CHA-Type Zeolite Prepared by Interzeolite Conversion Method Using FAU and LTL-Type Zeolite: Effect of the Raw Materials on the Crystallization Mechanism, and Physicochemical and Catalytic Properties
Abstract
:1. Introduction
2. Results
2.1. Synthesis of CHA-Type Zeolite
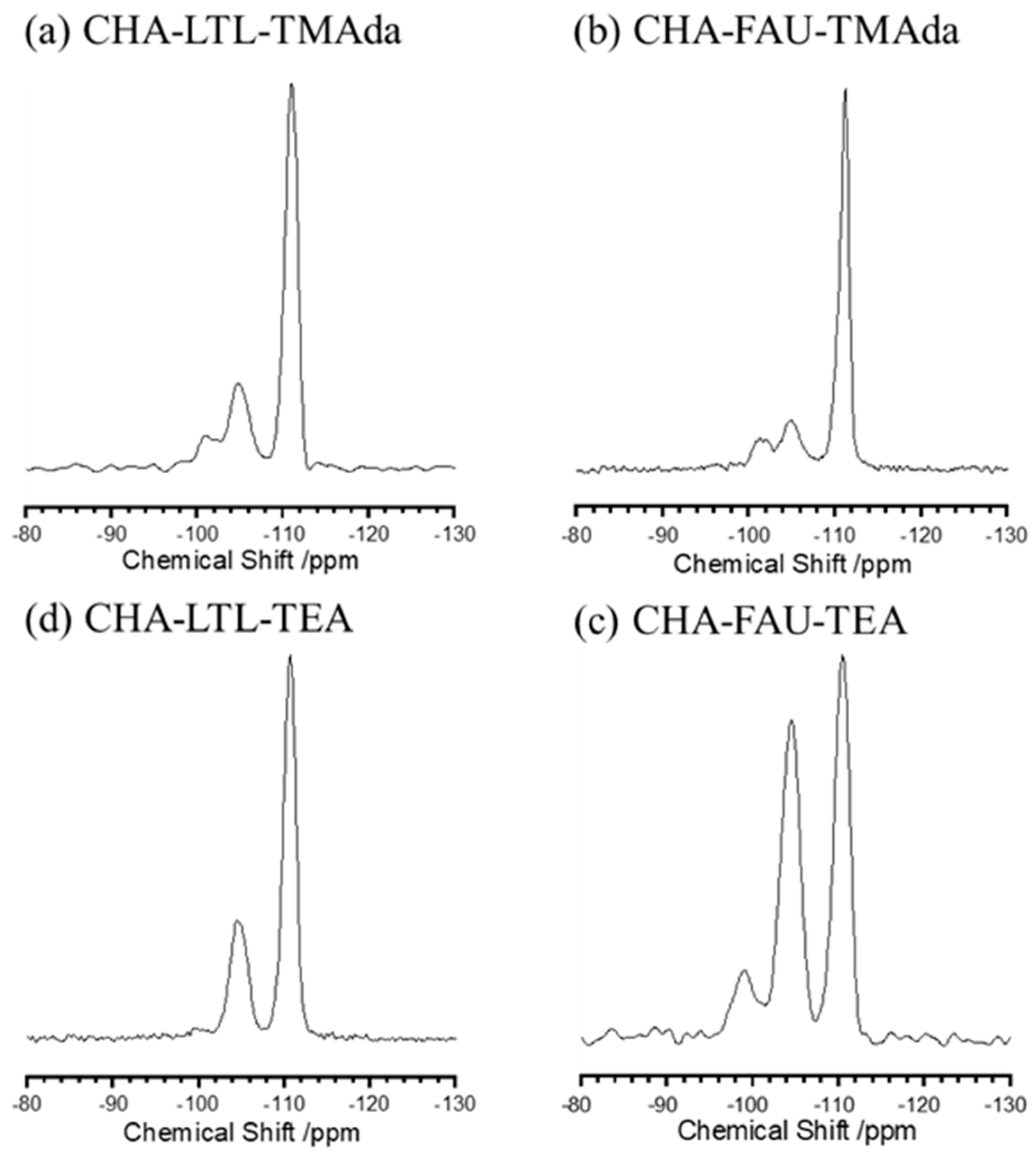
2.2. Evaluation of Al Species in CHA Structure
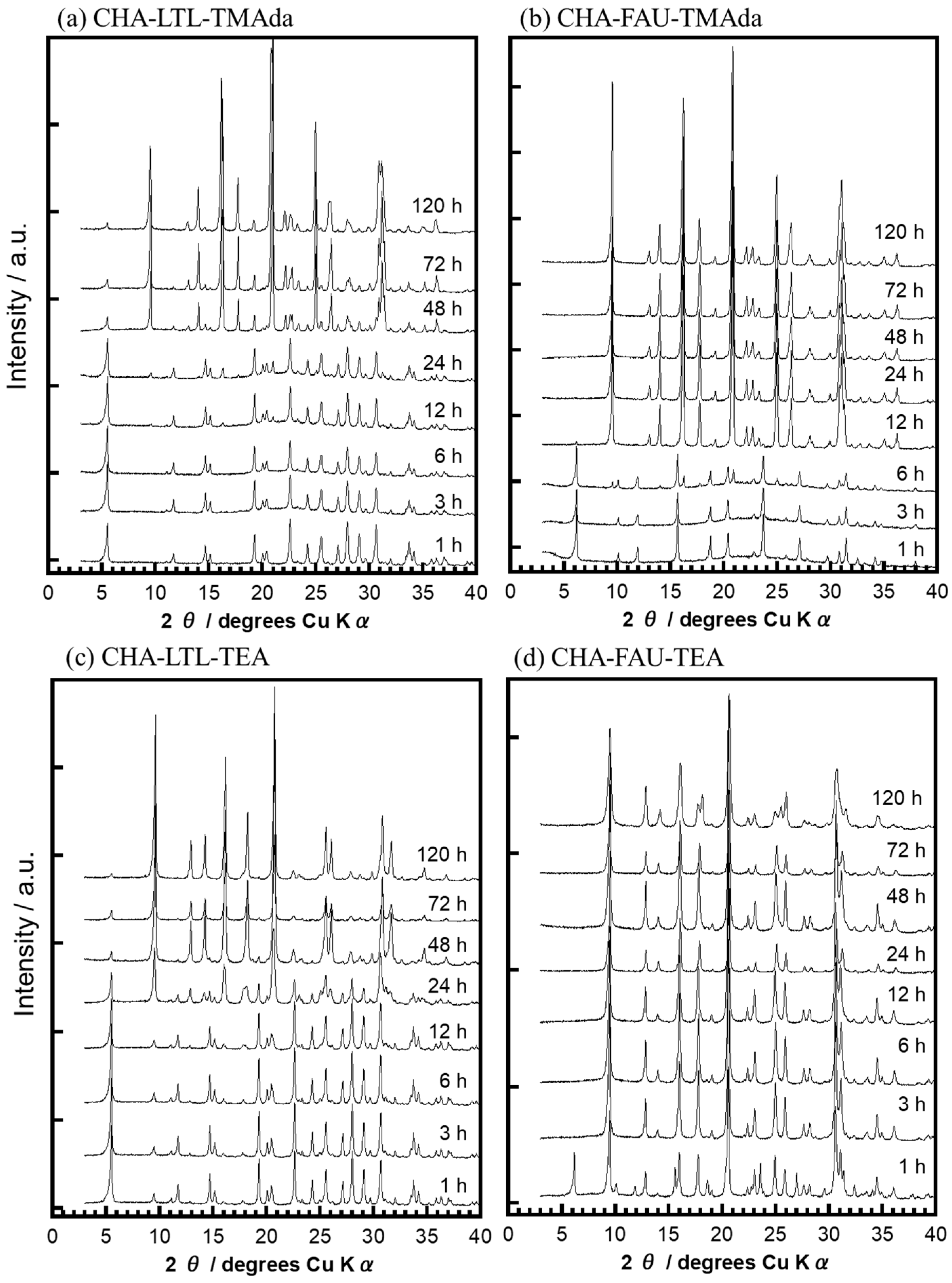
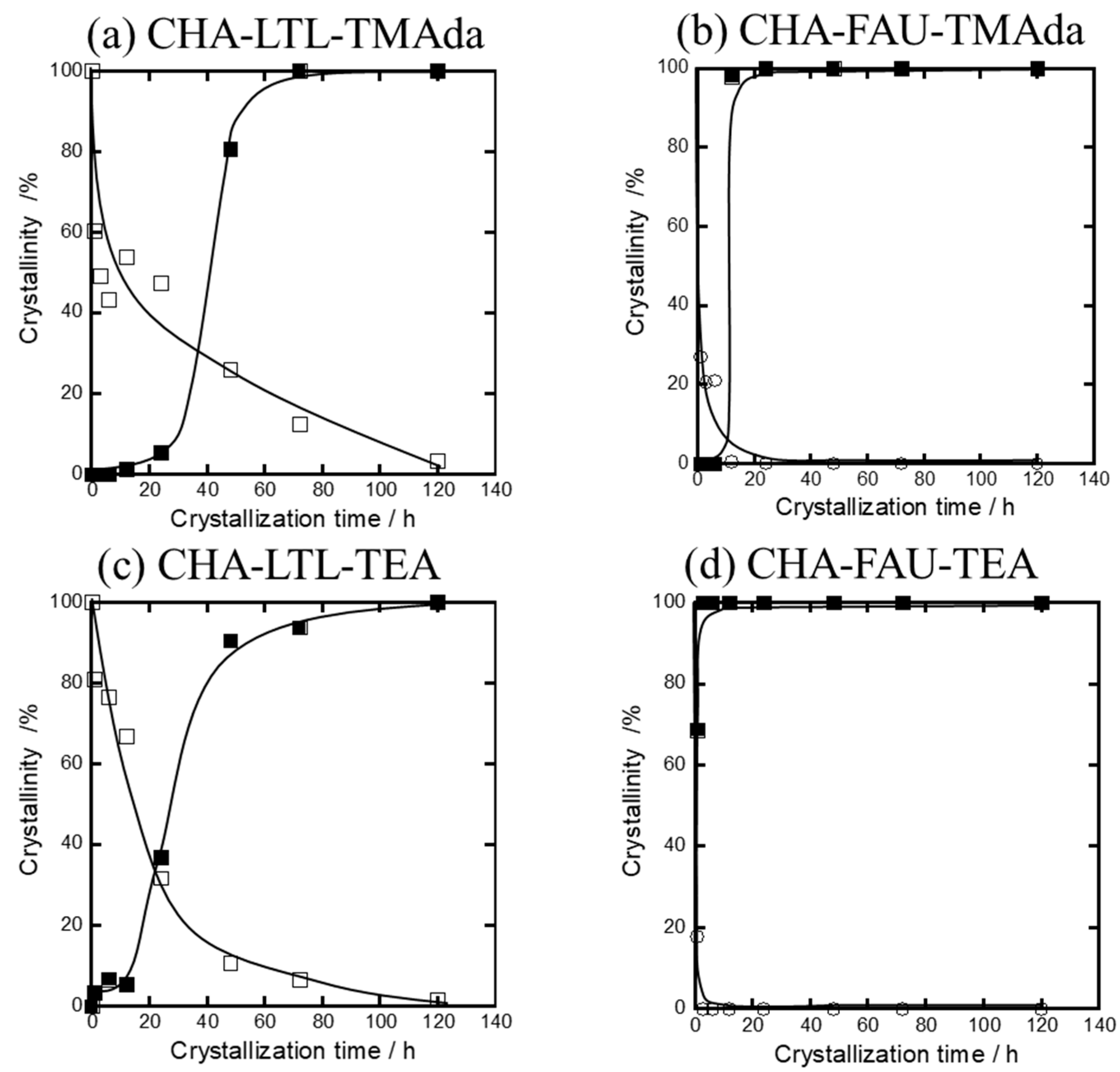
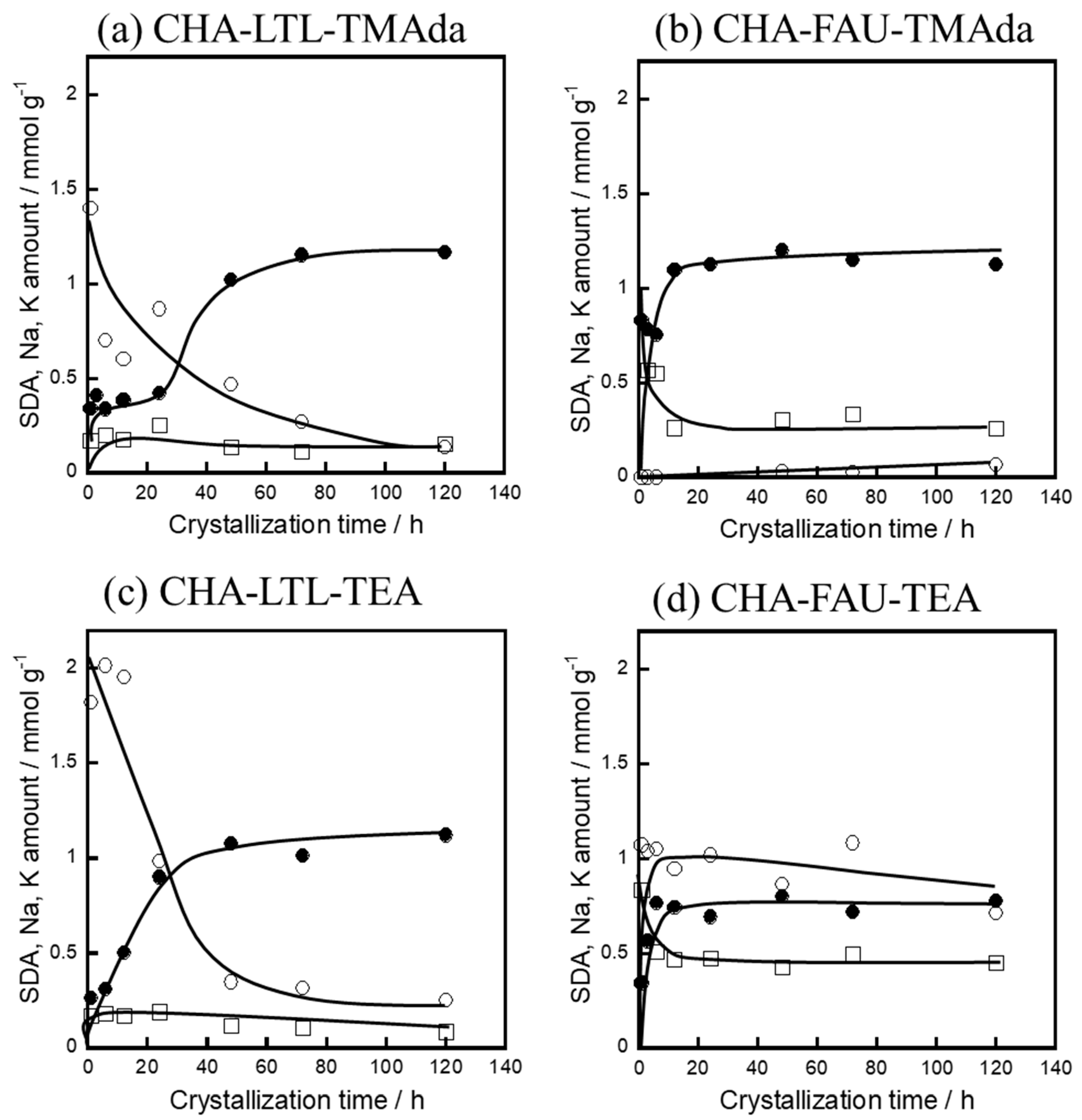
2.3. Crystallization Behavior of CHA via the IZC Method Using FAU and LTL
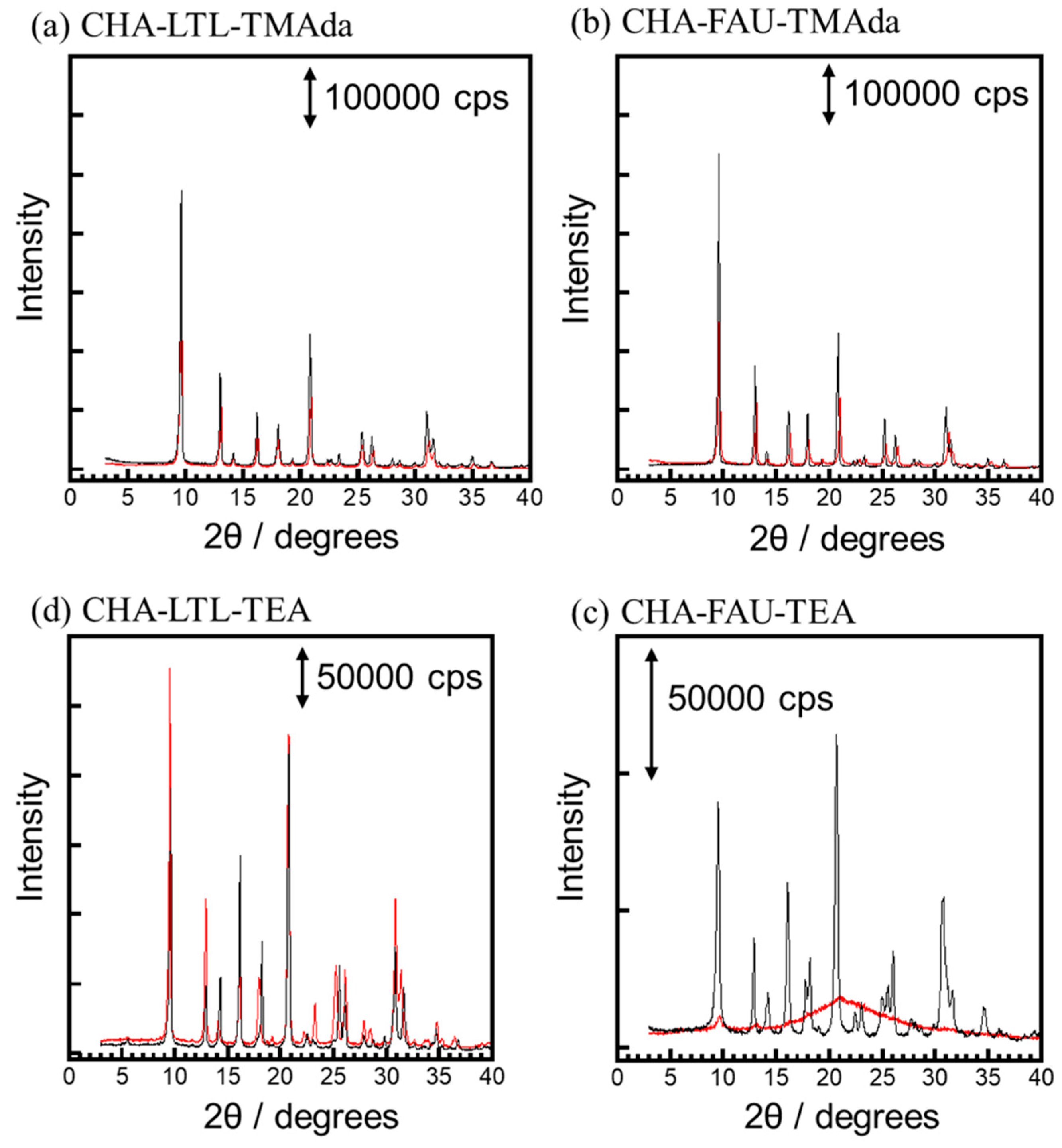
2.3.1. Crystallinity
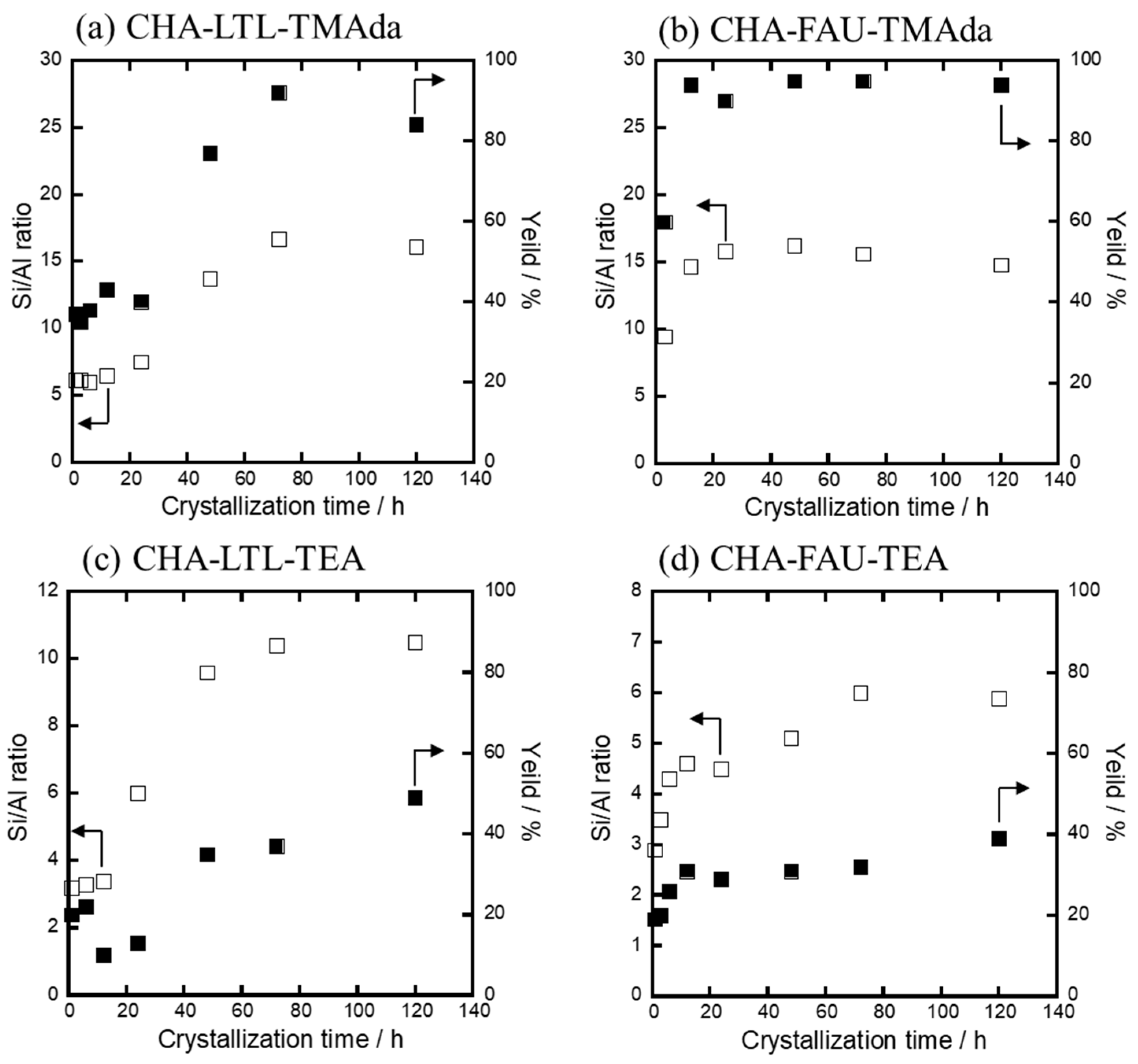
3.3.2. Solid Yield and Al Content
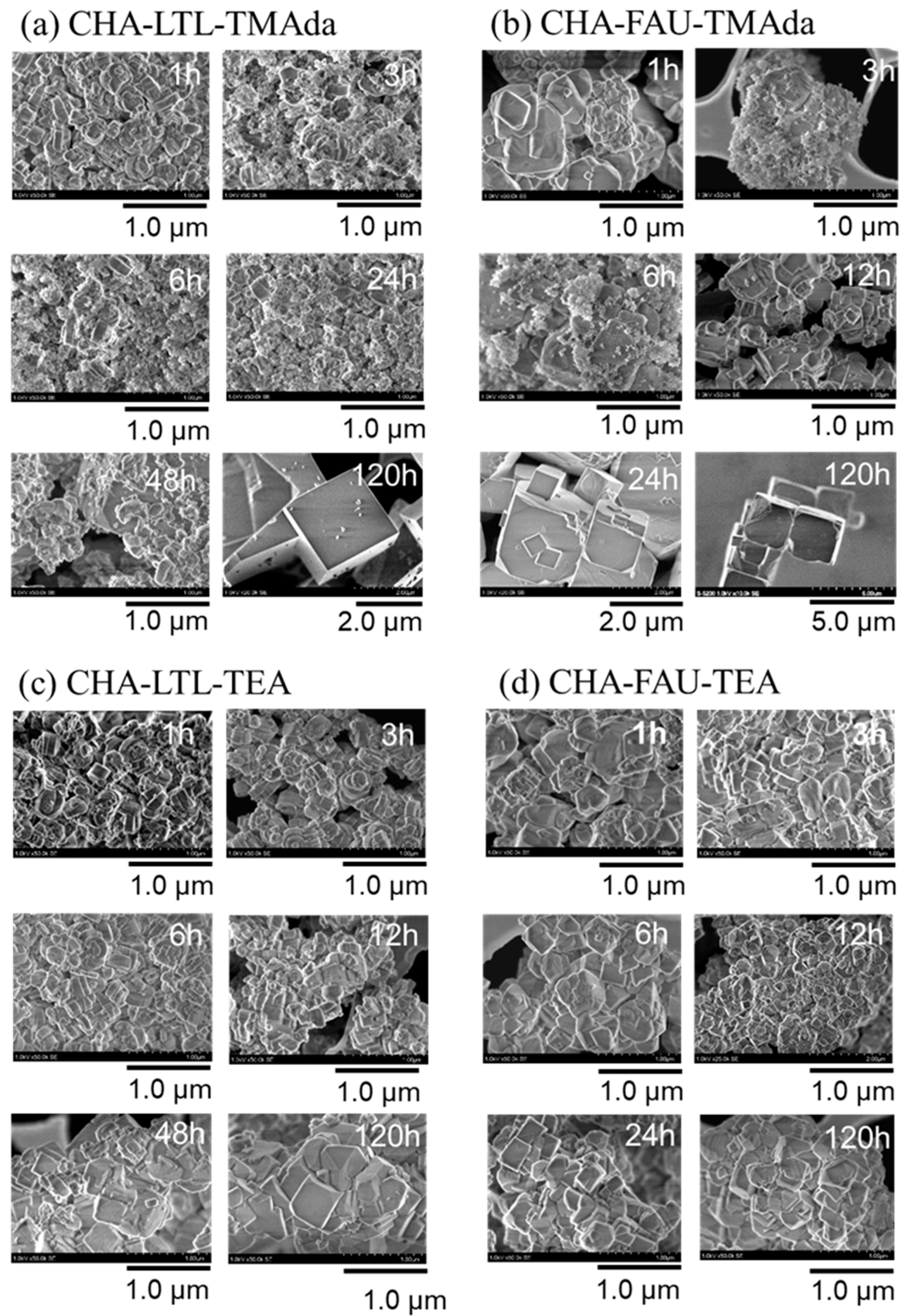
2.3.3. Particle Morphology
2.4. Hydrothermal Stability
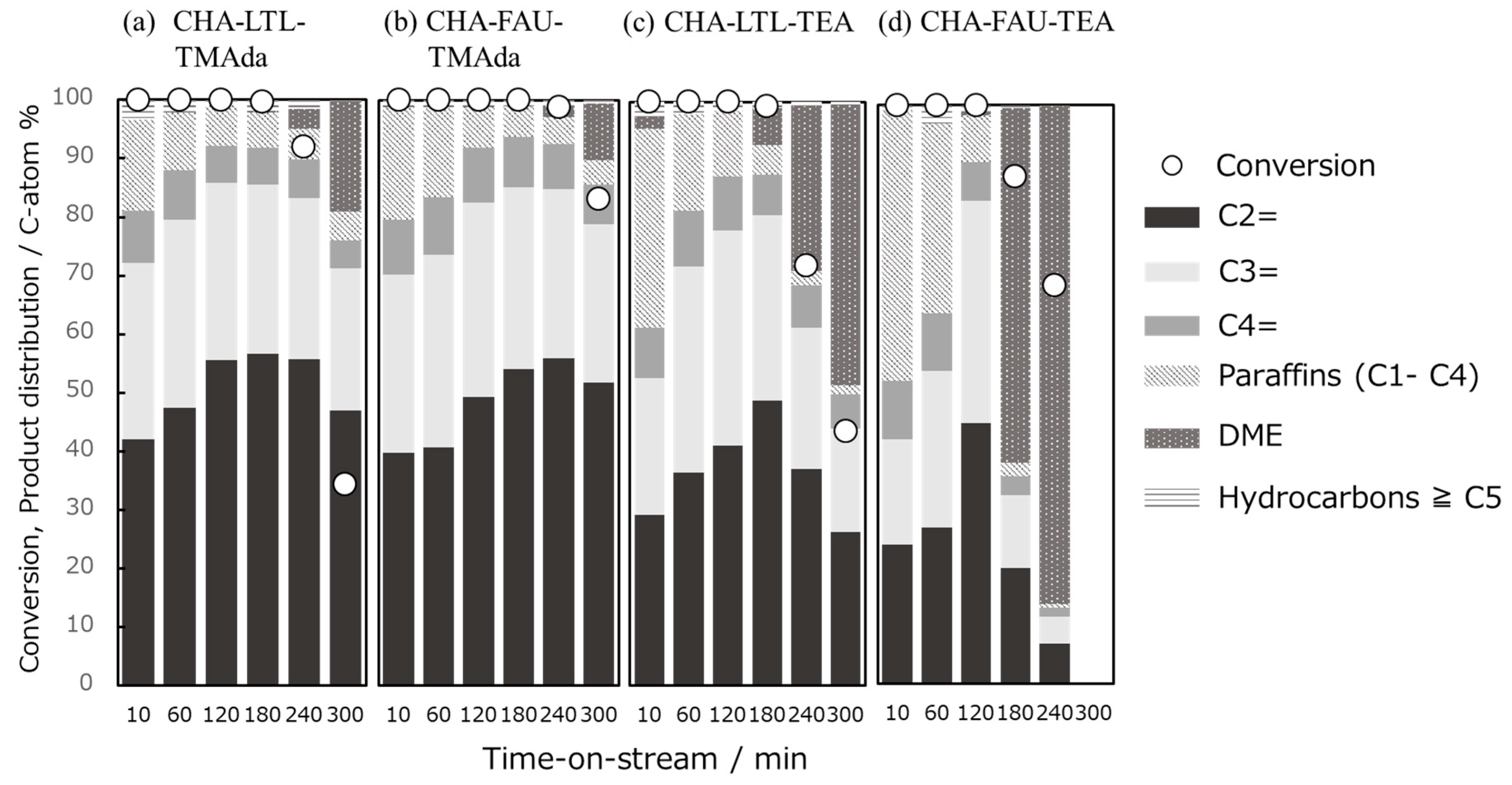
2.5. MTO Reaction
3. Materials and Methods
3.1. Synthesis of CHA-Type Zeolite from FAU- and LTL-Type Zeolites
3.2. Characterization
- LTL = 5.5°,11.7°,14.6°,19.2°,22.6°,24.2°,25.5°,28.0°,29.0° and 30.5°
- FAU = 6.0°,10.1°,11.8°,15.6°,18.7°,20.4°,23.7°,27.1° and 31.4°
- CHA = 9.8°,16.1°,18.2°,21.0°,25.3°,26.4° and 31.1°
3.3. Hydrothermal Stability
3.4. MTO Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Strohmaier, K.G.; Reyes, S.C.; Levin, D. Preparation of High-Silica Chabazite Zeolites and Their Use in the Conversion of Oxygenates into Olefins. U.S. Patent 7,435,863, 12 December 2003. [Google Scholar]
- Bhawe, Y.; Moliner-Marin, M.; Lunn, J.D.; Liu, Y.; Malek, A.; Davis, M. Effect of Cage Size on the Selective Conversion of Methanol to Light Olefins. ACS Catal. 2012, 2, 2490–2495. [Google Scholar] [CrossRef]
- Andersen, P.J.; Bailie, J.E.; Casci, J.L.; Chen, H.Y.; Fedeyko, J.M.; Foo, R.K.S.; Rajaram, R.R. Transition Metal/Zeolite SCR [Selective Catalytic Reduction] Catalysts. U.S. Patent 20,100,290,963 A1, 18 November 2010. [Google Scholar]
- Bull, I.; Boorse, R.S.; Jaglowski, W.M.; Koermer, G.S.; Moini, A.; Patchett, J.A.; Xue, W.; Burk, P.; Dettling, J.C.; Caudle, M.T. Copper CHA Zeolite Catalysts. WO 2,008,106,519 A1, 27 February 2008. [Google Scholar]
- Zhao, R.; Zhao, Z.; Li, S.; Zhang, W. Insights into the Correlation of Aluminum Distribution and Bronsted Acidity in H-Beta Zeolites from Solid-State NMR Spectroscopy and DFT Calculations. J. Phys. Chem. Lett. 2017, 8, 2323–2327. [Google Scholar] [CrossRef] [PubMed]
- Bourgogne, M.; Guth, J.L.; Wey, R. Process for the Preparation of Synthetic Zeolite, and Zeolites Obtained by Saeed Process. U.S. Patent 4,503,024, 5 March 1985. [Google Scholar]
- Zones, S.I. Zeolite SSZ-13 and Its Method of Preparation. U.S. Patent 4,544,538, 1 October 1985. [Google Scholar]
- Eilertsen, E.A.; Arstad, B.; Svelle, S.; Lillerud, K.P. Single parameter synthesis of high silica CHA zeolites from fluoride media. Microporous Mesoporous Mater. 2012, 153, 94–99. [Google Scholar] [CrossRef]
- Vattipalli, V.; Paracha, A.M.; Hu, W.; Chen, H.; Fan, W. Broadening the Scope for Fluoride-Free Synthesis of Siliceous Zeolites. Angew. Chem. Int. Ed. 2018, 57, 3607–3611. [Google Scholar] [CrossRef] [PubMed]
- Santilli, D.S.; Zones, S.I. Selective Conversion of Methanol to Low Molecular Weight Olefins over High Silica SSZ-13 Zeolite. U.S. Patent 4,496,786, 29 January 1985. [Google Scholar]
- Eilertsen, E.A.; Nilsen, M.H.; Wendelbo, R.; Olsbye, U.; Lillerud, K.P. Synthesis of high silica CHA zeolites with controlled Si/Al ratio. Stud. Suf. Sci. Catal. 2008, 174, 265–268. [Google Scholar]
- Deimund, M.A.; Harrison, L.; Lunn, J.D.; Liu, Y.; Malek, A.; Shayib, R.; Davis, M.E. Effect of Heteroatom Concentration in SSZ-13 on the Methanol-to Olefins Reaction. ACS Catal. 2016, 6, 542–550. [Google Scholar] [CrossRef]
- Zhu, Q.; Kondo, J.N.; Ohnuma, R.; Kubota, Y.; Yamaguchi, M.; Tatsumi, T. The study of methanol-to-olefin over proton type aluminosilicate CHA zeolite. Microporous Mesoporous Mater. 2008, 112, 153–161. [Google Scholar] [CrossRef]
- Kubota, Y.; Inagaki, S.; Fukuoka, T. Production of High Si/Al Ratio CHA-Type Zeolite. JP2016169118A, 23 September 2016. [Google Scholar]
- Bohstrom, Z.; Arstad, B.; Lillerud, K.P. Preparation of high silica chabazite with controllable particle size. Microporous Mesoporous Mater. 2014, 195, 294–302. [Google Scholar] [CrossRef]
- Zones, S.I. Conversion of Faujasites to High-silica Chabazite SSZ-13 in the Presence of N,N,N-Trimethyl-l -adamantammonium Iodide. J. Chem. Soc. Fraday Trans. 1991, 87, 3709–3716. [Google Scholar] [CrossRef]
- Zones, S.I.; Van Nordstrand, R.A. Novel zeolite transformations: The template-mediated conversion of Cubic P zeolite to SSZ-13. ZEOLITES 1988, 8, 166–174. [Google Scholar] [CrossRef]
- Zones, S.I. Preparation of molecular sieves using a structure directing agent and an N,N,N-triakyl benzyl quaternary ammonium cation. U.S. Patent 20,080,075,656, 25 September 2008. [Google Scholar]
- Itakura, M.; Inoue, T.; Takahashi, A.; Fujitani, T.; Oumi, Y.; Sano, T. Synthesis of High-silica CHA Zeolite from FAU Zeolite in the Presence of Benzyltrimethylammonium Hydroxide. Chem. Lett. 2008, 37, 908–909. [Google Scholar] [CrossRef]
- Itaura, M.; Goto, I.; Takahashi, A.; Fujitani, T.; Ida, Y.; Sadakane, M.; Sano, T. Synthesis of high silica CHA-type zeolite by inter zeolite conversion of FAU-type zeolite in the presence of seed crystals. Microporous Mesoporous Mater. 2011, 144, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, N.; Itakura, M.; Kiyozumi, Y.; Ide, Y.; Sadakane, M.; Sano, T. Acid stability evaluation of CHA-type zeolites synthesized by interzeolite conversion of FAU-type zeolite and their membrane application for dehydration of acetic acid aqueous solition. Microporous Mesoporous Mater. 2012, 158, 141–147. [Google Scholar] [CrossRef]
- Martin, N.; Moliner, M.; Corma, A. High yield synthesis of high-silica chabazite by combining the role of zeolite precursors and tetraethylammonium: SCR of NOx. Chem. Commun. 2015, 51, 9965–9968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhadraa, B.N.; Seoa, P.W.; Khana, N.A.; Junb, J.W.; Kimb, T.W.; Kimb, C.U.; Jhunga, S.H. Conversion of Y into SSZ-13 zeolite in the presence of tetraethylammonium hydroxide and ethylene-to-propylene reactions over SSZ-13 zeolites. Catal. Today 2017, 298, 53–60. [Google Scholar] [CrossRef]
- Takata, T.; Tsunoji, N.; Takamitsu, Y.; Sadakane, M.; Sano, T. Incorporation of various heterometal atoms in CHA zeolites by hydrothermal conversion of FAU zeolite and their performance for selective catalytic reduction of NOx with ammonia. Microporous Mesoporous Mater. 2017, 246, 89–101. [Google Scholar] [CrossRef]
- Takata, T.; Tsunoji, N.; Takamitsu, Y.; Sadakane, M.; Sano, T. Nanosized CHA zeolites with high thermal and hydrothermal stability derived from the hydrothermal conversion of FAU zeolite. Microporous Mesoporous Mater. 2016, 225, 524–533. [Google Scholar] [CrossRef]
- Xiong, X.; Yuan, D.; Wu, Q.; Chen, F.; Meng, X.; Lv, R.; Dai, D.; Maurer, S.; McGuire, R.; Feyen, M.; et al. Efficient and rapid transformation of high silica CHA zeolite from FAU zeolite in the absence of water. J. Mater. Chem. A 2017, 5, 9076–9080. [Google Scholar] [CrossRef]
- Suji, K.; Wagner, P.; Davis, M.E. High-silica molecular sieve syntheses using the sparteine related compounds as structure-directing agents. Microporous Mesoporous Mater. 1999, 28, 461–469. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Lan, A.; Bian, H.; Liu, R.; Li, X.; Han, P.; Dou, T. Synthesis of SSZ-13 zeolite with zeolite L-added synthesis gel absent from additional aluminum source. Microporous Mesoporous Mater. 2019, 279, 1–9. [Google Scholar] [CrossRef]
- Tang, L.; Haw, K.G.; Zhang, Y.; Fang, Q.; Qiu, S.; Valtchev, V. Fast and efficient synthesis of SSZ-13 by interzeolite conversion of Zeolite Beta and Zeolite L. Microporous Mesoporous Mater. 2019, 280, 306–314. [Google Scholar] [CrossRef]
- Geng, H.; Li, G.; Liu, D.; Liu, C. Rapid and efficient synthesis of CHA-type zeolite by interzeolite conversion of LTA-type zeolite in the presence of N, N, N-trimethyladamantammonium hydroxide. J. Solid State Chem. 2018, 265, 193–199. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Wagner, P.; Lovallo, M.; Tsuji, K.; Takewaki, T.; Chen, C.-Y.; Beck, L.W.; Jones, C.; Tsapatsis, M.; Zons, S.I.; et al. Synthesis, Characterization, and Structure Solution of CIT-5, a New, High-Silica, Extra-Large-Pore Molecular Sieve. J. Phys. Chem. B 1998, 102, 7139–7142. [Google Scholar] [CrossRef]
- Muraoka, K.; Chaikittisilp, W.; Tatsuya Okubo, T. Energy Analysis of Aluminosilicate Zeolites with Comprehensive Ranges of Framework Topologies, Chemical Compositions, and Aluminum Distributions. J. Am. Chem. Soc. 2016, 138, 6184–6193. [Google Scholar] [CrossRef]
- Honda, K.; Yashiki, A.; Itakura, M.; Ide, Y.; Sadakane, M.; Sano, T. Influence of seeding on FAU-*BEA interzeolite conversions. Microporous Mesoporous Mater. 2011, 142, 161–167. [Google Scholar] [CrossRef]
- Goel, S.; Zones, S.I.; Iglesia, E. Encapsulation of Metal Clusters within MFI via Interzeolite Transformations and Direct Hydrothermal Syntheses and Catalytic Consequences of Their Confinement. J. Am. Chem. Soc. 2014, 136, 15280–15290. [Google Scholar] [CrossRef]
- Li, C.; Moliner, M.; Corma, A. Building Zeolites from Precrystallized Units: Nanoscale Architecture. Angew. Chem. Inter. Ed. 2018, 57, 15330–15353. [Google Scholar] [CrossRef]
- Goel, S.; Zones, S.I.; Iglesia, E. Synthesis of Zeolites via Interzeolite Transformations without Organic Structure-Directing Agents. Chem. Mater. 2015, 27, 2056–2066. [Google Scholar] [CrossRef]
- Inoue, T.; Itakura, M.; Jon, H.; Oumi, Y.; Takahashi, A.; Fujitani, T.; Sano, T. Synthesis of LEV zeolite by interzeolite conversion method and its catalytic performance in ethanol to olefins reaction. Microporous Mesoporous Mater. 2009, 122, 149–154. [Google Scholar] [CrossRef]
- Joichi, Y.; Shimono, D.; Tsunoji, N.; Takamitsu, Y.; Sadakane, M.; Sano, T. Stepwise Gel Preparation for High-Quality CHA Zeolite Synthesis: A Common Tool for Synthesis Diversification. Cryst. Growth Des. 2018, 18, 5652–5662. [Google Scholar] [CrossRef]
- Nakazawa, N.; Inagaki, S.; Kubota, Y. Novel Technique to Synthesize AFX-Type Zeolite Uisng a Bulky and Rigid Diquaternary Ammonium Cation. Adv. Porous Mater. 2016, 4, 219–229. [Google Scholar] [CrossRef]
- Peng, C.; Liu, Z.; Horimoto, A.; Anand, C.; Yamada, H.; Ohara, K.; Sukenaga, S.; Ando, M.; Shibata, H.; Takewaki, T.; et al. Preparation of nanosized SSZ-13 zeolite with enhanced hydrothermal stability by a two-stage synthetic method. Microporous Mesoporous Mater. 2018, 255, 192–199. [Google Scholar] [CrossRef]
- Dusselier, M.; Davis, M.E. Small-Pore Zeolites: Synthesis and Catalysis. Chem. Rev. 2018, 118, 5265–5329. [Google Scholar] [CrossRef]
- Nishitoba, T.; Yoshida, N.; Kondo, J.N.; Yokoi, T. Control of Al Distribution in the CHA-Type Aluminosilicate Zeolites and Its Impact on the Hydrothermal Stability and Catalytic Properties. Ind. Eng. Chem. Res. 2018, 57, 3914–3922. [Google Scholar] [CrossRef]
- Lippmaa, E.; Satnoson, M.M.A.; Engelhardt, G.; Grimmer, A.R. Structural Studies of Silicates by Solid-state High-Resolution 29Si NMR. J. Am. Chem. Soc. 1980, 102, 4889–4893. [Google Scholar] [CrossRef]
- Iwama, M.; Suzuki, Y.; Plévert, J.; Itabashi, K.; Ogura, M.; Okubo, T. Location of Alkali Ions and their Relevance to Crystallization of Low Silica X Zeolite. Cryst. Growth Des. 2010, 10, 3471–3479. [Google Scholar] [CrossRef]
- Mousavi, S.F.; Jafari, M.; Kazemimoghadam, M.; Mohammadi, T. Template free crystallization of zeolite Rho via Hydrothermal synthesis: Effects of synthesis time, synthesis temperature, water content and alkalinity. Ceram. Int. 2013, 39, 7149–7158. [Google Scholar] [CrossRef]
- Bronic, J.; Palcic, A.; Subotic, B.; Itani, L.; Valtchev, V. Influence of alkalinity of the starting system on size and morphology of the zeolite A crystals. Mater. Chem. Phys. 2012, 132, 973–976. [Google Scholar] [CrossRef]
- Ong, L.H.; Domok, M.; Olindo, R.; van Veen, A.C.; Lercher, J.A. Dealumination of HZSM-5 via steam-treatment. Microporous Mesoporous Mater. 2012, 164, 9–20. [Google Scholar] [CrossRef]
- Wang, C.M.; Wang, Y.D.; Xie, Z.K. Insights into the reaction mechanism of methanol-to-olefins conversion in HSAPO-34 from first principles: Are olefins themselves the dominating hydrocarbon pool species? J. Catal. 2013, 301, 8–19. [Google Scholar] [CrossRef]
- Li, C.; Paris, C.; Triguero, J.M.; Boronat , M.; Moliner, M.; Corma, A. Synthesis of reaction-adapted zeolites as methanol-to-olefins catalysts with mimics of reaction intermediates as organic structure directing agents. Nat. Catal. 2018, 1, 547–554. [Google Scholar] [CrossRef]
- Kim, W.; So, J.; Choi, S.-W.; Liu, Y.; Dixit, R.S.; Sievers, C.; Sholl, D.S.; Nair, S.; Jones, C.W. Hierarchical Ga-MFI Catalysts for Propane Dehydrogenation. Chem. Mater. 2017, 29, 7213–7222. [Google Scholar] [CrossRef]
- Xie, Y.; Hua, W.; Yue, Y.; Gao, Z. Dehydrogenation of propane to propylene over Ga2O3 supported on mesoporous HZSM-5 in the presence of CO2. J. Chem. 2010, 28, 1559–1564. [Google Scholar]
- Svelle, S.; Joensen, F.; Nerlov, J.; Olsbye, U.; Lillerud, K.P.; Kolboe, S.; Bjorgen, M. Conversion of Methanol into Hydrocarbons over Zeolite H-ZSM-5: Ethene Formation Is Mechanistically Separated from the Formation of Higher Alkenes. J. Am. Chem. Soc. 2006, 128, 14770–14771. [Google Scholar] [CrossRef]
- Song, W.; Fu, H.; Haw, J.F. Supramolecular Origins of Product Selectivity for Methanol-to-Olefin Catalysis on HSAPO-34. J. Am. Chem. Soc. 2001, 123, 4749–4754. [Google Scholar] [CrossRef]
| Sample | in Gel | Yield/% | Si/Al | Na+/Al | K+/Al | (Na+ + K+)/Al | SDA/Al | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Si/Al | Na/Si | K/Si | SDA/Si | |||||||
| CHA–LTL–TMAda | 15 | 0.3 | 0.0 | 0.2 | 84 | 16.3 | 0.07 | 0.06 | 0.07 | 1.02 |
| CHA–FAU–TMAda | 15 | 0.3 | 0.0 | 0.2 | 94 | 14.6 | 0.12 | - | 0.12 | 1.10 |
| CHA–LTL–TEA | 15 | 0.3 | 0.1 | 0.55 | 49 | 10.5 | 0.05 | 0.16 | 0.21 | 0.78 |
| CHA–FAU–TEA | 15 | 0.3 | 0.1 | 0.55 | 40 | 6.3 | 0.36 | 0.18 | 0.54 | 0.23 |
| Sample | Si/Al (ICP) | Si/Al a (NMR) | Proportion of Q4(nAl) b and Q3(nAl) c/% | Q4(2Al)/Q4(1Al) | ||||
|---|---|---|---|---|---|---|---|---|
| Q4(3Al) | Q4(2Al) | Q4(1Al) | Q4(0Al) | Q3(0Al) | ||||
| CHA–LTL–TMAda | 16.3 | 14.7 | <0.1 | 2.1 | 22.9 | 67.0 | 7.9 | 0.091 |
| CHA–FAU–TMAda | 14.6 | 19.6 | <0.1 | 1.5 | 16.4 | 73.1 | 9 | 0.122 |
| CHA–LTL–TEA | 10.5 | 12.9 | <0.1 | 1.3 | 28.4 | 69.1 | 1.2 | 0.046 |
| CHA–FAU–TEA | 6.3 | 6.2 | <0.1 | 9.8 | 44.4 | 44.6 | 0.11 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishitoba, T.; Nozaki, T.; Park, S.; Wang, Y.; Kondo, J.N.; Gies, H.; Yokoi, T. CHA-Type Zeolite Prepared by Interzeolite Conversion Method Using FAU and LTL-Type Zeolite: Effect of the Raw Materials on the Crystallization Mechanism, and Physicochemical and Catalytic Properties. Catalysts 2020, 10, 1204. https://doi.org/10.3390/catal10101204
Nishitoba T, Nozaki T, Park S, Wang Y, Kondo JN, Gies H, Yokoi T. CHA-Type Zeolite Prepared by Interzeolite Conversion Method Using FAU and LTL-Type Zeolite: Effect of the Raw Materials on the Crystallization Mechanism, and Physicochemical and Catalytic Properties. Catalysts. 2020; 10(10):1204. https://doi.org/10.3390/catal10101204
Chicago/Turabian StyleNishitoba, Toshiki, Takuya Nozaki, Sungsik Park, Yong Wang, Junko N. Kondo, Hermann Gies, and Toshiyuki Yokoi. 2020. "CHA-Type Zeolite Prepared by Interzeolite Conversion Method Using FAU and LTL-Type Zeolite: Effect of the Raw Materials on the Crystallization Mechanism, and Physicochemical and Catalytic Properties" Catalysts 10, no. 10: 1204. https://doi.org/10.3390/catal10101204
APA StyleNishitoba, T., Nozaki, T., Park, S., Wang, Y., Kondo, J. N., Gies, H., & Yokoi, T. (2020). CHA-Type Zeolite Prepared by Interzeolite Conversion Method Using FAU and LTL-Type Zeolite: Effect of the Raw Materials on the Crystallization Mechanism, and Physicochemical and Catalytic Properties. Catalysts, 10(10), 1204. https://doi.org/10.3390/catal10101204





