Abstract
Anthocyanins from maqui (Aristotelia chilensis) and blackberry (Rubus glaucus) were used as light harvesters to improve the photocatalytic activity of titanium dioxide in visible light. Anthocyanins from both species were obtained using high-frequency ultrasound-assisted liquid-liquid extraction with methanol. Mixtures of anthocyanins were developed to study their effectiveness in the visible light/TiO2 reaction for the oxidation of aniline blue. For this purpose, stainless-steel foams were covered with TiO2 and anthocyanin and characterized by SEM. Different samples were fabricated by varying the ratio of the two anthocyanins in the mixture (100, 75, 50, 25 and 0 vol% of maqui-anthocyanin (delphinidin)). The mixtures of 25 vol% anthocyanin from maqui and 75 vol% anthocyanin from blackberry had higher total anthocyanin content and better photocatalytic activity in visible light: degradation of aniline blue was 40% at pH 7, 56% at pH 3 and 95% at pH 3 with the injection of oxygen for 2 h in comparison with TiO2-foam/UV light, which yielded values of 13% at pH 7 and 73% at pH 3 with and without the addition of oxygen. Natural dyes that are low-cost and environmentally friendly substances are shown to be capable of improving the visible-light photocatalytic activity of TiO2.
1. Introduction
Wastewater treatment is a major concern to everyone, especially today, given the lack of water in many communities around the world. Different wastewater treatments that are performed today can efficiently eliminate pollutants. However, some compounds are exceedingly difficult to remove, such as toxic organic compounds, which are present in pesticides, dyes and pharmaceutical products, among other materials. Aniline is one such compound, and one of the best techniques for removing this type of substance is an advanced oxidation process, more specifically the heterogeneous photocatalytic process.
Aniline is an organic compound (C6H5NH2) formed by a benzene ring bonded to an amino group. It has a characteristic lipid consistency, with a light-yellow tonality and moderate solubility in water. It can be oxidized in air, where it changes to a red-brown compound. It is volatile with a fatty odor and is a highly toxic substance that can cause cancer in humans and animals, as well as other illnesses, including cyanosis, anemia, loss of appetite, weight loss and damage to the kidneys, liver, bones and nervous system [1]. Aniline is used in a large number of product fabrications, such as polyurethane foam, rubber, paints, dye, pharmaceuticals, pesticides and explosives. Moreover, it is a by-product of petroleum, paper and coal processing fabrication [1], suggesting that it is abundant in water everywhere. Households are another source of aniline waste; for example, clothes with dye are recycled by residents without knowledge of its toxicity, and it is then discharged into sewage. Aniline, a blue dye used in textile industries, is a triphenylmethane compound, with a molecular orientation in which a carbon atom at the center of the molecule is bonded to two benzene rings and one p-quinoid group, which can be -NH2, NR2 or -OH [2].
Heterogeneous advanced oxidation with an oxide photocatalyst has been demonstrated to be a reliable technique for the removal of aniline. As the catalyst material, the most studied and used is titanium oxide, which has to be irradiated with UV light to achieve adequate performance [2,3,4,5]. However, the use of UV light (4% of sunlight) for catalyst activation has prevented this process from becoming a cleaner and low-cost option.
The anatase structure of titanium oxide has been demonstrated to have a higher catalytic capacity as a photocatalytic treatment [6,7,8,9,10]. These particles interact with UV photons that have the necessary energy (3.2 eV) to excite electrons from the valence band to the conduction band through the bandgap, generating two carriers. The electron and the hole can migrate to the surface of the particle and react with water molecules, oxidative substances (e.g., H2O2) or oxygen dissolved around the particles, producing radical species that oxidize toxic organic pollutants. This material can be used in slurry form and supported form [5].
The use of this technique for aniline degradation has been successfully performed, and the effect of parameters such as pH, concentration (both catalyst and toxic compound) and temperature, among others, were analyzed in the process. Other catalysts, such as ZnO, ZnS and SnO2, have also been studied using UV light irradiation, and the best result was with ZnO in acid conditions (pH 4) [2]. In other studies, with TiO2 coated on porous nickel foam irradiated by UV light, pH did not affect the process. However, the addition of oxygen and hydrogen peroxides to the polluted water had a considerable impact on the system due to an increase in radical molecules such as OH*, which improved the oxidation of the toxic substance; however, there was no clear conclusion about the best conditions for aniline degradation [3]. Another photocatalysis study used a rotating drum reactor equipped with glass cylinders coated with TiO2 and two irradiation sources: a UV lamp inside the reactor at the center and a solar light outside of it. The combination of UV/solar/H2O2/TiO2 achieved up to 85% mineralization of aniline in 120 min [5].
In all of these processes, the photocatalyst interacts with UV light, the main driving force of the generation of electron-hole carriers on semiconductor materials [1]. In order to overcome this barrier, several studies were performed to improve the absorption of visible light by the catalyst using doping methods with metals, metal oxides, N or C, or through surface functionalization with light-absorbing molecules that can act as electron donors [9,10,11,12,13]. The latter approach is primarily used in photovoltaic applications. Recently, a research group reported the first study on the use of light-harvesting molecules such as dyes or pigments to improve the visible-light activity of titanium dioxide by using a synthetic dye, in this case, methyl red, to oxidize diclofenac [14].
Catalyst surfaces functionalized with natural dyes as light-absorbing molecules have been mostly studied for their use in dye-sensitized solar cells (DSSCs). In these systems, the dye functions as a visible light harvester by reducing the bandgap with its molecular orbital energy levels (LUMO and HOMO), in which electrons can be excited with visible light photons and transported to TiO2 at higher energy and velocity to produce the e- and h+ carriers necessary for the photocatalyst process. One group of dyes studied in these DSSC studies was anthocyanins [15]. These can be obtained from forest fruits such as blackberry and maqui, vegetables such as eggplants and legumes such as black soybeans [16,17], among others. Anthocyanins from maqui (Aristotelia chilensis) used in DSSCs were found to be better light harvesters for this technology due to the presence of a broad visible light absorption range in the electromagnetic spectrum. Maqui absorbs light in two wavelength ranges, from 270 nm to 290 nm and 465 nm to 560 nm, which are the UV and visible ranges, respectively [15].
Anthocyanins are a group of phenolic compounds that are soluble in water. The colors of these molecules include purple, red and blue, and they are responsible for the colors of the fruit, vegetables and cereals in which they reside. Most of them have glucoside groups formed by polyhydroxy and polymethyl derivatives of 2-phenyl benzo pyrylium or flavylium salts with a sugar attached to the molecule. These flavonoids have mainly been studied as potential replacements of synthetic colorants in food, cosmetic and pharmaceutical products, as well as in the above-mentioned DSSCs, due to their non-toxic nature and low-cost fabrication process. Their use in DSSCs has shown a possible path to study the ability of these molecules to improve the photocatalytic activity of titanium dioxide using visible light. Maqui (Aristotelia chilensis) and blackberry (Rubus glaucus), as primary sources of anthocyanin pigments, have been combined in other DSSC studies, which demonstrated that a combination of the anthocyanins from both fruits resulted in better improvement in the efficiency of the solar cell, depending on the concentration of the pigment in the mixture. They also showed that this mixture presented a good ability to bind to TiO2 [18].
The aim of this research is to study the use of a mixture of anthocyanins from maqui and blackberry to improve the visible-light photocatalytic activity of TiO2 supported on stainless-steel foam with 93% porosity (which can provide more active sites than a thin film on a flat substrate). This system was used for the advanced oxidation of aniline blue. Different foam materials, including titanium and nickel, have been previously studied; for example, a titanium oxide support and stainless-steel mesh have been used. Stainless-steel foam was selected for this work because of its low cost and mechanical properties. It is the most commonly used material in the construction of wastewater plants, and it can provide mechanical and temperature stability to the TiO2 thin film. Anthocyanins were obtained by ultrasonic-assisted extraction of the juice, which was extracted from the pulp by maceration. The pH of the toxic solution, the concentration of aniline and the injection of oxygen were the studied parameters.
2. Results
2.1. Anthocyanin Extraction
Anthocyanins were obtained by ultrasound-assisted liquid-liquid extraction from the juices of both forest fruits. The ultrasound-assisted extraction technique is frequently employed to extract bioactive compounds from various food matrices [17], and the method is capable of significantly reducing the duration of the process by acting directly on the cells of food matrices through nanometer-sized cavitation bubbles created in the solvent, which penetrate the cells and accelerate the diffusion process. The total anthocyanin content obtained for each maqui-blackberry juice mixture and each fruit alone is in Table 1, in which the results are expressed as milligrams of cyanidin-3-glucoside equivalent per 100 g of fruit.

Table 1.
Total anthocyanin content (TA) of maqui-blackberry mixes.
The total anthocyanin (TA) increased with the increase in blackberry content in the mixture, as the anthocyanin content in blackberry is significant and only of the cyanidin-3-O-glucoside type.
2.2. Stainless-Steel Foam Coated with TiO2
Once the pigments were obtained, the surfaces of the stainless-steel foam were covered with two layers of TiO2 and one layer of the pigment for each type of anthocyanin mixture. Two oxide layers were used to prevent the complete blocking of the foam channels and to allow comparison with other results in the literature, in which low catalyst quantities have demonstrated good performance. At the same time, one pigment layer is expected to be satisfactory to harvest all of the light necessary for high photocatalytic activity. When natural dyes were used to functionalize the TiO2 surface in DSSCs, the process of adsorption was carried out from 30 min to 24 h, and the dye reached adsorption-desorption equilibrium in 2 h. In our case, after 1 min of soaking the TiO2-foam samples in anthocyanin mixtures, it was observed that all parts of the samples were well covered. It was decided to use them without reaching adsorption-desorption equilibrium to prevent the entire surface of the samples from becoming saturated with the pigment and leave room for the adsorption of aniline. Nevertheless, further studies in which the adsorption-desorption equilibrium of the dye is reached need to be performed to observe if the photocatalytic activity can be improved. As can be seen in the SEM image (see Figure 1), the walls of the porous foam were well-coated with titanium dioxide, and at the same time, some agglomerates of TiO2 nanoparticles were trapped in the intricate porous channels.
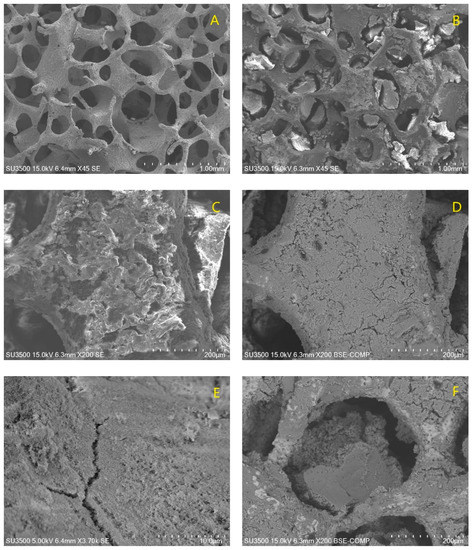
Figure 1.
SEM image of the stainless-steel foam as received (A); SS with TiO2 coating (B); SS with TiO2 (at 200X) on the walls of foam channels, SE (C) and BSE (D); typical crack formed on the TiO2 coating (E) and foam channel with TiO2 clusters formed during the dip-coating (F).
2.3. Stainless-Steel Foam Coated with TiO2-Anthocyanin
The stainless-steel foams were well coated with TiO2 catalyst and anthocyanin, as can be seen in Figure 2. Foams covered only with titanium dioxide had a mass gain of around 0.5996 ± 0.1916 g, while the samples with TiO2-anthocyanin had a mass of around 0.4431 ± 0.0812 g. Some of the TiO2 was lost from the samples during functionalization with the pigments. Nevertheless, the nanoparticles that agglomerated in the channels did not block them at all: a cross-section of the foam sample covered with TiO2-anthocyanin showed that the pigment was able to cover all of the TiO2 on the foam support (see Figure 2).
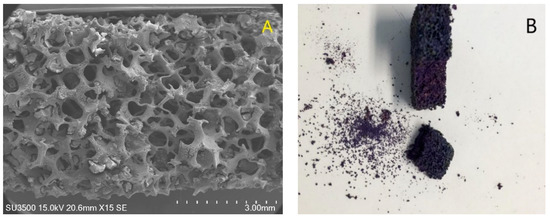
Figure 2.
(A) SEM image of the total size of the coated foam and (B) photograph of stainless-steel foam covered with TiO2-anthocyanin 25 Ma.
2.4. Photocatalytic Degradation of Aniline Blue
Aniline oxidation was performed in UV light for samples with TiO2 and visible light for TiO2-anthocyanin. In this work, 1 mM of aniline blue solution was used for the photocatalytic study at pH 3, pH 7 and pH 3 with the injection of air by a water pump. As can be seen in Figure 3, at pH 7 (the standard condition of aniline solution), TiO2-foam in UV light removed around 12% of the color in 120 min. In contrast, the best results of the TiO2-anthocyanin-foam samples in visible light were obtained with the 25 Ma sample; thus, 25 vol% anthocyanin from maqui (delphinidin) mixed with 75 vol% anthocyanin (cyanidin) from blackberry improved the photocatalytic activity of titanium dioxide in visible light, removing 46% of aniline blue in 120 min at pH 7.
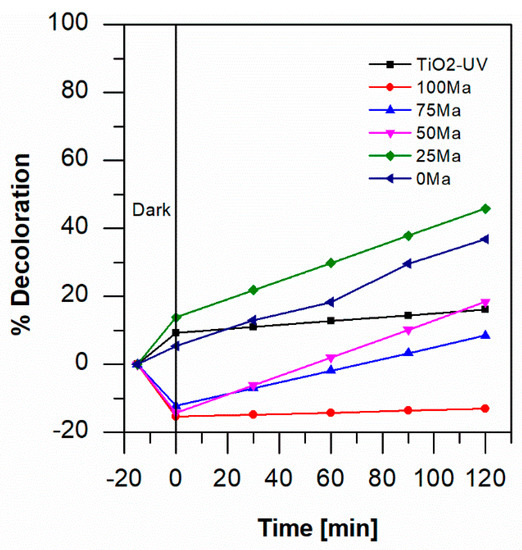
Figure 3.
Percentage of degradation by photocatalysis of 1 mM of aniline blue at pH 7 with TiO2-foam/UV light and with TiO2-anthocyanin-foam/visible light.
For foam/TiO2/anthocyanin systems, at pH 3, the adsorption in the dark was around 28 to 38%, and higher dye adsorption was obtained with blackberry anthocyanin in the mixture (see Figure 4). Anthocyanin is more stable in acidic conditions, so this time, the loss of pigment from the catalysts was avoided. The degradation of aniline blue in visible light was around 51 and 55% in 120 min with 100 Ma and 25 Ma, respectively.
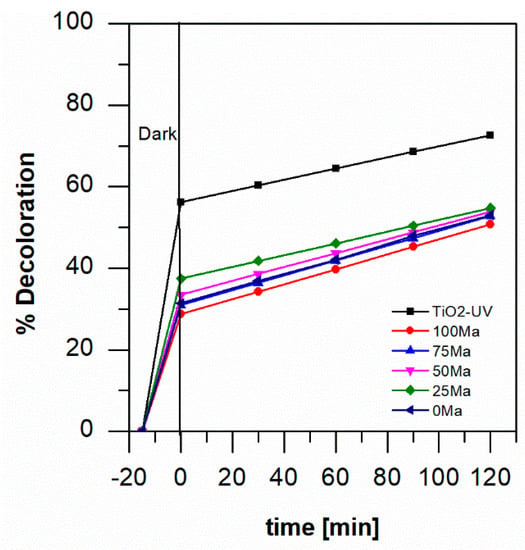
Figure 4.
Percentage of degradation by photocatalysis of 1 mM of aniline blue at pH 3 with TiO2-foam/UV light and with TiO2-anthocyanin-foam/visible light.
In photocatalytic studies, it has been demonstrated that the addition of H2O2 or injection of oxygen can improve the process due to the presence of more OH* and O2*− species, respectively. In this case, the air was injected into the reactor by a pump, and in Figure 5, it is possible to observe the effects of the oxygen addition at pH 3. The air was pumped during the dark and illumination steps. As can be seen in the figure, agitation of the solution generated by bubbles of injected air decreased the adsorption of aniline blue on most of the samples in the dark, except for 75 Ma and 100 Ma. However, after illumination, the degradation was higher for all anthocyanin-functionalized samples in visible light, and at 120 min, the 25 Ma sample achieved the best discoloration result, with the elimination of 95% of the absolute concentration of aniline. For TiO2-foam, the degradation of color was almost the same as that without air at 120 min, i.e., 73%.
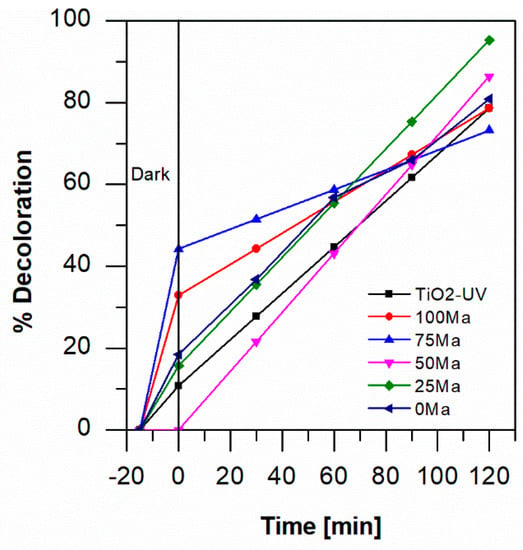
Figure 5.
Percentage of degradation by photocatalysis of 1 mM of aniline blue at pH 3 with the injection of air with TiO2-foam/UV light and with TiO2-anthocyanin-foam/visible light.
By comparing the results of the 25 Ma sample, which produced the greatest color degradation at all parameters examined in the study (see Figure 6), it is possible to observe that the addition of oxygen increased the rate of the reaction and resulted in almost the complete degradation of aniline blue. High total anthocyanin content due to the higher content of the cyanidin-type anthocyanin (from blackberry) with the addition of oxygen in acidic conditions improved the photocatalytic activity of titanium dioxide in visible light.
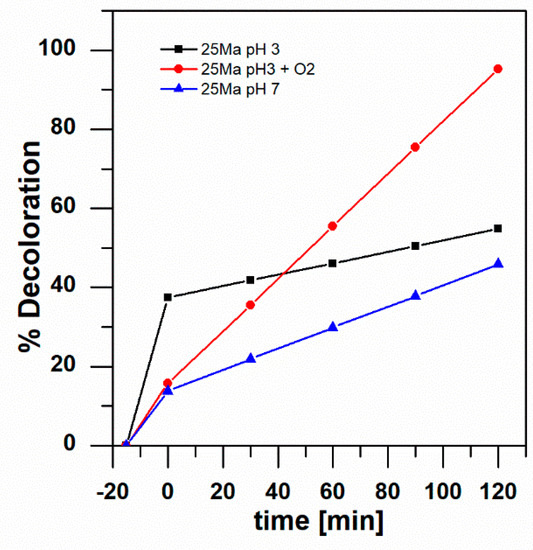
Figure 6.
Percentage of degradation by photocatalysis of 1 mM aniline blue for the 25 Ma sample at pH 7 and pH 3 with and without the injection of air.
The kinetic study of the degradation of aniline blue showed that the reaction follows a pseudo-first-order kinetic behavior (see Figure 7). As in most studies on the photocatalytic degradation of dye with metallic oxides, this system follows the Langmuir-Hinshelwood model, in which ln(C/C0) = −kt, where C0 is the initial concentration of the dye, C is the concentration every 30 min, t is the time, and k is the rate of degradation. At pH 7, the 25 Ma sample produced faster degradation, and at pH 3, the TiO2 foam was faster. At pH 3 with O2, 25 Ma and 50 Ma were the fastest (see Figure 7). In Table 2, it shows the rate values obtained for every type of sample in study.
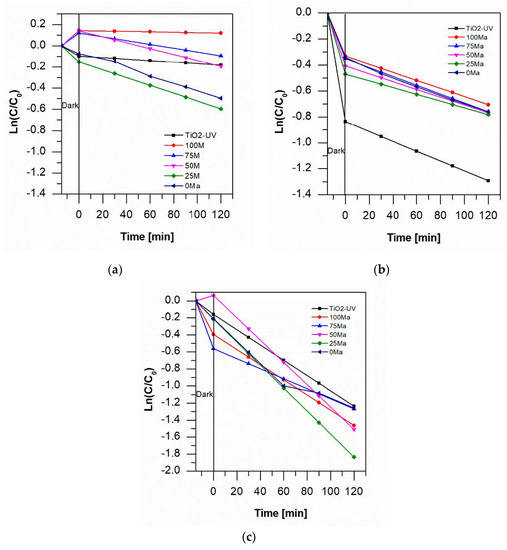
Figure 7.
Kinetic behavior of aniline degradation at (a) pH 7, (b) pH 3 and (c) pH 3 + O2.

Table 2.
Langmuir-Hinshelwood rate values for aniline blue degradation by the photocatalytic process with TiO2-foam/UV light and TiO2-anthocyanin-foam/visible light.
The UV-visible absorption spectra of 1 mM of aniline blue at pH 3 with and without oxygen are shown in Figure 8. Degradation in the color of aniline was observed after dark adsorption, which means that photocatalysis started at t = 0 min. Both pigments, from maqui and blackberry, can provide the necessary energy through electron injection to the semiconductor because of their visible and UV light absorption capacity, providing TiO2 with the ability to improve its photocatalytic activity in visible light.
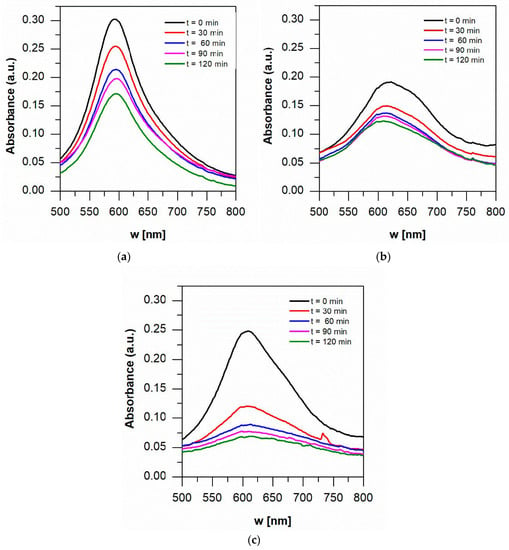
Figure 8.
Visible absorption spectra of 1 mM aniline blue in 25 Ma samples at (a) pH 7, (b) pH 3 and (c) pH 3 + O2. t = 0 is after dark adsorption.
3. Discussion
Maqui (Aristotelia chilensis) is a forest fruit from the south of Chile that mostly grows in the wild. In the last decade, different studies have found that it has functionalities that can be used for food and pharmaceutical purposes due to its high content of anthocyanin [16,17,18,19,20]. They have been predominantly studied in dye-sensitized solar cells (DSSCs), as discussed above. However, this study is the first to use maqui, as well as blackberry fruit, as a light harvester for the photocatalytic oxidation of toxic dye. The total content of anthocyanin, which was determined by the pH differential method, increased with the increase in blackberry content in the mixture, as shown in Table 1. In general, the TA content depends on the type of anthocyanin, and this depends on the source, extraction process and the genotype of the fruit, among other factors [21]. Maqui generally has the delphinidin type of anthocyanin, and blackberry has the cyanidin type structure (See Figure 9).
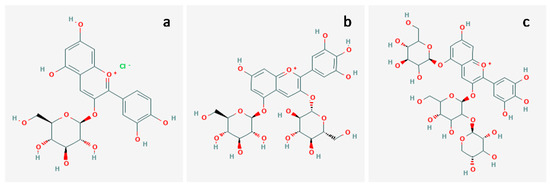
Figure 9.
Anthocyanins: (a) cyanidin-3-O-glucoside from blackberry [PubChem CID441667], (b) delphinidin-3,5-O-glucoside [PubChem CID101100906] and (c) delphinidin-3-sambubioside-5-glucoside from maqui [PubChem CID44256891].
Anthocyanin has the capability of absorbing light due to the positive charge of its molecules, and this is not changed by the pH; thus, when it is on the surface of titanium dioxide foam, it can adsorb aniline blue and, in consequence, oxidize it. The pH affects the bond stability of maqui-derived anthocyanins on the surface of titanium dioxide but not its electrostatic surface charge. The LUMO (lowest unoccupied molecular orbital) and the HOMO (highest occupied molecular orbital) levels of anthocyanins are reported to extend over the whole molecule, and the energy of the LUMO is higher than that of the conduction band of TiO2. In comparison, the HOMO level is lower than the redox potential, generating an improvement in the formation of electron–hole carriers on the catalyst and reducing its recombination [22]. The fraction of incident photons absorbed by the pigments is the reported light-harvesting efficiency, which, for the anthocyanins in the study, cyanidin and delphinidin, was around 0.82 and 0.83, respectively, showing that both can absorb a large quantity of light. The free energy of electron injection from these anthocyanins to the TiO2 substrate was negative, around −1.11 eV and −1.16 eV, respectively, demonstrating that the injection of an electron from the pigments to the catalyst can be spontaneous. All of these characteristics of anthocyanins make them appropriate molecules to improve the photocatalytic activity of TiO2 in visible light, as is demonstrated in this study.
Once the pigments were obtained, stainless-steel coated with TiO2 and anthocyanins was characterized and used for the photocatalytic oxidation of aniline blue. As observed in Figure 1, the excess TiO2 nanoparticles on the surfaces were almost the same for all samples; this did not have a remarkable effect on the photocatalytic activity. Nevertheless, the nanoparticle agglomerations in the channels did not block them at all: a cross-section of the foam sample covered with TiO2-anthocyanin showed that the pigment was able to cover all channels on the foam support (see Figure 2). The adsorption of anthocyanin on TiO2 was reported by Emildo Marcano [22], who proposed two possible mechanisms: physisorption (when the -H atoms of the pigment bond to the oxygen atoms on the TiO2 surface) or chemisorption (when the -H atoms dissociate from the anthocyanin, leaving an oxygen atom to bond to Ti atoms on the surface of the TiO2). It can be seen from the photocatalytic experiments that the pH affected the stability of the bond between the catalyst nanoparticles and anthocyanins, particularly for the maqui anthocyanin, called delphinidin, which may be because it has fewer hydroxyl groups in its structure (See Figure 9), making it, at neutral pH, less stable than cyanidin. A larger quantity of cyanidin (from the blackberry) appeared to be the most stable. It is possible to infer that the maqui anthocyanin was physiosorbed and blackberry anthocyanin was chemisorbed on the TiO2 surface. The combination of these anthocyanins in the 25 Ma sample demonstrated more stability on the surfaces of the catalyst. This could be due to the effect of association of the anthocyanin from maqui and from blackberry produced by the link created between the structures.
The photocatalytic oxidation of aniline started at pH 7, and as presented above, the highest percentage of discoloration was reached with the 25 Ma sample, with a higher content of anthocyanins, resulting in improvement in the visible-light activity of TiO2 for this process. As was described by Leyrer et al. [18], maqui and blackberry have remarkably similar UV and visible absorption spectra, with two peaks in the range of 200–300 nm and one peak in the range of 450–600 nm, and the absorptions of the peaks are relatively equivalent between the two. The visible range absorption for these pigments provides the electrons necessary for the enhancement of TiO2 visible-light activity. Another effect observed is that during the dark step of the process, the foam of these two types of samples adsorbs a large quantity of aniline blue (~9% and 14%, respectively). For the 50 Ma, 75 Ma and 100 Ma foam/TiO2/anthocyanin samples, a different reaction was observed: during the dark step, aniline was not adsorbed on the surface, and instead, the toxic wastewater was more contaminated, with the natural pigment desorbed and the wastewater color enriched. This phenomenon, whereby the intensity of the absorption peak increases in the 400–600 nm range, is commonly observed for anthocyanins and aniline. After that, when the visible light was on, the degradation started and showed the same pattern, demonstrating that higher content of anthocyanin in the form of cyanidin-3-O-glucoside from blackberry was better than that from maqui (delphinidin types). More than 550 different anthocyanins have been reported among all vegetables, fruits and cereals. In particular, for maqui, there are two types of anthocyanin-delphinidin 3,5-diglucoside and delphinidin 3-glucoside—while blackberry has cyanidin-3-glucoside; the difference in the chemical structure is the number of -OH substitutions. Blackberry anthocyanins have fewer -OH substitutions than anthocyanins from maqui. Leyre et al. [18] demonstrated that better absorption occurred between 200 and 300 nm for maqui and between 300 and 700 nm for blackberry; however, the best results for DSSCs were obtained with a mixture of the pigments rather than with each one alone, indicating an additive effect of the two types of anthocyanins. In our case, the mixture improved the TiO2 photocatalytic activity with visible-light absorption when higher anthocyanin content was present, which occurred with the 25 Ma mixture, with a larger quantity of anthocyanin from blackberry.
J. Díaz-Angulo et al. [14] presented the first work using a synthetic azo dye (methyl red) to improve light-harvesting for photocatalysis using low catalyst concentrations, between 0.3 and 0.5 g L−1 TiO2 in slurry form. They added both TiO2 powder and methyl red together to a toxic water solution. The TiO2-methyl red system was able to degrade both diclofenac and the azo dye, making the two molecules compete to react with the oxidized species. In our case, the amount of catalyst deposited on the stainless-steel foam was around 0.44 g, and SS-foam/TiO2/anthocyanin samples with high quantities of anthocyanin from maqui (100, 75 and 50 Ma) transferred the anthocyanin to the wastewater solution, which means that the pigment was desorbed from the catalyst surface. Nevertheless, after 30 min of illumination with visible light, both anthocyanins desorbed in the solution and aniline started to degrade, as in J. Díaz-Angulo et al.’s work, making them compete for the oxidized species and, as a consequence, reducing the efficiency of the process.
On the other hand, the oxidation of aniline at pH 3 (see Figure 4) was much better when using TiO2-foam, with around 73% degradation in UV light, due to the electrostatic interaction that occurs in acidic conditions between the catalyst and aniline blue. The zero-point charge of TiO2 is 6.5, which means that in acidic conditions, its surface is positively charged, and because the dye is an anion, there is a strong interaction between them at the surface of the titanium dioxide layer [2]. During the dark step, the adsorption of the aniline blue was around 8% at pH 7 and 55% at pH 3. This is due to this electrostatic interaction. The degradation of aniline blue in visible light was around 51 and 55% after 120 min following the same behavior of the dark step.
L. Wenhua et al. [3] demonstrated that UV light irradiation can cause slight aniline degradation in 2 h; in these conditions, it is around 3%, which is the same value obtained with TiO2/Ni foam at pH 7. However, Durán et al. showed that complete degradation of aniline with the solar/UV artificial lamp/H2O2/TiO2 system occurred at pH 4 in 10 min, and 85% degradation of aniline could be achieved in the solar/H2O2/TiO2 system in 120 min [5]. For other catalysts, such as ZnO, ZnS and SnO2, the best results for the degradation of aniline were obtained at pH 4 or pH 5, with zinc oxide being the better photocatalyst in aniline degradation [2]. In another study, M. Pirsaheb et al. [1] showed that Cr:ZnO nanoparticles could remove 93% of aniline under sunlight illumination at pH 9 for 6 h. In general, pH is an essential factor in this process, but it depends not only on the catalyst but also on the doped substance used to improve the photocatalytic activity of the catalyst. In this case, the surface of TiO2 maintained an acidic pH because of the functionalization by anthocyanin. In Table 3, a comparison of some of the photocatalytic studies referenced here, along with the results of the present work, shows that natural pigment is promising as a light harvester due to its non-toxicity and low cost.

Table 3.
Aniline oxidation studies on photocatalytic processes compared with the present study.
The photocatalytic reaction rate can be influenced by temperature, pH, the concentration of the catalyst, the oxidant, the formation of radicals (OH* and O2*−) and even the types of dyes to be oxidized. Possible intermediate compounds that can be generated throughout the degradation of dyes such as aniline include phenol, azobenzene, benzoquinone, nitrobenzene and oxalic acid [23]. In this particular case, the rates of all the samples tested are in Table 2, and it can be seen that the fastest process occurred under pH 3/O2 conditions for the 25 Ma and 50 Ma samples, indicating that aerobic photocatalysis was determined by the presence of oxygen, which was transformed into O2*− on the surface of the samples by the electrons transferred from anthocyanin to the titanium oxide surface. This O2*− can form hydroperoxides species with H atoms released from aniline and promote more aniline oxidation, forming an intermediate species such as nitrosobenzene [24]. In contrast, the best sample rates at pH 7 and pH 3 without oxygen were the same. The oxidation of natural pigments at pH 7 decreased the degradation of the contaminant and the rate of degradation. The analysis of the total mineralization of aniline by using the TiO2-anthocyanin-foam system will be studied in a pilot plant. However, in most of the literature, total mineralization of aniline by photocatalysis has generally required more time, from 6 to 10 h, depending on the substance used to dope TiO2, and this is due to the formation of secondary species during the oxidation process.
4. Materials and Methods
4.1. Chemicals and Materials
Anatase titanium dioxide (99.8% pure and particle size of 21 nm, Sigma-Aldrich), stainless-steel foam (AISI316, 93% porosity, 24 pore/cm3, Goodfellow), ethanol (C2H6O, 99%, Winkler), sodium cholate (Mw 430.55 g/mol, ≥99%, Sigma-Aldrich), sodium acetate (CH3COONa.3H2O, Merck), chloride acid (37%, Winkler), acetic acid (C2H4O2, 99.8%, Vimaroni) and methanol (CH3OH, Winkler) were used. Maqui and blackberry pulp were from Temuco, Chile, and regular dye (Blue dye, Montblanc) commonly used in households was used as the aniline source. Deionized water was used for TiO2 dispersion and aniline solutions. A home-made photocatalytic reactor with four lamp holders was used for the degradation experiments; UV lamps (20 W power, 365 nm maximum radiation, 606.09 μW/cm2) were used for SS-foam/TiO2, and a compact integrated fluorescent lamp (27 W power, 1850 lm, 70 lm/W) was used as the visible light source for SS-foam/TiO2-anthocyanin systems.
4.2. Methods
4.2.1. Anthocyanin Ultrasound-Assisted Extraction
For the extraction of anthocyanin, maqui and blackberry fruits were macerated in separate jars, and the solid residues were removed from the liquid extract by filtration with Whatman Nº1 filter paper. Then, the obtained liquid (around 10 mL for every 250 g of fruit) was mixed with methanol to 42 vol%. Ultrasound tip sonication (20 kHz) was performed for 15 min to complete the extraction of each fruit. Once anthocyanins were obtained, three mixtures of the extracts of both fruits were formed (see Table 4). After creating the mixtures, the anthocyanin content was determined by the pH differential method using the forest fruit extract diluted twofold with distilled water and then adjusted to 5 mL using two buffer solutions: one of potassium chloride 0.025 M at pH 1 (adjusted with 0.1% HCl) and the other one with acetic acid 0.4 M at pH 4.5 [19]. These solutions were tested using a UV-vis spectrophotometer (Halo RB-10) at 520 nm and 700 nm, respectively, and the total anthocyanin content of the extract was calculated using the following equations:
ΔAbs = [(A510 − A700)pH = 1 − (A510 − A700)pH = 4.5]
TA [mg/L] = (Abs ∙ PM ∙ FD ∙ 1000)/(ε ∙ 1)

Table 4.
Anthocyanin extract mixtures from maqui berry and blackberry.
The results are expressed as grams of cyanidin-3-glucoside equivalent per milligram per liter of the juice (g cy-3-glu equivalents, mg L−1), using a molar extinction coefficient of 26,900 cm−1 mol−1 and molecular weight of 449 g mol−1.
4.2.2. Stainless-Steel Foam Dip-Coating
A solution was fabricated by dissolving titanium dioxide powder (20 wt%) in ethanol with sodium cholate (1 wt%) as a dispersant and binding agent. The solution was homogenized by stirring for 5 h. After that, stainless-steel foam with a thickness of 0.5 cm was cut into 2 × 2 cm samples, which were cleaned in an ultrasound bath with ethanol-water (1:1) solution for 15 min and then dried in the furnace at 70 °C for 2 h. After weighing the samples, a dip-coating machine was used to coat them, first in titanium dioxide slurry with an immersion speed of 6.5 mm/s, a holding time of 1 min and a withdrawing speed of 1.5 mm/s. The foam samples were coated with two layers of TiO2, and after each coating layer, the samples were dried for 1 h at room temperature, followed by a sintering process at 400 °C for 15 min in a Nabertherm furnace. Finally, one layer of anthocyanin used to coat the TiO2/foam using the dip-coating machine with the same parameters. Three samples of each concentration of anthocyanin from maqui-blackberry were fabricated, including three samples of only TiO2-foam for every parameter in the study. The stainless-steel foam with and without TiO2 was studied by scanning electron microscopy (SEM, Hitachi).
4.2.3. Degradation Method
The photocatalytic degradation of aniline blue was performed in a home-made photo-chamber with the capacity for four lamps mounted 10 cm above the center of the chamber, with walls covered with aluminum paper to increase the light reflection and a computer fan in order to keep the temperature low. For UV photocatalysis, every lamp had 20 W power and 356 nm maximum radiation. The four lamps produced an intensity inside the box of approximately 606.09 μW/cm2. Visible photocatalysis was performed with energy-efficient compact fluorescent lamps (spiral form) with 27 W power, 1850 lm and 70 lm/W. A glass vessel with a jacket was placed in the center of the photo-chamber with 80 mL of aniline blue solution. For every foam sample, the process started with the dark step for 15 min inside the photocatalytic chamber. Then, the light was turned on, and the evolution of the photocatalytic degradation was tracked by collecting 5 mL of the solution every 30 min until reaching 120 min. Every sample was tested using the UV-visible spectrophotometer (Halo RB-10). These study parameters are shown in Table 5. In all the experiments, the aniline concentration was 1 mM, the temperature was maintained at 30 °C, and the degradation was studied for 2 h.

Table 5.
Parameters of the photocatalytic degradation process of aniline blue.
Finally, studies were performed to evaluate the effect of injecting oxygen into the system through an air pump in pH 3 conditions.
5. Conclusions
Anthocyanin mixtures of maqui berry and blackberry improved the photocatalytic activity of TiO2 in visible light. The best results were obtained with the mixture containing 25 vol% maqui (Aristotelia chilensis) and 75 vol% blackberry due to an association effect of the anthocyanin from both fruits and high total anthocyanin content, determined by the ratio between delphinidin and cyanidin anthocyanin types. These pigments were well adsorbed by the catalyst and showed good stability during the photocatalytic degradation of aniline in acidic conditions. This pH effect is vital in order to minimize the desorption of the pigment from the surface of the catalyst and its degradation during the process because this leads to the dye competing with aniline for the oxidizing species. The degradation of aniline blue reached 95% for TiO2-anthocyanin (25 Ma)-stainless-steel foam/visible light/injected oxygen in 120 min, while 73% degradation was obtained with TiO2 without functionalization and with UV light in 120 min. The kinetics of the reaction revealed that the system with higher anthocyanin content and injected oxygen was faster in the removal of aniline. The stainless-steel foam acts as an ideal support for the catalyst, providing a high superficial area for the photocatalytic process. The TiO2 layers (two) were well distributed, and the agglomerated particles inside the channels did not block the porous channels, so pigments and the aniline dye could pass through without hindering the photocatalytic process. Mineralization of aniline blue needs to be determined.
Author Contributions
Conceptualization, D.V.; methodology, D.V., F.P. and S.M.; validation, D.V., F.P. and S.M.; formal analysis, D.V.; investigation, F.P. and S.M.; resources, D.V.; data curation, S.M.; writing—original draft preparation, D.V.; writing—review and editing, D.V.; visualization, D.V.; supervision, D.V.; project administration, D.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any specific grant from external funding agancies.
Acknowledgments
Authors wants to thanks Eng. Rodrigo Ortiz for his comments and valuable guidance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pirsaheb, M.; Shahmoradi, B.; Beikmohammadi, M.; Azizi, E.; Hossini, H.; Ashraf, G.M. Photocatalytic degradation of aniline from aqueous solutions under sunlight illumination using immobilized Cr: ZnO nanoparticles. Sci. Rep. 2017, 7, 1473. [Google Scholar] [CrossRef] [PubMed]
- Egzar, H.K.; Mashkour, M.S.; Juda, A.M. Study the Photodegradation of Aniline Blue dye in aqueous Phase by using Different Photocatalysts. Asian Trans. Basic Appl. Sci. 2013, 3, 23–28. [Google Scholar]
- Wenhua, L.; Hong, L.; Sao’an, C.; Jianqing, Z.; Chunan, C. Kinetics of photocatalytic degradation of aniline in water over TiO2 supported on porous nickel. J. Photochem. Photobiol. A Chem. 2000, 131, 125–132. [Google Scholar] [CrossRef]
- Shahrezaei, F.; Mansouri, Y.; Zinatizadeh, A.A.L.; Akhbari, A. Photocatalytic degradation of aniline using TiO2 nanoparticles in a vertical circulating photocatalytic reactor. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef]
- Durán, A.; Monteagudo, J.M.; Martín, I.S.; Merino, S. Photocatalytic degradation of aniline using an autonomous rotating drum reactor with both solar and UV-C artificial radiation. J. Environ. Manag. 2018, 210, 122–130. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Jorgetto, A.; Milbrat, A.; Schneider, J.F.; Li, Z.; Giammaria, G.; Saeki, M.J.; Gianeti, T.M.R.; Lima, G.P.P.; de Albuquerque Pedrosa, V.; Mul, G.; et al. Magnetically-extractable hybrid of magnetite, mesoporous silica and titania for the photo-degradation of organic compounds in water. Appl. Surf. Sci. 2018, 457, 121–133. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [PubMed]
- Toe, E.D.; Kurniawan, W.; Mariquit, E.G.; Hinode, H. Synthesis of N-doped mesoporous TiO2 by a facile one-step solvothermal process for visible light photocatalytic degradation of organic pollutant. J. Environ. Chem. Eng. 2018, 6, 5125–5134. [Google Scholar] [CrossRef]
- Shukla, A.; Singha, R.K.; Sasaki, T.; Adak, S.; Bhandari, S.; Prasad, V.V.; Bordoloi, A.; Bal, R. Room temperature selective reduction of nitroarenes to azoxy compounds over Ni-TiO2 catalyst. Mol. Catal. 2020, 490, 110943. [Google Scholar] [CrossRef]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, non-metal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Han, X.; An, L.; Hu, Y.; Li, Y.; Hou, C.; Wang, H.; Zhang, Q. Ti3C2 MXene-derived carbon-doped TiO2 coupled with g-C3N4 as the visible-light photocatalysts for photocatalytic H2 generation. Appl. Catal. B Environ. 2020, 265, 118539. [Google Scholar] [CrossRef]
- Pedroza-Herrera, G.; Medina-Ramírez, I.E.; Lozano-Álvarez, J.A.; Rodil, S.E. Evaluation of the Photocatalytic Activity of Copper Doped TiO2 nanoparticles for the Purification and Disinfection of Industrial Effluents. Catal. Today 2020, 341, 37–48. [Google Scholar] [CrossRef]
- Diaz-Angulo, J.; Lara-Ramos, J.; Mueses, M.; Hernández-Ramírez, A.; Li Puma, G.; Machuca-Martínez, F. Enhancement of the oxidative removal of diclofenac and of the TiO2 rate of photon absorption in dye-sensitized solar pilot scale CPC photocatalytic reactors. Chem. Eng. J. 2020, 381, 122520. [Google Scholar] [CrossRef]
- Bohnenkamp, B.; Linnemann, J.H.; Junger, I.J.; Schwenzfeier-Hellkamp, E.; Ehrmann, A. Influence of different solvents on the electrical properties of dye-sensitized solar cells. J. Renew. Sustain. Energy 2018, 10, 282–286. [Google Scholar] [CrossRef]
- Fredes, C.; Yousef, G.G.; Robert, P.; Grace, M.H.; Lila, M.A.; Gómez, M.; Gebauer, M.; Montenegro, G. Anthocyanin profiling of wild maqui berries (Aristotelia chilensis [Mol.] Stuntz) from different geographical regions in Chile. J. Sci. Food Agric. 2014, 94, 2639–2648. [Google Scholar] [CrossRef]
- Ryu, D.; Koh, E. Optimization of Ultrasound-Assisted Extraction of Anthocyanins and Phenolic Compounds from Black Soybeans (Glycine max L.). Food Anal. Methods 2019, 12, 1382–1389. [Google Scholar] [CrossRef]
- Leyrer, J.; Rubilar, M.; Morales, E.; Pavez, B.; Leal, E.; Hunter, R. Factor Optimization in the Manufacturing Process of Dye-Sensitized Solar Cells Based on Naturally Extracted Dye from a Maqui and Blackberry Mixture (Aristotelia Chilensis and Rubus Glaucus). J. Electron. Mater. 2018, 47, 6136–6143. [Google Scholar] [CrossRef]
- Jaime Guerrero, C.; Luigi Ciampi, P.; Andrea Castilla, C.; Fernando Medel, S.; Heidi Schalchli, S.; Emilio Hormazabal, U.; Emma Bensch, T.; Miren Alberdi, L. Capacidad antioxidante, antocianinas y fenoles totales de berries silvestres y cultivados en Chile. Chil. J. Agric. Res. 2010, 70, 537–544. [Google Scholar] [CrossRef]
- Escribano-Bailón, M.T.; Alcalde-Eon, C.; Muñoz, O.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanins in berries of Maqui (Aristotelia chilensis (Mol.) Stuntz). Phytochem. Anal. 2006, 17, 8–14. [Google Scholar] [CrossRef]
- Fan-Chiang, H.J.; Wrolstad, R.E. Anthocyanin pigment composition of blackberries. J. Food Sci. 2005, 70, C198–C202. [Google Scholar] [CrossRef]
- Marcano, E. DFT study of anthocyanidin and anthocyanin pigments for Dye-Sensitized Solar Cells: Electron injecting from the excited states and adsorption onto TiO2 (anatase) surface. Phys. Sci. Rev. 2019, 2, 29–38. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, Y.; Huang, W.; Huang, S. Degradation kinetics and mechanism of aniline by heat-assisted persulfate oxidation. J. Environ. Sci. 2012, 24, 821–826. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Sakamoto, H.; Fujiwara, K.; Ichikawa, S.; Hirai, T. Selective Photocatalytic Oxidation of Aniline to Nitrosobenzene by Pt Nanoparticles Supported on TiO2 under Visible Light Irradiation. ACS Catal. 2014, 4, 2418–2425. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).