Abstract
N- and Ni-coated TiO2 (NNT) were prepared by a facile sol-gel method as a photosensitive photocatalyst to visible light. NNT sol was used to coat the surface of an LED lamp cap and body made of polycarbonate with a thin NNT film. The coated thin film was dried in an oven at 130 °C. This NNT thin film had an amorphous TiO2 structure and absorbed 600 nm of visible light. The decomposition properties of formaldehyde on the NNT photocatalyst after irradiation with visible light were investigated. The LED lamp was irradiated with visible light at 500–620 nm and 6 W. Formaldehyde was decomposed by a photocatalytic reaction by visible light irradiation on the NNT-coated polycarbonate surface. Escherichia coli (E. coli), Staphylococcus aureus, and Pseudomonas aeruginosa were also used to examine the sterilizing properties of pathogenic bacteria using an LED lamp kit. The pathogenic bacteria on the NNT-coated polycarbonate surface were sterilized by irradiation with visible light.
1. Introduction
Since the development of technology for decomposing water into hydrogen and oxygen by photoelectrochemical reactions using TiO2, a range of semiconductor photocatalysts, such as ZnO, Fe2O3, and WO3, have been reported [1,2,3,4,5]. TiO2 has high chemical stability and photocatalytic activity under UV light, making it the most widely applied material for photocatalytic reactions [6,7,8]. On the other hand, the TiO2 photocatalyst has a high bandgap (3.2 eV), meaning that it reacts only to wavelengths in the UV light range. A photocatalyst with excellent visible light sensitivity will allow a decrease in energy consumption by the light source in the photocatalytic reaction as well as the use of sunlight. Photocatalytic technology aims to complete the environmental purification technology by hydrogen production and organic decomposition through photocatalytic reactions using sunlight. Accordingly, in recent years, many studies have focused on the development of photocatalysts with high sensitivity in visible light [9,10,11,12,13,14,15].
Photocatalysts with high photocatalytic activity in visible light have been developed by doping with metal ions or by combining a semiconductor with a narrow bandgap and TiO2. In addition, many attempts have been made to modify TiO2 through a range of methods, such as doping the TiO2 surface with a precious metal [16,17]. The method of nitrogen doping on the TiO2 surface is effective in reducing the bandgap by mixing the p state of N and the O2p state [18]. The photocatalytic efficiency can be improved if the TiO2 structure is doped with a transition metal. Doping the TiO2 lattice with transition metals, such as V, Cr, Fe, Mg, Co, Zn, and Mo, causes a redshift and improves the photosensitivity in visible light [19,20,21,22,23,24,25].
Dopant incorporation leads to an increase or decrease in the bandgap. The redshift of the light absorption spectrum of TiO2 doped with Mn and N revealed a decrease in bandgap due to the formation of a new energy level between the Ti3d state of the conduction band and the O2p state of the valence band [26]. Therefore, the method of lowering the bandgap by doping chemically stable TiO2 with nitrogen or metal ions can be considered effective. Hence, a search for transition metals with excellent photosensitivity would be meaningful. In addition, a more convenient technique is needed to dope TiO2 with these metal ions and nitrogen ions.
The demand for clean energy has led to the development of various types of alternative energy technologies. The development of heterogeneous photocatalytic materials for hydrogen production through water splitting is considered a promising method. The use of a heterogeneous photocatalytic reaction to decompose and remove contaminants would be effective [27,28,29]. Photocatalysts are often used for reaction directly as the powder type. They are often applied by coating on a support, such as ore, ceramic, and metal. The supports can be heat-treated at high temperatures after coating, thereby making the thin film coating robust. On the other hand, the supports have limited applicability if the photocatalyst is to be applied in daily life products. This is because many daily life items are made of materials that are vulnerable to heat, such as plastics. Various materials, such as plastics, must be stable to the photocatalyst coating. Among various plastics, polycarbonate is used widely not only for industrial materials but also for daily life products. Despite this, its hardness is lower than that of metal, and it is limited in applications in various fields because of its weak physical properties, such as deterioration in ultraviolet light [30,31]. To compensate for these shortcomings, surface modification processes, such as surface heat treatment for polycarbonate, chemical surface treatment, electrochemical surface treatment, plasma surface treatment, surface thin-film coating, and coupling agent treatment, were also suggested as improvement methods [32,33,34].
Polycarbonate surface treatments not only can improve the physical properties of the raw material but also can add chemical functionality [35,36]. When a polycarbonate surface is coated with TiO2, deterioration of the base material due to ultraviolet absorption can be suppressed and contaminants on the surface can be photodegraded. In addition, hydrophilization of the surface results in a self-cleaning function in which contaminants are cleaned by rain [37,38]. When the surface on which the photocatalyst is coated is organic, a silica film is formed to prepare the substrate for deformation after a long time and the surface is then coated with the photocatalyst [39]. Indoor air purification and fine dust removal can be achieved by a photocatalytic reaction when the polycarbonate surface is coated with a photocatalyst thin film. For this, however, the technology of uniformly coating the photocatalytic thin film without affecting the appearance or color of the polycarbonate surface is important. In addition, as the glass transition temperature (Tg) of polycarbonate is 147 °C and the heat resistance is weak, it is essential to develop a photocatalyst capable of exhibiting activity by accurately coating the photocatalyst in the allowable temperature range. TiO2 shows photoreactive activity only in ultraviolet light. Therefore, it is necessary to develop a thin film coating technology that modifies it to exhibit photoactivity, even in visible light.
This paper proposes a visible light-sensitive photocatalyst and a thin film coating technology that can allow the coating of caps of LED lamps made of polycarbonate. A method of manufacturing a TiO2 visible light-sensitive photocatalyst is presented. The physicochemical and optical properties of the TiO2 photocatalyst were modified by doping with Ni ions and nitrogen. The photocatalytic decomposition of formaldehyde by visible-light LED irradiation through a lamp cap coated with a modified TiO2 photocatalyst was evaluated. The antibacterial performance against pathogenic bacteria was also investigated.
2. Results and Discussion
2.1. Physicochemical Properties of NNT
A polycarbonate surface coated with a thin film was made after dipping the polycarbonate in an N- and Ni-coated TiO2 (NNT) coating sol, followed by heat treatment at 130 °C. The physicochemical properties of the powder obtained after calcining at 500 °C are also compared with modified TiO2 (NNT) in the anatase form. Hereafter, the NNT thin film coated on the polycarbonate surface is NNT-F and the NNT powder obtained after calcining at 500 °C is NNT-P.
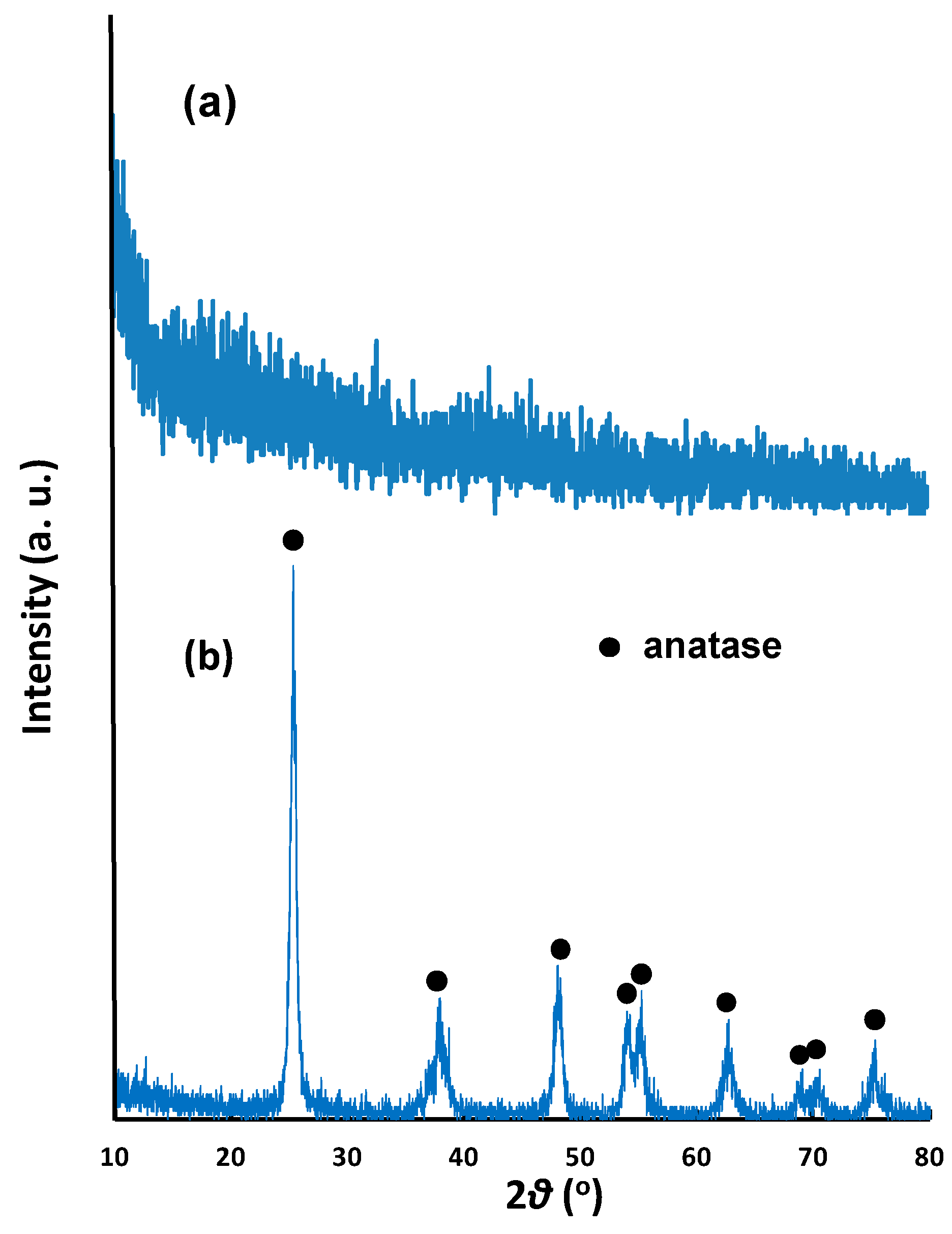
Figure 1a shows the X-ray diffraction (XRD) patterns of NNT-F and NNT-P. The NNT-F exhibited was observed as an amorphous type from the XRD pattern, whereas NNT-P showed a typical anatase pattern. In the XRD pattern, a peak for Ni-doped NNT was not observed, possibly due to the very low Ni content.
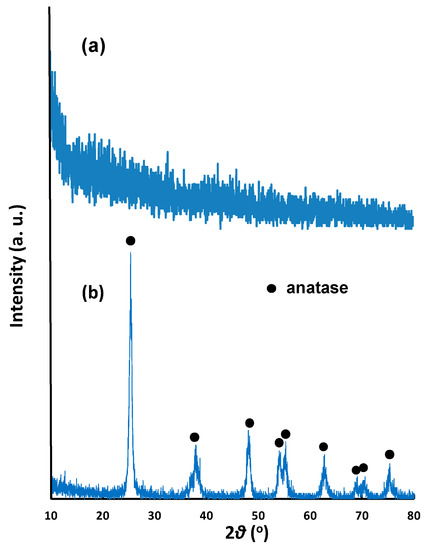
Figure 1.
XRD patterns of (a) N- and Ni-coated TiO2 thin film (NNT-F) and (b) N- and Ni-coated TiO2 powder (NNT-P).
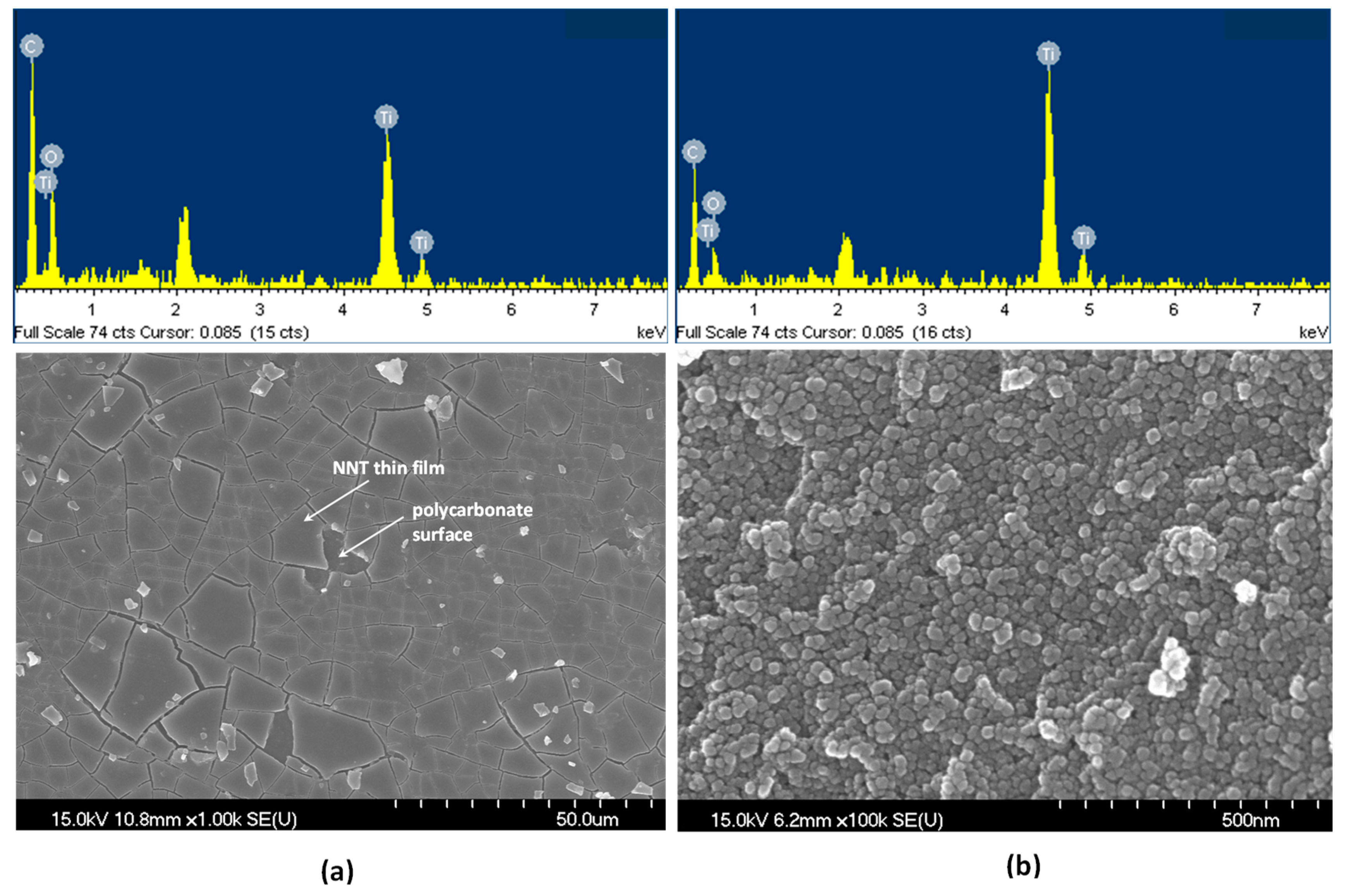
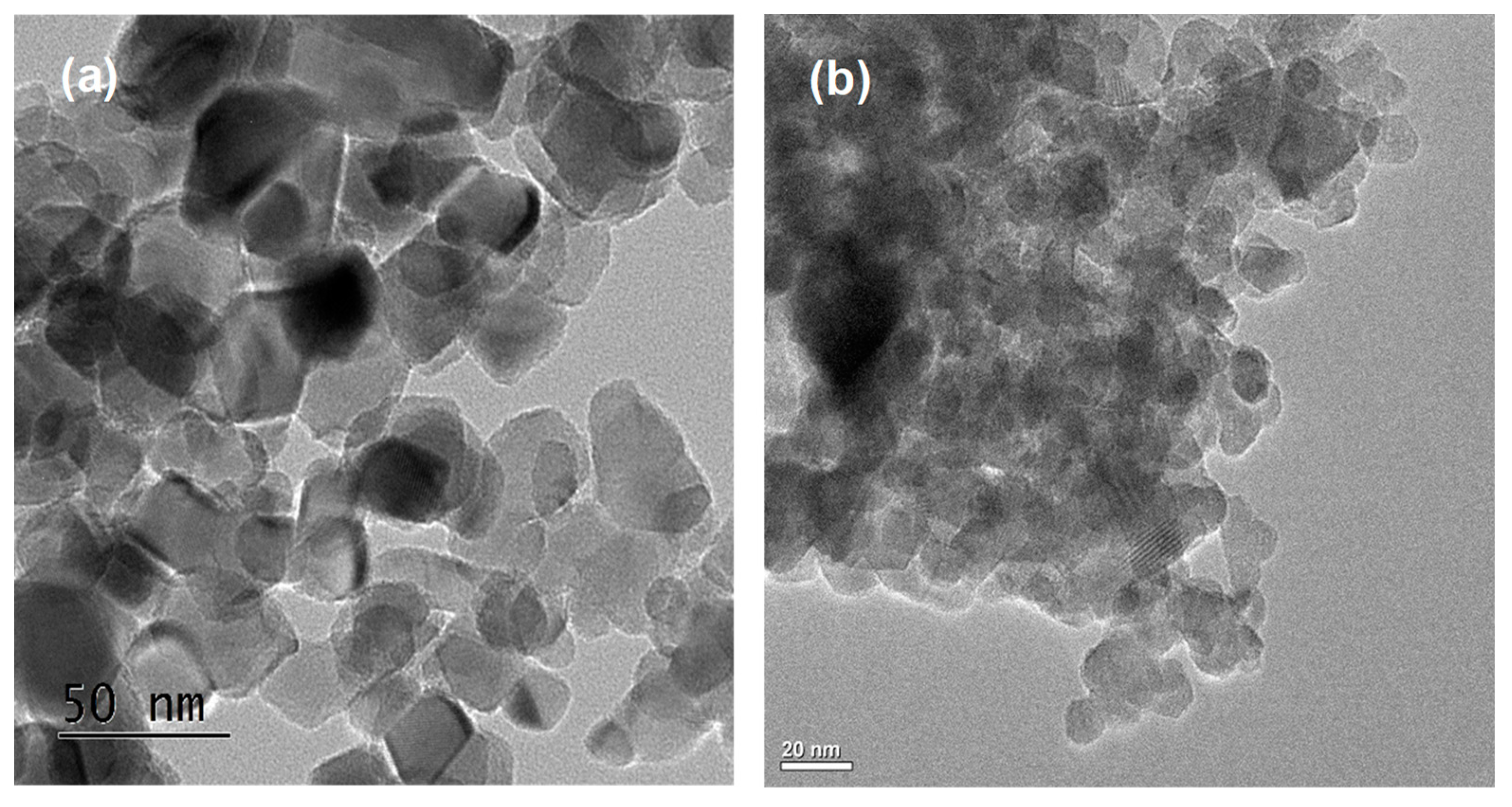
Figure 2 shows scanning electron microscopy (SEM) images and the energy-dispersive X-ray spectroscopy (EDS) results of NNT-F and NNT-P. The Figure 2a is an image of the surface of NNT-F coated on the polycarbonate surface. The surface of the polycarbonate was covered with a thin layer of NNT-F. When preparing a specimen to see the NNT thin film and the polycarbonate surface separately, a specimen was made by breaking a polycarbonate bulb coated with NNT. Therefore, in the SEM image, the NNT thin film coated on the polycarbonate surface appears to be broken. From this image, it was possible to see the NNT thin film and the polycarbonate surface separately. On the other hand, the crystals of NNT-P were very small and uniform, and they did not agglomerate. EDS revealed the two NNTs to be similar. Figure 3 shows transmission electron microscopy (TEM) images of TiO2 (P25) and NNT-P. The crystalline sizes of TiO2 (P25) and NNT-P were ca. 20 nm and ca. 10 nm, respectively. The NNT-P crystallites were much smaller with a more uniform crystal size than TiO2 (P25). The crystal shape was also more spherical than TiO2 (P25).
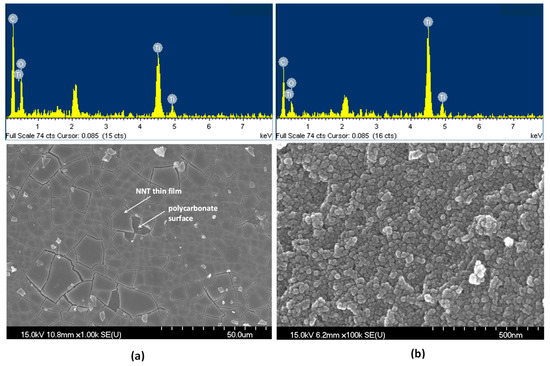
Figure 2.
SEM images and EDS of (a) NNT-F and (b) NNT-P.
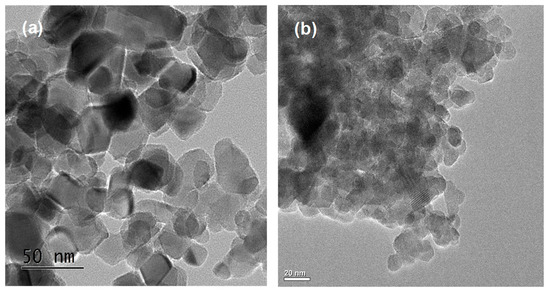
Figure 3.
TEM images of (a) TiO2 (P25) and (b) NNT-P photocatalysts.
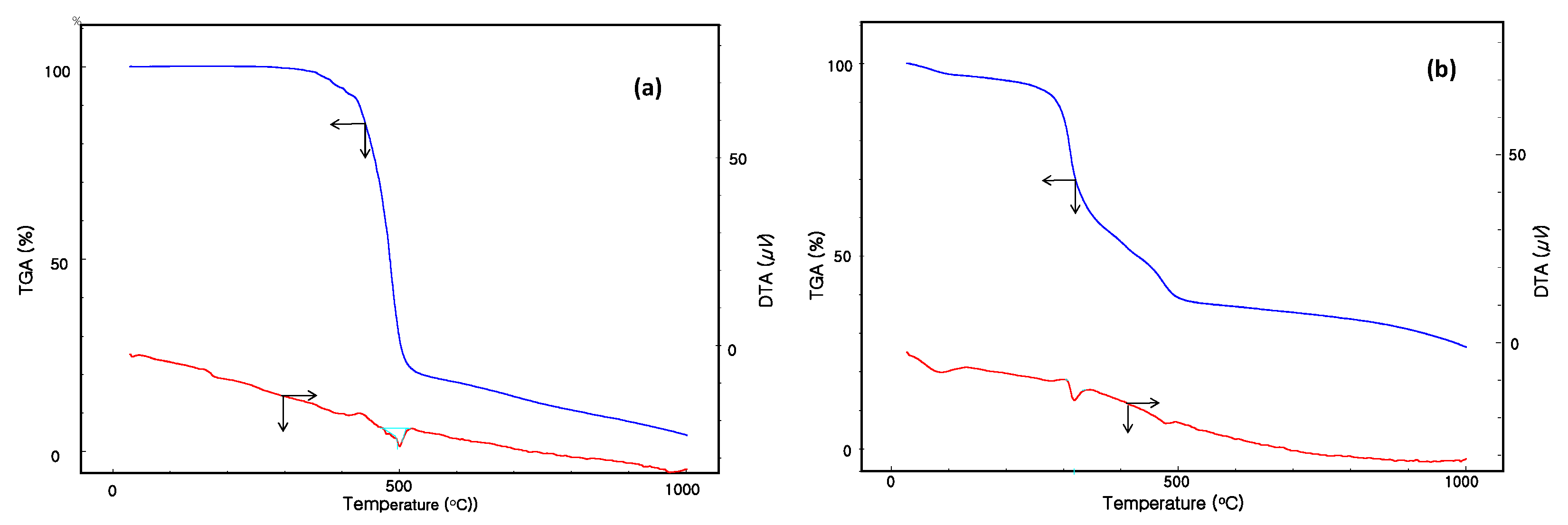
Figure 4 shows the thermogravimetric analysis (TGA) and differential thermal analysis (DTA) results for NNT-F and pure polycarbonate, as recorded in the temperature range 20–1000 °C. As shown in Figure 4a, the TGA/DTA curves of pure polycarbonate exhibited a thermal stability up to 500 °C, with a small mass loss corresponding to volatile material. Figure 4b shows the TGA/DTA results of the NNT-F. Two small endothermic peaks at 310 °C and 495 °C in the DTA curve of Figure 4b, associated with the first weight lost in the TGA, were attributed to the release of volatiles and organic materials of the NNT-F. The second endothermic peak at 495 °C was attributed to mass loss of polycarbonate.
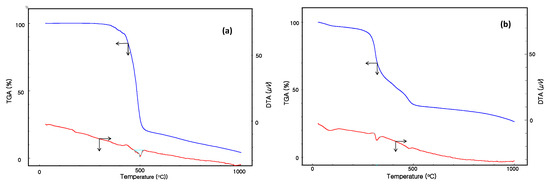
Figure 4.
Thermogravimetric analysis (TGA)/differential thermal analysis (DTA) results of (a) pure polycarbonate and (b) NNT-F.
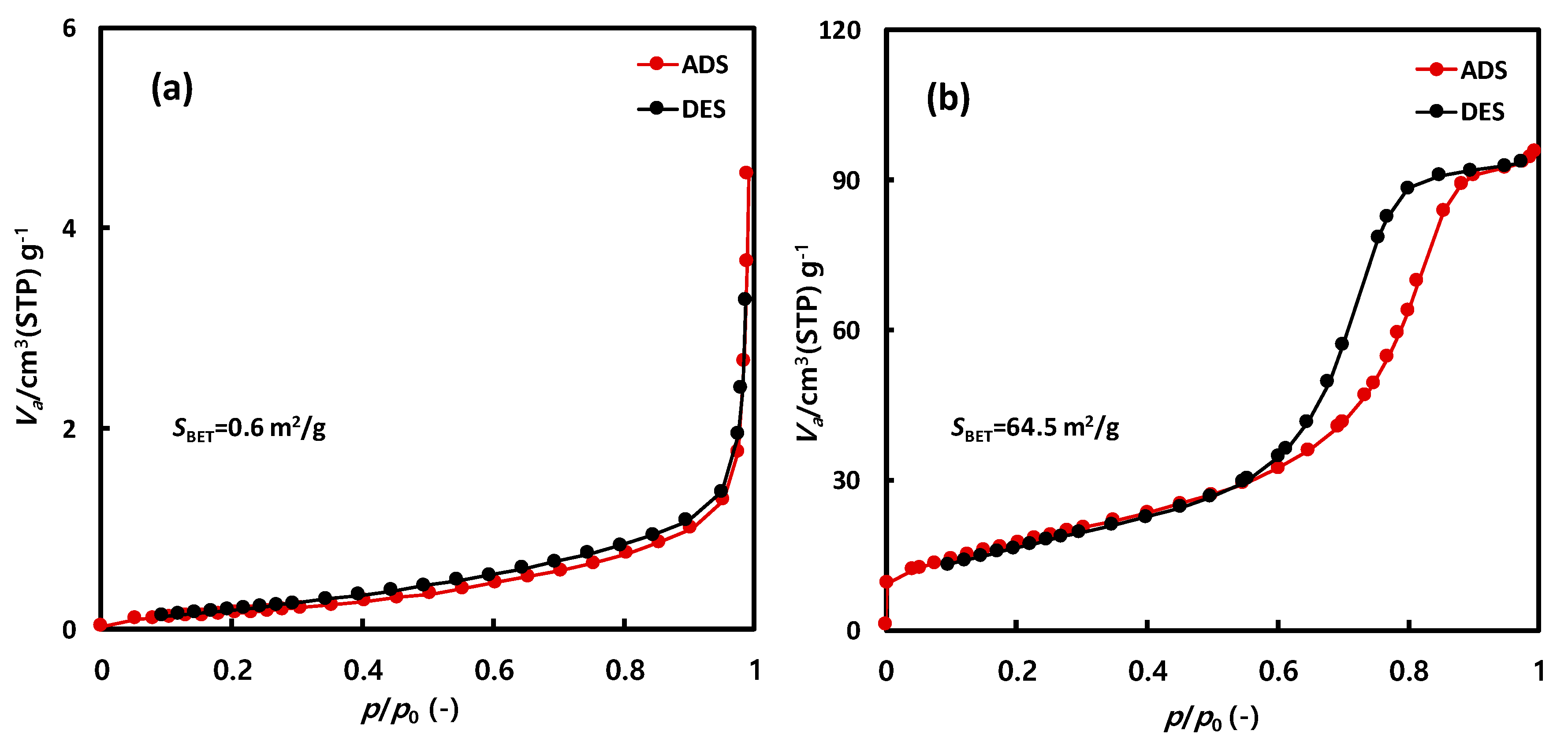
Figure 5 presents the nitrogen adsorption isotherms of NNT-F and NNT-P. The nitrogen adsorption amount of NNT-F was very small. The Brunauer–Emmett–Teller (BET) surface area was also very low, below 1 m2/g. In comparison, the surface area of NNT-P was 64.5 m2/g. The adsorption–desorption isotherm revealed hysteresis, which was attributed to the voids among the particles.
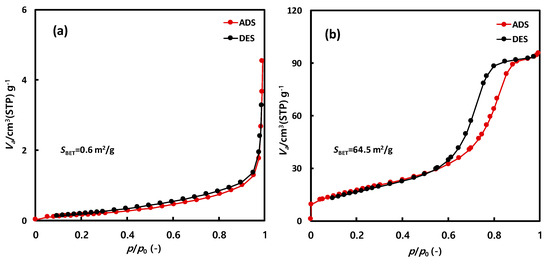
Figure 5.
N2 isotherm of (a) NNT-F and (b) NNT-P.
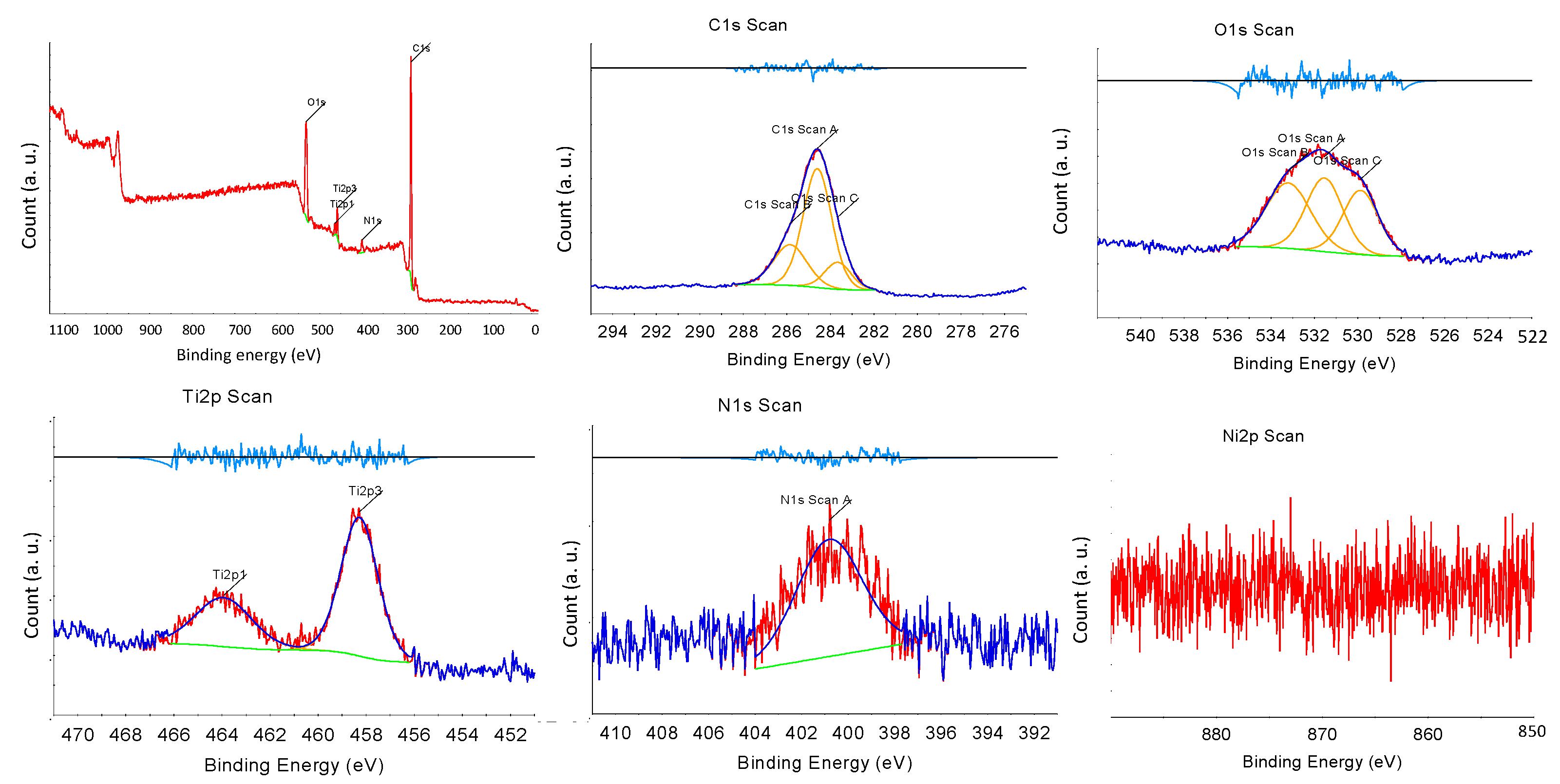
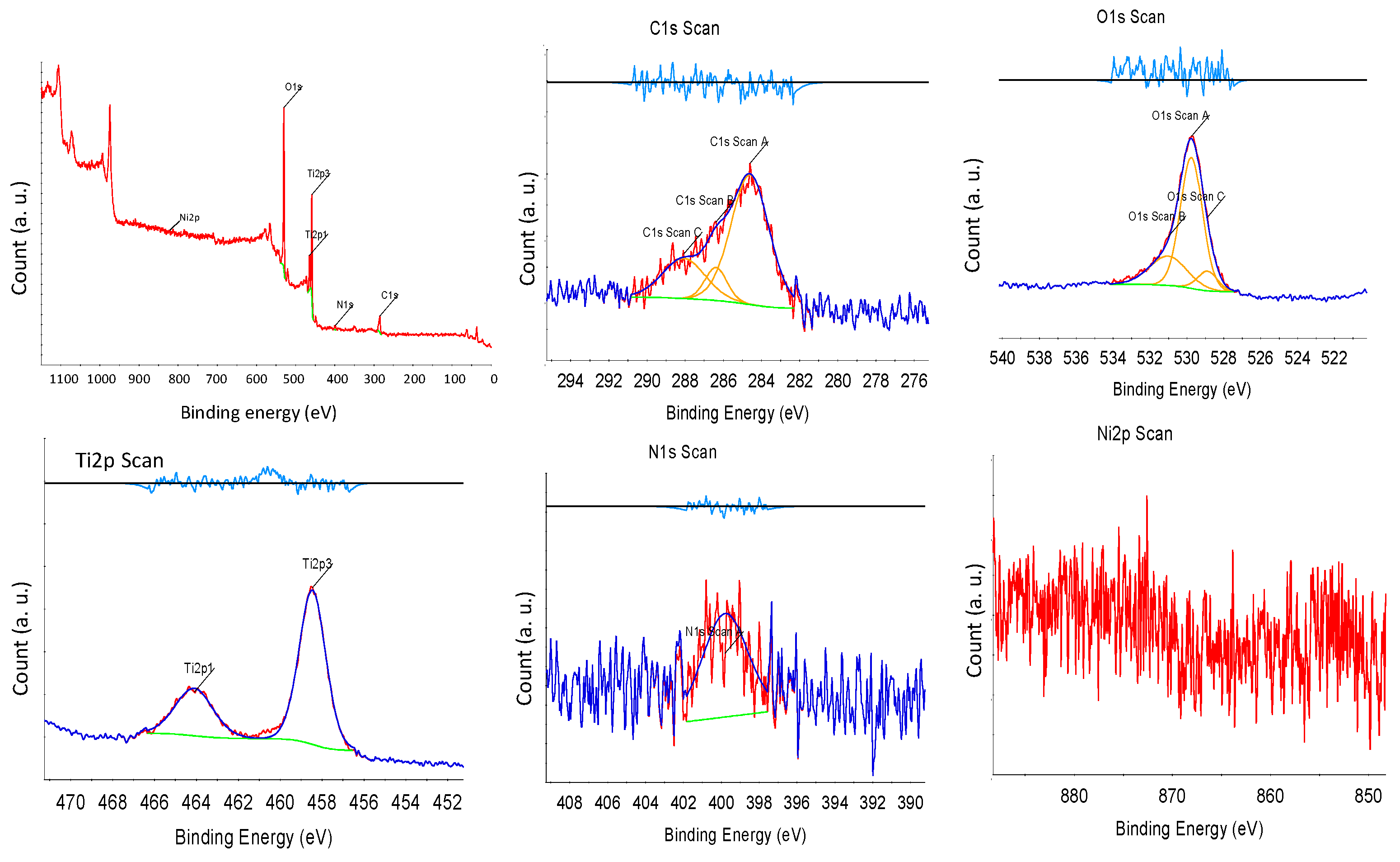
X-ray photoelectron spectroscopy (XPS) was performed to investigate the surface condition of the synthesized NNT photocatalysts. Figure 6 presents the XPS spectrum of the NNT-F. In XPS, the Tip3, Tip1 peaks, O1s, and N1s peaks were observed. The Ni2p peak was very weak. The Ti2p1/2 and Ti2p3/2 peaks were observed at binding energies of 463 eV and 457 eV, respectively. The N1s peak appeared at approximately 398 eV. The peak of N1s at 400 eV indicated the influence of chemically adsorbed nitrogen compounds, nitrogen located inside, and nitrogen contained in the raw materials used to produce TiO2 [40]. Figure 7 shows the XPS result of NNT-P. The XPS of NNT-P also revealed the Tip3 and Tip1 peaks and the O1s and N1s peaks. A very weak Ni2p peak was also observed. The Ti2p1/2 and Ti2p3/2 peaks were found at 464 eV and 458 eV, respectively, showing a slight shift compared to NNT-F. The N1s peak appeared around 400 eV. The size of the peak decreased slightly. The N1s peak appearing around 400 eV was assigned to the influence of chemically adsorbed nitrogen compounds or nitrogen located inside [40].
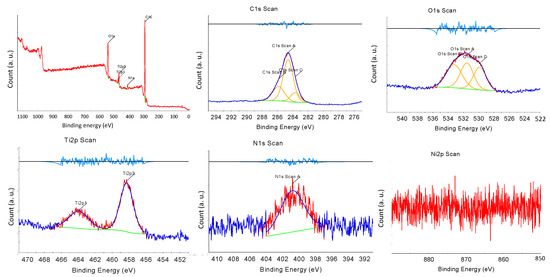
Figure 6.
XPS results of NNT-F.
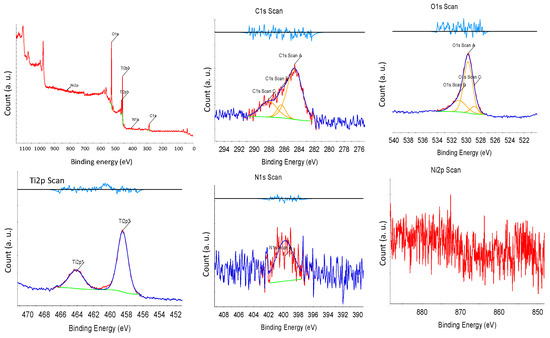
Figure 7.
XPS results of NNT-P.
Doping with nitrogen atoms is an effective approach to alter the electronic structure of metal oxides. Both substitutional and interstitial N-doping can reduce the oxygen-vacancy formation energy significantly. In addition, doping with N atoms may introduce new singly ionized oxygen vacancies. On the other hand, a comparison of substitutional and interstitial N-doping can effectively narrow the bandgap and improve electron transport, which is conducive to the formation of oxygen vacancies. Interstitial N-doped MnO2 showed improved catalytic activity to degrade carcinogenic HCHO(formaldehyde) compared to substitutional N-doped MnO2 because of the larger number of oxygen vacancies and stronger oxygen activation capacity [41]. N-doped NNT also appears to have nitrogen inside it. This type of nitrogen bond is described as interstitial [42]. The photocatalytic activity has been reported to be better in the interstitial type [43]. As a result, nitrogen doping allows the use of visible light because NNT absorbs 400–600 nm visible light.
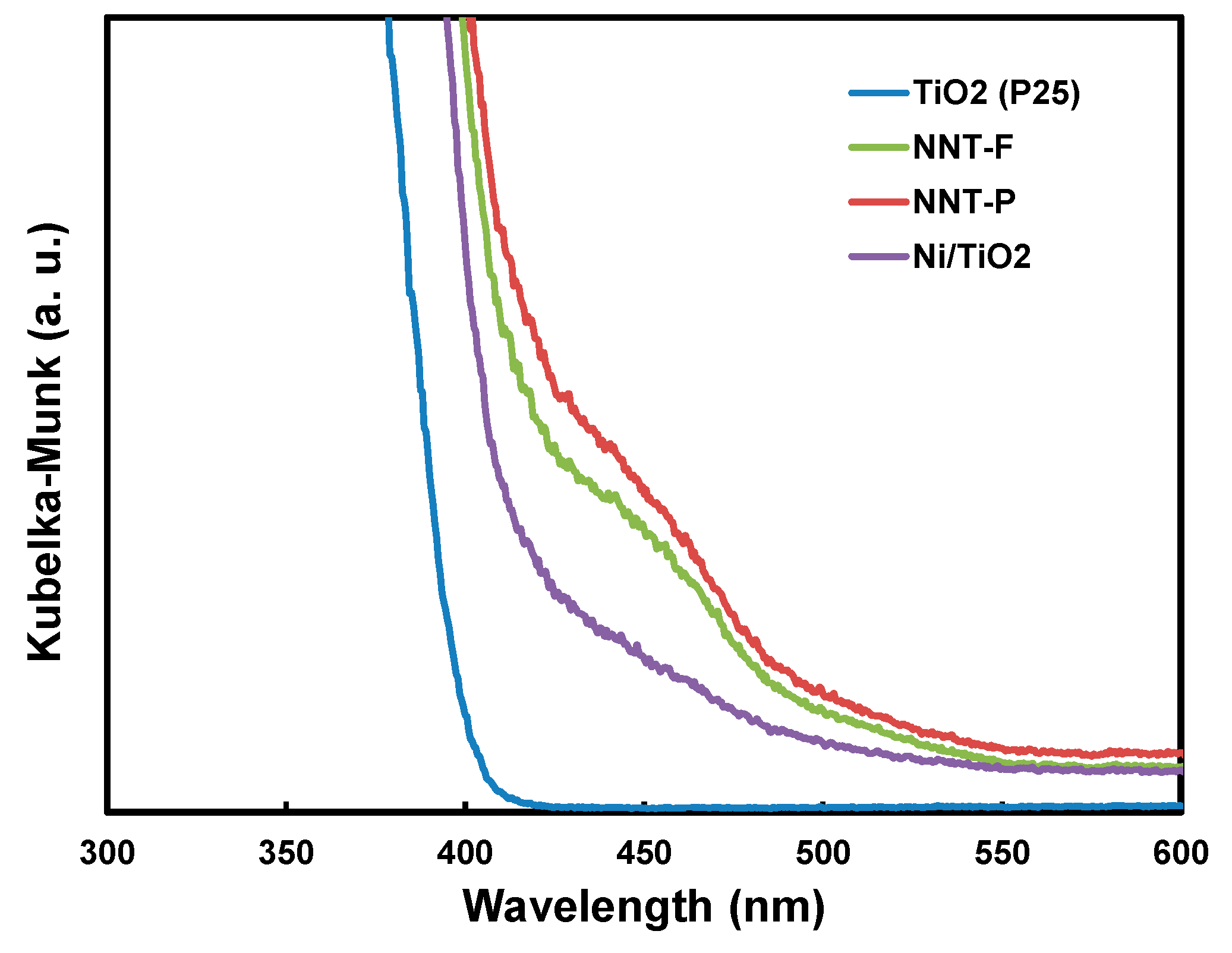
The UV-visible light absorption edge is related to the energy band of a semiconductor catalyst. Therefore, it is necessary to investigate the light absorption of the NNT nanoparticles to evaluate the photocatalytic activity of the NNT. Figure 8 shows the absorption spectrum of the NNT photocatalyst converted from the UV-visible diffusion reflectance spectroscopy (DRS) spectra according to the Kübelka–Münk theory [44]. According to this spectrum, NNT absorbs ultraviolet and visible light up to approximately 600 nm. The absorption edge of NNT-P was approximately 565 nm. The bandgap of the NNT was estimated from the square root plot of the Kübelka–Münk function F(R) for the photon energy. The bandgaps of NNT-F and NNT-P were ca. 2.5 eV. The narrower bandgap of the NNT nanoparticles suggests that it can exhibit photocatalytic activity in visible light.
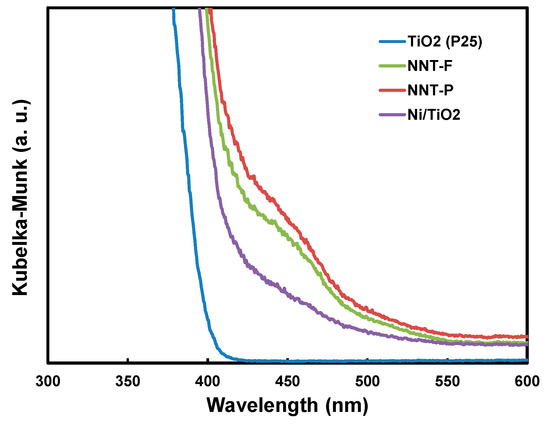
Figure 8.
UV-visible diffusion reflectance spectroscopy (DRS) spectra of TiO2 (P25), Ni/TiO2, and N- and Ni-coated TiO2 (NNTs).
2.2. Visible Light Photocatalytic Activity of NNT Photocatalysts
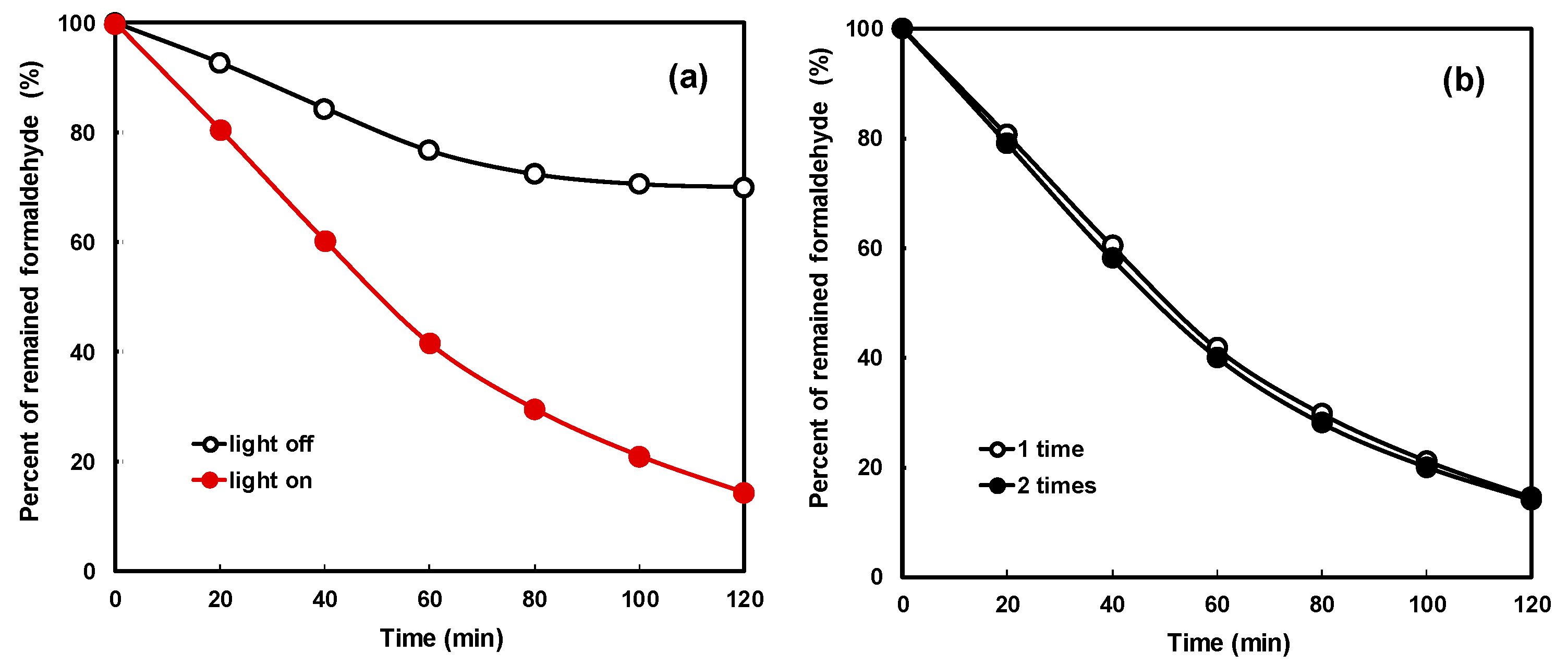
Figure 9a shows the results of formaldehyde decomposition with visible light LED lamps of thin film-coated NNT photocatalysts. In the figure, “light off” shows a curve of the change in the formaldehyde concentration without installing the LED lamp in the experiment box. No change in the concentration of formaldehyde due to a photocatalytic reaction was noted, but the concentration of formaldehyde decreased due to adsorption on the inner surface of the experiment box and the surface of the lamp. The formaldehyde concentration decreased linearly up to 60 min by adsorption and not by a photocatalytic reaction and gradually reached equilibrium. The rate of decrease in the concentration of formaldehyde by adsorption was approximately 28%. In the figure, the curve of the “light on” sign revealed a decrease in the concentration of formaldehyde as the photocatalytic reaction occurs by illuminating the LED lamp. The formaldehyde concentration decreased linearly with time; approximately 80% formaldehyde was removed after 120 min. Approximately 52% of formaldehyde was removed by the photoreaction of the LED visible light and the NNT photocatalyst coated on the polycarbonate surface. Formaldehyde was converted to carbon dioxide and water by the photocatalytic reaction.
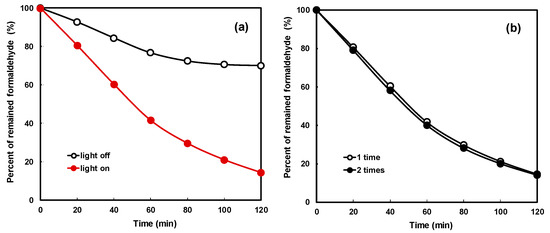
Figure 9.
Formaldehyde degradation (a) with adsorption effect and (b) with coating number.
Figure 9b compares the removal characteristics of formaldehyde by the photocatalytic reaction when the NNT photocatalyst was coated once and twice. The decomposition characteristics were similar regardless of the number of coatings. This is because the photocatalytic reaction is a reaction between light and the thin film photocatalyst surface. Hence, the photocatalyst thin film shows similar performance if it is formed uniformly and tightly with a single coating. This can also be achieved after multiple coatings.
2.3. Antibacterial Properties of NNT in Visible Light
Table 1 lists the results of examining the antibacterial properties of pathogenic bacteria on the surface of the NNT-coated thin film. E. coli, Staphylococcus aureus, and Pseudomonas aeruginosa increased 37-fold, 38-fold, and eightfold, respectively, after 24 h in the blank test compared to the initial concentration. On the other hand, the pathogenic bacteria inoculated on the surface of the NNT coated thin film were sterilized after 24 h by irradiation with the LED visible light lamp. Hence, the pathogenic bacteria were sterilized by the strong oxidizing ability of the photocatalyst in visible light.

Table 1.
Antibacterial properties of NNT thin films coated on polycarbonate in visible light.
3. Materials and Methods
3.1. Preparation of NNT Photocatalysts
NNT was prepared by a sol-gel reaction. A 0.1 M solution of titanium tetraisopropoxide (Sigma, St. Louis, MI, USA, 99%) and 1.2 M of diethanolamine (Sigma, St. Louis, MI, USA, 99%) was mixed with 800 mL of 2-propanol (Duksan, Ansan, Korea, 98.5%). The mixed solution was reacted with stirring at room temperature for 5 h to obtain a TiO2 sol. Nickel (II) chloride hexahydrate (NiCl2·6H2O; Wako, Osaka, Japan, 98%) was then injected into the sol. The amount of NiCl2·6H2O injected was adjusted so the amount of Ni was 2% relative to the mass of TiO2 in solution. The resulting mixture was stirred at room temperature for 5 h. For N-doping, 0.05 M ammonium carbonate ((NH4)2CO3, Samchun, Seoul, Korea, 30.0%) was injected and stirred for 12 h to obtain the NNT sol. The NNT-P was obtained by heating at 300 °C for 1 h, followed by calcination at 500 °C for 5 h. The cap and body of the LED lamp made from polycarbonate were coated using a dip-coating method. Dip coating was performed so that the lamp cap and body were dipped in the NNT photocatalyst sol and was slowly pulled up to form an NNT coating film on the surface. After coating, the cap and body were dried at room temperature for 12 h and then in a dryer at 130 °C for 5 h.
3.2. Photocatalytic Decomposition of Formaldehyde in Visible Light
The photocatalytic decomposition of formaldehyde on the NNT photocatalyst thin film was performed under visible light irradiation. The light source was an LED light source (6 W) combining a 585-nm LED lamp and a 613-nm LED lamp. Figure 10a shows a photograph of the cap and body of the LED lamp. The cap of the lamp was semispherical, with a diameter of 5 cm. The lamp cap and body were made of polycarbonate. The inside and outside of the polycarbonate cap and body were coated with an NNT thin film using the dip-coating method. Figure 10b shows the LED lamp kit after assembling the cap and body with the LED lamp and Figure 10c shows the emission spectrum of the LED lamp, respectively. The emission spectrum appeared in the range of 580–640 nm. The strongest emission spectrum was observed at 605 nm.

Figure 10.
(a) LED lamp cap and body, (b) assembled LED lamp kit, and (c) emission spectrum of the LED lamp.
Photocatalytic decomposition of formaldehyde was carried out by installing an LED lamp with a cap coated with the photocatalyst thin film inside an experimental box (35 × 50 × 30 cm, V = 52.5 L) blocked from external air inflow. The gas-phase formaldehyde was produced by vaporization of a formaldehyde solution (Duksan, Ansan, Korea, EP, 40%). The formaldehyde evaporator connected to the box was generated at 40 °C while generating formaldehyde vapor. This formaldehyde gas was injected into the reaction box through a tube. The internal gas of the reaction box was circulated using a fan. The initial concentration was measured using a gas detection tube and a gas chromatograph (GC, Younglin, M650D, Anyang, Korea). The visible-light photocatalytic reaction to the NNT thin film was performed by irradiating with an LED visible light lamp. The change in formaldehyde concentration was analyzed by GC equipped with an HP-1 column by taking a gas sample in a box according to the elapsed time of the photocatalytic reaction.
3.3. Estimation of Antibacterial Properties
The antibacterial abilities of pathogenic bacteria, such as E. coli, Staphylococcus aureus, and Pseudomonas aeruginosa, by irradiating the surfaces of NNT-coated polycarbonate with the visible light LED lamps were investigated. E. coli (ATCC 25922), Staphylococcus aureus (ATCC 6538), and Pseudomonas aeruginosa (ATCC 15442) were supplied by Korean Collecting for Type Culture (KCTC). Bacteria were inoculated on the cap surface of the LED lamp, and the LED lamp was operated for 24 h. The blank was inoculated with pathogens on the cap of the bulb, which was not coated with a photocatalyst. The sterilization effect by photocatalysis with NNT and visible light was investigated. After the reaction was complete, the bacteria on the surface of the lamp cap were inoculated in Trypticase soy agar (TSA) medium and cultured for 24 h. The number of living bacteria was counted using a colony counter. The number of bacteria in the medium was calculated by multiplying the dilution factor. The blank was performed under the same conditions in the sample without the photocatalyst coating.
3.4. Analysis
For SEM and XPS analysis, the NNT-F specimen was used by directly cutting polycarbonate coated with an NNT thin film into a size of 5 × 5 mm. For XRD and DRS analysis, a powder was obtained by scraping off a thin film of NNT coated on the polycarbonate surface. The crystallinity and structure of the NNT photocatalyst were determined by high-resolution XRD (Rigaku, D/Max Ultima III, Tokyo, Japan) using Ni-filtered CuKα X-ray radiation (λ = 1.5405 Å). The shape and microstructure of the photocatalyst were investigated by SEM (Hitachi, S-4700/EX-200, Tokyo, Japan). The samples were analyzed by a surface visual assessment and a microscope, and an EDS system for elemental analysis. The specimens were prepared by cutting the samples in the middle of their length using a puncher and by mounting them for polishing. The cross sections were then polished without etching.
The shape and size of the photocatalyst particles were measured by TEM (JEOL JEM-2100F, Tokyo, Japan). TEM was carried out using a LaB6 filament and operated at 200 kV. The TEM specimen was prepared by dispersing the sample powder well in a dispersion medium to prevent entanglement and coating on a mesh grid. The chemical composition of the photocatalyst was analyzed by EDS using an energy dispersive X-ray spectrometer (NORANS Z-MAXII 350, Tokyo, Japan). The nitrogen adsorption isotherm of the photocatalyst was measured using a volume adsorption device (Mirae SI, Nanoporosity XQ, Gwangju, Korea) at liquid nitrogen temperature. The photocatalyst sample was pretreated at 100 °C for two hours and exposed to nitrogen gas. The surface area was calculated by applying the BET equation [45].
The binding state of the NNT constituent elements was investigated by XPS (VG Systems, MultiLab 2000, East Grinstead, UK) at a chamber at a pressure of 10−6–10−7 Pa using Al-Kα X-rays. The resolution of the instrument was measured in the 0.35 eV range using a silver fermi edge. The light absorption properties of NNT were obtained by UV-visible diffusion reflectance spectroscopy (Shimadzu, UV-2510) using BaSO4 as a standard reflection material to obtain a DRS spectrum of NNT at 200–800 nm. The optical band gap of NNT was obtained by applying the Kübelka–Münk theory [44]. Simultaneous TGA and DTA measurements on the pure polycarbonate and NNT-F were carried out using a TGA/DSC (Shimadzu, TGA-50/DSC-60, Tokyo, Japan) instrument in nitrogen atmosphere with a heating rate of 10 °C/min.
4. Conclusions
N- and Ni-codoped TiO2 were prepared using a facile sol-gel method. After coating the polycarbonate surface with NNT sol using the dip-coating method and drying at 130 °C, a uniform thin film was produced. This thin film had an amorphous structure. The modified TiO2 thin film doped with nitrogen and Ni absorbed visible light at wavelengths longer than 600 nm. The removal characteristics of formaldehyde from visible light were investigated using the LED lamp kit assembled with the polycarbonate cap and body coated with the NNT thin film. Formaldehyde was removed by a decomposition reaction with visible light. The kit also sterilized E. coli, Staphylococcus aureus, and Pseudomonas aeruginosa by LED visible light.
Author Contributions
B.-G.P. and K.-H.C. conceived and designed the experiments; C.-H.L. and B.-G.P. performed the experiments; B.-G.P. analyzed the data; K.-H.C. wrote the paper; and B.-G.P. and C.-H.C. revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This paper was supported by Research Funds of Kwangju Women’s University in 2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semicounductor electorode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Wolcott, A.; Wang, G.M.; Sobo, A.; Fitzmorris, R.C.; Qian, F.; Zhang, J.Z.; Li, Y. Nitrogen-doped ZnO nanowire arrays for photoelectro chemical water splitting. Nano Lett. 2009, 9, 2331–2336. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, A.; Smith, W.A.; Kuykendall, T.R.; Zhao, Y.; Zhang, J.Z. Photoelectrochemical Study of Nanostructured ZnO Thin Films for Hydrogen Generation from Water Splitting. Adv. Funct. Mater. 2009, 19, 1849–1856. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Z. High photoelectrochemical water splitting performance on nitrogen doped double-wall TiO2 nanotube array electrodes. Int. J. Hydrogen Energy 2011, 36, 13481–13487. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic production of hydrogen from biomass-derived feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Navalon, S.; Corma, A.; Garcia, H. Photocatalytic CO2 reduction by TiO2 and related titanium containing solids. Energy Environ. Sci. 2012, 5, 9217–9233. [Google Scholar] [CrossRef]
- Tian, J.; Leng, Y.; Zhao, Z.; Xia, Y.; Sang, Y.; Hao, P. Carbon quantum dots/hydrogenated TiO2 nanobelt hetero structures and their broad spectrum photocatalytic properties under UV, visible, and near-infrared irradiation. Nano Energy 2015, 11, 419–427. [Google Scholar] [CrossRef]
- Sharotri, N.; Sud, D. A greener approach to synthesize visible light responsive nanoporous S-doped TiO 2 with enhanced photocatalytic activity. N. J. Chem. 2015, 39, 2217–2223. [Google Scholar] [CrossRef]
- Sharotri, N.; Sud, D. Ultrasound-assisted synthesis and characterization of visible light responsive nitrogen-doped TiO 2 nanomaterials for removal of 2-Chlorophenol. Desalin. Water Treat. 2015, 57, 1–13. [Google Scholar] [CrossRef]
- Yao, Z.; Jia, F.; Tian, S.; Li, C.; Jiang, Z.; Bai, X. Microporous Ni-Doped TiO2 film Photocatalyst by Plasma Electrolytic Oxidation. ACS Appl. Mater. Interfaces 2010, 2, 2617–2622. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Kim, B.; Kim, Y.K. Highly Enhanced Photoactivity of Anatase TiO2 Nanocrystals by Controlled Hydrogenation-Induced Surface Defects. ACS Catal. 2013, 3, 2479–2486. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, B.; Li, H.; Ming, H.; Bala, H.; Yao, S.; Zhang, J.; Fu, W.; Cao, J.; Sun, G.; et al. Visible light responsive CsPbBr3/TiO2 photocatalyst with long-term stability in aqueous solution. Mater. Lett. 2020, 274, 128041. [Google Scholar] [CrossRef]
- Shenoy, S.; Sridharan, K. Bismuth oxybromide nanoplates embedded on activated charcoal as effective visible light driven photocatalyst. Chem. Phys. Lett. 2020, 749, 137435. [Google Scholar] [CrossRef]
- Khan, H.; Usen, N.; Boffito, D.C. Spray-dried microporous Pt/TiO2 degrades 4-chlorophenol under UV and visible light. J. Environ. Chem. Eng. 2019, 7, 103267. [Google Scholar] [CrossRef]
- Qiu, H.; Ma, X.; Sun, C.; Zhao, B.; Chen, F. Surface oxygen vacancies enriched Pt/TiO2 synthesized with a defect migration strategy for superior photocatalytic activity. Appl. Surf. Sci. 2020, 506, 145021. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Yamashita, H.; Harada, M.; Misaka, J.; Takeuchi, M.; Ikeue, K.; Anpo, M. Degradation of propanol diluted in water under visible light irradiation using metal ion-implanted titanium dioxide photocatalysts. J. Photochem. Photobiol. A Chem. 2002, 148, 257–261. [Google Scholar] [CrossRef]
- Dvoranová, D.; Brezová, V.; Mazúr, M.; Malati, M.A. Investigations of metal-doped titanium dioxide photocatalysts. Appl. Catal. B Environ. 2002, 37, 91–105. [Google Scholar] [CrossRef]
- Kemp, T.J.; McIntyre, R.A. Transition metal-doped titanium (IV) dioxide: Characterization and influence on photodegradation of poly (vinyl chloride). Polym. Degrad. Stab. 2006, 91, 165–194. [Google Scholar] [CrossRef]
- Kapoor, P.; Uma, S.; Rodriguez, S.; Klabunde, K. Aerogel processing of MTi2O5 (M = Mg, Mn, Fe, Co, Zn, Sn) compositions using single source precursors: Synthesis, characterization and photocatalytic behavior. J. Mol. Catal. A Chem. 2005, 229, 145–150. [Google Scholar] [CrossRef]
- Rauf, M.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Kara, F.; Kurban, M.; Coşkun, B. Evaluation of electronic transport and optical response of two-dimensional Fe-doped TiO2 thin films for photodetector applications. Optik 2020, 210, 164605. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Detrimental cationic doping of titania in photocatalysis: Why chromium Cr3+-doping is a catastrophe for photocatalysis, both under UV- and visible irradiations. New J. Chem. 2012, 36, 883–890. [Google Scholar] [CrossRef]
- Jedsukontorn, T.; Ueno, T.; Saito, N.; Hunsom, M. Narrowing bandgap energy of defective black TiO2 fabricated by solution plasma process and its photocatalytic activity on glycerol transformation. J. Alloys Compd. 2018, 757, 188–199. [Google Scholar] [CrossRef]
- Kumar, A.; Raizada, P.; Singh, P.; Saini, R.V.; Saini, A.K.; Hosseini-Bandegharaei, A. Perspective and status of polymeric graphitic carbon nitride based Z-scheme photocatalytic systems for sustainable photocatalytic water purification. Chem. Eng. J. 2020, 391, 123496. [Google Scholar] [CrossRef]
- Huang, C.-W.; Nguyen, V.-H.; Zhou, S.-R.; Hsu, S.-Y.; Tan, J.-X.; Wu, K.C.-W. Metal–organic frameworks: Preparation and applications in highly efficient heterogeneous photocatalysis. Sustain. Energy Fuels 2020, 4, 504–521. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Singh, P.; Hosseini-Bandegharaei, A.; Thakur, P. Converting type II AgBr/VO into ternary Z scheme photocatalyst via coupling with phosphorus doped g-C3N4 for enhanced photocatalytic activity. Sep. Purif. Technol. 2019, 227, 115692. [Google Scholar] [CrossRef]
- Hozumi, A.; Takai, O. Effect of hydrolysis groups in fluoro-alkyl silanes on water repellency of transparent two-layer hard-coatings. Appl. Surf. Sci. 1996, 103, 431–441. [Google Scholar] [CrossRef]
- Moustaghfir, A.; Tomasella, E.; Rivaton, A.; Mailhot, B.; Jacquet, M.; Gardette, J.L.; Cellier, J. Sputtered zinc oxide coatings: Structural study and application to photoprotection of the polycarbonate. Surf. Coat. Technol. 2004, 180–181, 642–645. [Google Scholar] [CrossRef]
- Hetemi, D.; Pinson, J. Surface functionalisation of polymers. Chem. Soc. Rev. 2017, 46, 5701–5713. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wang, Z.K.; Zheng, H.; Thwe, A.; Lam, Y. Investigation on polycarbonate surface wetting property with femtosecond laser irradiation and ultrasonic treatment. Opt. Laser Technol. 2019, 115, 316–324. [Google Scholar] [CrossRef]
- Puga, A.V.; Garcia-Valls, R.; Fernández-Prieto, S.; Smets, J.; York, D. Dual xanthan gum/poly (vinyl acetate) or alkyl-functionalized poly (vinyl alcohol) films as models for advanced coatings. J. Appl. Polym. Sci. 2014, 131, 40870. [Google Scholar] [CrossRef]
- Kang, E.-T.; Tan, K.L.; Kato, K.; Uyama, Y.; Ikada, Y. Surface Modification and Functionalization of Polytetrafluoroethylene Films. Macromolecules 1996, 29, 6872–6879. [Google Scholar] [CrossRef]
- Hwang, D.; Moon, J.; Shul, Y.; Jung, K.; Kim, D.; Lee, D. Scratch Resistant and Transparent UV-Protective Coating on Polycarbonate. J. Solgel Sci. Technol. 2003, 26, 783–787. [Google Scholar] [CrossRef]
- Yaghoubi, H.; Taghavinia, N.; Alamdari, E.K. Self cleaning TiO2 coating on polycarbonate: Surface treatment, photocatalytic and nanomechanical properties. Surf. Coat. Technol. 2010, 204, 1562–1568. [Google Scholar] [CrossRef]
- Liu, H.; Feng, Y.; Shao, J.; Chen, Y.; Wang, Z.L.; Li, H.; Chen, X.; Bian, Z. Self-cleaning triboelectric nanogenerator based on TiO2 photocatalysis. Nano Energy 2020, 70, 104499. [Google Scholar] [CrossRef]
- Park, Y.-K.; Chung, K.-H.; Park, I.-S.; Kim, S.-C.; Kim, S.-J.; Jung, S.-C. Photocatalytic degradation of 1,4-dioxane using liquid phase plasma on visible light photocatalysts. J. Hazard Mater. 2020, 399, 123087. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- He, T.; Zeng, X.; Rong, S. The controllable synthesis of substitutional and interstitial nitrogen-doped manganese dioxide: The effects of doping sites on enhancing the catalytic activity. J. Mater. Chem. A 2020, 8, 8383–8396. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Kübelka, P.; Münk, F. Ein beitrag zür optik der farbanstriche. Z. Tech. Phys. 1931, 12, 593–596. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).