Large-Area Patterning of Oil-Based Inks on Superhydrophobic TiO2 Nanotubular Layers by Photocatalytic Wettability Conversion
Abstract
:1. Introduction
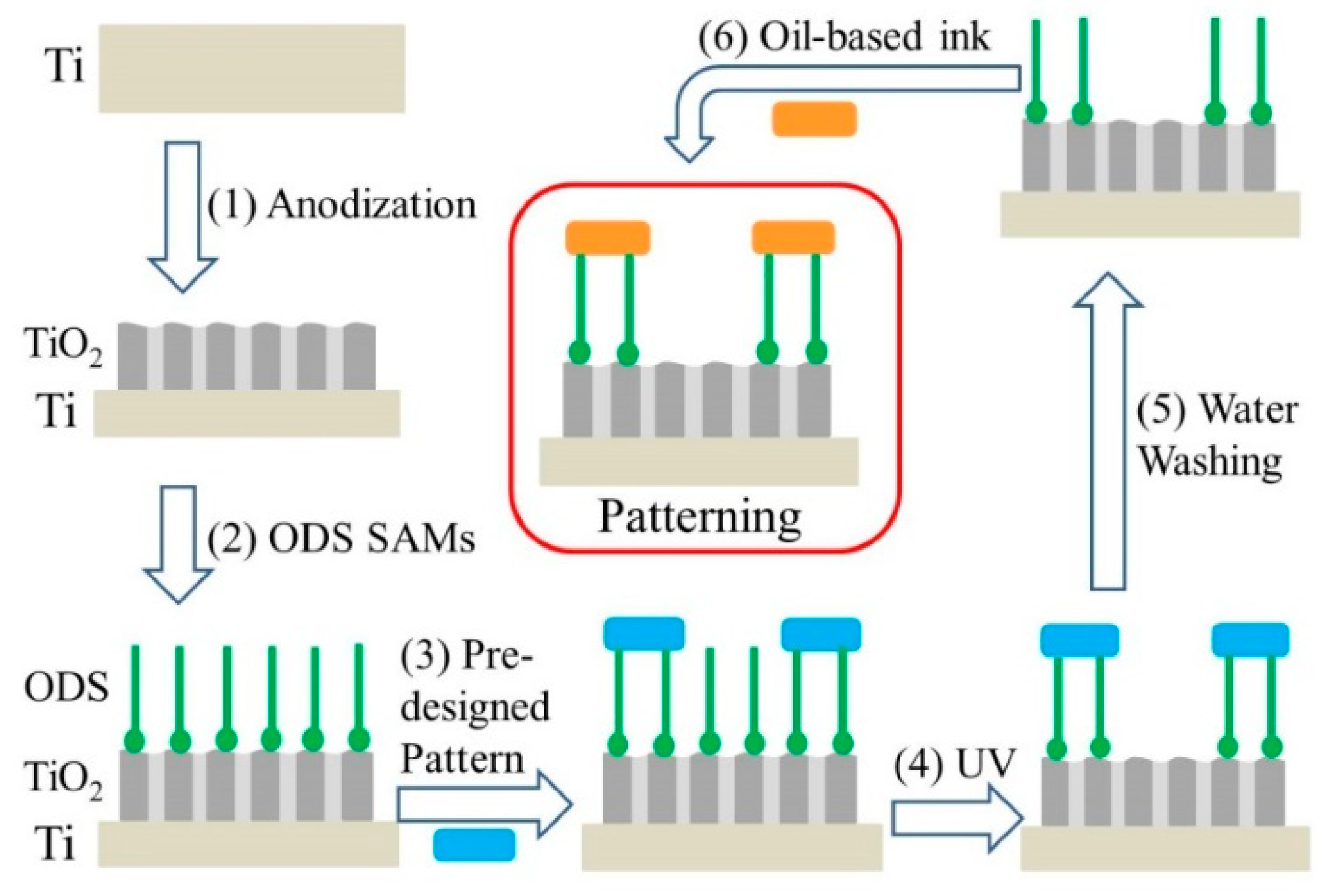
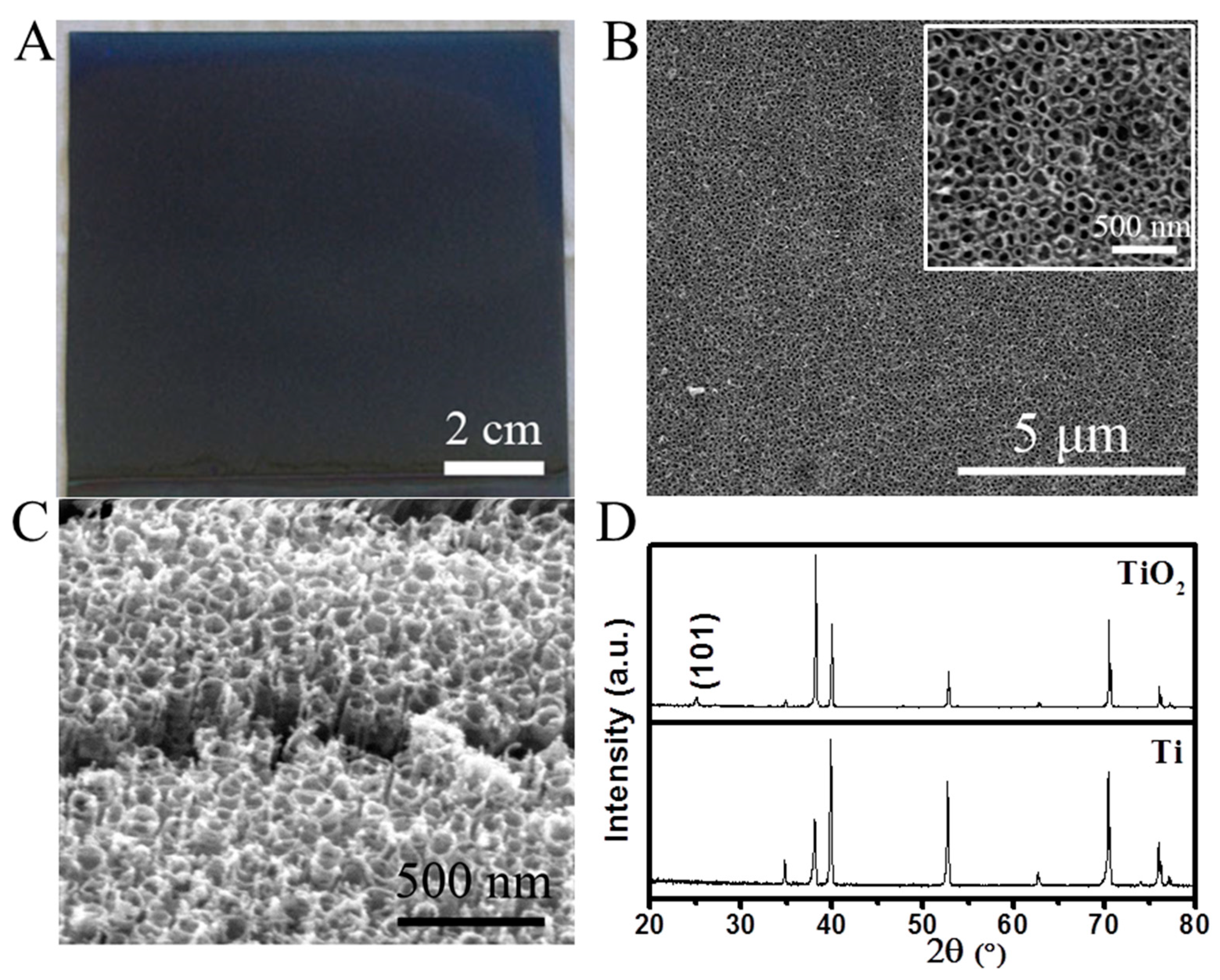
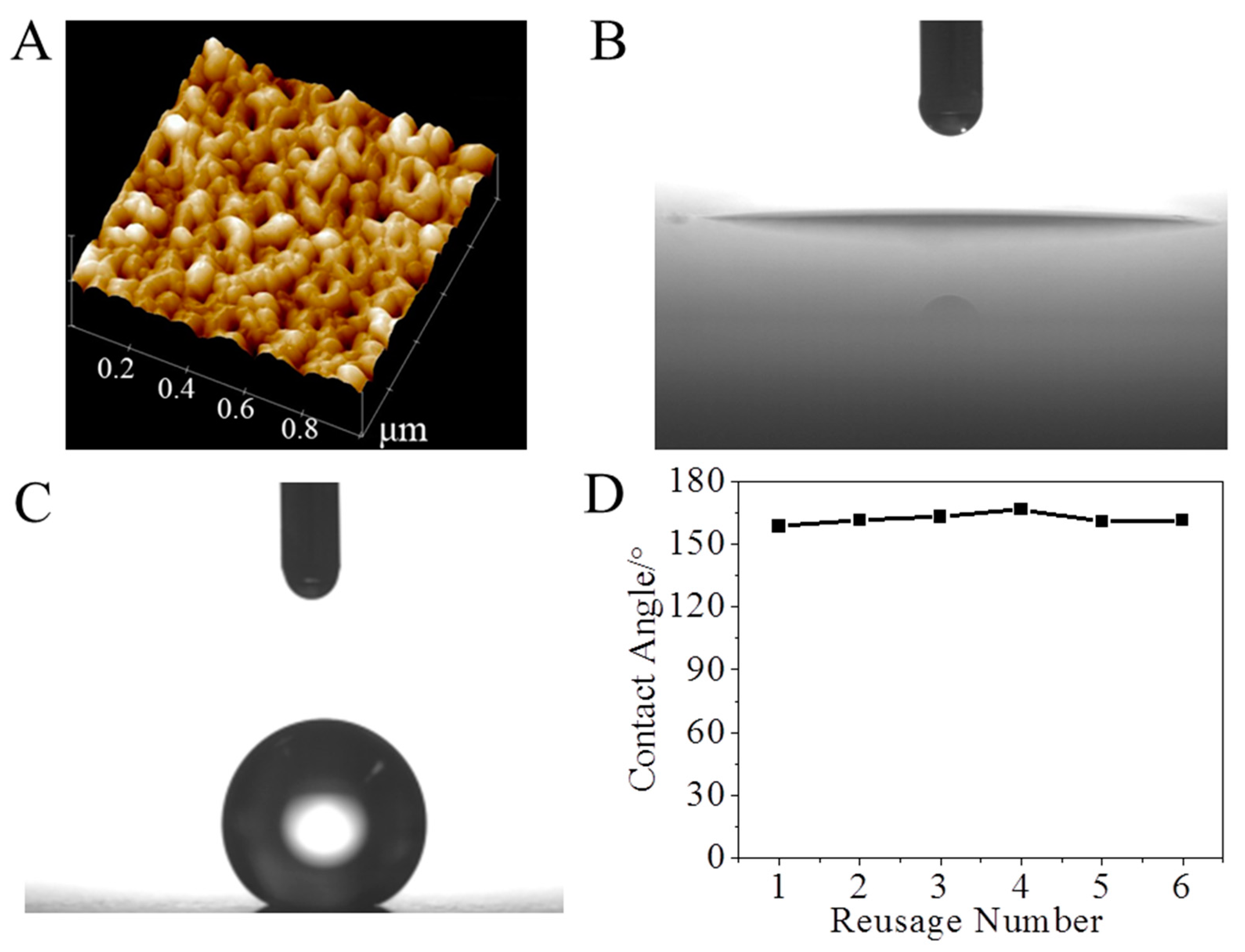
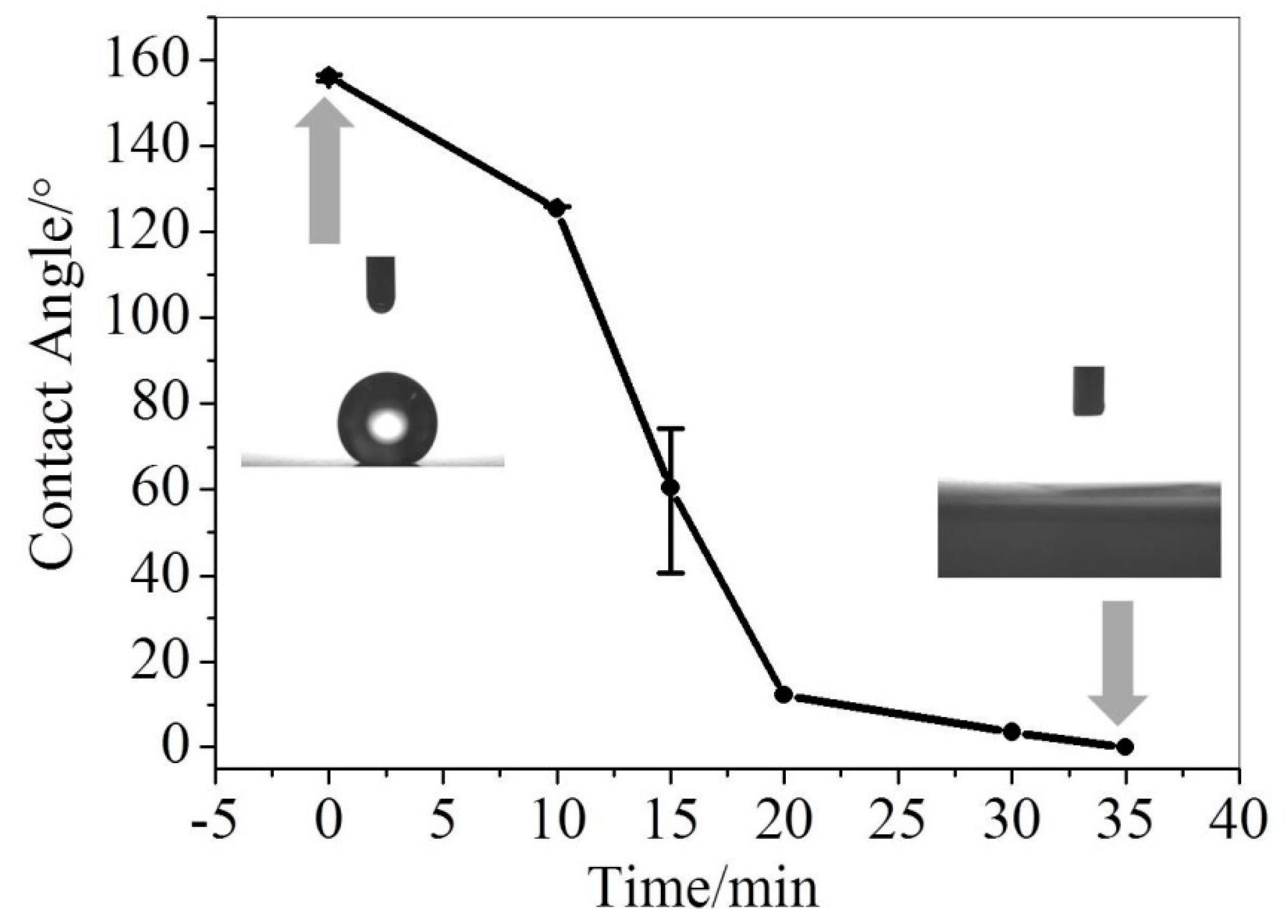
2. Results and Discussion
3. Materials and Methods
3.1. Fabrication of a Large-Area Superhydrophobic TiO2 Nanotubular Layer
3.2. Fabrication of a Pre-Designed Pattern and the Patterning of Oil-Based Ink
3.3. Characterizations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gau, H.; Herminghaus, S.; Lenz, P.; Lipowsky, R. Liquid morphologies on structured surfaces: From microchannels to microchips. Science 1999, 283, 46–49. [Google Scholar] [CrossRef] [Green Version]
- Burgess, I.B.; Mishchenko, L.; Hatton, B.D.; Kolle, M.; Lonĉar, M.; Aizenberg, J. Encoding complex wettability patterns in chemically functionalized 3D photonic crystals. J. Am. Chem. Soc. 2011, 133, 12430–12432. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y. Surface micropatterning to regulate cell functions. Biomaterials 1999, 20, 2333–2342. [Google Scholar] [CrossRef]
- Gu, Z.; Fujishima, A.; Sato, O. Patterning of a colloidal crystal film on a modified hydrophilic and hydrophobic surface. Angew. Chem. Int. Ed. 2002, 41, 2068–2070. [Google Scholar]
- Jokinen, V.; Sainiemi, L.; Franssila, S. Complex droplets on chemically modified silicon nanograss. Adv. Mater. 2008, 20, 3453–3456. [Google Scholar] [CrossRef]
- Feng, S.; Wang, S.; Gao, L.; Li, G.; Hou, Y.; Zheng, Y. Controlled directional water-droplet spreading on a high-adhesion surface. Angew. Chem. Int. Ed. 2014, 53, 6163–6167. [Google Scholar] [CrossRef]
- Bai, H.; Wang, L.; Ju, J.; Sun, R.; Zheng, Y.; Jiang, L. Efficient water collection on integrative bioinspired surfaces with star-shaped wettability patterns. Adv. Mater. 2014, 26, 5025–5030. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, J.; Gao, H.; Feng, X.; Jiang, L. Superwettability-based interfacial chemical reactions. Adv. Mater. 2019, 31, 1800718. [Google Scholar]
- Zhang, D.; Cheng, Z.; Kang, H.; Yu, J.; Liu, Y.; Jiang, L. A smart superwetting surface with responsivity in both surface chemistry and microstructure. Angew. Chem. Int. Ed. 2018, 57, 3701–3705. [Google Scholar] [CrossRef]
- Lim, H.S.; Han, J.T.; Kwak, D.; Jin, M.; Cho, K. Photoreversibly switchable superhydrophobic surface with erasable and rewritable pattern. J. Am. Chem. Soc. 2006, 128, 14458–14459. [Google Scholar] [CrossRef]
- Tian, D.; Chen, Q.; Nie, F.; Xu, J.; Song, Y.; Jiang, L. Patterned wettability transition by photoelectric cooperative and anisotropic wetting for liquid reprography. Adv. Mater. 2009, 21, 3744–3749. [Google Scholar] [CrossRef]
- Dai, H.; Dong, Z.; Jiang, L. Directional liquid dynamics of interfaces with superwettability. Sci. Adv. 2020, 6, eabb5528. [Google Scholar] [CrossRef]
- Tian, D.; Song, Y.; Jiang, L. Patterning of controllable surface wettability for printing techniques. Chem. Soc. Rev. 2013, 42, 5184–5209. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Dong, Z.; Jiang, L. Bioinspired designs of superhydrophobic and superhydrophilic materials. ACS Cent. Sci. 2018, 4, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Lin, C.; Wang, H.; Huang, J.; Zhuang, H.; Sun, L. Superhydrophilic-superhydrophobic micropattern on TiO2 nanotube films by photocatalytic lithography. Electrochem. Commun. 2008, 10, 387–391. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, M.; Liu, Z.; Tryk, D.A.; Nishimoto, S.; Murakami, T.; Fujishima, A. Superhydrophobic TiO2 surfaces: preparation, photocatalytic wettability conversion, and superhydrophobic−superhydrophilic patterning. J. Phys. Chem. C. 2007, 111, 14521–14529. [Google Scholar] [CrossRef]
- Zhang, X.; Kono, H.; Liu, Z.; Nishimoto, S.; Tryk, D.A.; Murakami, T.; Sakai, H.; Abe, M.; Fujishima, A. A transparent and photo-patternable superhydrophobic film. Chem. Commun. 2007, 46, 4949–4951. [Google Scholar] [CrossRef]
- Nakata, K.; Nishimoto, S.; Yuda, Y.; Ochiai, T.; Murakami, T.; Fujishima, A. Rewritable superhydrophilic-superhydrophobic patterns on a sintered titanium dioxide substrate. Langmuir 2010, 26, 11628–11630. [Google Scholar] [CrossRef]
- Nishimoto, S.; Sekine, H.; Zhang, X.; Liu, Z.; Nakata, K.; Murakami, T.; Koide, Y.; Fujishima, A. Assembly of self-assembled monolayer-coated Al2O3 on TiO2 thin films for the fabrication of renewable superhydrophobic-superhydrophilic structures. Langmuir 2009, 25, 7226–7228. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Kubo, A.; Zhang, X.; Liu, Z.; Taneichi, N.; Okui, T.; Murakami, T.; Komine, T.; Fujishima, A. Novel hydrophobic/hydrophilic patterning process by photocatalytic Ag nucleation on TiO2 thin film and electroless Cu deposition. Appl. Surf. Sci. 2008, 254, 5891–5894. [Google Scholar] [CrossRef]
- Nishimoto, S.; Kubo, A.; Nohara, K.; Zhang, X.; Taneichi, N.; Okui, T.; Liu, Z.; Nakata, K.; Sakai, H.; Murakami, T.; et al. TiO2-based superhydrophobic-superhydrophilic patterns: Fabrication via an ink-jet technique and application in offset printing. Appl. Surf. Sci. 2009, 255, 6221–6225. [Google Scholar] [CrossRef]
- Nakata, K.; Nishimoto, S.; Kubo, A.; Tryk, D.; Ochiai, T.; Murakami, T.; Fujishima, A. Fabrication and application of TiO2-based Superhydrophilic–superhydrophobic patterns on titanium substrates for offset printing. Chem. Asian J. 2009, 4, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Fujishima, A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-induced amphiphilic surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Feng, X.; Zhai, J.; Jiang, L. The fabrication and switchable superhydrophobicity of TiO2 nanorod films. Angew. Chem. Int. Ed. 2005, 44, 5115–5118. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Cao, M.; Fujishima, A.; Jiang, L. Bio-inspired titanium dioxide materials with special wettability and their applications. Chem. Rev. 2014, 114, 10044–10094. [Google Scholar] [CrossRef]
- Tadanaga, K.; Morinaga, J.; Matsuda, A.; Minami, T. Superhydrophobic-superhydrophilic micropatterning on flowerlike alumina coating film by the sol-gel method. Chem. Mater. 2000, 12, 590–592. [Google Scholar] [CrossRef]
- Zhang, X.; Jin, M.; Liu, Z.; Nishimoto, S.; Saito, H.; Murakami, T.; Fujishima, A. Preparation and photocatalytic wettability conversion of TiO2-based superhydrophobic surfaces. Langmuir 2006, 22, 9477–9479. [Google Scholar] [CrossRef]
- Järn, M.; Tåg, C.M.; Järnström, J.; Granqvist, B.; Rosenholm, J.B. Alternative models for determining the surface energy components in offset printing. J. Colloid Interface Sci. 2006, 301, 668–676. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef]
- Lee, K.; Mazare, A.; Schmuki, P. One-dimensional titanium dioxide nanomaterials: Nanotubes. Chem. Rev. 2014, 114, 9385–9454. [Google Scholar] [CrossRef] [Green Version]
- Cai, Q.; Paulose, M.; Varghese, O.K.; Grimes, C.A. The effect of electrolyte composition on the fabrication of self-organized titanium oxide nanotube arrays by anodic oxidation. J. Mater. Res. 2005, 20, 230–236. [Google Scholar] [CrossRef]
- Ren, H.; Xiao, T.; Zhang, Q.; Liu, Z. Photosynthesis-inspired bifunctional energy-harvesting devices that convert light and salinity gradients into electricity. Chem. Commun. 2018, 54, 12310–12313. [Google Scholar] [CrossRef]
- Mao, X.; Xiao, T.; Zhang, Q.; Liu, Z. An electrochemical anodization strategy towards high-activity porous MoS2 electrodes for the hydrogen evolution reaction. RSC Adv. 2018, 8, 15030–15035. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Liu, Z.; Zhang, Q.; Meng, C.; Zhang, T.; Zhai, J. Underwater superoleophobic porous membrane based on hierarchical TiO2 nanotubes: Multifunctional integration of oil-water separation, flow-through photocatalysis and selfcleaning. J. Mater. Chem. A 2015, 3, 1279–1286. [Google Scholar] [CrossRef]
- Balaur, E.; Macak, J.M.; Tsuchiya, H.; Schmuki, P. Wetting behaviour of layers of TiO2 nanotubes with different diameters. J. Mater. Chem. 2005, 15, 4488–4491. [Google Scholar] [CrossRef]
- Balaur, E.; Macak, J.M.; Taveira, L.; Schmuki, P. Tailoring the wettability of TiO2 nanotube layers. Electrochem. Commun. 2005, 7, 1066–1070. [Google Scholar] [CrossRef]
- Lai, Y.; Gao, X.; Zhuang, H.; Huang, J.; Lin, C.; Jiang, L. Designing superhydrophobic porous nanostructures with tunable water adhesion. Adv. Mater. 2009, 21, 3799–38032. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Zhang, X.; Liu, Z.; Huo, K.; Chu, P.K.; Zhai, J.; Jiang, L. Regulating water adhesion on superhydrophobic TiO2 nanotube arrays. Adv. Funct. Mater. 2014, 24, 6381–6388. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Nishimoto, S.; Murakami, T.; Fujishima, A. Efficient photocatalytic degradation of gaseous acetaldehyde by highly ordered TiO2 nanotube arrays. Environ. Sci. Technol. 2008, 42, 8547–8551. [Google Scholar] [CrossRef]
- Feng, X.; Jiang, L. Design and creation of superwetting/antiwetting surfaces. Adv. Mater. 2006, 18, 3063–3078. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–561. [Google Scholar] [CrossRef]
- Sun, T.; Feng, L.; Gao, X.; Jiang, L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 2005, 38, 644–652. [Google Scholar] [CrossRef]
- Tatsuma, T.; Tachibana, S.; Miwa, T.; Tryk, D.A.; Fujishima, A. Remote bleaching of methylene blue by UV-irradiated TiO2 in the gas phase. J. Phys. Chem. B 1999, 103, 8033–8035. [Google Scholar] [CrossRef]
- Tatsuma, T.; Tachibana, S.; Fujishima, A. Remote oxidation of organic compounds by UV-irradiated TiO2 via the gas phase. J. Phys. Chem. B 2001, 105, 6987–6992. [Google Scholar] [CrossRef]
- Tatsuma, T.; Kubo, W.; Fujishima, A. Patterning of solid surfaces by photocatalytic lithography based on the remote oxidation effect of TiO2. Langmuir 2002, 18, 9632–9634. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, X.; Murakami, T.; Fujishima, A. Sol–gel SiO2/TiO2 bilayer films with self-cleaning and antireflection properties. Sol. Energy Mater. Sol. C 2008, 92, 1434–1438. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Ren, H.; Liu, Z. Large-Area Patterning of Oil-Based Inks on Superhydrophobic TiO2 Nanotubular Layers by Photocatalytic Wettability Conversion. Catalysts 2020, 10, 1203. https://doi.org/10.3390/catal10101203
Jin J, Ren H, Liu Z. Large-Area Patterning of Oil-Based Inks on Superhydrophobic TiO2 Nanotubular Layers by Photocatalytic Wettability Conversion. Catalysts. 2020; 10(10):1203. https://doi.org/10.3390/catal10101203
Chicago/Turabian StyleJin, Jiao, Huihui Ren, and Zhaoyue Liu. 2020. "Large-Area Patterning of Oil-Based Inks on Superhydrophobic TiO2 Nanotubular Layers by Photocatalytic Wettability Conversion" Catalysts 10, no. 10: 1203. https://doi.org/10.3390/catal10101203



