalyst. | ~90% | [40] |
| Solution exchanged Fe/SSZ-39, Fe loading 0.9 wt% | Same as above | ~75% | [39] |
| Solution exchanged Fe/LTA, Fe/Al ratio 0.37 | A feed gas containing 500 ppm NH3, 500 ppm NO, 5% O2 and 10% H2O, balanced with N2; 600 mg catalyst; GHSV = 1 × 105 h−1. | ~80% | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F. Fe-Exchanged Small-Pore Zeolites as Ammonia Selective Catalytic Reduction (NH3-SCR) Catalysts. Catalysts 2020, 10, 1324. https://doi.org/10.3390/catal10111324
Gao F. Fe-Exchanged Small-Pore Zeolites as Ammonia Selective Catalytic Reduction (NH3-SCR) Catalysts. Catalysts. 2020; 10(11):1324. https://doi.org/10.3390/catal10111324
Chicago/Turabian StyleGao, Feng. 2020. "Fe-Exchanged Small-Pore Zeolites as Ammonia Selective Catalytic Reduction (NH3-SCR) Catalysts" Catalysts 10, no. 11: 1324. https://doi.org/10.3390/catal10111324
APA StyleGao, F. (2020). Fe-Exchanged Small-Pore Zeolites as Ammonia Selective Catalytic Reduction (NH3-SCR) Catalysts. Catalysts, 10(11), 1324. https://doi.org/10.3390/catal10111324





