Recent Advances in Glycerol Catalytic Valorization: A Review
Abstract
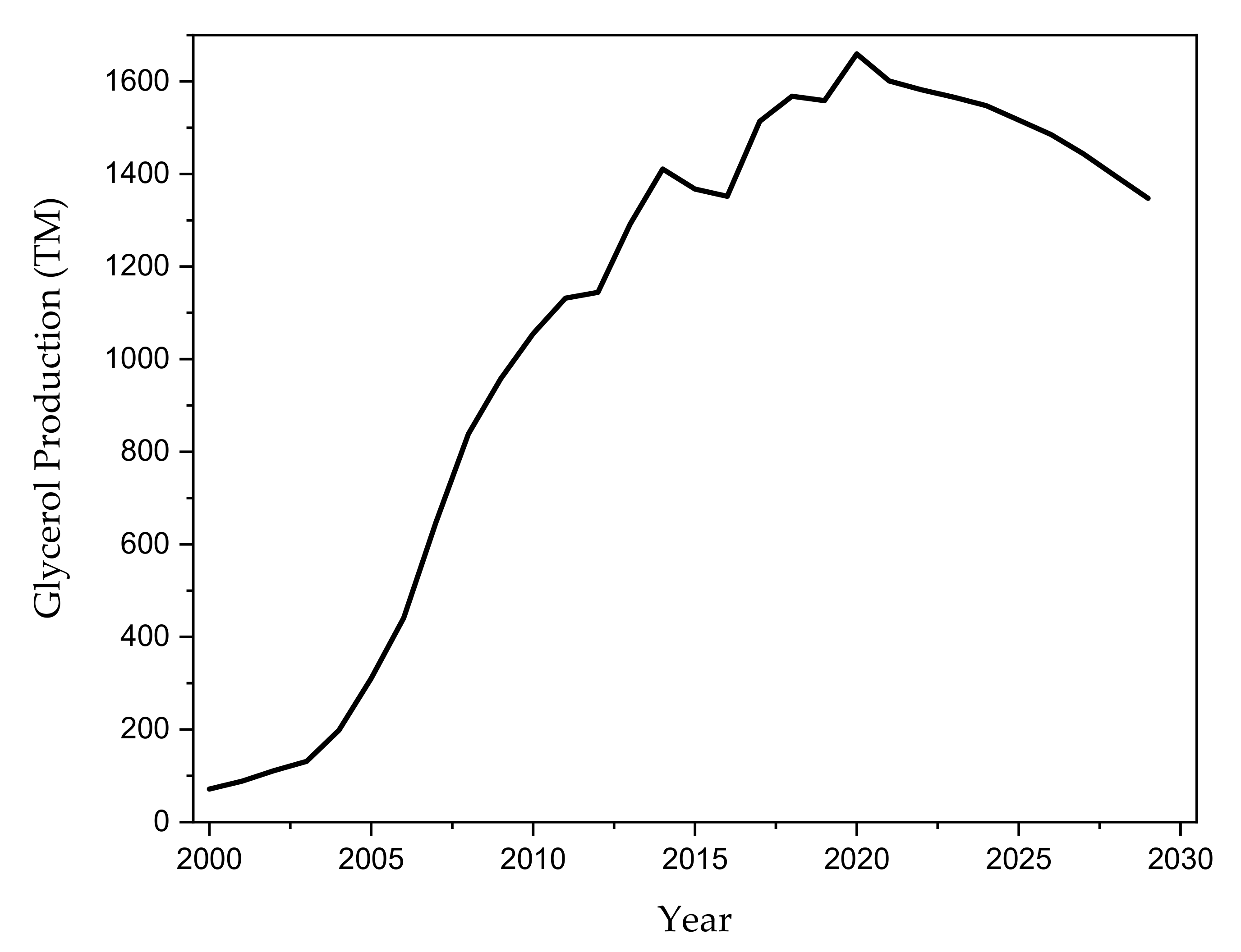
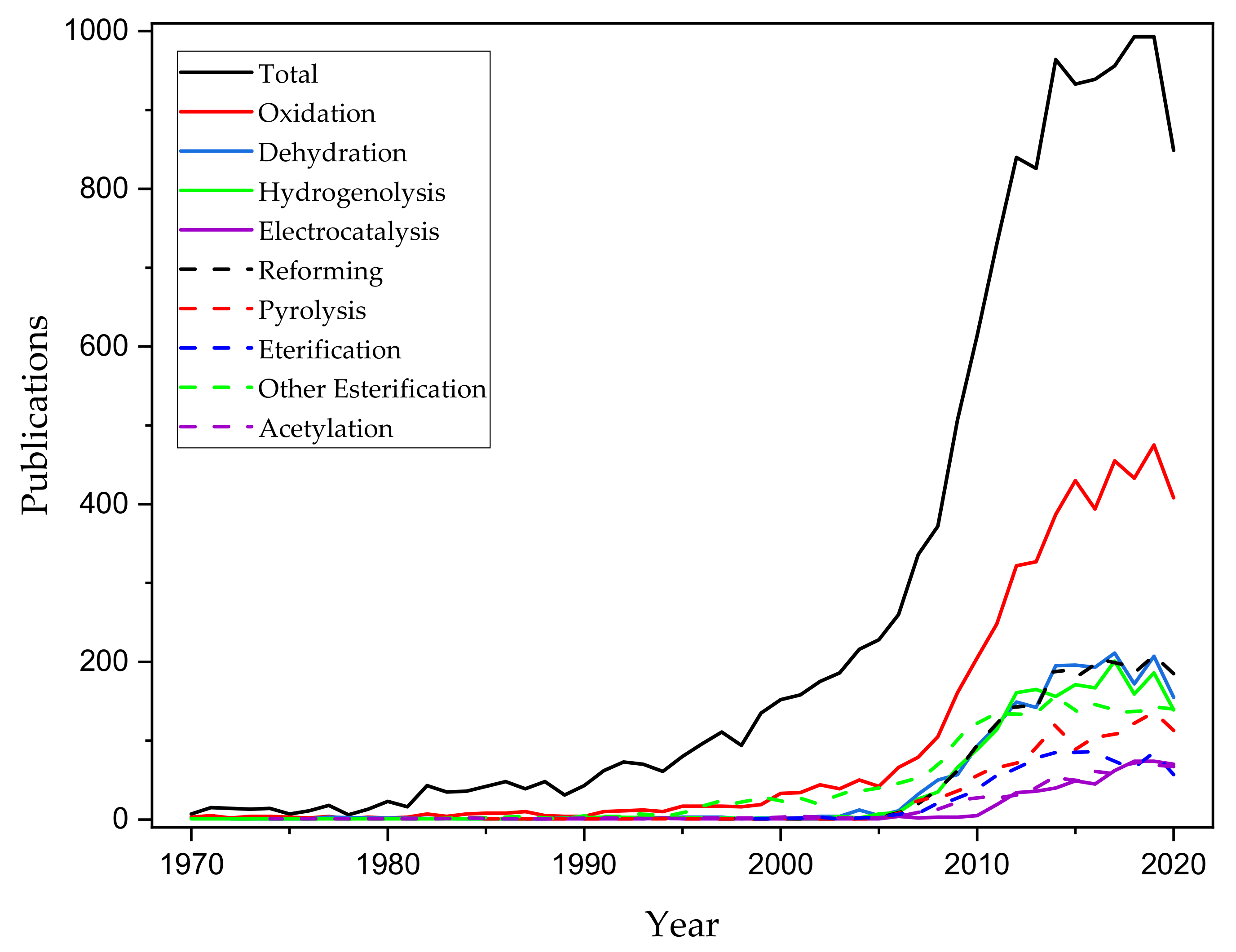
1. Introduction
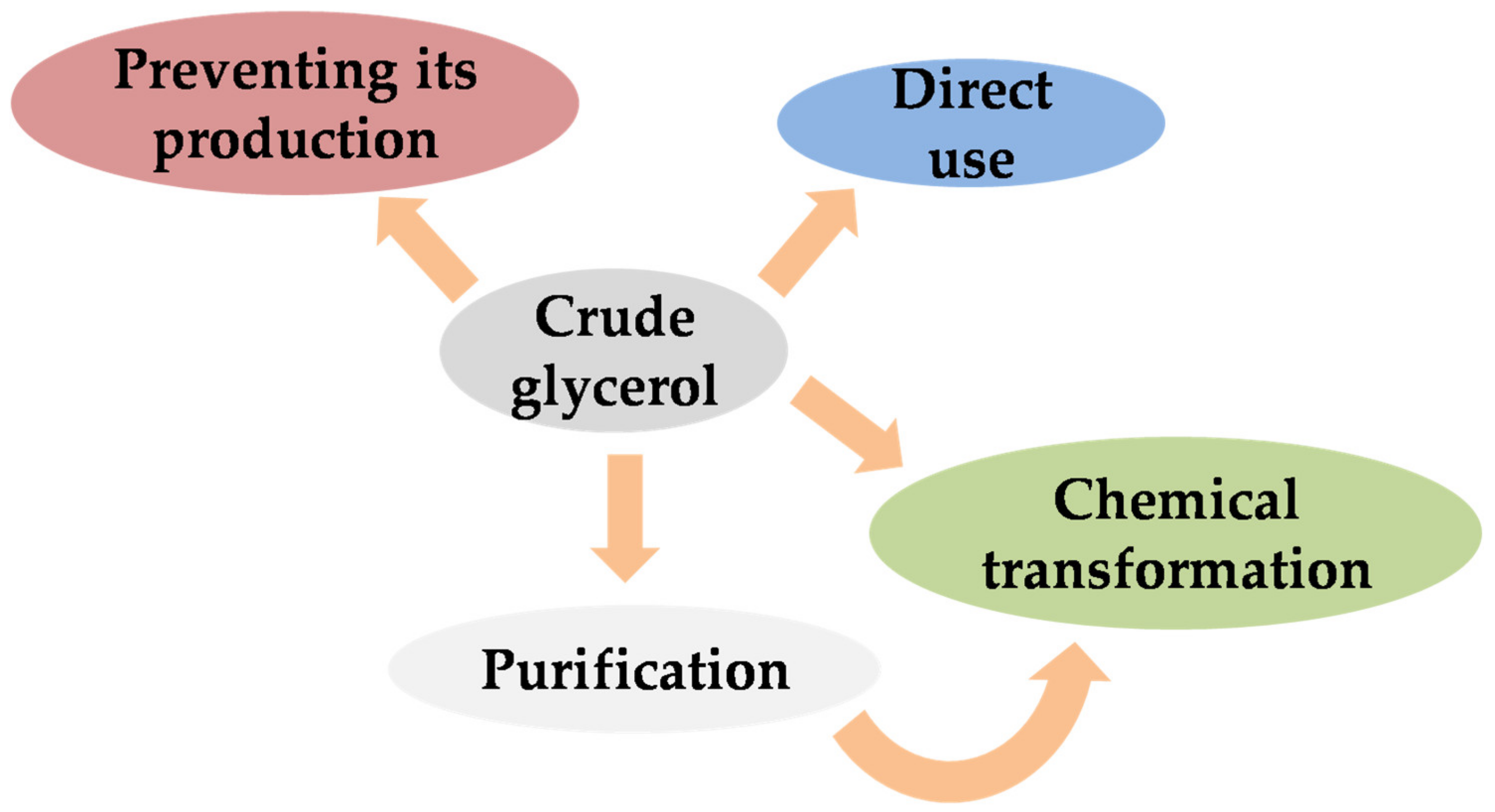
2. Treatment or Management of Crude Glycerol
- Direct use: The main advantage of the direct use of crude glycerol is the avoidance of further and more expensive steps for glycerol management. Depending on the global composition (methanol, water, soap, or organic matter content) and the purity of crude glycerol, different direct processes can be suitable, such as anaerobic digestion, animal feed, thermo-chemical conversion, etc. Nowadays, the direct combustion of crude glycerol is not advisable, due to its low heating value (and therefore its low energy efficiency) and the unsustainable nature of this process, implying the production of toxic products, such as acrolein [1]. However, in practice, there are a few alternatives that can be used instead of low-purity glycerol [38]. That is the reason why the rest of the options are more popular in research. Nevertheless, new trends are currently being considered for the direct use of crude or residual glycerol, such as its use as an additive to produce biohydrogen by the anaerobic biodigestion of cassava wastewater [39];
- Preventing its production: In this case, other derivatives of glycerol are produced during transesterification by different means, resulting in interesting compounds, such as glycerol triacetate (Glyperol), glycerol carbonate (DMC-Biod), and monoglyceride (Ecodiesel), which contribute to a better atom yield during the transesterification of fatty acids [40,41];
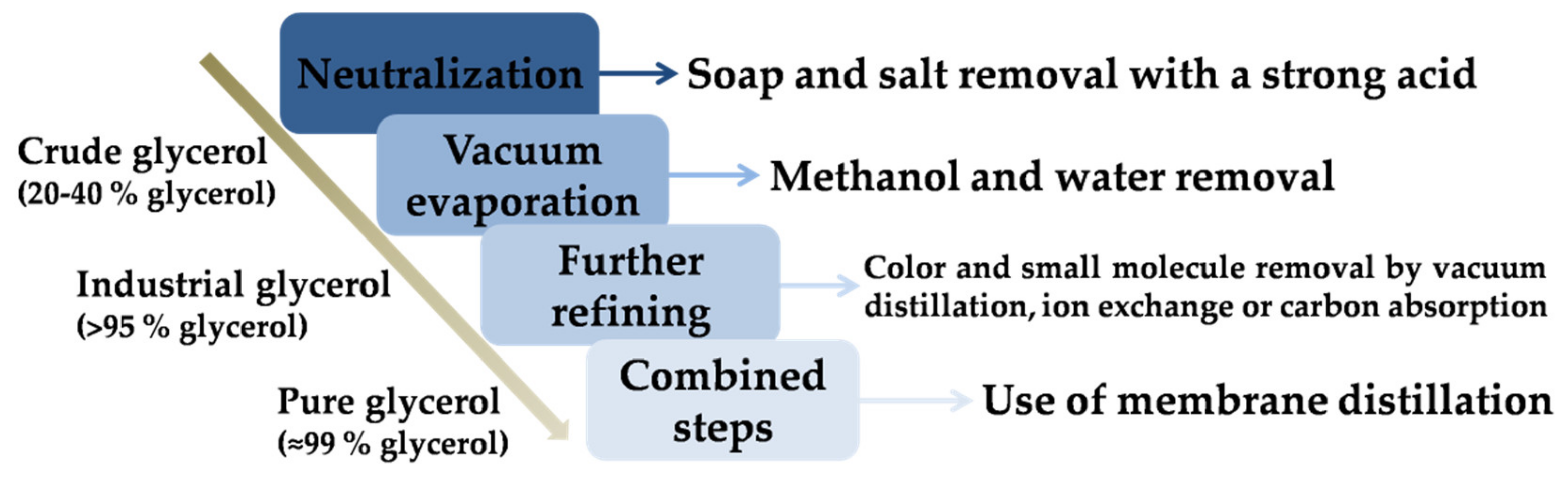
- Purification: As abovementioned, crude glycerol has low prices, with its purification being expensive for small companies. This fact can explain the existence of the two previous options mentioned, and the research related to glycerol purification to develop a cheaper and more competitive process. Therefore, the alternative is the use of cheap or simple purification steps, in order to obtain high-quality glycerol as a starting point for the production of plenty of products (up to 1500) for multiple purposes [1]. Depending on the kind of catalyst used during the transesterification to obtain fatty acid methyl esters, the purification process can vary. The use of homogeneous catalysts (which are commonly used in industry) requires the separation of glycerol through a settler unit, the removal of surplus/excess catalysts with mineral acids, etc. On the other hand, the use of heterogeneous catalysts (which has some advantages, but usually few cycles offer acceptable yields) requires a double separation process, without catalyst neutralization [3,33]. Figure 4 presents a brief schematic representation of the main separation stages and the glycerol purity corresponding to the use of homogeneous catalysts, following previous separation through decanting or centrifugation from biodiesel.It should be noted that further refining steps can imply high energy costs, especially in the case of vacuum distillation. Other alternatives are the use of cyclic distillation columns or dividing wall distillation. If ion exchange is used, the removal of fatty acids, inorganic salts, and free ions at room temperature is possible and therefore low energy consumption is possible. Carbon absorption is required to remove small molecules (like lauric and myristic acids) and decolorization. Finally, for a high level of purification or pure glycerol, the mentioned techniques are simultaneously used—commonly known as combined steps—alternatively with membrane distillation [1].Pure glycerol has a wider range of potential use than crude glycerol, due to its low toxicity, biodegradability, low vapor pressure, and high boiling point. For instance, it can be used in pharmaceutical, cosmetic, explosive, solvent, and food industries, among others [33,38]. Moreover, pure glycerol is normally used as starting material for further catalytic valorization, as it minimizes secondary reactions given or catalyzed by other compounds contained in crude glycerol. It should be underlined that the crude glycerol composition can vary due to the employment of different kinds of catalysts, their concentration, the alcohol/oil ratio, raw materials (especially vegetable oils, whose fatty acid profile considerably varies), and the yield of the transesterification reaction (and the subsequent crude glycerol purity). The wide range in composition may require some adjustments in the separation process (Figure 4), in order to be adequate for each crude glycerol obtained [38].Alternatively, it should be noted that the separation process included in Figure 4 might require extra steps or variation, according to the crude glycerol obtained in each process. For instance, vegetable oils, whose fatty acid profile considerably varies, depending on the raw material, lead to a wide range of transesterification yields and thus subsequent crude glycerol purities [38];
- Chemical transformation: Both in the case of crude or pure glycerol (especially for the latter), multiple chemical routes have been investigated to produce more valuable products. Among them, the most common or described chemical routes should be pointed out, such as glycerol carboxylation, hydrogenolysis, oxidation, acetylation, and reforming, among others, which will be thoroughly explained in the following sections [1,29].
3. Energy Use of Glycerol
3.1. Combustion
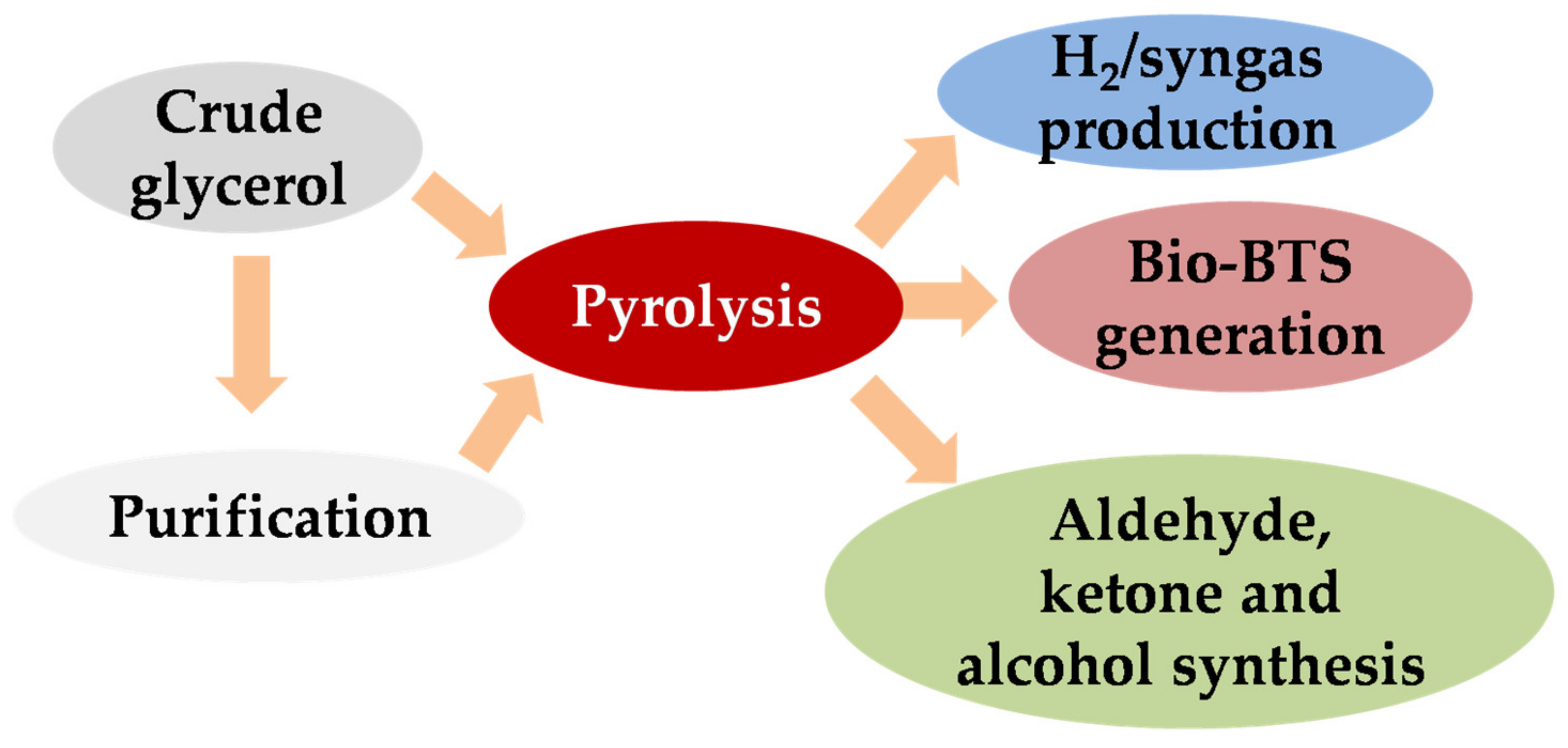
3.2. Pyrolysis
3.3. Glycerol Reforming
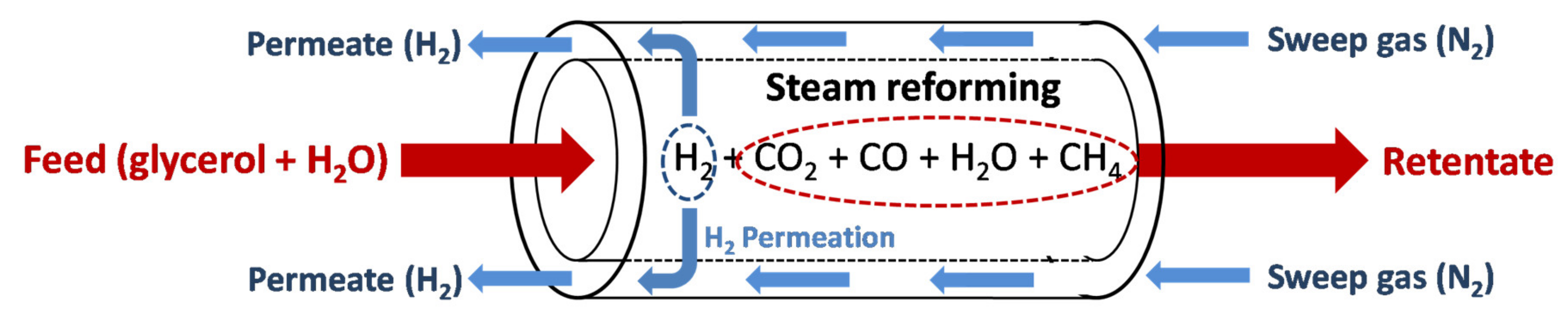
3.3.1. Steam Reforming (SR)
3.3.2. Aqueous Phase Reforming (APR)
- Glycerol should be adsorbed over the active metal sites of the catalyst;
- It should have good support properties (dispersion of the active phase, co-catalytic sites, etc.);
- If possible, its cost should be low.
3.3.3. Dry Reforming
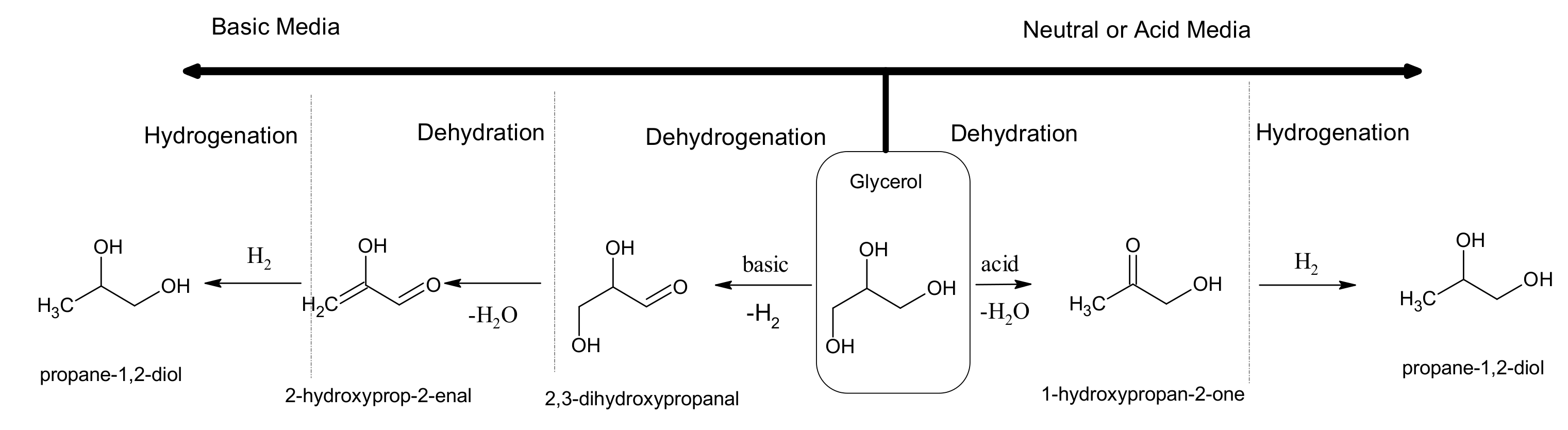
4. Hydrogenolysis and Reduction Processes
5. Glycerol Selective Oxidation
6. Production of Glycerol Carbonate
7. Production of Solketal or Acetalization of Glycerol
8. Emergent Valorization Routes
8.1. Emergent Strategy Developed to Obtain H2, CO, and Syngas
8.1.1. Photoreforming
8.1.2. Catalytic Transfer Hydrogenation (CTH)
8.2. Production of Valuable Chemicals
8.2.1. Conversion of Glycerol to Alkyl-Aromatics (Gasoline)
8.2.2. Production of Activated Carbon from Glycerol
8.3. Strategies of Glycerol Application
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodrigues, A.; Bordado, J.C.; Dos Santos, R.G. Upgrading the Glycerol from Biodiesel Production as a Source of Energy Carriers and Chemicals—A Technological Review for Three Chemical Pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef]
- Pagliaro, M.; Rossi, M. The Future of Glycerol, 2nd ed.; Clark, J.H., Kraus, G.A., Eds.; Green Chemistry Series; Royal Society of Chemistry: Cambridge, UK, 2010; ISBN 978-1-84973-046-4. [Google Scholar]
- Bagnato, G.; Iulianelli, A.; Sanna, A.; Basile, A. Glycerol Production and Transformation: A Critical Review with Particular Emphasis on Glycerol Reforming Reaction for Producing Hydrogen in Conventional and Membrane Reactors. Membranes 2017, 7, 17. [Google Scholar] [CrossRef]
- Semkiv, M.V.; Ruchala, J.; Dmytruk, K.V.; Sibirny, A.A. 100 Years Later, What Is New in Glycerol Bioproduction? Trends Biotechnol. 2020, 38, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2020, 262, 116553. [Google Scholar] [CrossRef]
- Callegari, A.; Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Production technologies, current role, and future prospects of biofuels feedstocks: A state-of-the-art review. Crit. Rev. Environ. Sci. Technol. 2019, 50, 384–436. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Sherwood, J. Opportunities for Bio-Based Solvents Created as Petrochemical and Fuel Products Transition towards Renewable Resources. Int. J. Mol. Sci. 2015, 16, 17101–17159. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Garlapati, V.K.; Kumar, S.P.J.; Hans, M.; Singh, A.K. The role of renewable chemicals and biofuels in building a bioeconomy. Biofuels Bioprod. Biorefin. 2020, 14, 830–844. [Google Scholar] [CrossRef]
- Sharma, B.; Larroche, C.; Dussap, C.-G. Comprehensive assessment of 2G bioethanol production. Bioresour. Technol. 2020, 313, 123630. [Google Scholar] [CrossRef] [PubMed]
- Kargbo, H.; Harris, J.S.; Phan, A.N. “Drop-in” fuel production from biomass: Critical review on techno-economic feasibility and sustainability. Renew. Sustain. Energy Rev. 2021, 135, 110168. [Google Scholar] [CrossRef]
- Dedes, G.; Karnaouri, A.; Topakas, E. Novel Routes in Transformation of Lignocellulosic Biomass to Furan Platform Chemicals: From Pretreatment to Enzyme Catalysis. Catalysts 2020, 10, 743. [Google Scholar] [CrossRef]
- Eisentraut, A.; Sustainable Production of Second-Generation Biofuels. Organisation for Economic Co-operation and Development—Inernational Energy Agency (OECD-IEA). 2010. Available online: https://www.oecd.org/berlin/44567743.pdf (accessed on 26 September 2020).
- United Nations Conference on Trade and Development. Second Generation Biofuel Markets: State of Play, Trade and Developing Country Perspectives; United Nations Conference on Trade and Development: Geneva, Switzerland, 2016; Available online: https://www.sdgfund.org/second-generation-biofuel-markets-state-play-trade-and-developing-country-perspectives (accessed on 26 September 2020).
- Wegenhart, B.L.; Liu, S.; Thom, M.; Stanley, D.; Abu-Omar, M.M. Solvent-Free Methods for Making Acetals Derived from Glycerol and Furfural and Their Use as a Biodiesel Fuel Component. ACS Catal. 2012, 2, 2524–2530. [Google Scholar] [CrossRef]
- Werpy, T.A.; Petersen, G. Results of Screening for Potential Candidates from Sugars and Synthesis Gas. In Top Value Added Chemicals from Biomass; Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 2004; Volume I, 76p. [Google Scholar]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From Glycerol to Value-Added Products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef]
- Jiménez, R.X.; Young, A.F.; Fernandes, H.L. Propylene glycol from glycerol: Process evaluation and break-even price determination. Renew. Energy 2020, 158, 181–191. [Google Scholar] [CrossRef]
- Iliuta, I.; Iliuta, M.C. Integration of sorption-enhanced steam glycerol reforming with methanation of in-situ removed carbon dioxide—An alternative for glycerol valorization. Int. J. Hydrogen Energy 2020, 45, 18574–18586. [Google Scholar] [CrossRef]
- Muelas, A.; Remacha, P.; Pina, A.; Barroso, J.; Sobrino, A.; Aranda, D.; Bayarri, N.; Estévez, C.; Ballester, J. Combustion of crude glycerol and its blends with acetals. Exp. Therm. Fluid Sci. 2020, 114, 110076. [Google Scholar] [CrossRef]
- Roslan, N.A.; Abidin, S.Z.; Ideris, A.; Vo, D.-V.N. A review on glycerol reforming processes over Ni-based catalyst for hydrogen and syngas productions. Int. J. Hydrogen Energy 2020, 45, 18466–18489. [Google Scholar] [CrossRef]
- Kosamia, N.M.; Samavi, M.; Uprety, B.K.; Rakshit, S.K. Valorization of Biodiesel Byproduct Crude Glycerol for the Production of Bioenergy and Biochemicals. Catalysts 2020, 10, 609. [Google Scholar] [CrossRef]
- Nda-Umar, U.I.; Ramli, I.; Taufiq-Yap, Y.H.; Muhamad, E.N. An Overview of Recent Research in the Conversion of Glycerol into Biofuels, Fuel Additives and other Bio-Based Chemicals. Catalysts 2018, 9, 15. [Google Scholar] [CrossRef]
- Simoes, M.; Baranton, S.; Coutanceau, C. Electrochemical Valorisation of Glycerol. ChemSusChem 2012, 5, 2106–2124. [Google Scholar] [CrossRef]
- Fiorani, G.; Perosa, A.; Selva, M. Dimethyl carbonate: A versatile reagent for a sustainable valorization of renewables. Green Chem. 2018, 20, 288–322. [Google Scholar] [CrossRef]
- New Glycerol Upgrading Processes. Available online: https://www.mdpi.com/journal/catalysts/special_issues/glycerol_processes (accessed on 26 September 2020).
- Pagliaro, M. Glycerol The Renewable Platform Chemical, 1st ed.; Else: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-812205-1. [Google Scholar]
- Mota, C.; Pinto, B.P.; Lima, A.L. De Glycerol A Versatile Renewable Feedstock for the Chemical Industry; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-59375-3. [Google Scholar]
- Konwar, L.J.; Mikkola, J.-P.; Bordoloi, N.; Saikia, R.; Chutia, R.S.; Kataki, R. Sidestreams From Bioenergy and Biorefinery Complexes as a Resource for Circular Bioeconomy. In Waste Biorefinery; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 85–125. [Google Scholar]
- Liu, B.; Gao, F. Navigating Glycerol Conversion Roadmap and Heterogeneous Catalyst Selection Aided by Density Functional Theory: A Review. Catalysts 2018, 8, 44. [Google Scholar] [CrossRef]
- European Union Production of Cyclic Carbonates from CO2 Using Renewable Feedstocks. Available online: https://cordis.europa.eu/project/id/309497/es (accessed on 26 September 2020).
- European Union Glycerol Biorefinery Approach for the Production of High Quality Products of Industrial Value. Available online: https://cordis.europa.eu/project/id/613667/es (accessed on 26 September 2020).
- OECD-FAO. Food and Agriculture Organization of the United Nations; OECD-FAO: Paris, France, 2020. [Google Scholar]
- Thanh, L.T.; Okitsu, K.; Van Boi, L.; Maeda, Y. Catalytic Technologies for Biodiesel Fuel Production and Utilization of Glycerol: A Review. Catalysts 2012, 2, 191–222. [Google Scholar] [CrossRef]
- Jun, C.-H. Transition metal-catalyzed carbon–carbon bond activation. Chem. Soc. Rev. 2004, 33, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Mizugaki, T.; Kaneda, K. Development of High Performance Heterogeneous Catalysts for Selective Cleavage of C–O and C–C Bonds of Biomass-Derived Oxygenates. Chem. Rec. 2018, 19, 1179–1198. [Google Scholar] [CrossRef] [PubMed]
- Santoro, S.; Kozhushkov, S.I.; Ackermann, L.; Vaccaro, L. Heterogeneous catalytic approaches in C–H activation reactions. Green Chem. 2016, 18, 3471–3493. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, B.; Zhang, M.; Chen, Y.; Wang, L. Mechanism of C–C and C–H bond cleavage in ethanol oxidation reaction on Cu2O(111): A DFT-D and DFT+U study. Phys. Chem. Chem. Phys. 2017, 19, 26210–26220. [Google Scholar] [CrossRef]
- Pitt, F.D.; Domingos, A.M.; Barros, A.C. Purification of residual glycerol recovered from biodiesel production. S. Afr. J. Chem. Eng. 2019, 29, 42–51. [Google Scholar] [CrossRef]
- Meier, T.R.W.; Cremonez, P.A.; Maniglia, T.C.; Sampaio, S.C.; Teleken, J.G.; Da Silva, E.A. Production of biohydrogen by an anaerobic digestion process using the residual glycerol from biodiesel production as additive to cassava wastewater. J. Clean. Prod. 2020, 258, 120833. [Google Scholar] [CrossRef]
- Estévez-Toledano, R.C.; Aguado-Deblas, L.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A. Biodiesel at the Crossroads: A Critical Review. Catalysts 2019, 9, 1033. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, E.Y. Environmentally-Benign Dimethyl Carbonate-Mediated Production of Chemicals and Biofuels from Renewable Bio-Oil. Energies 2017, 10, 1790. [Google Scholar] [CrossRef]
- Habaki, H.; Hayashi, T.; Sinthupinyo, P.; Egashira, R. Purification of glycerol from transesterification using activated carbon prepared from Jatropha Shell for biodiesel production. J. Environ. Chem. Eng. 2019, 7, 103303. [Google Scholar] [CrossRef]
- Setyawan, H.Y.; Zhu, M.; Zhang, Z.; Zhang, D. An Experimental Study of Effect of Water on Ignition and Combustion Characteristics of Single Droplets of Glycerol. Energy Procedia 2015, 75, 578–583. [Google Scholar] [CrossRef][Green Version]
- Menon, A.; Waller, N.; Hu, W.; Hayhurst, A.; Davidson, J.; Scott, S.A. The combustion of solid paraffin wax and of liquid glycerol in a fluidised bed. Fuel 2017, 199, 447–455. [Google Scholar] [CrossRef][Green Version]
- Presciutti, A.; Asdrubali, F.; Baldinelli, G.; Rotili, A.; Malavasi, M.; Di Salvia, G. Energy and exergy analysis of glycerol combustion in an innovative flameless power plant. J. Clean. Prod. 2018, 172, 3817–3824. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Ghorbani, B.; Abedi, H. Biodiesel production integrated with glycerol steam reforming process, solid oxide fuel cell (SOFC) power plant. Energy Convers. Manag. 2020, 206, 112467. [Google Scholar] [CrossRef]
- Lee, B.-H.; Sh, L.; Bae, J.-S.; Choi, Y.-C.; Jeon, C.H. Combustion behavior of low-rank coal impregnated with glycerol. Biomass Bioenergy 2016, 87, 122–130. [Google Scholar] [CrossRef]
- Gibson, I.; Slim, C.J.; Zheng, Y.; Scott, S.A.; Davidson, J.F.; Hayhurst, A. The continuous combustion of glycerol in a fluidised bed. Combust. Flame 2019, 200, 60–68. [Google Scholar] [CrossRef]
- Żukowski, W.; Berkowicz, G. Dataset on flue gas composition during combustion in the fluidised bed reactor. Glycerol combustion. Data Brief 2020, 30, 105418. [Google Scholar] [CrossRef]
- Fantozzi, F.; Frassoldati, A.; Bartocci, P.; Cinti, G.; Quagliarini, F.; Bidini, G.; Ranzi, E. An experimental and kinetic modeling study of glycerol pyrolysis. Appl. Energy 2016, 184, 68–76. [Google Scholar] [CrossRef]
- Almazrouei, M.; Janajreh, I. Model-fitting approach to kinetic analysis of non-isothermal pyrolysis of pure and crude glycerol. Renew. Energy 2020, 145, 1693–1708. [Google Scholar] [CrossRef]
- He, S.; Muizebelt, I.; Heeres, A.; Schenk, N.; Blees, R.; Heeres, H. Catalytic pyrolysis of crude glycerol over shaped ZSM-5/bentonite catalysts for bio-BTX synthesis. Appl. Catal. B Environ. 2018, 235, 45–55. [Google Scholar] [CrossRef]
- De OliveiraMaia, D.; de SouzaChagas, A.M.; Araújo, A.M.D.M.; De Mendonça, A.V.; Ferreira, I.M.D.L.; Lemos, F.; Araujo, A.S.; Fernandes, V.J.; Gondim, A.D. Catalytic pyrolysis of glycerol in the presence of Nickel (II) Schiff base complex supported in SBA-15: Kinetic and products (TG-FTIR and PY-CG/MS). Thermochim. Acta 2018, 669, 160–168. [Google Scholar] [CrossRef]
- Batista, L.M.B.; Oliveira, J.L.F.; Bezerra, F.A.; Araújo, A.M.D.M.; Júnior, V.J.F.; Araujo, A.S.; Alves, A.P.; Gondim, A.D. Synthesis, characterization and evaluation of niobium catalysts in the flash pyrolysis of glycerol. Solid State Sci. 2019, 97, 105977. [Google Scholar] [CrossRef]
- Shahirah, M.N.N.; Gimbun, J.; Ideris, A.; Khan, M.R.; Cheng, C.-K. Catalytic pyrolysis of glycerol into syngas over ceria-promoted Ni/α-Al2O3 catalyst. Renew. Energy 2017, 107, 223–234. [Google Scholar] [CrossRef]
- Shahirah, M.N.N.; Gimbun, J.; Lam, S.S.; Ng, Y.H.; Cheng, C.-K. Synthesis and characterization of a La Ni/α-Al2O3 catalyst and its use in pyrolysis of glycerol to syngas. Renew. Energy 2019, 132, 1389–1401. [Google Scholar] [CrossRef]
- Fasolini, A.; Cespi, D.; Tabanelli, T.; Cucciniello, R.; Cavani, F. Hydrogen from Renewables: A Case Study of Glycerol Reforming. Catalysts 2019, 9, 722. [Google Scholar] [CrossRef]
- Sabio, E.; Álvarez-Murillo, A.; González, J.; Ledesma, B.; Román, S. Modelling the composition of the gas obtained by steam reforming of glycerine. Energy Convers. Manag. 2017, 146, 147–157. [Google Scholar] [CrossRef]
- Román, S.; Ledesma, B.; Alvarez, A.; Al-Kassir, A.; Yusaf, T.; Cano, B.L. Glycerin, a Biodiesel By-Product with Potentiality to Produce Hydrogen by Steam Gasification. Energies 2015, 8, 12765–12775. [Google Scholar] [CrossRef]
- Charisiou, N.; Polychronopoulou, K.; Asif, A.; Goula, M. The potential of glycerol and phenol towards H2 production using steam reforming reaction: A review. Surf. Coat. Technol. 2018, 352, 92–111. [Google Scholar] [CrossRef]
- Bepari, S.; Kuila, D. Steam reforming of methanol, ethanol and glycerol over nickel-based catalysts-A review. Int. J. Hydrogen Energy 2020, 45, 18090–18113. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, Y.; Han, Y.; Hong, J.; Zhang, Y.; Li, J.; Jing, F.; Chu, W. Preparation of stable and highly active Ni/CeO2 catalysts by glow discharge plasma technique for glycerol steam reforming. Appl. Catal. B Environ. 2019, 249, 257–265. [Google Scholar] [CrossRef]
- Xiong, Y.; Wang, B.; Yan, J.; Hong, J.; Wang, L.; Zhang, Y.; Li, J.; Jing, F.; Chu, W. Plasma assisted preparation of nickel-based catalysts supported on CeO2 with different morphologies for hydrogen production by glycerol steam reforming. Powder Technol. 2019, 354, 324–332. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Li, Z.; Zhang, K.; Li, B. Enhancement of membrane hydrogen separation on glycerol steam reforming in a fluidized bed reactor. Int. J. Hydrogen Energy 2018, 43, 18863–18872. [Google Scholar] [CrossRef]
- Macedo, M.S.; Soria, M.; Madeira, L.M. Glycerol steam reforming for hydrogen production: Traditional versus membrane reactor. Int. J. Hydrogen Energy 2019, 44, 24719–24732. [Google Scholar] [CrossRef]
- Saidi, M.; Moradi, P. Conversion of biodiesel synthesis waste to hydrogen in membrane reactor: Theoretical study of glycerol steam reforming. Int. J. Hydrogen Energy 2020, 45, 8715–8726. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Ghahremani, M.; Amiri, T.Y.; Basile, A. Performance evaluation of Pd Ag membrane reactor in glycerol steam reforming process: Development of the CFD model. Int. J. Hydrogen Energy 2019, 44, 1000–1009. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; Liu, H.; Liu, G.; He, Y. Numerical studies of sorption-enhanced glycerol steam reforming in a fluidized bed membrane reactor at low temperature. Int. J. Hydrogen Energy 2020, 45, 8346–8356. [Google Scholar] [CrossRef]
- Bac, S.; Keskin, S.; Avci, A.K. Recent advances in materials for high purity H2 production by ethanol and glycerol steam reforming. Int. J. Hydrogen Energy 2019. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, Z.; Wang, Y.; Liang, T.; Li, X.; Yang, Z.; Chen, M.; Wang, J. Effects of attapulgite-supported transition metals catalysts on glycerol steam reforming for hydrogen production. Int. J. Hydrogen Energy 2018, 43, 20451–20464. [Google Scholar] [CrossRef]
- Dahdah, E.; Aouad, S.; Gennequin, C.; Estephane, J.; Nsouli, B.; Aboukaïs, A.; Abi-Aad, E. Glycerol steam reforming over Ru-Mg-Al hydrotalcite-derived mixed oxides: Role of the preparation method in catalytic activity. Int. J. Hydrogen Energy 2018, 43, 19864–19872. [Google Scholar] [CrossRef]
- Dobosz, J.; Cichy, M.; Zawadzki, M.; Borowiecki, T. Glycerol steam reforming over calcium hydroxyapatite supported cobalt and cobalt-cerium catalysts. J. Energy Chem. 2018, 27, 404–412. [Google Scholar] [CrossRef]
- Lima, D.S.; Calgaro, C.O.; Perez-Lopez, O.W. Hydrogen production by glycerol steam reforming over Ni based catalysts prepared by different methods. Biomass Bioenergy 2019, 130, 105358. [Google Scholar] [CrossRef]
- Moreira, R.; Moral, A.; Bimbela, F.; Portugal, A.; Ferreira, A.; Sanchez, J.L.; Gandía, L.M. Syngas production via catalytic oxidative steam reforming of glycerol using a Co/Al coprecipitated catalyst and different bed fillers. Fuel Process. Technol. 2019, 189, 120–133. [Google Scholar] [CrossRef]
- Silva, J.M.; Ribeiro, L.S.; Órfão, J.; Soria, M.; Madeira, L.M. Low temperature glycerol steam reforming over a Rh-based catalyst combined with oxidative regeneration. Int. J. Hydrogen Energy 2019, 44, 2461–2473. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, C.-H.; Wang, T. Production of methane from biomass glycerol through coupling of steam reforming and methanation on Ni-Mn/Al2O3. Sustain. Chem. Pharm. 2019, 13, 100150. [Google Scholar] [CrossRef]
- Al-Salihi, S.; Abrokwah, R.; Dade, W.; Deshmane, V.; Hossain, T.; Kuila, D. Renewable hydrogen from glycerol steam reforming using Co–Ni–MgO based SBA-15 nanocatalysts. Int. J. Hydrogen Energy 2020, 45, 14183–14198. [Google Scholar] [CrossRef]
- Charisiou, N.; Italiano, C.; Pino, L.; Sebastian, V.; Vita, A.; Goula, M. Hydrogen production via steam reforming of glycerol over Rh/γ-Al2O3 catalysts modified with CeO2, MgO or La2O3. Renew. Energy 2020, 162, 908–925. [Google Scholar] [CrossRef]
- Chen, D.; Wang, W.; Liu, C. Hydrogen production through glycerol steam reforming over beehive-biomimetic graphene-encapsulated nickel catalysts. Renew. Energy 2020, 145, 2647–2657. [Google Scholar] [CrossRef]
- Dahdah, E.; Estephane, J.; Gennequin, C.; Aboukaïs, A.; Abi-Aad, E.; Aouad, S. Zirconia supported nickel catalysts for glycerol steam reforming: Effect of zirconia structure on the catalytic performance. Int. J. Hydrogen Energy 2020, 45, 4457–4467. [Google Scholar] [CrossRef]
- Dahdah, E.; Estephane, J.; Gennequin, C.; Aboukaïs, A.; Aouad, S.; Abi-Aad, E. Effect of La promotion on Ni/Mg-Al hydrotalcite derived catalysts for glycerol steam reforming. J. Environ. Chem. Eng. 2020, 8, 104228. [Google Scholar] [CrossRef]
- Feng, P.; Huang, K.; Xu, Q.; Qi, W.; Xin, S.; Wei, T.; Liao, L.; Yan, Y. Ni supported on the CaO modified attapulgite as catalysts for hydrogen production from glycerol steam reforming. Int. J. Hydrogen Energy 2020, 45, 8223–8233. [Google Scholar] [CrossRef]
- Ismaila, A.; Chen, H.; Shao, Y.; Xu, S.; Jiao, Y.; Chen, X.; Gao, X.; Fan, X. Renewable hydrogen production from steam reforming of glycerol (SRG) over ceria-modified γ-alumina supported Ni catalyst. Chin. J. Chem. Eng. 2020, 28, 2328–2336. [Google Scholar] [CrossRef]
- Jing, F.; Liu, S.; Wang, R.; Li, X.; Yan, Z.; Luo, S.; Chu, W. Hydrogen production through glycerol steam reforming over the NiCexAl catalysts. Renew. Energy 2020, 158, 192–201. [Google Scholar] [CrossRef]
- Menezes, J.P.D.S.; Duarte, K.R.; Manfro, R.L.; Souza, M.M.V.M. Effect of niobia addition on cobalt catalysts supported on alumina for glycerol steam reforming. Renew. Energy 2020, 148, 864–875. [Google Scholar] [CrossRef]
- Moogi, S.; Nakka, L.; Potharaju, S.P.; Ahmed, A.; Farooq, A.; Jung, S.-C.; Rhee, G.H.; Park, Y.-K. Copper promoted Co/MgO: A stable and efficient catalyst for glycerol steam reforming. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- Moogi, S.; Lee, I.-G.; Hwang, K.-R. Catalytic steam reforming of glycerol over Ni–La2O3–CeO2/SBA-15 catalyst for stable hydrogen-rich gas production. Int. J. Hydrogen Energy 2020. [Google Scholar] [CrossRef]
- García, L.; Valiente, A.; Oliva, M.; Ruiz, J.; Arauzo, J. Influence of operating variables on the aqueous-phase reforming of glycerol over a Ni/Al coprecipitated catalyst. Int. J. Hydrogen Energy 2018, 43, 20392–20407. [Google Scholar] [CrossRef]
- Shabaker, J.; Huber, G.; Dumesic, J. Aqueous-phase reforming of oxygenated hydrocarbons over Sn-modified Ni catalysts. J. Catal. 2004, 222, 180–191. [Google Scholar] [CrossRef]
- Shabaker, J. Aqueous-phase reforming of methanol and ethylene glycol over alumina-supported platinum catalysts. J. Catal. 2003, 215, 344–352. [Google Scholar] [CrossRef]
- Bastan, F.; Kazemeini, M.; Larimi, A.; Maleki, H. Production of renewable hydrogen through aqueous-phase reforming of glycerol over Ni/Al2O3MgO nano-catalyst. Int. J. Hydrogen Energy 2018, 43, 614–621. [Google Scholar] [CrossRef]
- Reynoso, A.; Ayastuy, J.; Iriarte-Velasco, U.; Gutiérrez-Ortiz, M. Cobalt aluminate spinel-derived catalysts for glycerol aqueous phase reforming. Appl. Catal. B Environ. 2018, 239, 86–101. [Google Scholar] [CrossRef]
- Bossola, F.; Pereira-Hernández, X.I.; Evangelisti, C.; Wang, Y.; Santo, V.D. Investigation of the promoting effect of Mn on a Pt/C catalyst for the steam and aqueous phase reforming of glycerol. J. Catal. 2017, 349, 75–83. [Google Scholar] [CrossRef]
- Putra, R.D.D.; Trajano, H.L.; Liu, S.; Smith, K.J.; Smith, K.; Kim, C.-S. In-situ glycerol aqueous phase reforming and phenol hydrogenation over Raney Ni®. Chem. Eng. J. 2018, 350, 181–191. [Google Scholar] [CrossRef]
- Liu, S.; Tamura, M.; Shen, Z.; Su, Y.; Nakagawa, Y.; Tomishige, K. Hydrogenolysis of glycerol with in-situ produced H2 by aqueous-phase reforming of glycerol using Pt-modified Ir-ReOx/SiO2 catalyst. Catal. Today 2018, 303, 106–116. [Google Scholar] [CrossRef]
- Thirabunjongcharoen, S.; Bumroongsakulsawat, P.; Praserthdam, P.; Charojrochkul, S.; Assabumrungrat, S.; Kim-Lohsoontorn, P. Thermally double coupled reactor coupling aqueous phase glycerol reforming and methanol synthesis. Catal. Today 2020. [Google Scholar] [CrossRef]
- Callison, J.; Subramanian, N.; Rogers, S.; Chutia, A.; Gianolio, D.; Catlow, C.; Wells, P.; Dimitratos, N. Directed aqueous-phase reforming of glycerol through tailored platinum nanoparticles. Appl. Catal. B Environ. 2018, 238, 618–628. [Google Scholar] [CrossRef]
- Morales-Marín, A.; Ayastuy, J.; Iriarte-Velasco, U.; Gutiérrez-Ortiz, M. Nickel aluminate spinel-derived catalysts for the aqueous phase reforming of glycerol: Effect of reduction temperature. Appl. Catal. B Environ. 2019, 244, 931–945. [Google Scholar] [CrossRef]
- Reynoso, A.; Iriarte-Velasco, U.; Gutiérrez-Ortiz, M.; Ayastuy, J. Highly stable Pt/CoAl2O4 catalysts in Aqueous-Phase Reforming of glycerol. Catal. Today 2020. [Google Scholar] [CrossRef]
- Yu, J.; Odriozola, J.A.; Reina, T. Dry Reforming of Ethanol and Glycerol: Mini-Review. Catalysts 2019, 9, 1015. [Google Scholar] [CrossRef]
- Dang, C.; Wu, S.; Yang, G.; Cao, Y.; Wang, H.; Peng, F.; Yu, H. Syngas production by dry reforming of the mixture of glycerol and ethanol with CaCO3. J. Energy Chem. 2020, 43, 90–97. [Google Scholar] [CrossRef]
- Harun, N.; Abidin, S.Z.; Osazuwa, O.U.; Taufiq-Yap, Y.H.; Azizan, M.T. Hydrogen production from glycerol dry reforming over Ag-promoted Ni/Al2O3. Int. J. Hydrogen Energy 2019, 44, 213–225. [Google Scholar] [CrossRef]
- Tavanarad, M.; Meshkani, F.; Rezaei, M. Production of syngas via glycerol dry reforming on Ni catalysts supported on mesoporous nanocrystalline Al2O3. J. CO2 Util. 2018, 24, 298–305. [Google Scholar] [CrossRef]
- Bulutoglu, P.S.; Say, Z.; Bac, S.; Ozensoy, E.; Avci, A.K. Dry reforming of glycerol over Rh-based ceria and zirconia catalysts: New insights on catalyst activity and stability. Appl. Catal. A Gen. 2018, 564, 157–171. [Google Scholar] [CrossRef]
- Arif, N.N.M.; Abidin, S.Z.; Osazuwa, O.U.; Vo, D.N.; Azizan, M.T.; Taufiq-Yap, Y.H. Hydrogen production via CO2 dry reforming of glycerol over Re Ni/CaO catalysts. Int. J. Hydrogen Energy 2019, 44, 20857–20871. [Google Scholar] [CrossRef]
- El Doukkali, M.; Iriondo, A.; Gandarias, I. Enhanced catalytic upgrading of glycerol into high value-added H2 and propanediols: Recent developments and future perspectives. Mol. Catal. 2020, 490, 110928. [Google Scholar] [CrossRef]
- Zhou, C.H.; Zhao, H.; Tong, D.S.; Wu, L.M.; Yu, W.H. Recent Advances in Catalytic Conversion of Glycerol. Catal. Rev. 2013, 55, 369–453. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Xiao, G. Sustainable value-added C3 chemicals from glycerol transformations: A mini review for heterogeneous catalytic processes. Chin. J. Chem. Eng. 2019, 27, 1536–1542. [Google Scholar] [CrossRef]
- Feng, J.; Xu, B. Reaction Mechanisms for the Heterogeneous Hydrogenolysis of Biomass-Derived Glycerol to Propanediols. Prog. React. Kinet. Mech. 2014, 39, 1–15. [Google Scholar] [CrossRef]
- Zhu, S.; Gao, X.; Zhu, Y.; Fan, W.; Wang, J.; Li, Y. A highly efficient and robust Cu/SiO2 catalyst prepared by the ammonia evaporation hydrothermal method for glycerol hydrogenolysis to 1,2-propanediol. Catal. Sci. Technol. 2015, 5, 1169–1180. [Google Scholar] [CrossRef]
- Vila, F.; Granados, M.L.; Ojeda, M.; Fierro, J.L.G.; Mariscal, R. Glycerol hydrogenolysis to 1,2-propanediol with Cu/γ-Al2O3: Effect of the activation process. Catal. Today 2012, 187, 122–128. [Google Scholar] [CrossRef]
- Xia, S.; Yuan, Z.; Wang, L.; Chen, P.; Hou, Z. Catalytic production of 1,2-propanediol from glycerol in bio-ethanol solvent. Bioresour. Technol. 2012, 104, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.D.; Park, J.R.; Park, D.S.; Kwak, B.K.; Yi, J. Promoter effect of Pd in CuCr2O4 catalysts on the hydrogenolysis of glycerol to 1,2-propanediol. Green Chem. 2012, 14, 2638–2646. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, M.; Yang, X.; Zhao, X.; Sun, L.; Deng, W.; Wang, A.; Li, J.; Zhang, T. On the mechanism of H2 activation over single-atom catalyst: An understanding of Pt1/WOx in the hydrogenolysis reaction. Chin. J. Catal. 2020, 41, 524–532. [Google Scholar] [CrossRef]
- Mane, R.; Patil, S.; Shirai, M.; Rayalu, S.S.; Rode, C.V. Influence of carbon based supports on selectivity behavior of diols and propanol in Ru catalyzed glycerol hydrogenolysis. Appl. Catal. B Environ. 2017, 204, 134–146. [Google Scholar] [CrossRef]
- Gallegos-Suarez, E.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I.; Arcoya, A. Comparative study of the hydrogenolysis of glycerol over Ru-based catalysts supported on activated carbon, graphite, carbon nanotubes and KL-zeolite. Chem. Eng. J. 2015, 262, 326–333. [Google Scholar] [CrossRef]
- Seguel, J.; García, R.; Chimentão, R.J.; Fierro, J.L.G.; Ghampson, I.T.; Escalona, N.; Sepúlveda, C. Thermal Modification Effect on Supported Cu-Based Activated Carbon Catalyst in Hydrogenolysis of Glycerol. Materials 2020, 13, 603. [Google Scholar] [CrossRef]
- Costa, J.D.R.M.; Santos, R.C.; Coutinho, L.P.; Silva, O.R.; Barros, H.O.; Freire, V.N.; Valentini, A. CO2 role on the glycerol conversion over catalyst containing CaO-SiO2 doped with Ag and Pt. Catal. Today 2020, 344, 199–211. [Google Scholar] [CrossRef]
- Mane, R.; Rode, C. Simultaneous glycerol dehydration and in situ hydrogenolysis over Cu-Al oxide under an inert atmosphere. Green Chem. 2012, 14, 2780–2789. [Google Scholar] [CrossRef]
- Checa, M.; Montes, V.; Hidalgo-Carrillo, J.; Marinas, A.; Urbano, F.J. Influence of Boron, Tungsten and Molybdenum Modifiers on Zirconia Based Pt Catalyst for Glycerol Valorization. Nanomaterials 2019, 9, 509. [Google Scholar] [CrossRef]
- Kuljiraseth, J.; Kumpradit, T.; Leungcharoenwattana, T.; Poo-Arporn, Y.; Jitkarnka, S. Integrated glycerol- and ethanol-based chemical synthesis routes using Cu–Mg–Al LDH-derived catalysts without external hydrogen: Intervention of bio-ethanol co-fed with glycerol. Renew. Energy 2020, 156, 975–985. [Google Scholar] [CrossRef]
- Shan, J.; Liu, H.; Lu, K.; Zhu, S.; Li, J.; Wang, J.; Fan, W. Identification of the dehydration active sites in glycerol hydrogenolysis to 1,2-propanediol over Cu/SiO2 catalysts. J. Catal. 2020, 383, 13–23. [Google Scholar] [CrossRef]
- Żelazny, A.; Samson, K.; Grabowski, R.; Śliwa, M.; Ruggiero-Mikołajczyk, M.; Kornas, A. Hydrogenolysis of glycerol to propylene glycol over Cu/oxide catalysts: Influence of the support and reaction conditions. React. Kinet. Mech. Catal. 2017, 121, 329–343. [Google Scholar] [CrossRef]
- Ke, Y.; Li, X.; Li, J.; Liu, C.; Xu, C.; Dong, W. Conversion of glycerol to dihydroxyacetone over Au catalysts on various supports. J. Chem. Technol. Biotechnol. 2019, 95, 1153–1162. [Google Scholar] [CrossRef]
- Checa-Gómez, M.; Marinas, A.; Marinas, J.M.; Urbano, F.J. Deactivation study of supported Pt catalyst on glycerol hydrogenolysis. Appl. Catal. A Gen. 2015, 507, 34–43. [Google Scholar] [CrossRef]
- García-Fernández, S.; Gandarias, I.; Tejido-Núñez, Y.; Requies, J.; Arias, P.L. Influence of the Support of Bimetallic Platinum Tungstate Catalysts on 1,3-Propanediol Formation from Glycerol. ChemCatChem 2017, 9, 4508–4519. [Google Scholar] [CrossRef]
- De Andrade, T.S.; Souza, M.M.; Manfro, R.L. Hydrogenolysis of glycerol to 1,2-propanediol without external H2 addition in alkaline medium using Ni-Cu catalysts supported on Y zeolite. Renew. Energy 2020, 160, 919–930. [Google Scholar] [CrossRef]
- Priya, S.S.; Bhanuchander, P.; Kumar, V.P.; Dumbre, D.K.; Periasamy, S.R.; Bhargava, S.K.; Mannepalli, L.K.; Chary, K.V.R. Platinum Supported on H-Mordenite: A Highly Efficient Catalyst for Selective Hydrogenolysis of Glycerol to 1,3-Propanediol. ACS Sustain. Chem. Eng. 2016, 4, 1212–1222. [Google Scholar] [CrossRef]
- Li, X.; Xiang, M.; Wu, D. Hydrogenolysis of glycerol over bimetallic Cu Ni catalysts supported on hierarchically porous SAPO-11 zeolite. Catal. Commun. 2019, 119, 170–175. [Google Scholar] [CrossRef]
- Li, X.; Wu, D. Synthesis of Co-doped micro-mesoporous SAPO-11 zeolite for glycerol hydrogenolysis. Korean J. Chem. Eng. 2020, 37, 216–223. [Google Scholar] [CrossRef]
- Gebretsadik, F.B.; Llorca, J.; Salagre, P.; Cesteros, Y. Hydrogenolysis of Glycidol as an Alternative Route to Obtain 1,3-Propanediol Selectively Using MOx -Modified Nickel-Copper Catalysts Supported on Acid Mesoporous Saponite. ChemCatChem 2017, 9, 3670–3680. [Google Scholar] [CrossRef]
- Li, X.; Wu, Q.; Zhang, B.; Zhang, C.; Lin, W.; Cheng, H.; Zhao, F. Efficient conversion of glycerol to 1, 2-propenadiol over ZnPd/ZnO-3Al catalyst: The significant influences of calcination temperature. Catal. Today 2018, 302, 210–216. [Google Scholar] [CrossRef]
- Lei, N.; Miao, Z.; Liu, F.; Wang, H.; Pan, X.; Wang, A.; Zhang, T. Understanding the deactivation behavior of Pt/WO3/Al2O3 catalyst in the glycerol hydrogenolysis reaction. Chin. J. Catal. 2020, 41, 1261–1267. [Google Scholar] [CrossRef]
- Liang, Y.; Shi, G.; Jin, K. Promotion Effect of Al2O3 on Pt–WOx/SiO2 Catalysts for Selective Hydrogenolysis of Bioglycerol to 1,3-Propanediol in Liquid Phase. Catal. Lett. 2020, 150, 2365–2376. [Google Scholar] [CrossRef]
- Xi, Z.; Jia, W.; Zhu, Z. WO3–ZrO2–TiO2 Composite Oxide Supported Pt as an Efficient Catalyst for Continuous Hydrogenolysis of Glycerol. Catal. Lett. 2020, 1–14. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Wang, X.; Yao, D.; Wang, Y.; Huang, S.; Li, W.; Zhao, Y.; Wang, S.; Ma, X. Insight into the nature of Brönsted acidity of Pt-(WOx)n-H model catalysts in glycerol hydrogenolysis. J. Catal. 2020, 388, 154–163. [Google Scholar] [CrossRef]
- Vasiliadou, E.S.; Lemonidou, A.A. Glycerol transformation to value added C3 diols: Reaction mechanism, kinetic, and engineering aspects. Wiley Interdiscip. Rev. Energy Environ. 2014, 4, 486–520. [Google Scholar] [CrossRef]
- Chimentão, R.; Miranda, B.; Ruiz, D.; Gispert-Guirado, F.; Medina, F.; Llorca, J.; Santos, J. Catalytic performance of zinc-supported copper and nickel catalysts in the glycerol hydrogenolysis. J. Energy Chem. 2020, 42, 185–194. [Google Scholar] [CrossRef]
- Kandasamy, S.; Samudrala, S.P.; Bhattacharya, S. The route towards sustainable production of ethylene glycol from a renewable resource, biodiesel waste: A review. Catal. Sci. Technol. 2019, 9, 567–577. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Perspective on catalyst development for glycerol reduction to C3 chemicals with molecular hydrogen. Res. Chem. Intermed. 2018, 44, 3879–3903. [Google Scholar] [CrossRef]
- Pandey, D.K.; Pandhare, N.N.; Biswas, P. Production of propylene glycol (propane-1,2-diol) in vapor phase over Cu–Ni/γ-Al2O3 catalyst in a down flow tubular reactor: Effect of catalyst calcination temperature and kinetic study. React. Kinet. Mech. Catal. 2019, 127, 523–542. [Google Scholar] [CrossRef]
- Raju, N.; Rekha, V.; Abhishek, B.; Kumar, P.M.; Sumana, C.; Lingaiah, N. Studies on continuous selective hydrogenolysis of glycerol over supported Cu–Co bimetallic catalysts. New J. Chem. 2020, 44, 3122–3128. [Google Scholar] [CrossRef]
- Mitta, H.; Devunuri, N.; Sunkari, J.; Mutyala, S.; Putrakumar, B.; Perupogu, V.; Jyothi, S. A highly active dispersed copper oxide phase on calcined MgAlGaO catalysts in glycerol hydrogenolysis. Catal. Today 2020. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, G.; Feng, H.; Chen, L.; Wang, H.; Wang, B.; Zheng, L.; Hong, S.; Wei, M. Platinum–copper single atom alloy catalysts with high performance towards glycerol hydrogenolysis. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gatti, M.N.; Cerioni, J.L.; Pompeo, F.; Santori, G.F.; Nichio, N.N. High Yield to 1-Propanol from Crude Glycerol Using Two Reaction Steps with Ni Catalysts. Catalysts 2020, 10, 615. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic materials for the hydrogenolysis of glycerol to 1,3-propanediol. J. Mater. Chem. A 2014, 2, 6688–6702. [Google Scholar] [CrossRef]
- Greish, A.A.; Finashina, E.D.; Tkachenko, O.P.; Nikul’Shin, P.A.; Ershov, M.A.; Kustov, L.M. Hydrodeoxygenation of glycerol into propanols over a Ni/WO3–TiO2 catalyst. Mendeleev Commun. 2020, 30, 119–120. [Google Scholar] [CrossRef]
- Mizugaki, T.; Yamakawa, T.; Arundhathi, R.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Selective Hydrogenolysis of Glycerol to 1,3-Propanediol Catalyzed by Pt Nanoparticles–AlOx/WO3. Chem. Lett. 2012, 41, 1720–1722. [Google Scholar] [CrossRef]
- Varghese, J.J.; Cao, L.; Robertson, C.; Yang, Y.; Gladden, L.; Lapkin, A.A.; Mushrif, S.H. Synergistic Contribution of the Acidic Metal Oxide–Metal Couple and Solvent Environment in the Selective Hydrogenolysis of Glycerol: A Combined Experimental and Computational Study Using ReOx–Ir as the Catalyst. ACS Catal. 2018, 9, 485–503. [Google Scholar] [CrossRef]
- Aihara, T.; Miura, H.; Shishido, T. Effect of perimeter interface length between 2D WO3 monolayer domain and γ-Al2O3 on selective hydrogenolysis of glycerol to 1,3-propanediol. Catal. Sci. Technol. 2019, 9, 5359–5367. [Google Scholar] [CrossRef]
- Wang, C.; Chen, C. Stabilized hydrogenolysis of glycerol to 1,3-propanediol over Mg modified Pt/WOx–ZrO2 catalysts. React. Kinet. Mech. Catal. 2019, 128, 461–477. [Google Scholar] [CrossRef]
- López, A.; Aragón, J.; Hernández-Cortez, J.; Mosqueira, M.; Martínez-Palou, R. Study of hydrotalcite-supported transition metals as catalysts for crude glycerol hydrogenolysis. Mol. Catal. 2019, 468, 9–18. [Google Scholar] [CrossRef]
- Mallesham, B.; Sudarsanam, P.; Reddy, B.V.S.; Reddy, B.M. Development of cerium promoted copper–magnesium catalysts for biomass valorization: Selective hydrogenolysis of bioglycerol. Appl. Catal. B Environ. 2016, 181, 47–57. [Google Scholar] [CrossRef]
- Kant, A.; He, Y.; Jawad, A.; Li, X.; Rezaei, F.; Smith, J.D.; Rownaghi, A.A. Hydrogenolysis of glycerol over Ni, Cu, Zn, and Zr supported on H-beta. Chem. Eng. J. 2017, 317, 1–8. [Google Scholar] [CrossRef]
- Shozi, M.L.; Dasireddy, V.D.; Singh, S.; Mohlala, P.; Morgan, D.J.; Friedrich, H.B. Hydrogenolysis of Glycerol to Monoalcohols over Supported Mo and W Catalysts. ACS Sustain. Chem. Eng. 2016, 4, 5752–5760. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Shui, H.; Shui, H. Selective Hydrogenolysis of Glycerol and Crude Glycerol (a By-Product or Waste Stream from the Biodiesel Industry) to 1,2-Propanediol over B2O3 Promoted Cu/Al2O3 Catalysts. Catalysts 2017, 7, 196. [Google Scholar] [CrossRef]
- Zhang, G.; Jin, X.; Zhang, Q.; Cheng, Y.; Chen, X.; Liu, Y.; Feng, X.; Yang, C. PtRu/Zn3Ce1Ox catalysts with Lewis acid–base pairs show synergistic performances for the conversion of glycerol in the absence of externally added H2. Catal. Sci. Technol. 2020, 10, 4386–4395. [Google Scholar] [CrossRef]
- Cai, F.; Jin, F.; Hao, J.; Xiao, G. Selective hydrogenolysis of glycerol to 1,2-propanediol on Nb-modified Pd–Zr–Al catalysts. Catal. Commun. 2019, 131, 105801. [Google Scholar] [CrossRef]
- Singh, B.; Kim, Y.; Kwon, S.; Na, K. Selective Catalytic Transfer Hydrogenolysis of Glycerol to 2-Isopropoxy-Propan-1-Ol over Noble Metal Ion-Exchanged Mordenite Zeolite. Catalysts 2019, 9, 885. [Google Scholar] [CrossRef]
- Xi, Z.; Hong, Z.; Huang, F.; Zhu, Z.; Jia, W.; Li, J. Hydrogenolysis of Glycerol on the ZrO2-TiO2 Supported Pt-WOx Catalyst. Catalysts 2020, 10, 312. [Google Scholar] [CrossRef]
- Cheng, S.; Zeng, Y.; Pei, Y.; Fan, K.; Qiao, M.; Zong, B. Synthesis and Catalysis of Pt/W-s-SBA-15 Catalysts with Short Channel for Glycerol Hydrogenolysis to 1,3-Propanediol. Acta Chim. Sin. 2019, 77, 1054–1062. [Google Scholar] [CrossRef]
- Lei, N.; Zhao, X.; Hou, B.; Yang, M.; Zhou, M.; Liu, F.; Wang, A.; Zhang, T. Effective Hydrogenolysis of Glycerol to 1,3-Propanediol over Metal-Acid Concerted Pt/WOx/Al2O3 Catalysts. ChemCatChem 2019, 11, 3903–3912. [Google Scholar] [CrossRef]
- Feng, S.; Zhao, B.; Liang, Y.; Liu, L.; Dong, J. Improving Selectivity to 1,3-Propanediol for Glycerol Hydrogenolysis Using W- and Al-Incorporated SBA-15 as Support for Pt Nanoparticles. Ind. Eng. Chem. Res. 2019, 58, 2661–2671. [Google Scholar] [CrossRef]
- Zhou, W.; Luo, J.; Wang, Y.; Liu, J.; Zhao, Y.; Wang, S.; Ma, X. WOx domain size, acid properties and mechanistic aspects of glycerol hydrogenolysis over Pt/WOx/ZrO2. Appl. Catal. B Environ. 2019, 242, 410–421. [Google Scholar] [CrossRef]
- Liu, L.; Asano, T.; Nakagawa, Y.; Tamura, M.; Okumura, K.; Tomishige, K. Selective Hydrogenolysis of Glycerol to 1,3-Propanediol over Rhenium-Oxide-Modified Iridium Nanoparticles Coating Rutile Titania Support. ACS Catal. 2019, 9, 10913–10930. [Google Scholar] [CrossRef]
- Modvig, A.; Kumpidet, C.; Riisager, A.; Albert, J. Ru-Doped Wells-Dawson Polyoxometalate as Efficient Catalyst for Glycerol Hydrogenolysis to Propanediols. Materials 2019, 12, 2175. [Google Scholar] [CrossRef] [PubMed]
- Chaminand, J.; Djakovitch, L.; Gallezot, P.; Marion, P.; Pinel, C. Glycerol hydrogenolysis on heterogeneous catalysts. Green Chem. 2004, 6, 359–361. [Google Scholar] [CrossRef]
- Fan, Y.; Cheng, S.; Wang, H.; Ye, D.; Xie, S.; Pei, Y.; Hu, H.; Hua, W.; Li, Z.H.; Qiao, M.-H.; et al. Nanoparticulate Pt on mesoporous SBA-15 doped with extremely low amount of W as a highly selective catalyst for glycerol hydrogenolysis to 1,3-propanediol. Green Chem. 2017, 19, 2174–2183. [Google Scholar] [CrossRef]
- Zhou, W.; Guan, Y.; Zhou, Q.; Xie, H.; Yu, X.; Xia, T. Preparation Method of Platinum-Tungsten-Zirconia Catalyst and Its Application in Hydrogenation of Glycerol to Produce 1,3-Propanediol. China Patent CN111389397A, 19 February 2020. [Google Scholar]
- Zhang, C.; Wu, Y.; Zheng, R.; Jin, C.; Sun, X.; Hou, C.; Xia, G.; Li, M. Glycerol Hydrogenolysis Catalyst, Its Preparation Method and Application, and Glycerol Hydrogenolysis Method with It to Produce 1,3-Propanediol. China Patent CN111036208A, 21 April 2020. [Google Scholar]
- Wolski, L. Factors affecting the activity and selectivity of niobia-based gold catalysts in liquid phase glycerol oxidation. Catal. Today 2020, 354, 36–43. [Google Scholar] [CrossRef]
- Wu, S.T.; She, Q.M.; Tesser, R.; Di Serio, M.; Zhou, C.H. Catalytic glycerol dehydration-oxidation to acrylic acid. Catal. Rev. 2020, 1–43. [Google Scholar] [CrossRef]
- Babaei, Z.; Chermahini, A.N.; Dinari, M. Glycerol adsorption and mechanism of dehydration to acrolein over TiO2 surface: A density functional theory study. J. Colloid Interface Sci. 2020, 563, 1–7. [Google Scholar] [CrossRef]
- Lewicka, L.; Kalisz, D.; Migdal, A.; Plesnar, M.; Kedziora, A.; Dabrowski, Z. Process for Producing Acrylic Acid via Oxidation of Acrolein. Poland Patent PL227246B1, 2017. [Google Scholar]
- Dubois, J.-L.; Patience, G. Process for Preparation of Acrylic Acid from Aqueous Glycerol Solution. Patent Number WO2008087315A2, 24 July 2008. [Google Scholar]
- Xu, W.; Yang, B.; Wang, W.; Yao, Q. Method for Synthesizing Acrylic Acid from Glycerol. China Patent CN109305908A, 2019. [Google Scholar]
- Belliere-Baca, V.; Loridant, S.; Millet, J.-M.; Lauriol-Garbey, P. Method for Preparing Acrolein from Glycerol or Glycerine. Patent Number WO2010076510A2, 8 July 2010. [Google Scholar]
- Liu, R.; Wang, T.; Wei, F.; Jin, Y. Highly Efficient Production of Acrylic Acid by Sequential Dehydration and Oxidation of Glycerol. Ind. Eng. Chem. Res. 2014, 53, 8667–8674. [Google Scholar] [CrossRef]
- Prati, L.; Rossi, M. Gold on Carbon as a New Catalyst for Selective Liquid Phase Oxidation of Diols. J. Catal. 1998, 176, 552–560. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef]
- Somorjai, G.A.; Zhou, B. Nanotechnology in Catalysis. In Nanostructure Science and Technology; Zhou, B., Han, S., Raja, R., Somorjai, G.A., Eds.; Springer: New York, NY, USA, 2007; Volume 3, ISBN 978-0-387-34687-8. [Google Scholar]
- Kimura, H.; Tsuto, K.; Wakisaka, T.; Kazumi, Y.; Inaya, Y. Selective oxidation of glycerol on a platinum-bismuth catalyst. Appl. Catal. A Gen. 1993, 96, 217–228. [Google Scholar] [CrossRef]
- Prati, L.; Spontoni, P.; Gaiassi, A. From Renewable to Fine Chemicals Through Selective Oxidation: The Case of Glycerol. Top. Catal. 2009, 52, 288–296. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Rodrigues, E.G.; Delgado, J.J.; Chen, X.; Pereira, M.F.R.; Órfão, J.J.M. Pd, Pt, and Pt–Cu Catalysts Supported on Carbon Nanotube (CNT) for the Selective Oxidation of Glycerol in Alkaline and Base-Free Conditions. Ind. Eng. Chem. Res. 2016, 55, 8548–8556. [Google Scholar] [CrossRef]
- Evans, C.D.; Douthwaite, M.; Carter, J.H.; Pattisson, S.; Kondrat, S.A.; Bethell, D.; Knight, D.W.; Taylor, S.H.; Hutchings, G.J. Enhancing the understanding of the glycerol to lactic acid reaction mechanism over AuPt/TiO2 under alkaline conditions. J. Chem. Phys. 2020, 152, 134705. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, R.K.P.; Van Haveren, J.; Van Es, D.; Melián-Cabrera, I.; Meeldijk, J.; Heeres, H.J. An efficient one pot conversion of glycerol to lactic acid using bimetallic gold-platinum catalysts on a nanocrystalline CeO2 support. Appl. Catal. B Environ. 2014, 147, 92–100. [Google Scholar] [CrossRef]
- He, Z.; Ning, X.; Yang, G.; Wang, H.; Cao, Y.; Peng, F.; Yu, H. Selective oxidation of glycerol over supported noble metal catalysts. Catal. Today 2020. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, P.; Liu, Y.; Pan, J.; Li, D.; Wang, B.; Feng, J. Support morphology effect on the selective oxidation of glycerol over AuPt/CeO2 catalysts. J. Catal. 2020, 385, 146–159. [Google Scholar] [CrossRef]
- Palacio, R.; Álvaro, A.; Blach, D.; Torres, S.; Hernández, D.; López, D.; Martinez, F. Influence of the Acid Properties of the Support on Au-Based Catalysts for Glycerol Oxidation in Aqueous Medium. ChemistrySelect 2020, 5, 7789–7796. [Google Scholar] [CrossRef]
- Tao, M.; Li, Y.; Li, Y.; Zhang, X.; Geletii, Y.V.; Wang, X.; Hill, C.L. Heterogenization of polyoxometalates as solid catalysts in aerobic oxidation of glycerol. Catal. Sci. Technol. 2020, 10, 3771–3781. [Google Scholar] [CrossRef]
- Choi, Y.-B.; Nunotani, N.; Imanaka, N. Glyceraldehyde production from glycerol over Pt/CeO2-ZrO2-Fe2O3/SBA-16 catalysts around room temperature in open air system. Mater. Lett. 2020, 278, 128392. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, H.; Dai, Y.; Zheng, J.; Yu, H.; Zhou, C.; Yang, Y. Modulating the electronic property of Pt nanocatalyst on rGO by iron oxides for aerobic oxidation of glycerol. Catal. Commun. 2020, 144, 106073. [Google Scholar] [CrossRef]
- Pakrieva, E.; Kolobova, E.; German, D.; Stucchi, M.; Villa, A.; Prati, L.; Carabineiro, S.; Bogdanchikova, N.; Corberán, V.C.; Pestryakov, A. Glycerol Oxidation over Supported Gold Catalysts: The Combined Effect of Au Particle Size and Basicity of Support. Processes 2020, 8, 1016. [Google Scholar] [CrossRef]
- Liu, M.; Yan, W.; Wu, J.; Wang, S.; Xia, Q.; Fang, T.; Jin, X. Electronically Coupled PtCo/MgAl Hydrotalcite Catalysts Display Tunable Selectivity Toward Glyceric Acid and Lactic Acid for Glycerol Conversion. Catal. Lett. 2020, 150, 2590–2598. [Google Scholar] [CrossRef]
- Sever, B.; Yildiz, M. Conversion of glycerol to lactic acid over Au/bentonite catalysts in alkaline solution. React. Kinet. Mech. Catal. 2020, 130, 863–874. [Google Scholar] [CrossRef]
- Detoni, C.; Da Silva, A.R.P.; Souza, M.M.V.M. Effect of Pt/HZSM-5 dealumination by high temperature reduction on glycerol oxidation. J. Porous Mater. 2020, 27, 707–717. [Google Scholar] [CrossRef]
- Mitran, G.; Neațu, F.; Neațu, S.; Trandafir, M.M.; Florea, M. VAlPOs as Efficient Catalysts for Glycerol Conversion to Methanol. Catalysts 2020, 10, 728. [Google Scholar] [CrossRef]
- Yan, H.; Yao, S.; Yin, B.; Liang, W.; Jin, X.; Feng, X.; Liu, Y.; Chen, X.; Yang, C. Synergistic effects of bimetallic PtRu/MCM-41 nanocatalysts for glycerol oxidation in base-free medium: Structure and electronic coupling dependent activity. Appl. Catal. B Environ. 2019, 259, 118070. [Google Scholar] [CrossRef]
- Cherni, D.; Moussa, N.; Nsib, M.F.; Evangelisti, C.; Prati, L.; Villa, A. Base-free glycerol oxidation over N-TiO2 supported Au–Pt catalysts. React. Kinet. Mech. Catal. 2019, 128, 979–990. [Google Scholar] [CrossRef]
- Oliveira, H.S.; Oliveira, L.C.A.; Chagas, P.; Sangiorge, D.L.; Figueiredo, M.P.; Siqueira, K.P.F.; Hensen, E.J.; Portilho, M.F. A bifunctional catalyst based on Nb and V oxides over alumina: Oxidative cleavage of crude glycerol to green formic acid. New J. Chem. 2020, 44, 8538–8544. [Google Scholar] [CrossRef]
- Douthwaite, M.; Powell, N.; Taylor, A.; Ford, G.; López, J.M.; Solsona, B.; Yang, N.; Sanahuja-Parejo, O.; He, Q.; Morgan, D.J.; et al. Glycerol Selective Oxidation to Lactic Acid over AuPt Nanoparticles; Enhancing Reaction Selectivity and Understanding by Support Modification. ChemCatChem 2020, 12, 3097–3107. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S.; Kouamé, R.S.B. Selective Electrooxidation of Glycerol Into Value-Added Chemicals: A Short Overview. Front. Chem. 2019, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Klaas, L.; Modibedi, M.; Mathe, M.; Su, H.; Khotseng, L. Electrochemical Studies of Pd-Based Anode Catalysts in Alkaline Medium for Direct Glycerol Fuel Cells. Catalysts 2020, 10, 968. [Google Scholar] [CrossRef]
- Falase, A.; Garcia, K.; Lau, C.; Atanassov, P. Electrochemical and in situ IR characterization of PtRu catalysts for complete oxidation of ethylene glycol and glycerol. Electrochem. Commun. 2011, 13, 1488–1491. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.W.; Lee, S.; Han, J.; Lee, D.; Kim, J.-R.; Kim, T.-W.; Kim, C.-U.; Jeong, S.-Y.; Chae, H.-J.; et al. The Role of Ruthenium on Carbon-Supported PtRu Catalysts for Electrocatalytic Glycerol Oxidation under Acidic Conditions. ChemCatChem 2017, 9, 1683–1690. [Google Scholar] [CrossRef]
- Alaba, P.A.; Lee, C.S.; Abnisa, F.; Aroua, M.K.; Cognet, P.; Pérès, Y.; Daud, W.M.A.W. A review of recent progress on electrocatalysts toward efficient glycerol electrooxidation. Rev. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Ji, Y. Recent Development of Heterogeneous Catalysis in the Transesterification of Glycerol to Glycerol Carbonate. Catalysts 2019, 9, 581. [Google Scholar] [CrossRef]
- Leão, R.A.C.; De Souza, R.O.M.A.; Nogueira, D.O.; Silva, G.M.A.; Silva, M.V.M.; Gutarra, M.L.E.; Miranda, L.S.M.; De Castro, A.M.; Itabaiana, I.; De Souza, R.O.M.A. Consecutive lipase immobilization and glycerol carbonate production under continuous-flow conditions. Catal. Sci. Technol. 2016, 6, 4743–4748. [Google Scholar] [CrossRef]
- Varma, R.S.; Len, C. Glycerol valorization under continuous flow conditions-recent advances. Curr. Opin. Green Sustain. Chem. 2019, 15, 83–90. [Google Scholar] [CrossRef]
- Corporation, H. JEFFSOL Alkylene Carbonates; JEFFSOL: The Woodlands, TX, USA, 2001. [Google Scholar]
- Saeidabad, N.G.; Noh, Y.S.; Eslami, A.A.; Song, H.T.; Kim, H.D.; Fazeli, A.; Moon, D.J. A Review on Catalysts Development for Steam Reforming of Biodiesel Derived Glycerol; Promoters and Supports. Catalysts 2020, 10, 910. [Google Scholar] [CrossRef]
- Bartoli, M.; Zhu, C.; Chae, M.; Bressler, D.C. Value-Added Products from Urea Glycerolysis Using a Heterogeneous Biosolids-Based Catalyst. Catalysts 2018, 8, 373. [Google Scholar] [CrossRef]
- Manikandan, M.; Sangeetha, P. Optimizing the Surface Properties of MgO Nanoparticles Towards the Transesterification of Glycerol to Glycerol Carbonate. ChemistrySelect 2019, 4, 6672–6678. [Google Scholar] [CrossRef]
- De Caro, P.; Bandres, M.; Urrutigoïty, M.; Cecutti, C.; Thiebaud-Roux, S. Recent Progress in Synthesis of Glycerol Carbonate and Evaluation of Its Plasticizing Properties. Front. Chem. 2019, 7, 308. [Google Scholar] [CrossRef]
- Das, B.; Mohanty, K. A green and facile production of catalysts from waste red mud for the one-pot synthesis of glycerol carbonate from glycerol. J. Environ. Chem. Eng. 2019, 7, 102888. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Okoye, P.U.; Chen, S.; Li, X.; Duan, L.; Zhou, H.; Li, S.; Tang, T.; Zhang, L.; et al. Application of corncob residue-derived catalyst in the transesterification of glycerol with dimethyl carbonate to synthesize glycerol carbonate. BioResources 2019, 15, 142–158. [Google Scholar] [CrossRef]
- Chotchuang, A.; Kunsuk, P.; Phanpitakkul, A.; Chanklang, S.; Chareonpanich, M.; Seubsai, A. Production of glycerol carbonate from glycerol over modified sodium-aluminate-doped calcium oxide catalysts. Catal. Today 2020. [Google Scholar] [CrossRef]
- Rittiron, P.; Niamnuy, C.; Donphai, W.; Chareonpanich, M.; Seubsai, A. Production of Glycerol Carbonate from Glycerol over Templated-Sodium-Aluminate Catalysts Prepared Using a Spray-Drying Method. ACS Omega 2019, 4, 9001–9009. [Google Scholar] [CrossRef] [PubMed]
- Phadtare, D.; Kondawar, S.; Athawale, A.; Rode, C.V. Crystalline LaCoO3 perovskite as a novel catalyst for glycerol transesterification. Mol. Catal. 2019, 475, 110496. [Google Scholar] [CrossRef]
- Chaves, D.M.; Da Silva, M.J. A selective synthesis of glycerol carbonate from glycerol and urea over Sn(OH)2: A solid and recyclable in situ generated catalyst. New J. Chem. 2019, 43, 3698–3706. [Google Scholar] [CrossRef]
- Arora, S.; Gosu, V.; Kumar, U.K.A.; Subbaramaiah, V. A Facile Approach to Develop Rice Husk Derived Green Catalyst for One-pot Synthesis of Glycerol Carbonate from Glycerol. Int. J. Chem. React. Eng. 2020, 18, 20190078. [Google Scholar] [CrossRef]
- Mallesham, B.; Rangaswamy, A.; Rao, B.G.; Rao, T.V.; Reddy, B.M. Solvent-Free Production of Glycerol Carbonate from Bioglycerol with Urea Over Nanostructured Promoted SnO2 Catalysts. Catal. Lett. 2020, 1–16. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Zhang, J.; He, D. Glycerol carbonylation with CO2 to glycerol carbonate over CeO2 catalyst and the influence of CeO2 preparation methods and reaction parameters. Appl. Catal. A Gen. 2016, 513, 9–18. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Liu, H.; He, D. Photo-thermal synergistically catalytic conversion of glycerol and carbon dioxide to glycerol carbonate over Au/ZnWO4-ZnO catalysts. Appl. Catal. B Environ. 2019, 244, 836–843. [Google Scholar] [CrossRef]
- García, H.; García, J.I.; Fraile, J.M.; Mayoral, J.A. Solketal: Green and catalytic synthesis and its classification as a solvent: 2,2-dimethyl-4-hidroxymethyl-1,3-dioxolane, an interesting green solvent produced through heterogeneous catalysis. Chim. Oggi Chem. Today 2008, 26, 10–12. [Google Scholar]
- Li, L.; Korányi, T.I.; Sels, B.F.; Pescarmona, P.P. Highly-efficient conversion of glycerol to solketal over heterogeneous Lewis acid catalysts. Green Chem. 2012, 14, 1611–1619. [Google Scholar] [CrossRef]
- Nanda, M.R.; Zhang, Y.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Xu, C. Catalytic conversion of glycerol for sustainable production of solketal as a fuel additive: A review. Renew. Sustain. Energy Rev. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Najaafi, N.; Tarighi, S. A Review on the Catalytic Acetalization of Bio-renewable Glycerol to Fuel Additives. Front. Chem. 2018, 6, 573. [Google Scholar] [CrossRef]
- Fatimah, I.; Sahroni, I.; Fadillaha, G.; Musawwa, M.M.; Mahlia, T.M.I.; Muraza, O. Glycerol to Solketal for Fuel Additive: Recent Progress in Heterogeneous Catalysts. Energies 2019, 12, 2872. [Google Scholar] [CrossRef]
- Deutsch, J.; Martin, A.; Lieske, H. Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals. J. Catal. 2007, 245, 428–435. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuan, Z.; Qin, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C. A new continuous-flow process for catalytic conversion of glycerol to oxygenated fuel additive: Catalyst screening. Appl. Energy 2014, 123, 75–81. [Google Scholar] [CrossRef]
- Chen, L.; Nohair, B.; Zhao, D.; Kaliaguine, S. Glycerol acetalization with formaldehyde using heteropolyacid salts supported on mesostructured silica. Appl. Catal. A Gen. 2018, 549, 207–215. [Google Scholar] [CrossRef]
- Fernández, P.; Fraile, J.M.; Garcia-Bordeje, E.; Pires, E.; Bordejé, G. Sulfonated Hydrothermal Carbons from Cellulose and Glucose as Catalysts for Glycerol Ketalization. Catalysts 2019, 9, 804. [Google Scholar] [CrossRef]
- Kowalska-Kus, J.; Held, A.; Frankowski, M.; Nowinska, K. Solketal formation from glycerol and acetone over hierarchical zeolites of different structure as catalysts. J. Mol. Catal. A Chem. 2017, 426, 205–212. [Google Scholar] [CrossRef]
- Da Silva, M.J.; Teixeira, M.G.; Chaves, D.M.; Siqueira, L. An efficient process to synthesize solketal from glycerol over tin (II) silicotungstate catalyst. Fuel 2020, 281, 118724. [Google Scholar] [CrossRef]
- Shimura, K.; Yoshida, H. Heterogeneous photocatalytic hydrogen production from water and biomass derivatives. Energy Environ. Sci. 2011, 4, 2467–2481. [Google Scholar] [CrossRef]
- Clarizia, L.; Spasiano, D.; Di Somma, I.; Marotta, R.; Andreozzi, R.; Dionysiou, D.D. Copper modified-TiO2 catalysts for hydrogen generation through photoreforming of organics. A short review. Int. J. Hydrogen Energy 2014, 39, 16812–16831. [Google Scholar] [CrossRef]
- López-Tenllado, F.; Hidalgo-Carrillo, J.; Montes, V.; Marinas, A.; Urbano, F.; Marinas, J.; Ilieva, L.; Tabakova, T.; Reid, F. A comparative study of hydrogen photocatalytic production from glycerol and propan-2-ol on M/TiO2 systems (M=Au, Pt, Pd). Catal. Today 2017, 280, 58–64. [Google Scholar] [CrossRef]
- Deas, R.; Pearce, S.; Goss, K.; Wang, Q.; Chen, W.-T.; Waterhouse, G.I.N. Hierarchical Au/TiO2 nanoflower photocatalysts with outstanding performance for alcohol photoreforming under UV irradiation. Appl. Catal. A Gen. 2020, 602, 117706. [Google Scholar] [CrossRef]
- Stelmachowski, M.; Marchwicka, M.; Grabowska-Musiał, E.; Diak, M.; Zaleska, A. The Photocatalytic Conversion of (Biodiesel Derived) Glycerol to Hydrogen - A Short Review and Preliminary Experimental Results Part 2: Photocatalytic Conversion of Glycerol to Hydrogen in Batch and Semi-batch Laboratory Reactors. J. Adv. Oxid. Technol. 2014, 17, 179–186. [Google Scholar] [CrossRef]
- You, B.; Han, G.; Sun, Y. Electrocatalytic and photocatalytic hydrogen evolution integrated with organic oxidation. Chem. Commun. 2018, 54, 5943–5955. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T.; Kawai, T.S.T. Hydrogen evolution from water using solid carbon and light energy. Nat. Cell Biol. 1979, 282, 283–284. [Google Scholar] [CrossRef]
- Ma, D.; Zhai, S.; Wang, Y.; Liu, A.; Chen, C. TiO2 Photocatalysis for Transfer Hydrogenation. Molecules 2019, 24, 330. [Google Scholar] [CrossRef]
- Al-Azri, Z.H.; Chen, W.-T.; Chan, A.; Jovic, V.; Ina, T.; Idriss, H.; Waterhouse, G.I. The roles of metal co-catalysts and reaction media in photocatalytic hydrogen production: Performance evaluation of M/TiO2 photocatalysts (M = Pd, Pt, Au) in different alcohol–water mixtures. J. Catal. 2015, 329, 355–367. [Google Scholar] [CrossRef]
- Slamet, R.; Gunlazuardi, J.; Dewi, E.L. Enhanced photocatalytic activity of Pt deposited on titania nanotube arrays for the hydrogen production with glycerol as a sacrificial agent. Int. J. Hydrogen Energy 2017, 42, 24014–24025. [Google Scholar] [CrossRef]
- Chen, W.-T.; Dosado, A.G.; Chan, A.; Sun-Waterhouse, D.; Waterhouse, G.I. Highly reactive anatase nanorod photocatalysts synthesized by calcination of hydrogen titanate nanotubes: Effect of calcination conditions on photocatalytic performance for aqueous dye degradation and H2 production in alcohol-water mixtures. Appl. Catal. A Gen. 2018, 565, 98–118. [Google Scholar] [CrossRef]
- O’Rourke, C.; Wells, N.; Mills, A. Photodeposition of metals from inks and their application in photocatalysis. Catal. Today 2019, 335, 91–100. [Google Scholar] [CrossRef]
- Sadanandam, G.; Lalitha, K.; Kumari, V.D.; Shankar, M.V.; Subrahmanyam, M. Cobalt doped TiO2: A stable and efficient photocatalyst for continuous hydrogen production from glycerol: Water mixtures under solar light irradiation. Int. J. Hydrogen Energy 2013, 38, 9655–9664. [Google Scholar] [CrossRef]
- Hidalgo-Carrillo, J.; Martín-Gómez, J.; Morales, J.; Espejo, J.C.; Urbano, F.J.; Marinas, A. Hydrogen Photo-Production from Glycerol Using Nickel-Doped TiO2 Catalysts: Effect of Catalyst Pre-Treatment. Energies 2019, 12, 3351. [Google Scholar] [CrossRef]
- Lalitha, K.; Sadanandam, G.; Kumari, V.D.; Subrahmanyam, M.; Sreedhar, B.; Hebalkar, N.Y. Highly Stabilized and Finely Dispersed Cu2O/TiO2: A Promising Visible Sensitive Photocatalyst for Continuous Production of Hydrogen from Glycerol: Water Mixtures. J. Phys. Chem. C 2010, 114, 22181–22189. [Google Scholar] [CrossRef]
- Kumar, D.P.; Reddy, N.L.; Kumari, M.M.; Srinivas, B.; Kumari, V.D.; Sreedhar, B.; Roddatis, V.V.; Bondarchuk, O.; Karthik, M.; Neppolian, B.; et al. Cu2O-sensitized TiO2 nanorods with nanocavities for highly efficient photocatalytic hydrogen production under solar irradiation. Sol. Energy Mater. Sol. Cells 2015, 136, 157–166. [Google Scholar] [CrossRef]
- Chen, W.-T.; Chan, A.; Sun-Waterhouse, D.; Llorca, J.; Idriss, H.; Waterhouse, G.I. Performance comparison of Ni/TiO2 and Au/TiO2 photocatalysts for H2 production in different alcohol-water mixtures. J. Catal. 2018, 367, 27–42. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, D.; Chen, Y.; Fu, W.-F.; Lv, X.-J. Single-Atom Catalysts for Photocatalytic Reactions. ACS Sustain. Chem. Eng. 2019, 7, 6430–6443. [Google Scholar] [CrossRef]
- Pan, H.; Steiniger, A.; Heagy, M.D.; Chowdhury, S. Efficient production of formic acid by simultaneous photoreduction of bicarbonate and oxidation of glycerol on gold-TiO2 composite under solar light. J. CO2 Util. 2017, 22, 117–123. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.I.; Papageridis, K.; Motta, D.; Dimitratos, N.; Jaoude, M.A.; Polychronopoulou, K.; Goula, M.A. The Effect of Noble Metal (M: Ir, Pt, Pd) on M/Ce2O3-γ-Al2O3 Catalysts for Hydrogen Production via the Steam Reforming of Glycerol. Catalysts 2020, 10, 790. [Google Scholar] [CrossRef]
- Bac, S.; Keskin, S.; Avci, A.K. Recent advances in sustainable syngas production by catalytic CO2 reforming of ethanol and glycerol. Sustain. Energy Fuels 2020, 4, 1029–1047. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Lopez-Sanchez, J.A. Review of Hydrogen Production by Catalytic Aqueous-Phase Reforming. ChemistrySelect 2017, 2, 6563–6576. [Google Scholar] [CrossRef]
- Jin, W.; Pastor-Pérez, L.; Shen, D.; Sepulveda-Escribano, A.; Gu, S.; Reina, T.R. Catalytic Upgrading of Biomass Model Compounds: Novel Approaches and Lessons Learnt from Traditional Hydrodeoxygenation—A Review. ChemCatChem 2019, 11, 924–960. [Google Scholar] [CrossRef]
- Jin, X.; Yin, B.; Xia, Q.; Fang, T.; Shen, J.; Kuang, L.; Yang, C. Catalytic Transfer Hydrogenation of Biomass-Derived Substrates to Value-Added Chemicals on Dual-Function Catalysts: Opportunities and Challenges. ChemSusChem 2018, 12, 71–92. [Google Scholar] [CrossRef]
- Toledano, A.; Serrano, L.; Labidi, J.; Pineda, A.; Balu, A.M.; Luque, R. Heterogeneously Catalysed Mild Hydrogenolytic Depolymerisation of Lignin Under Microwave Irradiation with Hydrogen-Donating Solvents. ChemCatChem 2012, 5, 977–985. [Google Scholar] [CrossRef]
- Crotti, C.; Farnetti, E.; Licen, S.; Barbieri, P.; Pitacco, G. Iridium-catalyzed N-alkylation of diamines with glycerol. J. Mol. Catal. A Chem. 2014, 382, 64–70. [Google Scholar] [CrossRef]
- Cui, S.; Borgemenke, J.; Liu, Z.; Keener, H.M.; Li, Y. Innovative sustainable conversion from CO2 and biodiesel-based crude glycerol waste to bio-based polycarbonates. J. CO2 Util. 2019, 34, 198–206. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Montes, V.; Caballero, A.; Bautista, F. Sulfonated carbons from olive stones as catalysts in the microwave-assisted etherification of glycerol with tert-butyl alcohol. Mol. Catal. 2020, 488, 110921. [Google Scholar] [CrossRef]
- Calmanti, R.; Amadio, E.; Perosa, A.; Selva, M. Reaction of Glycerol with Trimethyl Orthoformate: Towards the Synthesis of New Glycerol Derivatives. Catalysts 2019, 9, 534. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, T.; Zhao, J. Study on the conversion of glycerol to nitriles over a Fe19.2K0.2/γ-Al2O3 catalyst. J. Catal. 2014, 313, 92–103. [Google Scholar] [CrossRef]
- Du, F.; Jin, X.; Yan, W.; Zhao, M.; Thapa, P.S.; Chaudhari, R.V. Catalytic H2 auto transfer amination of polyols to alkyl amines in one pot using supported Ru catalysts. Catal. Today 2018, 302, 227–232. [Google Scholar] [CrossRef]
- Pandya, R.; Mane, R.B.; Rode, C.V. Cascade dehydrative amination of glycerol to oxazoline. Catal. Sci. Technol. 2018, 8, 2954–2965. [Google Scholar] [CrossRef]
- Wormann, M.; Maier, M.E. Synthesis of allyl alcohol as a method to valorise glycerol from the biodiesel production. RSC Adv. 2019, 9, 15314–15317. [Google Scholar] [CrossRef]
- L’Hostis, C.; Fredon, E.; Thévenon, M.-F.; Santiago-Medina, F.-J.; Gérardin, P. Beech wood treated with polyglycerol succinate: A new effective method for its protection and stabilization. Holzforschung 2020, 74, 351–361. [Google Scholar] [CrossRef]
- Zamboulis, A.; Nakiou, E.A.; Christodoulou, E.; Bikiaris, D.N.; Kontonasaki, E.; Liverani, L.; Boccaccini, A.R. Polyglycerol Hyperbranched Polyesters: Synthesis, Properties and Pharmaceutical and Biomedical Applications. Int. J. Mol. Sci. 2019, 20, 6210. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.J.S.; Cecilia, J.A.; Moreno-Tost, R.; De Oliveira, M.F.; Soto, J.; Luna, F.M.T.; Vieira, R.S. Glycerol Oligomerization Using Low Cost Dolomite Catalyst. Waste Biomass Valoriz. 2018, 11, 1499–1512. [Google Scholar] [CrossRef]
- Sotto, N.; Cazorla, C.; Villette, C.; Billamboz, M.; Len, C. Toward the Sustainable Synthesis of Biosourced Divinylglycol from Glycerol. ACS Sustain. Chem. Eng. 2016, 4, 6996–7003. [Google Scholar] [CrossRef]
- Yu, H.; Xue, Z.; Lan, X.; Liu, Q.; Shi, R.; Mu, T. Highly efficient dissolution of xylan in ionic liquid-based deep eutectic solvents. Cellulose 2020, 27, 6175–6188. [Google Scholar] [CrossRef]
- Saputra, R.; Walvekar, R.; Khalid, M.; Mubarak, N.M. Synthesis and thermophysical properties of ethylammonium chloride-glycerol-ZnCl2 ternary deep eutectic solvent. J. Mol. Liq. 2020, 310, 113232. [Google Scholar] [CrossRef]
- Hoang, T.Q.; Zhu, X.; Danuthai, T.; Lobban, L.L.; Resasco, D.E.; Mallinson, R.G. Conversion of Glycerol to Alkyl-aromatics over Zeolites. Energy Fuels 2010, 24, 3804–3809. [Google Scholar] [CrossRef]
- Xu, W.; Gao, L.; Xiao, G. Effects of Additives and Metals on Crystallization of Nano-Sized HZSM-5 Zeolite for Glycerol Aromatization. Catalysts 2019, 9, 899. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Ji, Y.; Chen, F.; Xiao, F.-S. Mesoporous zeolites for biofuel upgrading and glycerol conversion. Front. Chem. Sci. Eng. 2017, 12, 132–144. [Google Scholar] [CrossRef]
- Possato, L.G.; Diniz, R.N.; Garetto, T.; Pulcinelli, S.H.; Santilli, C.V.; Martins, L. A comparative study of glycerol dehydration catalyzed by micro/mesoporous MFI zeolites. J. Catal. 2013, 300, 102–112. [Google Scholar] [CrossRef]
- Kumar, D.; Anand, N.; Pant, K.K. Glycerol conversion over palladium- and alumina-impregnated KIT-6 for the production of gasoline range hydrocarbons. Clean Technol. Environ. Policy 2017, 20, 751–757. [Google Scholar] [CrossRef]
- He, S.; Zuur, K.; Santosa, D.S.; Heeres, A.; Liu, C.; Pidko, E.A.; Heeres, H.J. Catalytic conversion of pure glycerol over an un-modified H-ZSM-5 zeolite to bio-based aromatics. Appl. Catal. B Environ. 2021, 281, 119467. [Google Scholar] [CrossRef]
- Ismail, H.; Zaaba, N. The mechanical properties, water resistance and degradation behaviour of silica-filled sago starch/PVA plastic films. J. Elastomers Plast. 2012, 46, 96–109. [Google Scholar] [CrossRef]
- Devi, B.L.A.P.; Gangadhar, K.N.; Prasad, P.S.S.; Jagannadh, B.; Prasad, R.B.N. A Glycerol-based Carbon Catalyst for the Preparation of Biodiesel. ChemSusChem 2009, 2, 617–620. [Google Scholar] [CrossRef]
- Ribeiro, R.S.; Silva, A.M.; Pinho, M.T.; Figueiredo, J.L.; Faria, J.L.; Gomes, H.T. Development of glycerol-based metal-free carbon materials for environmental catalytic applications. Catal. Today 2015, 240, 61–66. [Google Scholar] [CrossRef]
- Cui, Y.; Atkinson, J.D. Tailored activated carbon from glycerol: Role of acid dehydrator on physiochemical characteristics and adsorption performance. J. Mater. Chem. A 2017, 5, 16812–16821. [Google Scholar] [CrossRef]
- Estes, C.S.; Gerard, A.Y.; Godward, J.D.; Hayes, S.B.; Liles, S.H.; Shelton, J.L.; Stewart, T.S.; Webster, R.I.; Webster, H.F. Preparation of highly functionalized carbon nanoparticles using a one-step acid dehydration of glycerol. Carbon 2019, 142, 547–557. [Google Scholar] [CrossRef]
- Medeiros, M.A.; Ardisson, J.D.; Lago, R.M. Preparation of magnetic mesoporous composites from glycerol and Fe (III) salt. J. Chem. Technol. Biotechnol. 2019, 95, 1038–1045. [Google Scholar] [CrossRef]
- Antunes, E.; Jacob, M.V.; Brodie, G.; Schneider, P.A. Microwave pyrolysis of sewage biosolids: Dielectric properties, microwave susceptor role and its impact on biochar properties. J. Anal. Appl. Pyrolysis 2018, 129, 93–100. [Google Scholar] [CrossRef]
- Cui, Y.; Atkinson, J.D. Glycerol-derived magnetic mesoporous Fe/C composites for Cr(VI) removal, prepared via acid-assisted one-pot pyrolysis. Chemosphere 2019, 228, 694–701. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z.; Liu, H. Development of carbon adsorbents with high surface acidity and basicity from polyhydric alcohols with phosphoric acid activation for Ni(II) removal. Chemosphere 2018, 206, 115–121. [Google Scholar] [CrossRef]
- Gonçalves, M.; Oliveira, C.C.; Boas, I.K.; Soler, F.C.; Pinto, E.D.C.; Lavall, R.L.; Carvalho, W.A. Glycerin waste as sustainable precursor for activated carbon production: Adsorption properties and application in supercapacitors. J. Environ. Chem. Eng. 2019, 7, 103059. [Google Scholar] [CrossRef]
- Uchôa, I.M.A.; Neto, A.A.D.; Santos, E.D.S.; De Lima, L.F.; Neto, E.L.D.B. Evaluation of Lubricating Properties of Diesel Based Fuels Micro Emulsified With Glycerin. Mater. Res. 2017, 20, 701–708. [Google Scholar] [CrossRef]
- Mikhailenko, P.; Bertron, A.; Boussambe, G.N.M.; Valentin, R.; Mouloungui, Z.; Ringot, E. Recycled bio-sourced glycerol and diglycerol for asphalt release agents (ARA). Road Mater. Pavement Des. 2018, 21, 201–216. [Google Scholar] [CrossRef]
- Mishra, V.; Singh, D.; Habal, A. Investigating the condition number approach to select probe liquids for evaluating surface free energy of bitumen. Int. J. Pavement Res. Technol. 2019, 13, 10–19. [Google Scholar] [CrossRef]
- Campos, W.E.O.; Nobre, F.X.; Filho, G.N.D.R.; Da Silva, M.A.R.; Da Costa, C.E.F.; Nascimento, L.A.S.D.; Zamian, J.R. High Photocatalytic Activity under Visible Light for a New Morphology of Bi2WO6 Microcrystals. Catalysts 2019, 9, 667. [Google Scholar] [CrossRef]
- Ye, L.; Jin, X.; Liu, C.; Ding, C.; Xie, H.; Chu, K.H.; Wong, P.K. Thickness-ultrathin and bismuth-rich strategies for BiOBr to enhance photoreduction of CO2 into solar fuels. Appl. Catal. B Environ. 2016, 187, 281–290. [Google Scholar] [CrossRef]
- Kim, M.; Son, W.-S.; Ahn, K.H.; Kim, D.S.; Lee, H.-S.; Lee, Y.-W. Hydrothermal synthesis of metal nanoparticles using glycerol as a reducing agent. J. Supercrit. Fluids 2014, 90, 53–59. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Park, E.D. Preferential CO Oxidation in H2 over Au/La2O3/Al2O3 Catalysts: The Effect of the Catalyst Reduction Method. Catalysts 2018, 8, 183. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Upare, P.P.; Le, N.-T.; Hwang, Y.K.; Hwang, D.W.; Lee, U.-H.; Kim, H.R.; Chang, J.-S. Facile synthesis of CeO2-supported gold nanoparticle catalysts for selective oxidation of glycerol into lactic acid. Appl. Catal. A Gen. 2013, 468, 260–268. [Google Scholar] [CrossRef]

| Metal | Support | Observations | Reference |
|---|---|---|---|
| Ce-Ni | αAl2O3 | H2, CO, CO2, and CH4 production with H2:CO ratios below 2 (suitable for Fischer–Tropsch synthesis), obtained from primary glycerol decomposition | [55] |
| La-Ni | αAl2O3 | High catalytic activity on glycerol decomposition, due to its high acidity, to produce H2, CO, CO2, and CH4 | [56] |
| ZSM-5 | Bentonite | Experiments in a bench scale unit to produce bio-BTX, with coke formation on the catalyst as the main cause of deactivation | [52] |
| Ni Schiff base complex | SBA-15 silica | CO2, aldehyde, ketone, and alcohol production through fast pyrolysis. There were reductions in the decomposition temperature of glycerol | [53] |
| Nb2O5 | Vermiculite | Used in flash pyrolysis to mainly produce organic acids, esters, and aldehydes | [54] |
| Catalyst | Mechanism |
|---|---|
| Ni-based | Glycerol is absorbed at the catalytic site and is dissociated in H2 and hydroxyl. Afterwards, hydroxyl reacts with H2 to generate and release H2 and CO2 |
| Ru-based | Glycerol is absorbed on the surface of the catalyst with water, creating a complex that reacts to generate H2 and CO2 |
| Co-based | Co acts as a precursor, promoting H2 production and CH4 and CO2 generation |
| Pt-based | Dehydrogenation of glycerol to form acetol, a second dehydrogenation step with a first breaking of the C-C bond, and the formation of acetic acid, which decomposes into H2 and CO2 |
| Metal | Support | Observations | Reference |
|---|---|---|---|
| Ni, Co, Cu, Fe | Attapulgite | Catalytic activity increased with temperature (up to 700 °C), with Ni and Co showing the best glycerol conversion due to their capacity to promote the cleavage of C-C and C-H. The main inactivation problems were derived from metal sintering and coke deposition. | [70] |
| Ru | Hydrotalcite | High catalytic activity exceeding 600 °C. Deactivation caused by coke deposition. | [71] |
| Co and Co-Ce | Hydroxyapatite | High selectivity toward H2 (83%) at 650 °C. Cerium addition improved H2 selectivity and glycerol conversion, avoiding Co sintering. | [72] |
| Ni and Mg | Al2O3 | Mg contributed to the dispersion of Ni, increasing its resistance of Ni to sintering and coke deposition. | [73] |
| Co/Al | SiO2, SiC, and γ-Al2O3 | Oxidative steam reforming in a fixed bed reactor was carried out, reaching heterogeneous thermal decomposition of glycerol (at least 83%) with the three supports. | [74] |
| Rh | Al2O3 | The catalyst activity of the catalyst decreased over time, solving this problem by oxidative regeneration to gasify coke deposition. | [75] |
| Ni | CeO2 | Glow discharge plasma was used to prepare the catalyst, increasing Ni dispersion, promoting the Ni-O-Ce composite, and enhancing H2 production and resistance to coke deposition. | [62] |
| Ni | CeO2 | Glow discharge plasma improved Ni dispersion, hindering coke deposition and improving H2 selectivity and stability. | [63] |
| Ni | Al2O3 | In this work, glycerol steam reforming was used as a starting point to produce methane, showing good stability for a 40% glycerol aqueous solution. | [76] |
| Co-Ni | SBA-15 | Different mesoporous catalysts were prepared, being resistant to deactivation and yielding 100% glycerol conversion. | [77] |
| Rh | γ-Al2O3 | The catalyst was modified with CeO2, MgO, and La2O3, producing high glycerol conversions (over 90%) and sintering stability. | [78] |
| Ni | Graphene | Graphene-encapsulated nickel catalysts were synthesized and attached to an SiO2 skeleton, with rice husk char as the carbonaceous source. Multilayered graphene avoided oxidation and sintering of Ni, not reducing its catalytic activity. | [79] |
| Ni | ZrO2 | Tetragonal zirconia support was compared to a commercial monoclinic zirconia support, with the latter providing a better H2 yield and glycerol conversion. | [80] |
| Ni | Hydrotalcite | The effect of La promotion on this catalyst was studied, influencing coke deposition. | [81] |
| Ni | CaO-modified attapulgite | CaO increased the activity of the catalyst and the inhibition of coke deposition, and attapulgite showed a good carbon deposition resistance. | [82] |
| Ni | Ce-modified γ-Al2O3 | Ceria improved Ni dispersion, producing highly active small Ni particles in the catalyst, showing high glycerol conversion (99%) and H2 yield (62%). | [83] |
| NiCexAl | Metal oxides | Ce addition restricted Ni agglomeration, enhancing oxygen mobility, which improved the properties of Ni active sites, suppressing coke deposition. | [84] |
| Co | Al2O3 | The effect of niobia addition on this catalyst was studied, decreasing alumina acidity, improving catalyst reducibility, and reducing the formation of spinel phases. | [85] |
| Co and Cu | MgO and Al2O3 | MgO addition promoted stable H2 yields. Cu addition suppressed coke deposition due to the smaller Co particles obtained. | [86] |
| Ni, La and Ce | SAB-15 | La2O3 addition to Ni/SAB-15 avoided crystalline growth of Ni, and CeO2 addition facilitated carbon removal. The highest H2 yield was obtained for the 10% Ni/5% La/5% Ce catalyst. | [87] |
| Metal | Support | Observations | Reference |
|---|---|---|---|
| Pt-Mn | C | The promoting effect of Mg on H2 production was studied in steam and aqueous phase reforming, showing a good performance, especially for SR, and being a cheap option. | [93] |
| Ni | Al2O3/MgO | MgO addition improved the catalytic activity. The Al/Mg ratio was vital in the performance of the catalyst. No evidence of deactivation by carbon deposition was observed. | [91] |
| Pt | Al2O3 | A Pt nanoparticle supported on alumina was used, varying the particle size. Therefore, the best conversion of glycerol was obtained for the larger particle size. | [97] |
| Ir-ReOx | SiO2 | H2 generated in aqueous steam reforming was used for the selective C-O hydrogenolysis of glycerol (without external H2 sources). | [95] |
| Raney Ni® | -- | In-situ glycerol aqueous phase reforming and phenol hydrogenation. | [94] |
| Co aluminate spinel | -- | High performance when methanation and Fischer–Tropsch are inhibited. | [92] |
| Ni aluminate spinel | -- | Bulk nickel aluminate spinel prepared by co-precipitation at 850 °C, reaching 93% glycerol conversion and 57% conversion to gas. | [98] |
| Pt/CoAl2O4 | -- | Bimetallic catalyst showed higher activity (with synergistic effect between Pt and Co) than monometallic catalysts. | [99] |
| Pt | Al2O3/MgO | Aqueous phase glycerol reforming coupled to methanol synthesis. | [96] |
| Catalyst | Reaction Conditions | Glycerol Conversion % | 1,2-PDO Yield% | 1,3-PDO Yield% | Other Main Products’ (Yield, %) | Ref. |
|---|---|---|---|---|---|---|
| Ni/WO3–TiO2 | Vcat. = 2 mL. 30% glycerol aq. 3.0 MPa H2. 250 °C. LHSV = 0.46 h−1. gas flow 15 mL min−1. | 100 | monopropanols (94.0) | [147] | ||
| Ni-Cu/Y-zeolite | 1.25 g catalyst. 20 vol% glycerol aq. feed rate 0.041 mL min−1. 260 °C. 46 bar. 30 h. | 96.4 | 31.8 | LA (29.4) | [127] | |
| Cu–Ni/γ-Al2O3 | 20 wt% glycerol aq. feed rate = 0.03 mL/min. 220 °C. 0.75 MPa. WHSV = 1.3 h−1. H2 flow rate = 100 mL/min. N2 flow rate = 20 mL/min. 14 h. | 99.9 | 89.4 | [141] | ||
| Hydrotalcite-Cu | Glycerol/catalyst ratio of 12:1 w/w 200 °C. 3.4 MPa. 24 h | 66 | 63.1 | [152] | ||
| Cu/Ce3/Mg | 1 g of reduced catalyst, 50 g of 20 wt.% Glycerol aq. 473 K. 60 bar of H2 pressure, 10 h. | 56.4 | 54.9 | [153] | ||
| Ni-Zr/H-beta zeolite | 0.5 g catalyst. 40 mL 50 wt% glycerol aq. 200 °C. 600 psi H2. 10 h. | 77 | 20 | 10.8 | Monopropanols (39.3) | [154] |
| W/Al2O3 | 3 mL catalyst bed. 60 wt% glycerol/H2O 10 h−1 LHSV. 60 bar H2 1860 h−1 GHSV. 325 °C | 73 | 7.3 | Monopropanols (51.5) | [155] | |
| Ni/γ-Al2O3 and Ni/CS-P | Catalyst/glycerol = 0.16 (mass ratio). Ni/γ-Al2O3 catalyst 5 h 220 °C (a), followed by Ni/CS-P catalyst 2 h at 260 °C (b). 6.5 MPa H2. Crude glycerol Oleomud. | 100 | (a) 87 | (b) 1-PrOH (79) | [145] | |
| 5Cu-1B/Al2O3 | 2.0 g catalyst. 0.1 h–1 WHSV 10% crude glycerol aq. 250 °C, 6 MPa H2. | 70 | 56 | Acetol (3.5) | [156] | |
| 10%Cu/SBA-15(G) | Catalyst/glycerol = 1:10 weight ratio. 6.67 g n-butanol solution of glycerol (12 wt%). 4.0 MPa H2. 483 K. 6 h | 90 | 88.2 | [122] | ||
| Ni/ZnAl | 100 mg of catalyst. Feed: 3.5 mL h−1 of 3% glycerol aq. gas feed 5% H2/Ar. atmospheric pressure. 573 K. | 100 | Methane (100) | [138] | ||
| 10Cu-7Co/γ-Al2O3 | 1 g of catalyst. 200 °C. flow rate LHSV of 2.5 h−1. 20 wt% Glycerol aq. 3 h | 100 | 77 | [142] | ||
| 5CuO/Mg9Al2.7-Ga2.3O2 | 0.5 g catalyst, 20 wt.% glycerol 220 °C. 0.5 MPa H2 at 40 mL·min−1. | 100 | 98 | [143] | ||
| 5Co-MSAPO-11 | Catalyst 1 g, 5 g glycerol, 60 g water. 4.0 MPa H2. 210 °C, 8 h. | 67.1 | 63.6 | [130] | ||
| Cu2Ni/M-SAPO-11 | 0.5 g catalyst. 75 mL of 8 wt% glycerol aq. 4 MPa H2. 230 °C. 12 h | 95 | 94 | [129] | ||
| PtCu(Single Atom Alloy)/MgAl | 0.14 g catalyst. 10 mL of glycerol alcoholic solution (10 wt.%). 200 °C. and 2.0 MPa of H2, 8 h. | 100 | 99.2 | [144] | ||
| PtRu/Zn3Ce1 | 0.05 g catalyst. 15 mL 0.3 M glycerol aq. 1 MPa N2. 220 °C, 4 h | 46.4 | 13.92 | LA (9.28) Acetol (11.6) | [157] | |
| 8Nb/Pd−Zr−Al | 0.20 g catalyst, 20 g of 10 wt% glycerol aq., 200 °C; initial H2 pressure. 3.5 MPa; 8 h | 69.2 | 58.4 | [158] | ||
| Ru/Mordenite | 0.4 g catalyst. 0.5 g glycerol. 9 g i-PrOH. 0.5 MPa N2. 140 °C. 72 h. | 70 | 2-isopropoxy-propan-1-ol (70.0) | [159] | ||
| PtWZr3Ti7 | 4 g catalyst. 50 wt% glycerol aq. Feed: 0.03 mL/min. H2:glycerol:H2O = 10:1:5.1 (molar ratio). 5 MPa. 140 °C. 15 h. | 73.8 | 25.3 | 1-PrOH (42.3) | [135,160] | |
| 2 wt% Pt/WOx | 0.3 g catalyst. 12 g 5 wt% glycerol aq. 140 °C. 1 MPa H2. 800 rpm. 12 h. | 37.4 | 0.8 | 13.1 | 1-PrOH (18.8) | [114] |
| 5Pt/W–s-SBA-15 | 0.4 g of catalyst. 4.0 g of 30 wt% glycerol aq. 433 K. 4.0 MPa H2. 24 h. | 76.8 | 47.4 | 1-PrOH (22.0) | [161] | |
| Pt/0.50Mg/WOx–ZrO2 | 3.5 g catalyst. 4 MPa. 150 °C. Feed: 40 wt% glycerol aq. WHSV = 0.2 h−1. H2 flow 50 mL min−1. Time on stream 25 h | 52.9 | 3.9 | 32.1 | 1-PrOH (8.3) 2-PrOH (7.9) | [151] |
| 6Pt/12.9W/Al2O3 | Catalyst volume 2.0 mL, 50 wt% GLY. GHSV = 1000 h−1. 5.0 MPa H2, 180 °C. | 80.4 | 4 | 28.4 | 1-PrOH (33.6) 2-PrOH (9.9) | [162] |
| 2Pt/20(W+Al)-SBA-15 | 0.5 g of catalyst. 25 g 3 wt% glycerol aq. 160 °C. 6 MPa H2 for initial pressure. 12 h. | 65 | 32.5 | 1-PrOH (29.2) | [163] | |
| Pt/ZrW38Mn3 | 0.5 g catalyst. 4.0 g glycerol. 36.0 g water. 180 °C. 8 MPa. 18 h. | 55 | 5.5 | 24.8 | (22) | [164] |
| Ir-ReOx/Rutile. Re/Ir = 0.30 | Catalyst amount = 150 mg. 4 g glycerol. 2 g H2O. 8 MPa H2. T = 393 K. t = 4 h. | 52 | 29.6 | 1-PrOH (17.7) | [165] |
| Catalyst | Reaction Conditions | Glycerol Conversion % | Main Product (% Yield) | Ref. |
|---|---|---|---|---|
| Au- Nb2O5 | Glycerol (0.23 g), NaOH (0.20 g), catalyst (0.05 g), water (25 mL), pressure of oxygen (6 atm), 15 h. | 80 | GlyAc (72) | [171] |
| Pt/CeO2-ZrO2-Fe2O3/SBA-16 | 1 wt% glycerol aq. (10 mL), catalyst (0.3 g), 30 °C in an open-air system for 4 h. | 70.3 | GlyAc (28) | [191] |
| Pt-Fe3O4/rGO-8.5 | Glycerol (3 mmol), Pt/glycerol (molar ratio, 1:1000), H2O (30 mL), O2 flow (150 sccm), 60 °C, 2 h. | 29.5 | GlyAd (14.8) | [192] |
| Au/MgO/TiO2 | T = 50 °C, 0.3 M glycerol aq., NaOH/glycerol = 4 eqv., glycerol/gold ratio = 1000, 1 h | 100 | GlyAc (56) | [193] |
| PtCo/Mg3Al(OH)y(CO3)z | Glycerol: 0.3 M aq., NaOH/glycerol molar ratio: 2/1, volume: 15 mL, catalyst: 0.05 g, T: 100 °C, PO2: 0.5 MPa, 6 h | 96 | LA (64) | [194] |
| 1 wt% Au/bentonite | T = 90 °C, 50 mL NaOH/glycerol = 8 mol ratio, 0.25 g catalyst, 4 h | 82 | LA (76.1) | [195] |
| AlPMo12O40 | 5.0 mL of 1.0 M glycerol aq., 1 MPa O2, 4.0 mM catalyst, 60 °C for 5 h | 98.6 | LA (96.1) | [190] |
| AlPMo12O40 | 5.0 mL synthetic crude glycerol (71% Gly) in methanol, 1 MPa O2, 4.0 mM catalyst, 60 °C for 5 h | 92.1 | LA (86.8) | [190] |
| 3%Pt/HZSM-5 | 70 °C, 4 h, air flow (60 mL min−1) | 67.9 | GlyAc (30) | [196] |
| 2.9 V7.5APO(0.2) | 10% glycerol aq., T= 350 °C, 0.2 g catalyst, air flow 1200 mL h−1 and feed flow rate 3.6 mL h−1 | 98.3 | Methanol (83) | [197] |
| Pt0.8Ru0.8/MCM-41 | 0.2 catalyst, 25 mL aqueous solution of glycerol (0.22 M), glycerol/Pt molar ratio 700, 80 °C, 1 MPa O2, 12 h | 78.2 | GlyAc (63) | [198] |
| AuPt/N-TiO2 | 0.3 M aqueous glycerol solution, glycerol/M = 500, T = 100 °C, pO2 = 3 atm, 6 h | 92 | GlyAc (73) | [199] |
| VNb/Al2O3 | 50 mL Min−1 of neutralized crude glycerol: 50% H2O2 (1:1), 600 mg of catalyst at WHSV = 0.5 h−1 and 200 °C, t = 15 h measure | 100 | Formic acid (45) | [200] |
| AuPt/TiO2-NC | 20 mL, 0.3 M glycerol, 1.2 M NaOH; 3 bar O2; 110 °C; catalyst (0.029 g), 7 h | 99 | LA (82) | [201] |
| Molecule | Use as | Ref |
|---|---|---|
| Tert-butyl ethers | Gasoline additives | [263] |
| Orthoesters | Platform molecule | [264] |
| Alkyl amines | Pharmaceutical, agrochemicals, paints, etc. | [265,266] |
| Oxazoline | Biomedical polymers | [267] |
| Allyl alcohol | Pesticide | [268] |
| Polyglycerol succinate | Polymer production | [269] |
| Hyperbranched polyesters | Polymers | [270] |
| Di/tri-glycerol | Polymer production | [271] |
| Divinylglycol | Polymer production | [272] |
| Eutectic solvents | Solvents | [273,274] |
| Polycarbonates | Polymers | [262] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1279. https://doi.org/10.3390/catal10111279
Checa M, Nogales-Delgado S, Montes V, Encinar JM. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts. 2020; 10(11):1279. https://doi.org/10.3390/catal10111279
Chicago/Turabian StyleCheca, Manuel, Sergio Nogales-Delgado, Vicente Montes, and José María Encinar. 2020. "Recent Advances in Glycerol Catalytic Valorization: A Review" Catalysts 10, no. 11: 1279. https://doi.org/10.3390/catal10111279
APA StyleCheca, M., Nogales-Delgado, S., Montes, V., & Encinar, J. M. (2020). Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts, 10(11), 1279. https://doi.org/10.3390/catal10111279