Mono vs. Difunctional Coumarin as Photoinitiators in Photocomposite Synthesis and 3D Printing
Abstract
1. Introduction
2. Results
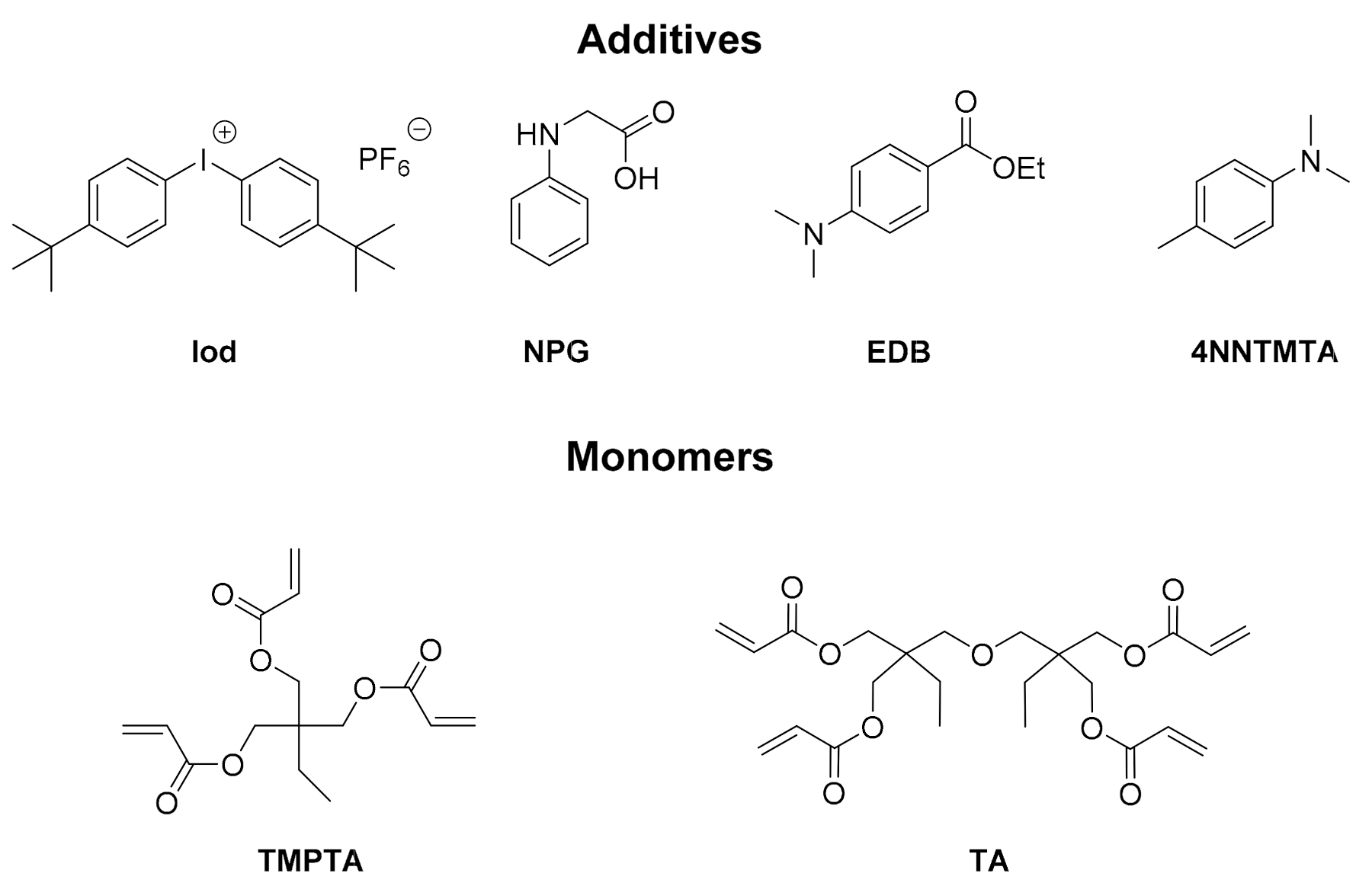
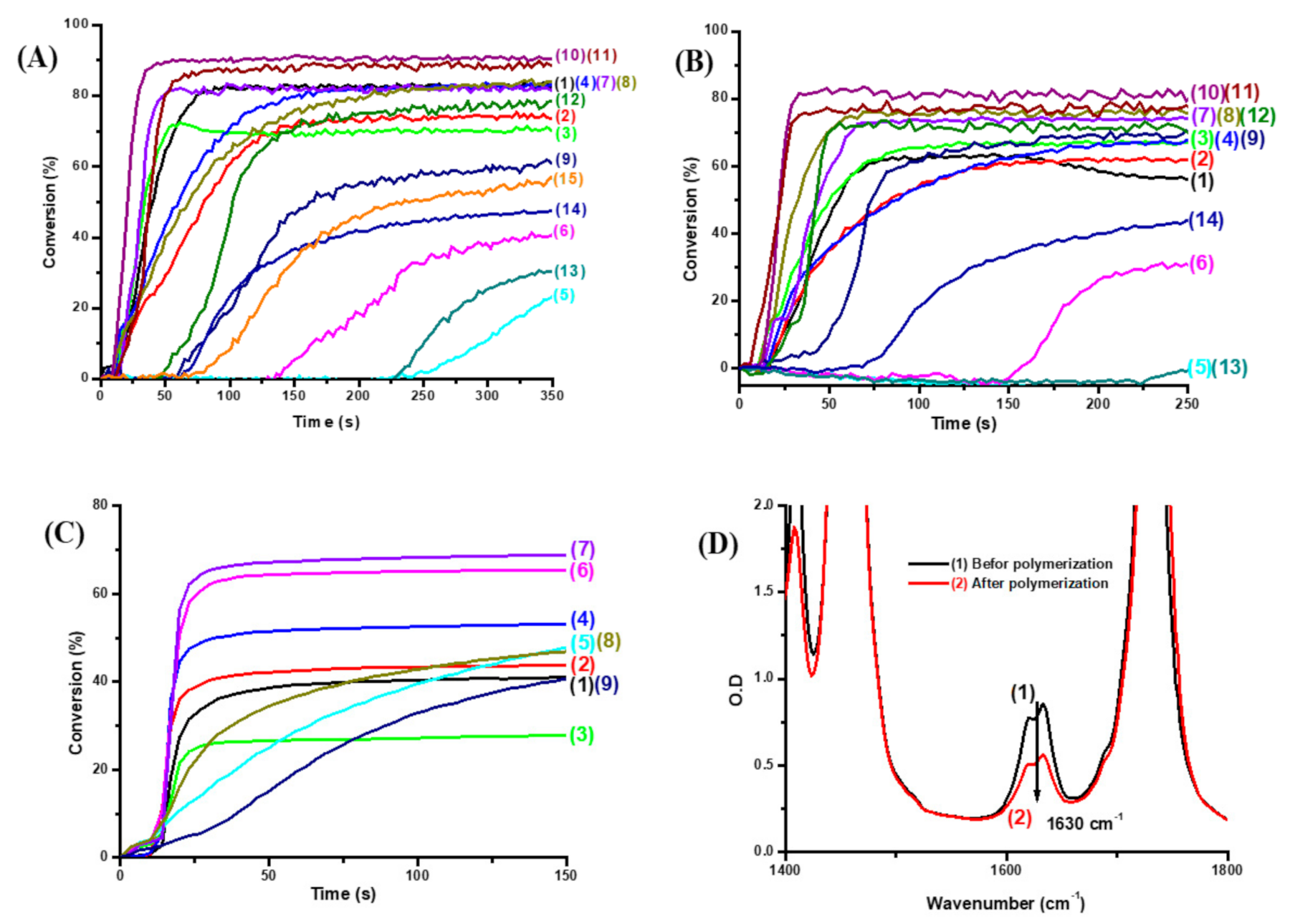
2.1. Free Radical Photopolymerization (FRP) of Acrylate Monomers (TMPTA or Di(trimethylolpropane) Tetraacrylate (TA))
2.2. 3D-Printing Experiments Using Coum/Iod or Coum/Iod/4-N,N,TMA Systems
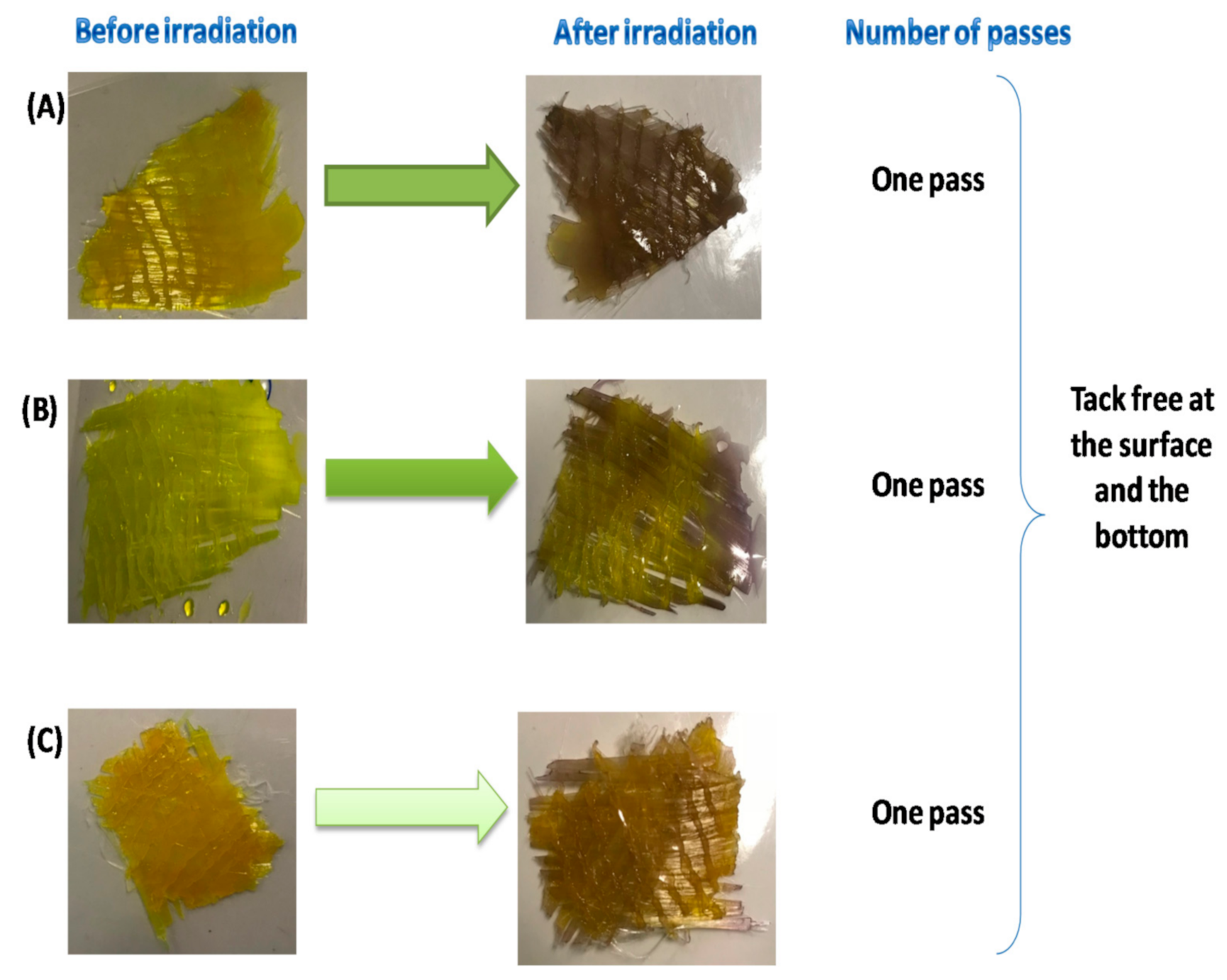
2.3. LED Conveyor Experiments for Composite Preparation
3. Discussion
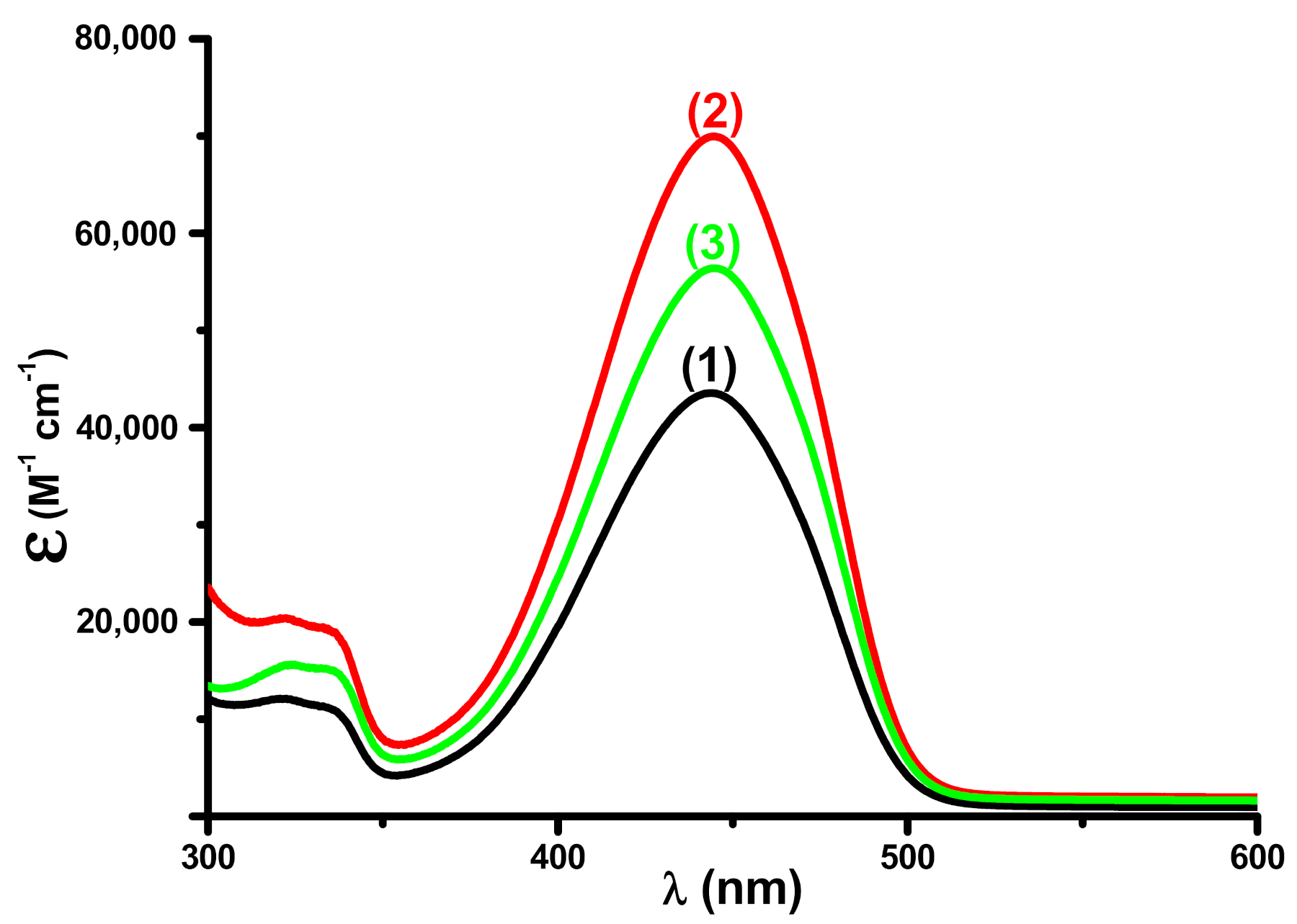
3.1. Light Absorption Properties of the Different Dyes
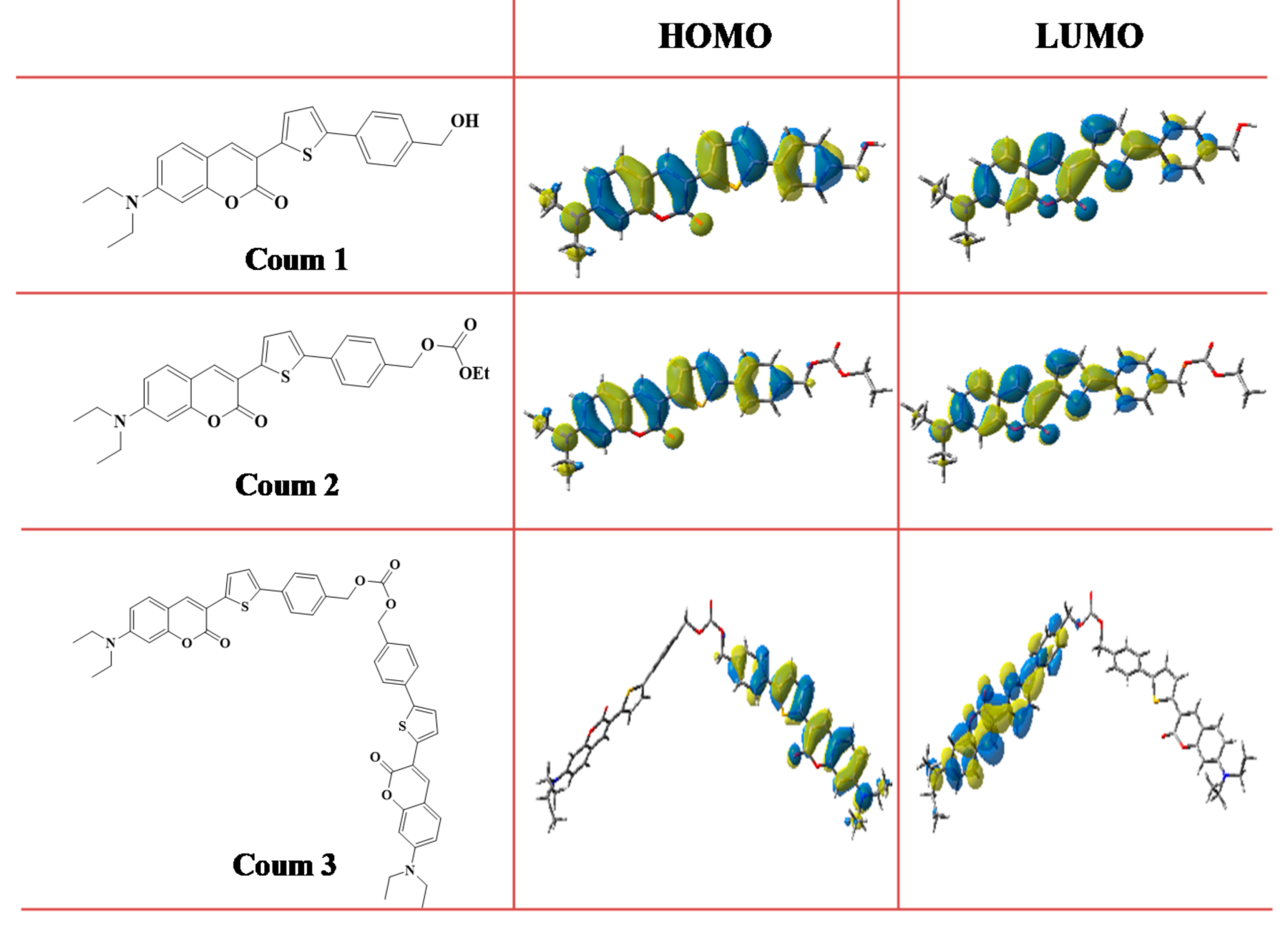
3.2. (Photo)Chemical Mechanisms
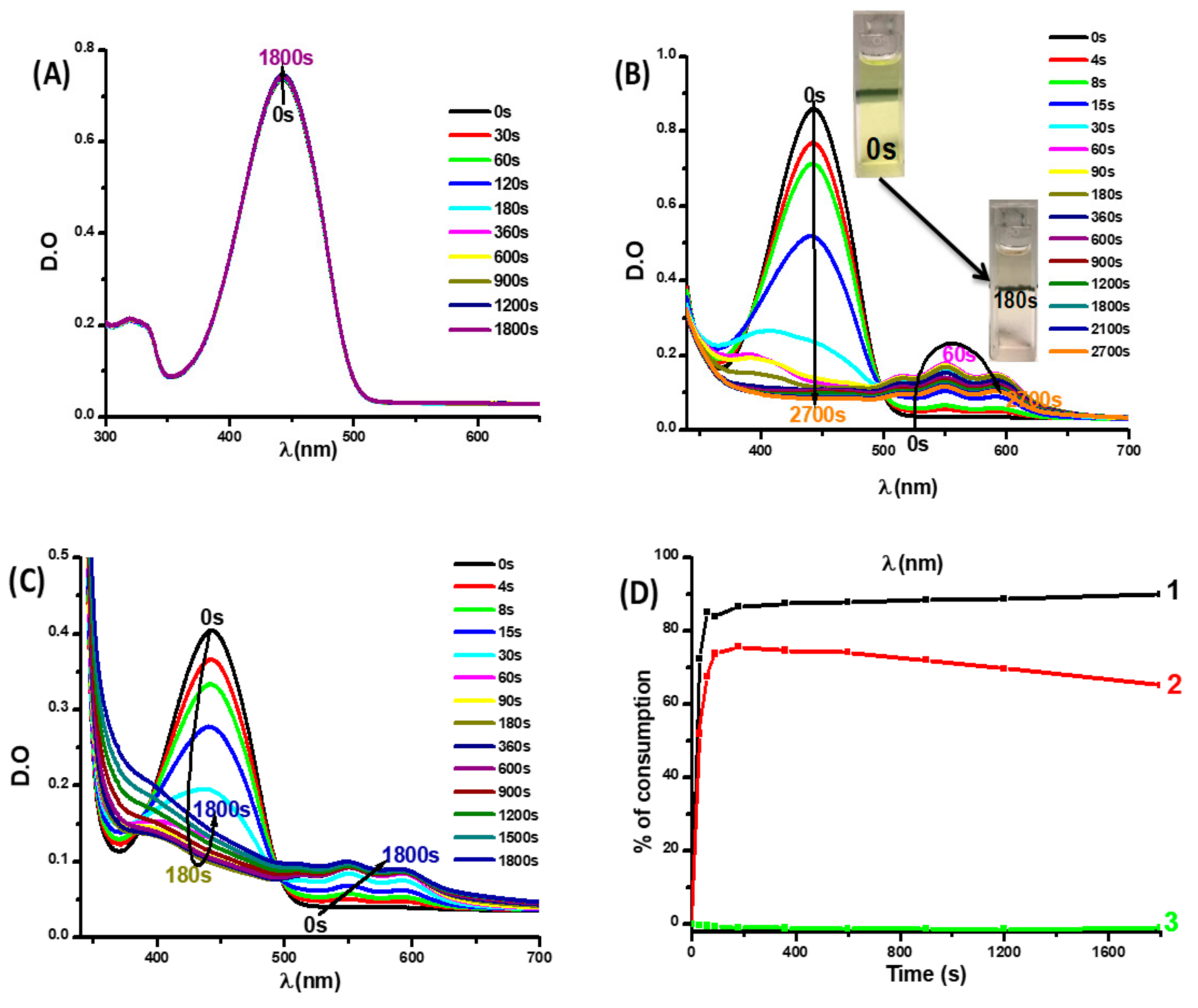
3.2.1. Photophysical and Photochemical Properties of Coum3
3.2.2. Photophysical Properties of Coumarins Coum1 and Coum2
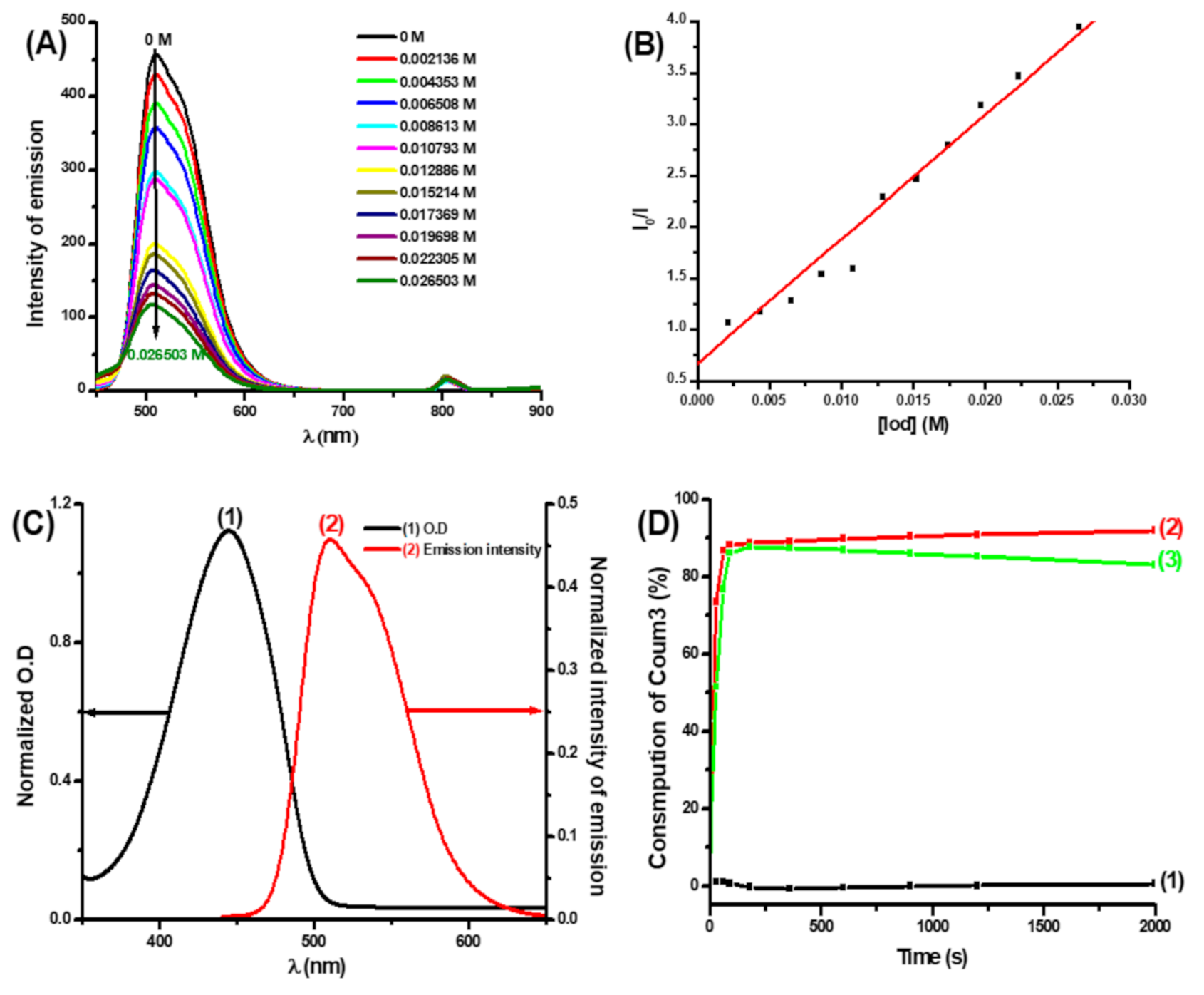
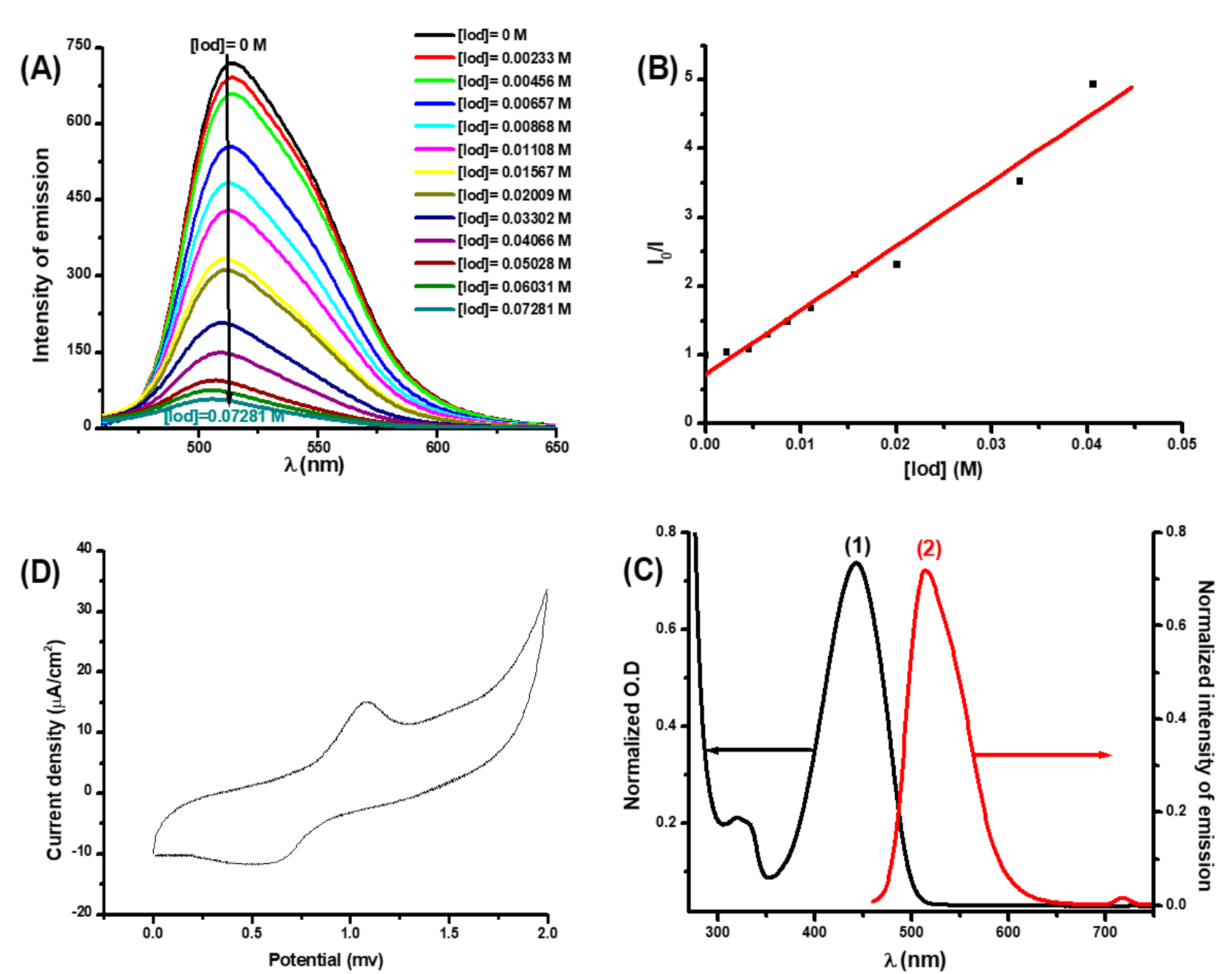
3.2.3. Fluorescence Quenching Experiments and Cyclic Voltammetry Measurements
3.2.4. Structure/Reactivity/Efficiency Relationship
4. Experimental Part
4.1. Synthesis of Coumarins
4.1.1. Synthesis of 7-(Diethylamino)-3-(thiophen-2-yl)-2H-chromen-2-one 3

4.1.2. Synthesis of 3-(5-Bromothiophen-2-yl)-7-(Diethylamino)-2H-chromen-2-one 4

4.1.3. Synthesis of 4-(5-(7-(Diethylamino)-2-oxo-2H-chromen-3-yl)thiophen-2-yl)benzaldehyde 5
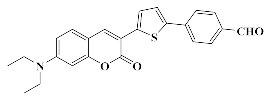
4.1.4. Synthesis of 7-(Diethylamino)-3-(5-(4-(hydroxymethyl)phenyl)thiophen-2-yl)-2H-chromen-2-one Coum1
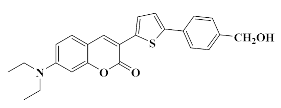
4.1.5. Synthesis of 4-(5-(7-(Diethylamino)-2-oxo-2H-chromen-3-yl) thiophen-2-yl) benzyl ethyl carbonate Coum2
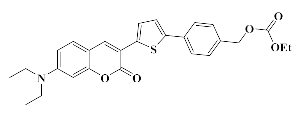
4.1.6. Synthesis of bis (4-(5-(7-(Diethylamino)-2-oxo-2H-chromen-3-yl) thiophen-2-yl)benzyl)carbonate Coum3

4.2. Other Chemicals
4.3. Light Sources
4.4. Free Radical Photopolymerization (FRP)
4.5. Redox Potentials
4.6. Fluorescence Experiments
4.7. UV-Visible Absorption and Photolysis Experiments
4.8. Computational Procedure
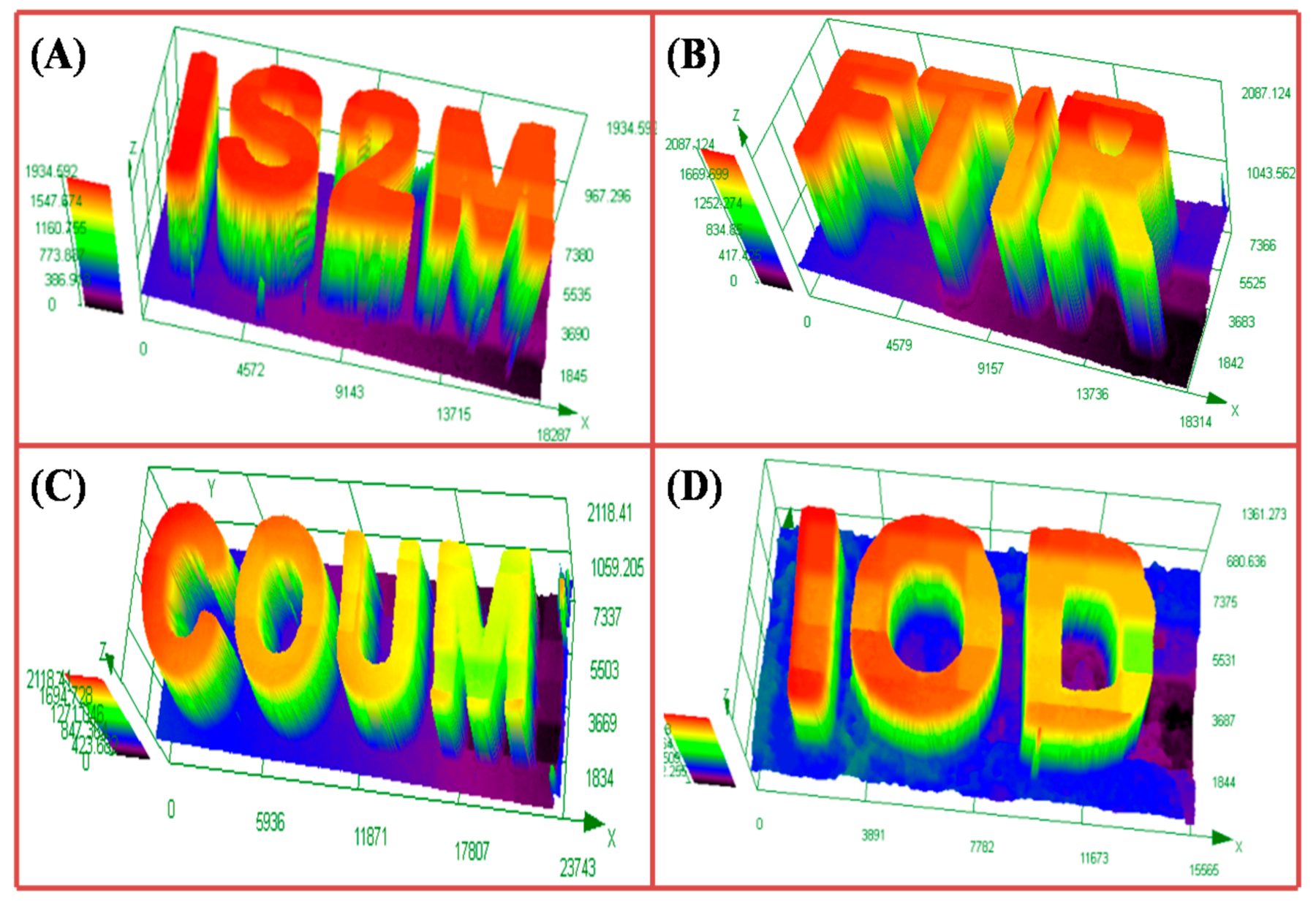
4.9. 3D Printing Experiments
4.10. Near-UV Conveyor
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ueno, K.; Oshikiri, T.; Sun, Q.; Shi, X.; Misawa, H. Solid-State Plasmonic Solar Cells. Chem. Rev. 2018, 118, 2955–2993. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Morlet-Savary, F.; Lalevée, J.; Allonas, X.; Ley, C. Dyes as photoinitiators or photosensitizers of polymerization reactions. Materials 2010, 3, 5130–5142. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Lalevee, J. Photoinitiators for Polymer Synthesis, Scope, Reactivity, and Efficiency; Wiley-VCH Verlag GmbH & Co.KGaA: Weinheim, Germany, 2012. [Google Scholar]
- Fouassier, J.-P. Photoinitiator, Photopolymerization and Photocuring: Fundamentals and Applications; Hanser Gardner: New York, NY, USA, 1995. [Google Scholar]
- Xiao, P.; Zhang, J.; Dumur, F.; Tehfe, M.-A.; Morlet-Savary, F.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Photoinitiating systems: Recent progress in visible light induced cationic and radical photopolymerization reactions under soft conditions. Prog. Polym. Sci. 2015, 41, 32–66. [Google Scholar] [CrossRef]
- Trykowska Konc, J.; Hejchman, E.; Kruszewska, H.; Wolska, I.; Maciejewska, D. Synthesis and pharmacological activity of O-aminoalkyl derivatives of 7-hydroxycoumarin. Eur. J. Med. Chem. 2011, 46, 2252–2263. [Google Scholar] [CrossRef]
- Wattenberg, L.W.; Low, L.K.T.; Fladmoe, A.V. Inhibition of chemical carcinogeninduced neoplasia by coumarins and a-angelicalactone. Cancer Res. 1979, 39, 1651–1654. [Google Scholar]
- Manolov, I.; Kostova, I.; Netzeva, T.; Konstantinov, S.; Karaivanova, M. Cytotoxic activity of cerium complexes with coumarin derivatives. Molecular modeling of the ligands. Archiv. Pharm. Med. Chem. 2000, 333, 93–98. [Google Scholar] [CrossRef]
- Lake, B.G. Coumarin metabolism, toxicity and carcinogenicity: Relevance for human risk assessment. Food Chem. Toxicol. 1999, 37, 423–453. [Google Scholar] [CrossRef]
- Manojkumar, P.; Ravi, T.K.; Subbuchettiar, G. Synthesis of coumarin heterocyclic derivatives with antioxidant activity and in vitro cytotoxic activity against tumour cells. Acta Pharm. 2009, 59, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Mayekar, S.A.; Chaskar, A.C.; Mulwad, V.V. Facile synthesis of coumarinyl isothiocyanate from amino coumarin. Synth. Commun. 2009, 40, 46–51. [Google Scholar] [CrossRef]
- Jiang, Z.-J.; Lv, H.-S.; Zhu, J.; Zha, B.-X. New fluorescent chemosensor based on quinoline and coumarin for Cu2+. Synth. Met. 2012, 162, 2112–2116. [Google Scholar] [CrossRef]
- Aldakov, D.; Anzenbacher, P. Dipyrrolyl quinoxalines with extended chromophores are efficient fluorimetric sensors for pyrophosphate. Chem. Commun. 2003, 12, 1394–1395. [Google Scholar] [CrossRef]
- Hunger, K. Industrial Dyes, WILEY-VCH Verlag; Gmbh & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Su, K.; Ming, J.; Zhang, L.; Xiang, S.; Cui, M.; Yang, H. A novel tricyclic heterocyclic fluorescent probe for Pd2+ in aqueous solution: Synthesis, properties and theoretical calculations. Opt. Mater. 2019, 93, 25–29. [Google Scholar] [CrossRef]
- Nasser Mabkhot, Y.; Barakat, A.; Mohammed Al-Majid, A.; Alshahrani, S.; Yousuf, S.; Iqbal Choudhary, M. Synthesis, reactions and biological activity of some new bis-heterocyclic ring compounds containing sulphur atom. Chem. Cent. J. 2013, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Cabanetos, C.; Blanchard, P.; Roncali, J. Arylamine Based Photoactive Push-Pull Molecular Systems: A Brief Overview of the Chemistry “Made in Angers”. Chem. Rec. 2019, 19, 1123–1130. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Graff, B.; Morlet-Savary, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Push-pull (thio)barbituric acid derivatives in dye photosensitized radical and cationic polymerization reactions under 457/473 nm Laser beams or blue LEDs. Polym. Chem. 2013, 4, 3866–3875. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Graff, B.; Vidal, L.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Structural effects in the indanedione skeleton for the design of low intensity 300-500 nm light sensitive initiators. Macromolecules 2014, 47, 26–34. [Google Scholar] [CrossRef]
- Xiao, P.; Dumur, F.; Tehfe, M.-A.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Red-light-induced cationic photopolymerization: Perylene derivatives as efficient photoinitiators Macromol. Rapid Commun. 2013, 34, 1452–1458. [Google Scholar] [CrossRef]
- Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Organic Electronics: An El Dorado in the quest of new photoCatalysts as photoinitiators of polymerization. Acc. Chem. Res. 2016, 49, 1980–1989. [Google Scholar] [CrossRef]
- Xiao, P.; Hong, W.; Li, Y.; Dumur, F.; Graff, B.; Fouassier, J.-P.; Gigmes, D.; Lalevée, J. Green light sensitive diketopyrrolopyrrole derivatives used in versatile photoinitiating systems for photopolymerizations. Polym. Chem. 2014, 5, 2293–2300. [Google Scholar] [CrossRef]
- Dietlin, C.; Schweizer, S.; Xiao, P.; Zhang, J.; Morlet-Savary, F.; Graff, B.; Fouassier, J.-P.; Lalevée, J. Photopolymerization upon LEDs: New photoinitiating systems and strategies. Polym. Chem. 2015, 6, 3895–3912. [Google Scholar] [CrossRef]
- Zhang, J.; Zivic, N.; Dumur, F.; Guo, C.; Li, Y.; Xiao, P.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Panchromatic photoinitiators for radical, cationic and thiol-ene polymerization reactions: A search in the diketopyrrolopyrrole or indigo dye series Mater. Today Commun. 2015, 4, 101–108. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Poriel, C.; Dumur, F.; Toufaily, J.; Hamieh, T.; Fouassier, J.-P.; Lalevée, J. Zinc tetraphenylporphyrin as high performance visible-light photoinitiator of cationic photosensitive resins for LED projector 3D printing applications. Macromolecules 2017, 50, 746–753. [Google Scholar] [CrossRef]
- Telitel, S.; Dumur, F.; Campolo, D.; Poly, J.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Iron complexes as potential photocatalysts for controlled radical photopolymerizations: A tool for modifications and patterning of surfaces. J. Polym. Sci. A Polym. Chem. 2016, 54, 702–713. [Google Scholar] [CrossRef]
- Gualandi, A.; Rodeghiero, G.; Della Rocca, E.; Bertoni, F.; Marchini, M.; Perciaccante, R.; Jansen, T.P.; Ceroni, P.; Cozzi, P.G. Application of coumarin dyes for organic photoredox catalysis. Chem. Commun. 2018, 54, 10044–10047. [Google Scholar] [CrossRef]
- Liu, L.; Huang, D.; Draper, S.M.; Yi, X.; Wu, W.; Zhao, J. Visible light-harvesting trans bis(alkylphosphine)platinum(II)-alkynyl complexes showing long-lived triplet excited states as triplet photosensitizers for triplet–triplet annihilation upconversion. Dalton Trans. 2013, 42, 10694–10706. [Google Scholar] [CrossRef]
- Kotchapadist, P.; Prachumrak, N.; Sunonnam, T.; Namuangruk, S.; Sudyoadsuk, T.; Keawin, T.; Jungsuttiwong, S.; Promarak, V. Synthesis, characterisation, and electroluminescence properties of n-coumarin derivatives containing peripheral triphenylamine. Eur. J. Org. Chem. 2015, 3, 496–505. [Google Scholar] [CrossRef]
- Yu, T.; Zhu, Z.; Bao, Y.; Zhao, Y.; Liu, X.; Zhang, H. Investigation of novel carbazole-functionalized coumarin derivatives as organic luminescent materials. Dyes Pigm. 2017, 147, 260–269. [Google Scholar] [CrossRef]
- Kochapradist, P.; Sunonnam, T.; Prachumrak, N.; Namuangruk, S.; Keawin, T.; Jungsuttiwong, S.; Sudyoadsuk, T.; Promarak, V. Synthesis, characterization, and properties of novel bis(aryl)carbazole-containing N-coumarin derivatives. Tetrahedron Lett. 2014, 55, 6689–6693. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Kermagoret, A.; Versace, D.-L.; Toufaily, J.; Hamieh, T.; Graff, B.; Dumur, F.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Copper photoredox catalysts for polymerization upon near UV or visible light: Structure/reactivity/efficiency relationships and use in LED projector 3D printing resins. Polym. Chem. 2016, 8, 568–580. [Google Scholar] [CrossRef]
- Al Mousawi, A.; Dietlin, C.; Graff, B.; Morlet-Savary, F.; Toufaily, J.; Fouassier, J.-P.; Chachaj-Brekiesz, A.; Ortyl, J.; Lalevée, J. Meta- terphenyl derivative/iodonium salt/9H-carbazole-9-ethanol photoinitiating systems for free radical promoted cationic polymerization upon visible lights. Macromol. Chem. Phys. 2016, 217, 1955–1965. [Google Scholar] [CrossRef]
- Abdallah, M.; Hijazi, A.; Graff, B.; Fouassier, J.-P.; Rodeghiero, G.; Gualandi, A.; Dumur, F.; Cozzi, P.G.; Lalevée, J. Coumarin derivatives as versatile photoinitiators for 3D printing, polymerization in water and photocomposite synthesis. Polym. Chem. 2019, 10, 872–884. [Google Scholar] [CrossRef]
- Bonardi, A.-H.; Bonardi, F.; Noirbent, G.; Dumur, F.; Dietlin, C.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Different NIR dye scaffolds for polymerization reactions under NIR light. Polym. Chem. 2019, 10, 6505–6514. [Google Scholar] [CrossRef]
- Tehfe, M.-A.; Dumur, F.; Xiao, P.; Delgove, M.; Graff, B.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Chalcone derivatives as highly versatile photoinitiators for radical, cationic, thiol-ene and IPN polymerization reactions upon visible lights. Polym. Chem. 2014, 5, 382–390. [Google Scholar] [CrossRef]
- Zhang, J.; Dumur, F.; Xiao, P.; Graff, B.; Bardelang, D.; Gigmes, D.; Fouassier, J.-P.; Lalevée, J. Structure design of naphthalimide derivatives: Toward versatile photoinitiators for near-UV/visible LEDs, 3D printing, and water-soluble photoinitiating systems. Macromolecules 2015, 48, 2054–2063. [Google Scholar] [CrossRef]

| Two-Component Photoinitiating System Coum/Iod (0.05%/1% w/w) and Coum/Iod (0.1%/1% w/w) | Three-Component Photoinitiating System Coum/Iod/EDB and Coum/Iod/NPG (0.1%/1%/1% w/w/w) | ||||
|---|---|---|---|---|---|
| Coum1/Iod 83% 1 73% 2 | Coum2/Iod 71% 1 83% 2 | Coum3/Iod n.p 1 32% 2 | Coum1/Iod/amine 84% 3 90% 4 | Coum2/Iod/amine 83% 3 89% 4 | Coum3/Iod/amine 59% 3 78% 4 |
| - | λmax (nm) | εmax (M−1 cm−1) | ε@ 405 nm (M−1 cm−1) |
|---|---|---|---|
| Coum1 | 444 | 43,500 | 23,000 |
| Coum2 | 444 | 69,900 | 35,900 |
| Coum3 | 445 | 56,400 | 28,900 |
| - | Eox (eV) | ES1 (eV) | ΔGS1 (eV) | KSV | ϕet(Coum/Iod) |
|---|---|---|---|---|---|
| Coum1 | 0.87 | 2.54 | −1.46 | 20 | 0.37 |
| Coum2 | 0.86 | 2.56 | −1.49 | 93 | 0.87 |
| Coum3 | n.o | 2.53 | - | 121 | 0.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahal, M.; Mokbel, H.; Graff, B.; Toufaily, J.; Hamieh, T.; Dumur, F.; Lalevée, J. Mono vs. Difunctional Coumarin as Photoinitiators in Photocomposite Synthesis and 3D Printing. Catalysts 2020, 10, 1202. https://doi.org/10.3390/catal10101202
Rahal M, Mokbel H, Graff B, Toufaily J, Hamieh T, Dumur F, Lalevée J. Mono vs. Difunctional Coumarin as Photoinitiators in Photocomposite Synthesis and 3D Printing. Catalysts. 2020; 10(10):1202. https://doi.org/10.3390/catal10101202
Chicago/Turabian StyleRahal, Mahmoud, Haifaa Mokbel, Bernadette Graff, Joumana Toufaily, Tayssir Hamieh, Frédéric Dumur, and Jacques Lalevée. 2020. "Mono vs. Difunctional Coumarin as Photoinitiators in Photocomposite Synthesis and 3D Printing" Catalysts 10, no. 10: 1202. https://doi.org/10.3390/catal10101202
APA StyleRahal, M., Mokbel, H., Graff, B., Toufaily, J., Hamieh, T., Dumur, F., & Lalevée, J. (2020). Mono vs. Difunctional Coumarin as Photoinitiators in Photocomposite Synthesis and 3D Printing. Catalysts, 10(10), 1202. https://doi.org/10.3390/catal10101202