Abstract
Today, climate change caused by global warming has become a worldwide problem with increasing greenhouse gas (GHG) emissions. Carbon capture and storage technologies have been developed to capture carbon dioxide (CO2); however, CO2 storage and utilization technologies are relatively less developed. In this light, we have reported efficient CO2 decomposition results using a nonperovskite metal oxide, SrFeCo0.5Ox, in a continuous-flow system. In this study, we report enhanced efficiency, reliability under isothermal conditions, and catalytic reproducibility through cyclic tests using SrFeO3−δ. This ferrite needs an activation process, and 3.5 vol% H2/N2 was used in this experiment. Activated oxygen-deficient SrFeO3−δ can decompose CO2 into carbon monoxide (CO) and carbon (C). Although SrFeO3−δ is a well-known material in different fields, no studies have reported its use in CO2 decomposition applications. The efficiency of CO2 decomposition using SrFeO3−δ reached ≥90%, and decomposition (≥80%) lasted for approximately 170 min. We also describe isothermal and cyclic experimental data for realizing commercial applications. We expect that these results will contribute to the mitigation of GHG emissions.
1. Introduction
Climate change caused by global warming has emerged as a problem worldwide owing to the increase in greenhouse gas (GHG) emissions. Carbon dioxide (CO2) emissions account for more than 90% of global GHG emissions [1,2]. According to the Intergovernmental Panel on Climate Change report in 2018, CO2 emissions were estimated to be at 32 and 40 billion tons in 2010 and 2020, respectively [3]. Carbon capture and storage technologies have been developed to capture CO2 emissions, especially those from power plants [4,5,6]. However, CO2 storage is vulnerable to earthquakes and can cause pollution and is, therefore, recognized as only a temporary method [7]. Therefore, there is an urgent need to develop and implement techniques for utilizing captured CO2. For example, CO2 reforming of methane has been proposed; however, it has high cost and energy requirements [8,9].
One of the treatment methods for captured CO2 is catalytic decomposition using oxygen-deficient metal oxides. Tamauara et al. reported that oxygen-deficient magnetite (Fe3+δO4, δ = 0.127) decomposed up to 100% of CO2 and H2O at 290 °C [10]. Subsequently, CO2 decomposition using ferrites with divalent metals such as Ni2+ and Cu2+ was investigated [11]. Mn2+- and Zn2+-based ferrites were also reported as having CO2 decomposition efficiencies of 66% and 90%, respectively [12,13]. Even trivalent, Ni-Cu ferrites were tested for an identical purpose [14]. It was reported that nickel and copper substitutions at the A-site of ferrites were the most beneficial for reduction and oxidation reactions and demonstrated meaningful results. However, all these approaches are difficult to apply in practical applications because the experimental data were obtained from a small stagnant batch-type reactor.
The first attempt to go beyond the batch system was made in 2001. Shin et al. reported CO2 decomposition data obtained through thermogravimetric analysis using activated CuFe2O4 [11]. Furthermore, Kim et al. investigated CO2 decomposition using activated Ni0.5Zn0.5Fe2O4−δ in a continuous flow of 10% CO2-balanced N2 [15,16]. They reported that trivalent ferrites (i.e., (NixZn1−x)Fe2O4, x = 0.3, 0.5, 0.7, and 1) showed a higher CO2 decomposition efficiency than divalent NiFe2O4 ferrite. They ascertained that the ferrites could completely decompose 10% CO2 for 5 to 7 min. They also asserted that Ni/Zn-ferrite synthesized by the hydrothermal method displayed better CO2 decomposition performance than that synthesized by the coprecipitation method. However, they did not perform a blank test and quantitative analysis. Although their results were elementary and had some weaknesses, their trials were invaluable in that they can be applied in practical applications. Therefore, the accumulated data of CO2 decomposition in a continuous system should be obtained for realizing economically efficient CO2 treatment.
In our previous work [17], we demonstrated the possibility of continuous CO2 decomposition by using oxygen-deficient metal oxides and suggested its reaction mechanism. Compared to Ni-ferrites, a nonperovskite-type metal oxide (i.e., SrFeCo0.5Ox) was much more effective for CO2 decomposition: Ni-ferrites decomposed only up to 20% of CO2, whereas SrFeCo0.5Ox displayed a CO2 decomposition efficiency of up to 90%. These results were obtained based on our suggested mechanism that high electrical and ionic conductivities affect CO2 decomposition. Currently, the obtainment of suitable isothermal and regeneration data will be more helpful for practical applications. In our ongoing research project, we have found that another material, SrFeO3−δ, shows greater promise for this purpose.
Originally, SrFeO3−δ was used as an oxygen transport material [18,19,20,21,22,23] and as a catalyst for methane combustion and chemical looping processes [24,25]. Perovskite-type SrFeO3−δ (0 ≤ δ ≤ 0.5) is a nonstoichiometric metal oxide containing Fe ions in a mixed valence, such as Fe4+ and Fe3+ [26]. Under reducing conditions, SrFeO3−δ produces oxygen vacancies; the number of oxygen vacancies depends on the temperature and the oxygen partial pressure [27]. Recently, Marek et al. reported the stable use of SrFeO3−δ in chemical looping systems; the material reduced above δ = 0.5 could be reoxidized with either CO2 or air, resulting in SrFeO3−δ (0 ≤ δ ≤ 0.5) [25]. Therefore, we consider it a promising material for CO2 decomposition. Several studies have reported on the use of SrFeO3−δ in various fields. However, no study has reported the use of SrFeO3−δ for CO2 decomposition in a continuous-flow system. In this report, we describe the reduction behavior and redox reaction of SrFeO3−δ. Furthermore, through cyclic experiments, we demonstrate that it exhibits consistently high CO2 decomposition performance under isothermal conditions. We also demonstrate its structural stability as a catalytic material for practical applications.
2. Results
2.1. Characterization
The crystal structure of SrFeO3−δ was analyzed by X-ray powder diffraction (XRD) at 40 kV and 200 mA. The XRD powder patterns of the samples were obtained in 0.02° steps over the range of 20° ≤ 2θ ≤ 80°. It has been reported that the structure of SrFeO3−δ could be changed susceptibly by δ values, namely, cubic at δ = 0–0.12, tetragonal at δ = 0.16–0.24, and orthorhombic at δ = 0.25 [28,29]. The lattice constant of SrFeO3−δ obtained from XRD data was a = 5.479(5) Å, b = 7.729(8) Å, c = 5.521(2) Å, and V = 233.8(5) Å3. It was determined to be an orthorhombic perovskite, which is in good agreement with the reported value (PDF# 01-077-9154). Figure 1a shows XRD powder patterns of as-synthesized SrFeO3−δ. We also obtained secondary electron images. These are discussed with those measured after CO2 decomposition tests at the end of this section. The chemical composition was reasonably acceptable, and the surface area of SrFeO3−δ was determined to be 3.19 m2/g.
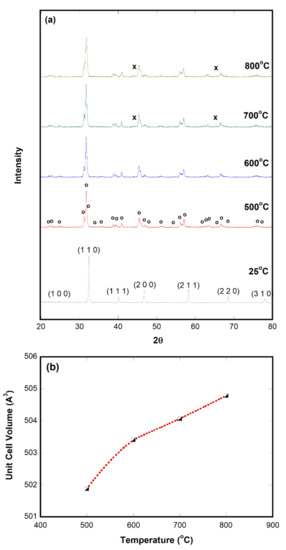
Figure 1.
In-situ XRD results of SrFeO3−δ: (a) In-situ XRD powder pattern and (b) unit cell volume at 500 ≤ T ≤ 800 °C. The symbols indicate Fe metal (x) and brownmillerite (o).
2.2. Oxygen-Deficient SrFeO3−δ
As discussed in our previous report [17], sample activation is an essential step to decompose CO2. An understanding of the reduction behavior is also needed because CO2 decomposition could mainly be affected by the number of oxygen vacancies and their mobility. CO2 decomposition is induced by the incorporation of O2− into oxygen vacancies. In-situ XRD was performed during high-temperature reduction with 3.5 vol% H2/Ar to identify structural changes occurring in the metal oxide. Figure 1 includes the in-situ X-ray powder patterns of SrFeO3−δ obtained at 500 ≤ T ≤ 800 °C.
SrFeO3−δ was observed in the perovskite phase at room temperature, and the pattern was very similar to that of SrFeO2.75 (PDF# 01-077-9154). As the temperature increased, the perovskite would lose more oxygen and change to a brownmillerite phase. Phase changes from SrFeO3−δ to SrFeO2.5 at 500 °C are attributed to the partial reduction of Fe4+ to Fe3+ [30]. An almost pure brownmillerite phase (SrFeO2.5, PDF# 01-070-0836) was observed at 500–600 °C. The XRD pattern at 500 °C was completely indexed with an orthorhombic unit cell with lattice parameter a = 5.69(9) Å, b = 15.80(2) Å, c = 5.57(2) Å, and V = 501.8(0) Å3. This indicated that the cell volume increased by more than twice via the expansion of one side in the orthorhombic unit cell, especially the b-axis. Srn+1FenO3n+1 and Fe0 peaks have been reported to appear when SrFeO2.5 was reduced further by increasing temperature and reaction time [25]. SrO and Fe0 peaks are considered the final products of the reduction. In our patterns, only a trace of Fe metal (PDF# 01-080-3817) peaks appeared at 2θ ≈ 44.1° and 65.4° at ≥700 °C. Typical Fe0 peaks could be observed at 2θ ≈ 44.0° and 65.3°. The Sr3Fe2O6.14 phase might be possible; however, it overlaps with brownmillerite peaks. In addition, based on our thermogravimetric result (not shown), the change in the nonstoichiometric value (δ) was determined to be ~0.8 at 25 °C ≤ T ≤ 800 °C. Figure 1b shows the calculated unit cell volumes as a function of temperature. They demonstrate linearity over 600 °C [31]. It has been noted that SrFeO3−δ should be activated without complete structural collapse. If these phase changes are reversible, it would be beneficial for catalyst redox reactions or in a chemical looping system.
2.3. Effect of Conductivity on CO2 Decomposition
As we suggested a mechanism in our previous report, CO2 decomposition is considered to be affected significantly by the conductivity of the sample [17]. To decompose CO2 effectively, the metals in the ferrite should easily provide electrons for CO2; therefore, the electronic conductivity plays an important role at first. As the oxygen in neutral CO2 has sufficient electrons, electron transfer from metals on the surface of the ferrite to CO2 is not likely to occur naturally. Therefore, the activation process should be performed before exposure to CO2, and the produced oxygen vacancies become the driving force of the redox reaction. Based on such reasoning, the amount of oxygen vacancies could be the most important factor in this reaction. In addition, the activation process would be easier if the metal oxide contained metals with variable oxidation number.
Once the oxygen ions (i.e., O2−) fill the oxygen vacant sites on the sample surface, the ability to decompose CO2 to CO or C is lost owing to the saturation (or deactivation) of the sample surface. However, if oxygen ions migrate well inside the lattice and oxygen vacancies are reformed on the sample surface, CO2 decomposition could be continued until all vacancies are filled. This is why oxygen ionic conductivity should also be considered. It would be interesting to determine how much of a role oxygen ion conductivity plays in the decomposition of carbon dioxide; however, this will be reported in a separate paper. After all, the oxygen ions accepted through the electronic conductivity effect could move to inside defects via oxygen ionic conducting properties. Therefore, samples with good electrical and ionic conductivity would decompose CO2 more effectively. The total conductivity of SrFeO3−δ is 31.6 S cm−1 at 800 °C [32], which is higher than that of SrFeCo0.5Ox (17 S cm−1) [33]. The total conductivity of SrFeO3−δ shows good agreement with the reference value, and it was determined to be 33.9 S cm−1 at 800 °C from our own measurement. This feature was the reason that SrFeO3−δ was selected for the CO2 decomposition experiment in this paper.
2.4. CO2 Decomposition
To the best of our knowledge, only two studies have reported CO2 decomposition in a continuous gas-flow reactor before our previous report [17]. One [11] provided TGA measurement results, and the other [15] provided extremely limited information. We have used several metal oxides for CO2 decomposition experiments in a continuous-flow reactor. In our previous report, we reported CO2 decomposition with SrFeCo0.5Ox using data obtained under nonisothermal conditions. Even if nonisothermal data are insufficient to cover all practical applications, they can serve as a cornerstone to determine the most economically efficient temperature region for CO2 decomposition. Furthermore, temperature fluctuates during both activation and decomposition processes. Considering these applications, we performed nonisothermal tests, isothermal tests, and cyclic experiments for CO2 decomposition with a noncobalt metal oxide, SrFeO3−δ. A comparison of nonisothermal data for SrFeCo0.5Ox and SrFeO3−δ is presented below.
Nonisothermal CO2 decomposition: Figure 2 shows a comparison of the results of nonisothermal CO2 decomposition using SrFeO3−δ and SrFeCo0.5Ox for temperatures ranging between 25 and 800 °C. Data for SrFeCo0.5Ox were extracted from our previous report [17], and the same experimental conditions were applied. Initially, we started CO2 decomposition with NiFe2O4 as an oxygen-deficient ferrite; however, it decomposed only up to 20% of CO2 in the continuous gas-flow system. We obtained a ~90% efficiency of CO2 decomposition using SrFeCo0.5Ox selected based on our proposed mechanism. Here, we demonstrated several enhanced CO2 decomposition results obtained by using SrFeO3−δ. First, SrFeO3−δ could be activated at a much lower temperature and for shorter duration. SrFeO3−δ was primarily activated at 280 ≤ T ≤ 600 °C, as shown in Figure 2a. The H2 concentration during reduction decreased rapidly up to ≈460 °C, which indicated the phase changes from perovskite to brownmillerite. This behavior can be confirmed from the in-situ XRD data shown in Figure 1. It is believed that oxygen vacancies are created the most at these temperatures. Second, the amount of CO2 decomposed using SrFeO3−δ is approximately 2.2 times higher than that decomposed using SrFeCo0.5Ox based on the calculated result of ≥50% CO2 decomposition, as shown in Figure 2b. As the temperature increased, the CO2 decomposition efficiencies of both metal oxides increased by up to ~90%. After reaching 800 °C, the CO2 decomposition efficiency of SrFeCo0.5Ox decreased, whereas the high decomposition efficiency of SrFeO3−δ was maintained over 100 min. This indicates that SrFeO3−δ might be a more appropriate material for CO2 decomposition than SrFeCo0.5Ox. The amount of CO produced using SrFeO3−δ was also slightly higher than that produced using SrFeCo0.5Ox.
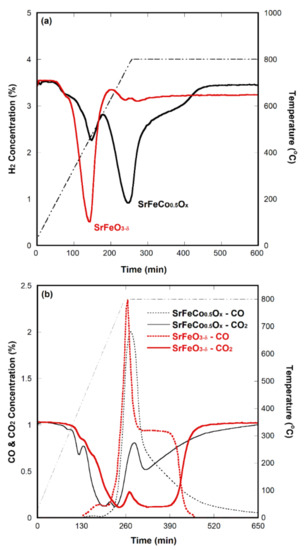
Figure 2.
CO2 decomposition results: (a) Consumed H2 concentration and (b) CO2 and CO concentrations during sample activation and CO2 decomposition tests, respectively. The black lines indicate the results of SrFeCo0.5Ox extracted from our previous work [17], and the straight dotted lines indicate temperature profiles (i.e., 3 °C/min).
The CO2 decomposition ability could be expressed in units of millimoles of decomposed CO2 and generated CO per gram of sample loaded (i.e., mmol g−1). This calculation was made using several assumptions. For example, the final decomposition was determined to be the point at which CO2 decomposition ceased, resulting in the revelation of the initial CO2 concentration. The final CO2 decomposition time was determined during the point at which SrFeO3−δ started to decompose at 200 °C and continued for over 4 h, even reaching 800 °C. This calculation was made using the decomposition time limit that ranged between 54 and 500 min (see Figure 2b). Secondly, in spite of nonisothermal CO2 decomposition, the ideal gas law was used to calculate the decomposed amount (i.e., mmol g−1) of input CO2. The exact same conditions were applied for the NiFe2O3−δ and SrFeCo0.5Ox samples. The results are summarized in Table 1. Both CO2 decomposition and CO generation using a perovskite (SrFeO3−δ) demonstrated enhanced performance compared to those using a spinel (NiFe2O3−δ) or a nonperovskite (SrFeCo0.5Ox). In addition, SrFeO3−δ is a cobalt-free compound that is economical and environmentally friendly. Generally, cobalt-containing metal oxides display good catalytic behavior but have several shortcomings, such as structural instability even at intermediate operating temperatures (500 to 800 °C) in a long-term test [34]. In the case of NiFe2O3−δ, other shortcomings were observed, such as too long an oxidation time.

Table 1.
The rate of decomposed CO2 and generated CO in nonisothermal experiments.
Isothermal CO2 decomposition: For practical applications, data for CO2 decomposition using SrFeO3−δ at constant temperature should be determined. Figure 3 shows the isothermal results. The measurements were performed at 500, 600, 625, 650, 700, and 800 °C. The temperatures for sample activation and decomposition were identically controlled, and a blank test was also performed in the same reactor. GC was used to determine data points every ~4 min after switching the gas with 1 vol% CO2/He. Fresh powder samples were used in each measurement. As the temperature increased, the amounts of CO2 decomposition and CO production increased (see Figure 3a,b). This is probably attributable to the amount and high mobility of oxygen vacancies at higher temperatures. The ionic conductivities are proportional to the mobility of perovskite metal oxides (i.e., σ = n × e × μ, where σ is the specific conductivity; n, the number of charge carriers of a species; e, its charge; and μ, its mobility) and generally increases with the temperature [35].
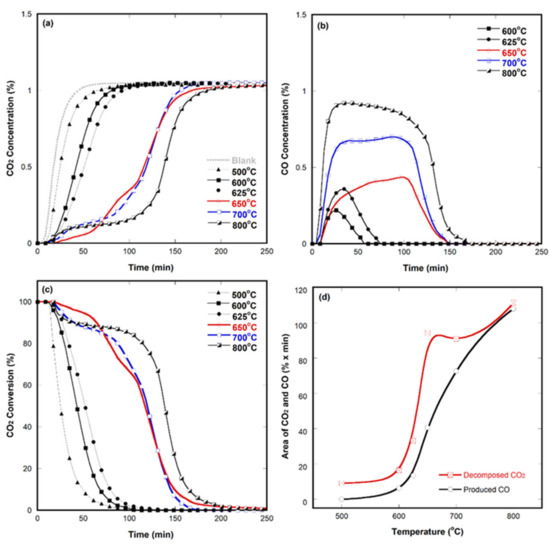
Figure 3.
Isothermal CO2 decomposition results obtained using SrFeO3−δ at various temperatures: (a) Decomposed CO2 concentration, (b) produced CO concentration, (c) CO2 conversion as a function of time, and (d) decomposed CO2 and produced CO area extracted from (a) and (b).
The CO2 decomposition results between 600 and 700 °C were noteworthy. As the operating temperature increased from 625 to 650 °C, the amount of CO2 decomposition doubled. We performed in-situ XRD and TGA experiments to analyze this unusual behavior in this temperature region (not shown). However, no special structural phase or weight changes were seen in the sample activation process. The amounts of consumed hydrogen for sample activation and cell parameters also demonstrated no considerable difference. The reason for the sudden increase in CO2 decomposition upon increasing temperature by only 25 °C remains unclear. We presume that the thermal energy at 650 °C might boost CO2 decomposition and the reverse Boudouard reaction (i.e., C(s) + CO2 → 2CO). The mobility increase caused by the thermal energy might be an important factor because other factors, such as the unit cell volume, oxygen ion vacancy concentration, and weight change from TGA, did not change abruptly. These issues are discussed further by comparing sample characteristics before and after performing measurements in Section 2.5.
Based on the obtained data, CO2 conversion rates were calculated using Equation (1).
Although Figure 3c illustrates the same data in the same format as Figure 3a, the conversion degree is easier to distinguish from the CO2 conversion plot. Furthermore, ≥90% of CO2 conversion lasted for ≈65 min at 650 °C. This drastic change was much more evident from the area plots of the decomposed CO2 and produced CO shown in Figure 3d. We calculated these areas by subtracting those obtained in the isothermal blank tests. It should be noted that the area for CO2 (i.e., amount of CO2 decomposition) was unusually high at 650 °C. It was even slightly higher than that at 700 °C. Further, CO production increased rapidly until the temperature was increased up to 800 °C. The shape of the isothermal CO2 decomposition curve at 650 °C also slightly differed from those of the others. We plan to analyze these behaviors using temperature-programed reduction and temperature-programed oxidation in a separate paper.
2.5. Stability Tests
Irrespective of how high the efficiency is, samples should have reproducibility and long-term stability, especially in severe redox reactions. This is the most important criterion for practical applications. In this report, we performed CO2 decomposition in five reproducibility tests. We also performed cyclic CO2 decomposition tests with partially activated SrFeO3−δ; however, we did not include the data, owing to their overlapped content. After these stability tests, the changes in the structural behavior and surface morphology of the used SrFeO3−δ were investigated.
Figure 4 shows the results of the five cyclic reproducibility tests for CO2 decomposition using SrFeO3−δ at 700 °C. The sample was activated for 800 min in each cycle. The CO2 decomposition of each cycle was subtracted from the amount of the blank test. The measurement data of the last cycle (i.e., the fifth cycle) did not perfectly match those of the first. However, they were reasonably close, and the difference could be attributed to the annealing effect of lasting temperatures. Average amounts of decomposed CO2 and generated CO were determined to be 1.38 and 1.02 mmol per gram of catalyst, respectively. This result indicated a certain level of coke generation or possible adsorption of part of the carbon dioxide instead of decomposition. Although SrFeO3−δ demonstrated good reproducibility even after repeating the redox experiment several times, it still took too long to activate the sample. In these days, we are using coke oven gas from the steel industry for sample activation. As it contains 55% to 60% hydrogen, the activation time will be much faster. In addition, SrFeO3−δ impregnated with a small amount of precious metals such as Ru and Rh is tested for low-temperature CO2 decomposition. Table 2 summarizes the test results.
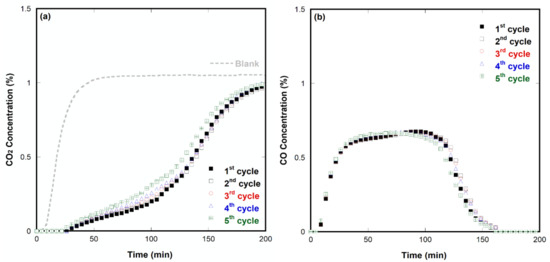
Figure 4.
Five cyclic reproducibility tests for CO2 decomposition using SrFeO3−δ at 700 °C: (a) Decomposed CO2 concentration and (b) produced CO concentration.

Table 2.
Results of isothermal cyclic experiments with SrFeO3−δ.
To investigate the structural changes, XRD measurements were performed with the tested SrFeO3−δ powders. Figure 5a shows the sample used for the nonisothermal CO2 decomposition experiment at up to 800 °C. Figure 5b,d illustrate the powder patterns of SrFeO3−δ that underwent cyclic tests at 700 and 650 °C, respectively. The brownmillerite phase remained, and it was hard to find impurities, including even traces of Fe-metal peaks. This indicates that the redox reaction is reversible and that SrFeO3−δ can presumably serve as an excellent reactant. For comparison, the in-situ XRD result extracted from Figure 1 was added in Figure 5c. Three oxidized XRD patterns (i.e., Figure 5a,b,d) shifted to a higher 2θ angle; Figure 5c shows the pattern of the reduced sample. When the sample reduced, the oxygen vacancy concentration increased, resulting in its increased volume. The unit cell volume of reduced SrFeO3−δ at 700 °C was 504.1 Å3, and that of the oxidized sample decreased to 489.1 Å3. Table 3 lists the fully indexed unit cell parameters for SrFeO3−δ tested at 700 °C.
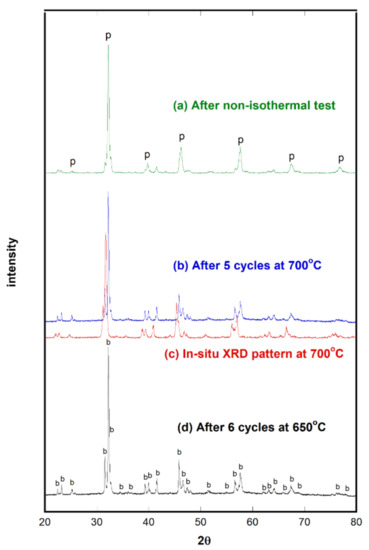
Figure 5.
XRD powder patterns of SrFeO3−δ tested for CO2 decomposition measurements: (a) After nonisothermal test, (b) after five cycles at 700 °C, (c) in-situ XRD at 700 °C, and (d) after six cycles at 650 °C. The symbols indicate perovskite (p) and brownmillerite (b).

Table 3.
Experimental conditions of nonisothermal and isothermal tests with SrFeO3−δ.
It should be noted that the powder pattern of the sample tested at 800 °C shows a mixed phase, that is, perovskite and brownmillerite, whereas the sample tested at ≤700 °C primarily shows a brownmillerite phase. This result is relevant to the increased amounts of decomposed CO2 at 800 °C (see Figure 3a). For a perovskite phase to exist at 800 °C, more oxygen vacancies need to be filled. This condition is induced by CO2 decomposition. It should also be noted that three oxidized samples were slightly reduced with N2 because 1 vol% CO2/He gas was switched with N2 after decomposition tests when cooling down to room temperature.
The microstructure of SrFeO3−δ was examined using SEM before and after CO2 decomposition experiments. Figure 6a shows a secondary electron image of pristine SrFeO3−δ, indicating that many small-sized (≤100 nm) particles are attached and dispersed on bigger ones. These small particles grew twice as large after the redox tests at 650 °C, and some large particles appeared to aggregate to each other (see Figure 6b). Even larger agglomerates developed and were observed in the sample tested at 700 °C, as shown in Figure 6c. Furthermore, the agglomerated particles (even ≥1 μm) displayed distinct grain boundaries. As the activity of samples is generally believed to depend on their surface area, a detailed microstructural investigation will be conducted in a separate study.
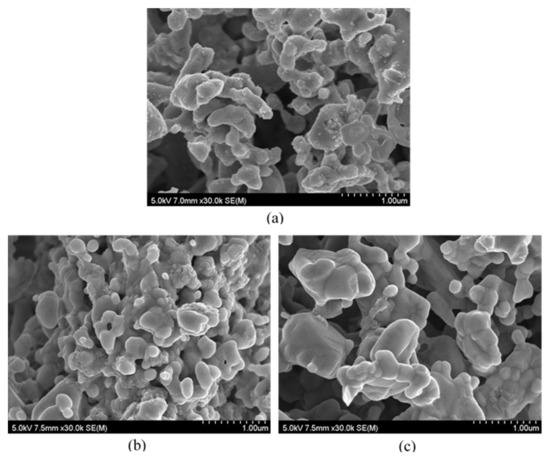
Figure 6.
SEM images of SrFeO3−δ: (a) Before the redox test, (b) after six cycles of redox measurements at 650 °C, and (c) after five cycles of reproducibility tests at 700 °C.
3. Materials and Methods
3.1. SrFeO3−δ Preparation and Characterization
SrFeO3−δ perovskite-type metal oxide was prepared using solid-state synthesis for use as a CO2 decomposition material. The metal oxide was purchased from K-ceracell Co. Ltd. (Taejeon, Korea), and SrCO3 (Alfa Aesar, >99%, Ward Hill, MA, USA) and Fe2O3 (Alfa Aesar, >99.9%, Ward Hill, MA, USA) were used as starting materials. After weighing the appropriate amount of materials, the powders were mixed in ethanol (Samchun Chemicals, >99.9%, Seoul, Korea). The mixed powder was ball-milled with zirconia balls (φ3–5 mm) for 48 h and then dried to remove the solvent. SrFeO3−δ powder was heated at 1000 °C for 3 h in ambient air. The final powders were obtained by ball-milling with ethanol for 24 h and then drying in an oven at 80 °C for 24 h.
The synthesized powders were characterized using X-ray powder diffraction (XRD, Rigaku D/MAX-2500, Tokyo, Japan), scanning electron microscopy (SEM, S-4800 Hitachi, Hitachi, Japan), and energy-dispersive X-ray spectroscopy (EDS, Thermo Scientific, Waltham, MA, USA) to analyze the phase structure and purity, surface morphology, and chemical composition, respectively. In-situ XRD (Rigaku D/MAX-2500, Tokyo, Japan) and thermogravimetric analysis (TGA, TA Instruments, Milford, MA, USA) were used to investigate the reduction behaviors of the synthesized powder. In-situ XRD and the change in weight were measured with ≈100 mL min−1 of 3.5 vol% H2/Ar.
3.2. CO2 Decomposition Experiments
We used a continuous-flow reactor to investigate the products of the CO2 decomposition reaction in the tests. Sample activation and CO2 decomposition were performed with 3.5 vol% H2/N2 and 1 vol% CO2/He, respectively. Approximately 1.5 g of sample powder with zirconia balls (φ2–3 mm, 10 g) was placed at the center of a quartz tube (I.D.: 12 mm, O.D.: 16 mm, height: 600 mm). The flow rate for the sample activation and CO2 decomposition was 50 mL min−1. The CO2 decomposition experiments were performed under increasing temperature at 25 ≤ T ≤ 800 °C (nonisothermal). The ramp rate was 3 °C min−1. The same measurements were performed at certain fixed temperatures between 500 and 800 °C (isothermal). The residence time was calculated to be 3.393 s. CO2 decomposition cycle tests were also performed to check the regeneration, reproducibility, and stability of the sample. The temperature was selected with the results based on the isothermal CO2 decomposition results. Five cyclic tests under a fixed condition were performed at 700 °C. The blank tests were carried out under identical conditions by using only zirconia balls instead of the sample powder. Table 3 summarizes the experimental conditions for both activation and decomposition. The produced gas concentrations were measured with an Agilent 8890 gas chromatograph (GC, ShinCarbon ST 100/120 micropacked column, Bellefonte, PA, USA). The details of this experiment have been described in a previous report [27].
4. Conclusions
We demonstrated that SrFeO3−δ could serve as a promising material for effective CO2 decomposition in a continuous gas-flow system. In this study, we performed three categorized CO2 treatment experiments using SrFeO3−δ: nonisothermal, isothermal, and stability tests. In nonisothermal experiments, the maximum CO2 decomposition rate reached ~90% and the decomposition rate (≥80%) lasted for around 170 min. Both CO2 decomposition and CO generation by SrFeO3−δ exhibited better performance than those of NiFe2O3−δ or SrFeCo0.5Ox. The results of isothermal tests indicated that the optimized temperature for CO2 decomposition should range between 650 and 700 °C. As the operating temperature increased from 625 to 650 °C, the amount of CO2 decomposition increased unusually, even though no special structural phase or weight changes occurred. In addition, SrFeO3−δ maintained the CO2 decomposition efficiency during isothermal cyclic and reproducibility experiments. Therefore, SrFeO3−δ is expected to have the capacity to contribute to the mitigation of CO2, a greenhouse gas. Nonetheless, studies of CO2 utilization should be continued because many unsolved problems remain in this field.
Author Contributions
Conceptualization, C.Y.P. and S.-C.N.; methodology, J.S.; investigation, S.-H.K. and J.-Y.K.; data curation, C.Y.P. and J.S.; writing—original draft preparation, J.S.; writing—review and editing, K.B.L. and C.Y.P.; supervision, C.Y.P.; project administration, S.-C.N.; funding acquisition, S.-C.N. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the financial support of the National Research Foundation under the “Next Generation Carbon Upcycling Project” (Project No. 2017M1A2A2043109) of the Ministry of Science and ICT, Republic of Korea.
Acknowledgments
The analytical support from the Platform Technology Laboratory at Korea Institute of Energy Research is much appreciated, especially the invaluable assistance provided for in-situ XRD and surface analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meinshausen, M.; Meinshausen, N.; Hare, W.; Raper, S.C.B.; Frieler, K.; Knutti, R.; Frame, D.J.; Allen, M.R. Greenhouse-gas emission targets for limiting global warming to 2 °C. Nat. Cell Biol. 2009, 458, 1158–1162. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.H. Potential sources of CO2 and the options for its large-scale utilization now and in the future. Catal. Today 1995, 23, 59–66. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.O.; Roberts, D.; Skea, J.; Shukla, P.R.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R.; et al. Summary for policymakers: Global warming of 1.5 °C. IPCC 2018, 1, 1–32. [Google Scholar]
- Bergman, P.D.; Winter, E.M. Disposal of carbon dioxide in aquifers in the U.S. Energy Convers. Manag. 1995, 36, 523–526. [Google Scholar] [CrossRef]
- Rubin, E.S.; Rao, A.B. A Technical, Economic and Environmental Assessment of Amine-Based CO2 Capture Technology for Power Plant Greenhouse Gas Control. In A Technical, Economic and Environmental Assessment of Amine-Based CO2 Capture Technology for Power Plant Greenhouse Gas Control; Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 2002; Volume 36, pp. 4467–4475. [Google Scholar]
- Desideri, U.; Paolucci, A. Performance modelling of a carbon dioxide removal system for power plants. Energy Convers. Manag. 1999, 40, 1899–1915. [Google Scholar] [CrossRef]
- Leung, D.Y.; Caramanna, G.; Maroto-Valer, M.M. An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Ferraro, M.; Antonucci, P. Supported catalysts for CO2 methanation: A review. Catalysts 2017, 7, 59. [Google Scholar] [CrossRef]
- Mark, M.F.; Maier, W.F. CO2-Reforming of methane on supported Rh and Ir catalysts. J. Catal. 1996, 164, 122–130. [Google Scholar] [CrossRef]
- Tamaura, Y.; Tahata, M. Complete reduction of carbon dioxide to carbon using cation-excess magnetite. Nat. Cell Biol. 1990, 346, 255–256. [Google Scholar] [CrossRef]
- Shin, H.C.; Choi, S.C.; Jung, K.D.; Han, S.H. Mechanism of M ferrites (M = Cu and Ni) in the CO2 Decomposition reaction. Chem. Mater. 2001, 13, 1238–1242. [Google Scholar] [CrossRef]
- Tabata, M.; Nishida, Y.; Kodama, T.; Mimori, K.; Yoshida, T.; Tamaura, Y. CO2 decomposition with oxygen-deficient Mn(II) ferrite. J. Mater. Sci. 1993, 28, 971–974. [Google Scholar] [CrossRef]
- Kodama, T.; Tabata, M.; Tominaga, K.; Yoshida, T.; Tamaura, Y. Decomposition of CO2 and CO into carbon with active wustite prepared form Zn(II)-bearing ferrite. J. Mater. Sci. 1993, 28, 547–552. [Google Scholar] [CrossRef]
- Shin, H.C.; Oh, J.H.; Lee, J.C.; Han, S.H.; Choi, S.C. The carbon dioxide decomposition reaction with (NixCu1-x)Fe2O4 solid solution. Phys. Stat. Sol. A 2002, 189, 741–745. [Google Scholar] [CrossRef]
- Kim, J.S.; Ahn, J.R. Characterization of wet processed (Ni, Zn)-ferrites for CO2 decomposition. J. Mater. Sci. 2001, 36, 4813–4816. [Google Scholar] [CrossRef]
- Kim, J.S.; Ahn, J.R.; Lee, C.W.; Murakami, Y.; Shindo, D. Morphological properties of ultra-fine (Ni,Zn)-ferrites and their ability to decompose CO2. J. Mater. Chem. 2001, 11, 3373–3376. [Google Scholar] [CrossRef]
- Kim, S.H.; Jang, J.T.; Sim, J.; Lee, J.H.; Nam, S.C.; Park, C.Y. Carbon dioxide decomposition using SrFeCo0.5Ox, a nonperovskite-type metal oxide. J. CO2 Util. 2019, 34, 709–715. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, H.; Liu, X.; Shen, Y.; Xu, L. Bismuth doping effects on the structure, electrical conductivity and oxygen permeability of Ba0.6Sr0.4Co0.7Fe0.3O3−δ ceramic membranes. Int. J. Hydrogen Energy 2012, 37, 12694–12699. [Google Scholar] [CrossRef]
- Li, X.; Kerstiens, T.; Markus, T. Oxygen permeability and phase stability of Ba0.5Sr0.5Co0.8Fe0.2O3−δ perovskite at intermediate temperatures. J. Membr. Sci. 2013, 438, 83–89. [Google Scholar] [CrossRef]
- Kovalevsky, A. Processing and oxygen permeability of asymmetric ferrite-based ceramic membranes. Solid State Ionics 2008, 179, 61–65. [Google Scholar] [CrossRef]
- Leo, A.; Liu, S.; Da Costa, J.C.D. Development of mixed conducting membranes for clean coal energy delivery. Int. J. Greenh. Gas Control. 2009, 3, 357–367. [Google Scholar] [CrossRef]
- Shao, Z.; Haile, S.M. A high-performance cathode for the next generation of solid-oxide fuel cells. Chemin 2004, 35. [Google Scholar] [CrossRef]
- Fuks, D.; Mastrikov, Y.A.; Kotomin, E.A.; Maier, J. Ab initio thermodynamic study of (Ba,Sr)(Co,Fe)O3 perovskite solid solutions for fuel cell applications. J. Mater. Chem. A 2013, 1, 14320. [Google Scholar] [CrossRef]
- Falcón, H.; Barbero, J.A.; Alonso, J.A.; Martínez-Lope, M.J.; Fierro, J.L.G. SrFeO3−δ Perovskite oxides: Chemical features and performance for methane combustion. Chem. Mater. 2002, 14, 2325–2333. [Google Scholar] [CrossRef]
- Marek, E.; Hu, W.; Gaultois, M.; Grey, C.P.; Scott, S.A. The use of strontium ferrite in chemical looping systems. Appl. Energy 2018, 223, 369–382. [Google Scholar] [CrossRef]
- Takeda, Y.; Kanno, K.; Takada, T.; Yamamoto, O.; Takano, M.; Nakayama, N.; Bando, Y. Phase relation in the oxygen nonstoichiometric system, SrFeOx (2.5 ≤ × ≤ 3.0). J. Solid State Chem. 1986, 63, 237–249. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, Q.; Wang, S.; Komvokis, V.G.; Amiridis, M.D.; Heyden, A.; Ma, S.; Chen, F. Synthesis and characterization of Mo-doped SrFeO3−δ as cathode materials for solid oxide fuel cells. J. Power Sources 2012, 202, 63–69. [Google Scholar] [CrossRef]
- Ji, K.; Dai, H.; Deng, J.; Zhang, L.; Wang, F.; Jiang, H.; Au, C.T. Three-dimensionally ordered macroporous SrFeO3−δ with high surface area: Active catalysts for the complete oxidation of toluene. Appl. Catal. A Gen. 2012, 425, 153–160. [Google Scholar] [CrossRef]
- Hombo, J.; Matsumoto, Y.; Kawano, T. Electrical conductivities of SrFeO3−δ and BaFeO3−δ perovskites. J. Solid State Chem. 1990, 84, 138–143. [Google Scholar] [CrossRef]
- Hodges, J.; Short, S.; Jorgensen, J.; Xiong, X.; Dabrowski, B.; Mini, S.; Kimball, C. Evolution of oxygen-vacancy ordered crystal structures in the perovskite series SrnFenO3n−1 (n = 2, 4, 8, and ∞), and the relationship to electronic and magnetic properties. J. Solid State Chem. 2000, 151, 190–209. [Google Scholar] [CrossRef]
- Khare, A.; Lee, J.; Park, J.; Kim, G.Y.; Choi, S.Y.; Katase, T.; Roh, S.; Yoo, T.S.; Hwang, J.; Ohta, H.; et al. Directing oxygen vacancy channels in SrFeO2.5 epitaxial thin films. ACS Appl. Mater. Interfaces 2018, 10, 4831–4837. [Google Scholar] [CrossRef]
- Vashuk, V.V.; Kokhanovskii, L.V.; Yushkevich, I.I. Electrical conductivity and oxygen stoichiometry of SrFeO3−δ. Inorg. Mater. 2000, 36, 79–83. [Google Scholar] [CrossRef]
- Ma, B.; Victory, N.I.; Balachandran, U.; Mitchell, B.J.; Richardson, J.W. Study of the mixed-conducting SrFeCo0.5Oy system. J. Am. Ceram. Soc. 2004, 85, 2641–2645. [Google Scholar] [CrossRef]
- Park, C.; Lee, T.; Dorris, S.; Park, J.H.; Balachandran, U. Ethanol reforming using Ba0.5Sr0.5Cu0.2Fe0.8O3−δ/Ag composites as oxygen transport membranes. J. Power Sources 2012, 214, 337–343. [Google Scholar] [CrossRef]
- West, A.R. Solid State Chemistry and Its Application; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1984. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).