High-Loaded Nickel Based Sol–Gel Catalysts for Methylcyclohexane Dehydrogenation
Abstract
1. Introduction
2. Results and Discussion
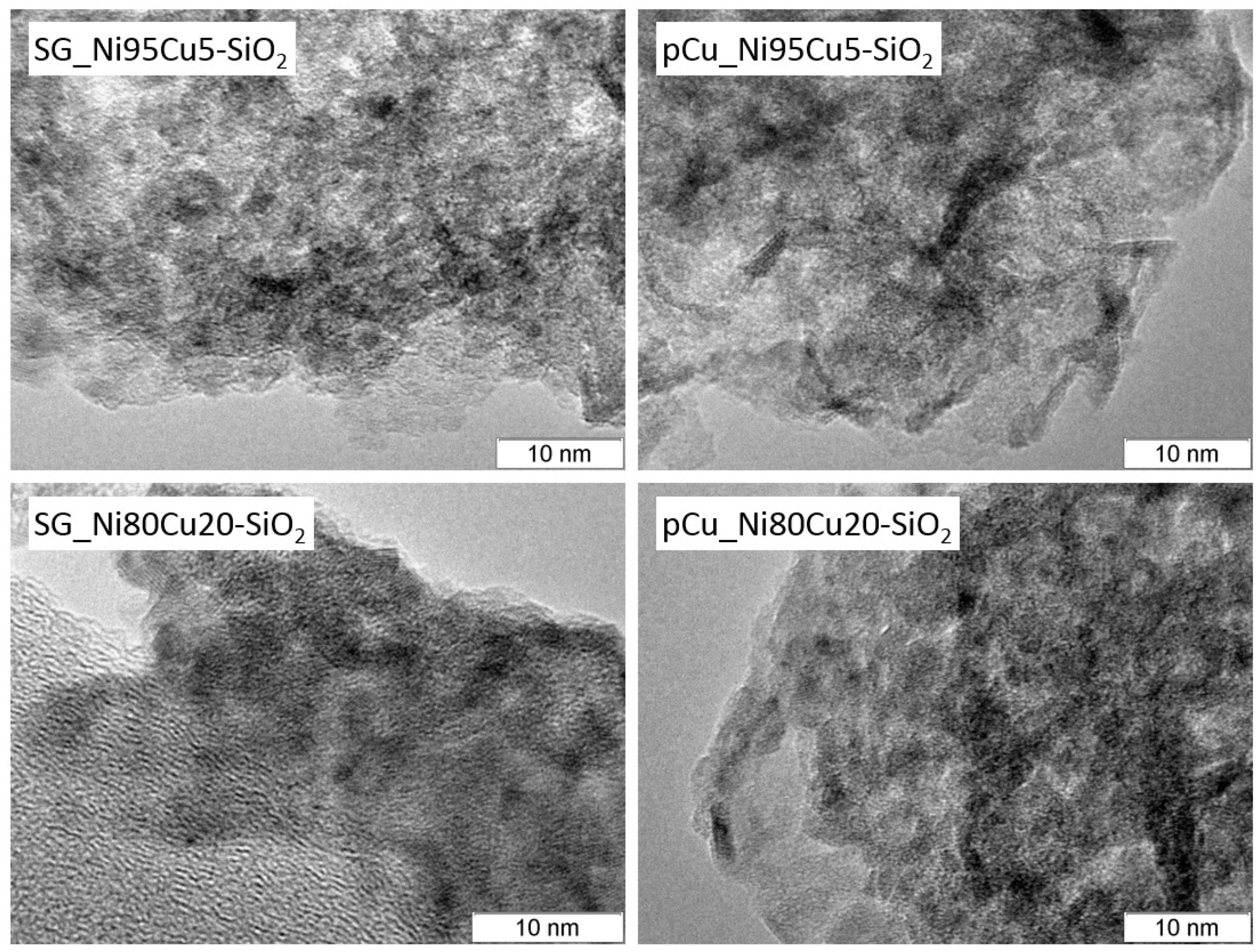
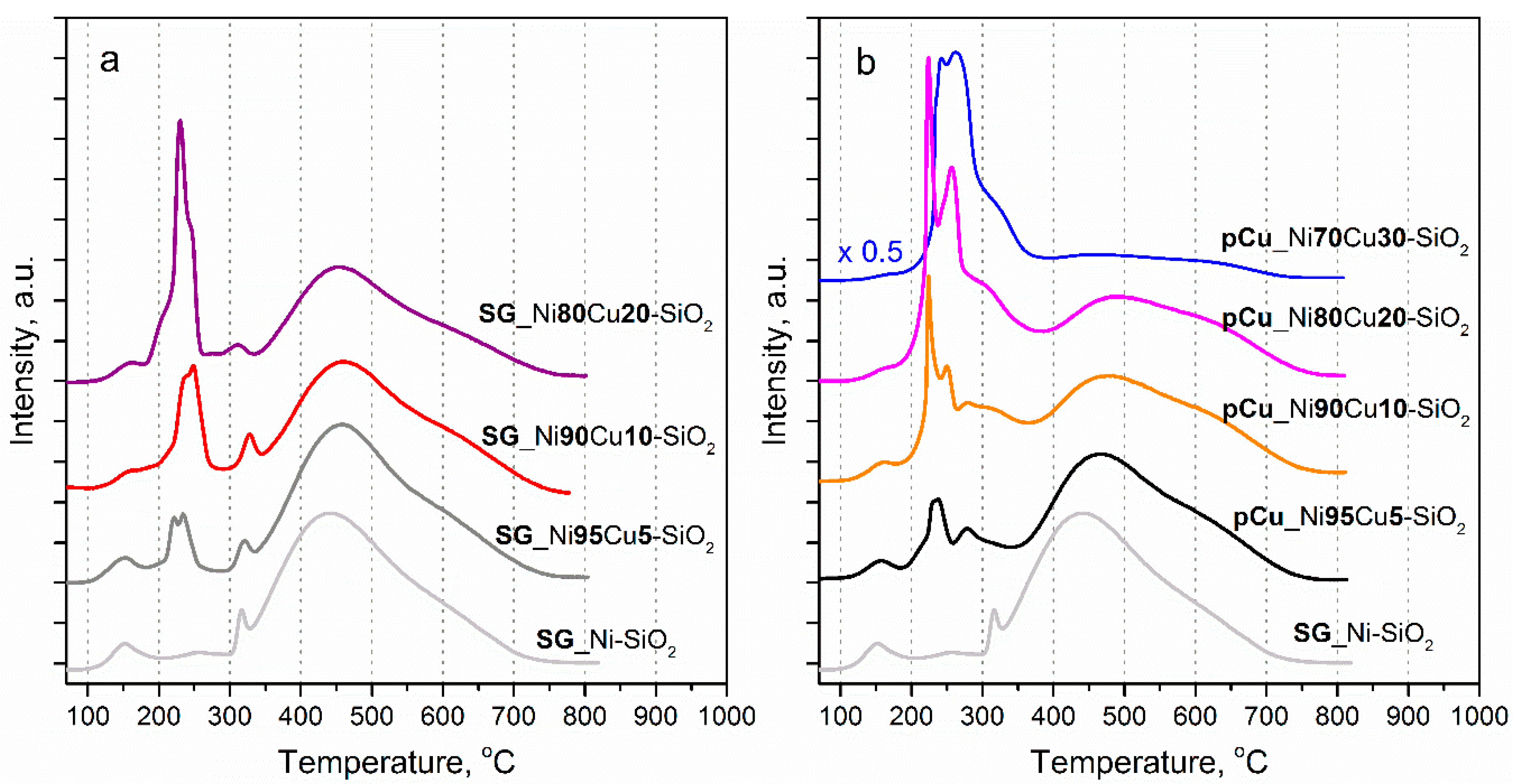
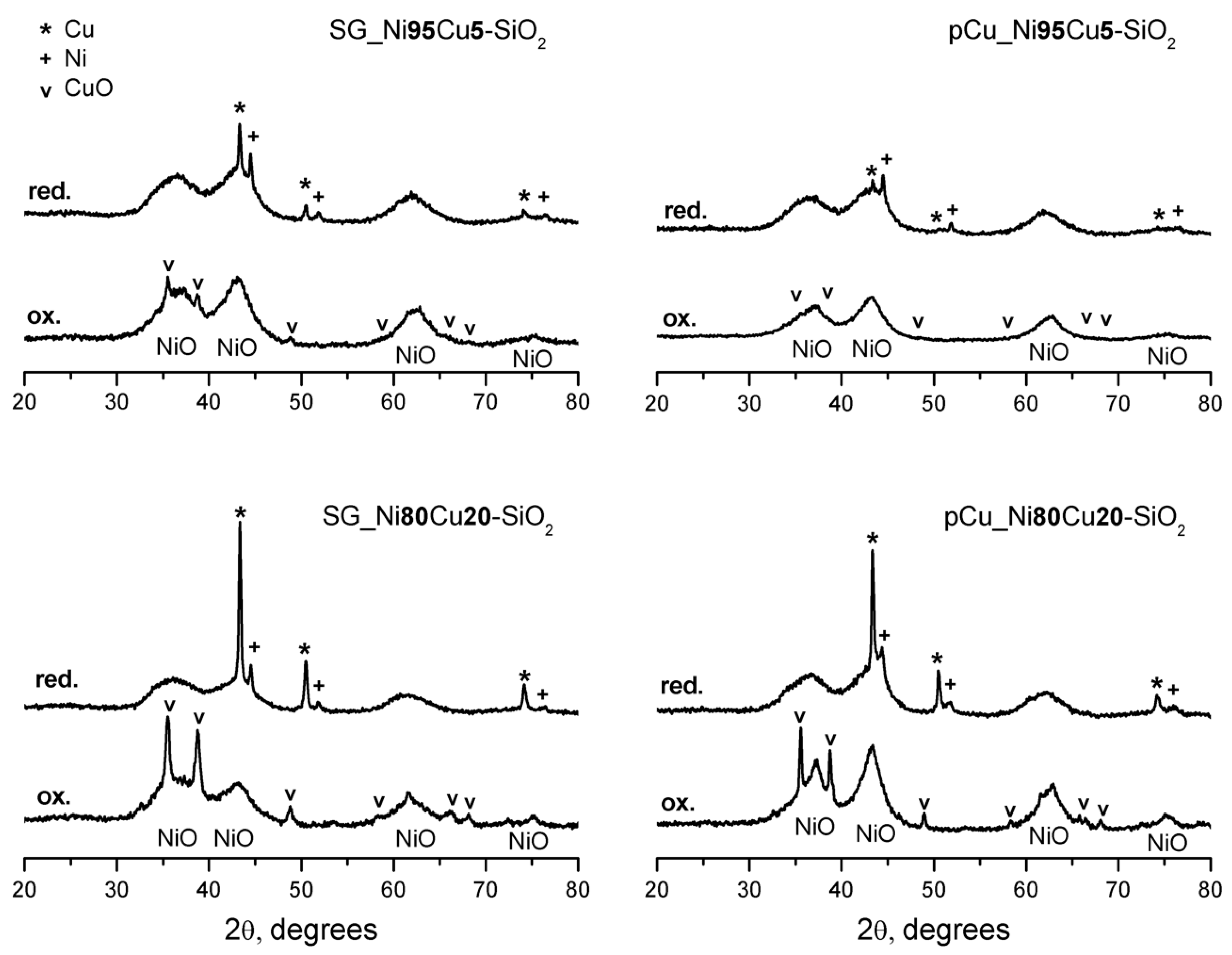
2.1. Catalyst Characterization
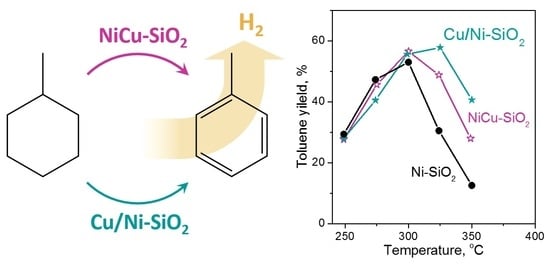
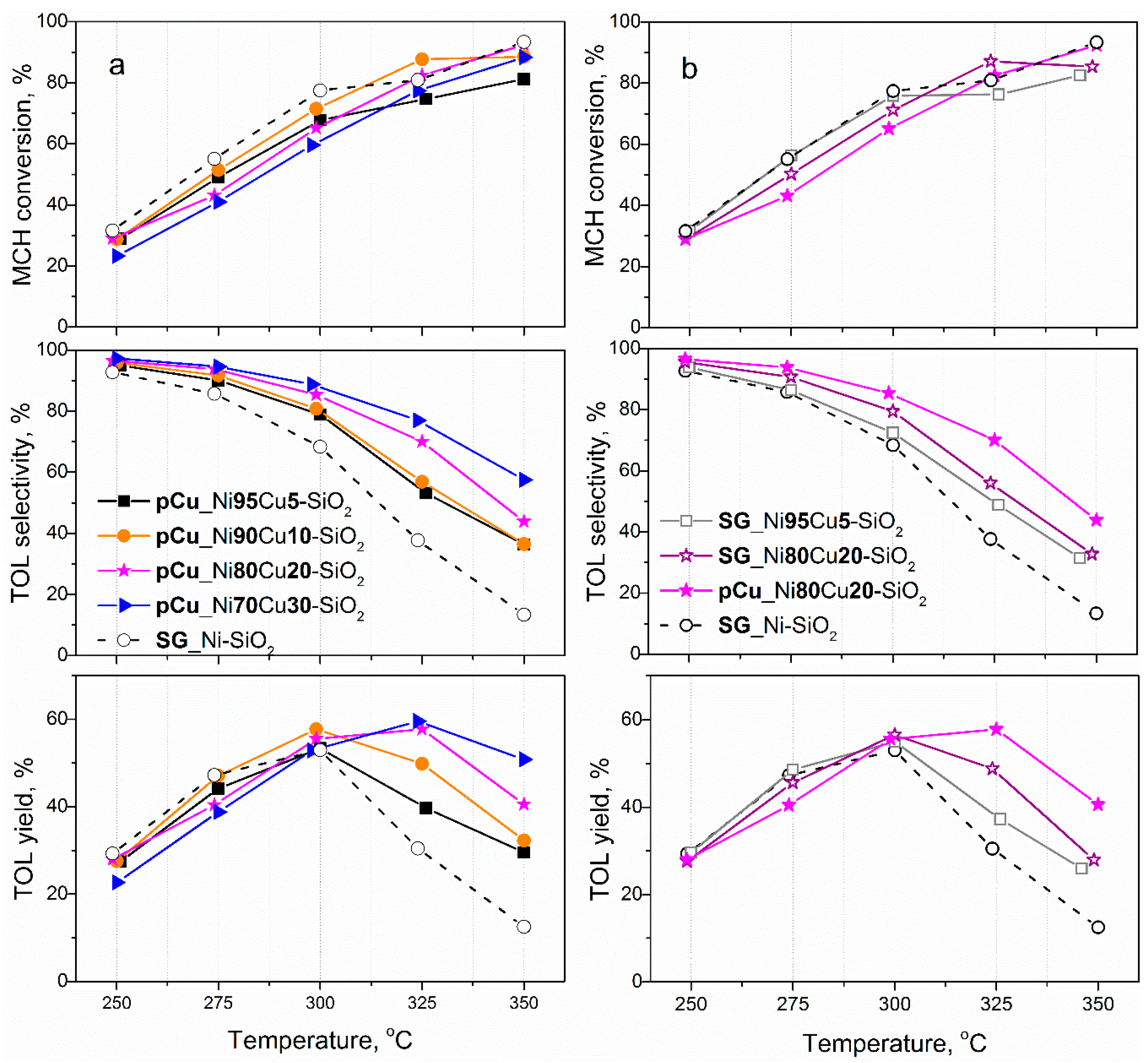
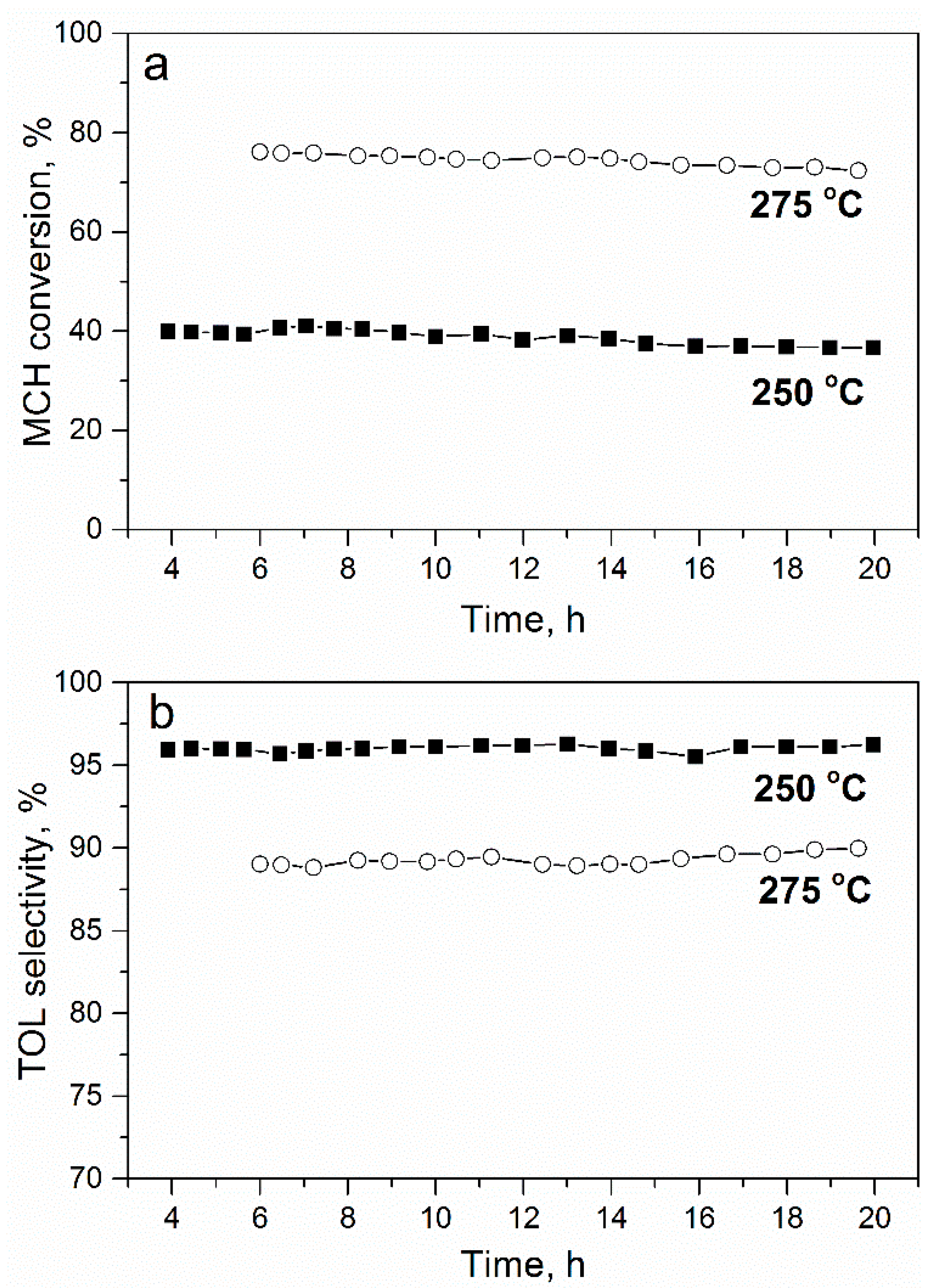
2.2. Catalyst Activity in MCH Dehydrogenation
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.2.1. Nitrogen Physisorption
3.2.2. CO Pulse Chemisorption Measurements
3.2.3. Temperature-Programmed Reduction
3.2.4. High-Resolution Transmission Electron Microscopy
3.2.5. X-ray Diffraction (XRD)
3.2.6. X-ray Fluorescence Analysis (XRF)
3.3. Testing in MCH Dehydrogenation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dalebrook, A.F.; Gan, W.; Grasemann, M.; Moret, S.; Laurenczy, G. Hydrogen storage: Beyond conventional methods. Chem. Commun. 2013, 49, 8735–8751. [Google Scholar] [CrossRef] [PubMed]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Biniwale, R.B.; Rayalu, S.; Devotta, S.; Ichikawa, M. Chemical hydrides: A solution to high capacity hydrogen storage and supply. Int. J. Hydrogen Energy 2008, 33, 360–365. [Google Scholar] [CrossRef]
- Gianotti, E.; Taillades-Jacquin, M.; Rozière, J.; Jones, D.J. High-Purity Hydrogen Generation via Dehydrogenation of Organic Carriers: A Review on the Catalytic Process. ACS Catal. 2018, 8, 4660–4680. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy—Review and discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Alhumaidan, F.; Tsakiris, D.; Cresswell, D.; Garforth, A. Hydrogen storage in liquid organic hydride: Selectivity of MCH dehydrogenation over monometallic and bimetallic Pt catalysts. Int. J. Hydrogen Energy 2013, 38, 14010–14026. [Google Scholar] [CrossRef]
- Okada, Y.; Shimura, M. Development of Large-Scale H2 Storage and Transportation Technology with Liquid Organic Hydrogen Carrier (LOHC); Joint GCC-JAPAN Environment Symposium: Doha, Qatar, February 2013. [Google Scholar]
- Itoh, N.; Watanabe, S.; Kawasoe, K.; Sato, T.; Tsuji, T. A membrane reactor for hydrogen storage and transport system using cyclohexane–methylcyclohexane mixtures. Desalination 2008, 234, 261–269. [Google Scholar] [CrossRef]
- Wunsch, A.; Mohr, M.; Pfeifer, P. Intensified LOHC-Dehydrogenation Using Multi-Stage Microstructures and Pd-Based Membranes. Membranes 2018, 8, 112. [Google Scholar] [CrossRef]
- Cholewa, M.; Zehner, B.; Kreuder, H.; Pfeifer, P. Optimization of membrane area to catalyst mass in a microstructured membrane reactor for dehydrogenation of methylcyclohexane. Chem. Eng. Process. Process Intensif. 2018, 125, 325–333. [Google Scholar] [CrossRef]
- Juangsa, F.B.; Prananto, L.A.; Mufrodi, Z.; Budiman, A.; Oda, T.; Aziz, M. Highly energy-efficient combination of dehydrogenation of methylcyclohexane and hydrogen-based power generation. Appl. Energy 2018, 226, 31–38. [Google Scholar] [CrossRef]
- Jothimurugesan, K.; Bhatia, S.; Srivastava, R.D. Kinetics of dehydrogenation of methylcyclohexane over a platinum-rhenium-alumina catalyst in the presence of added hydrogen. Ind. Eng. Chem. Fundam. 1985, 24, 433–438. [Google Scholar] [CrossRef]
- Niermann, M.; Beckendorff, A.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carrier (LOHC)—Assessment based on chemical and economic properties. Int. J. Hydrogen Energy 2019, 44, 6631–6654. [Google Scholar] [CrossRef]
- Coughlin, R.W.; Kawakami, K.; Hasan, A. Activity, yield patterns, and coking behavior of Pt and PtRe catalysts during dehydrogenation of methylcyclohexane: I. In the absence of sulfur. J. Catal. 1984, 88, 150–162. [Google Scholar] [CrossRef]
- Alhumaidan, F.; Cresswell, D.; Garforth, A. Hydrogen Storage in Liquid Organic Hydride: Producing Hydrogen Catalytically from Methylcyclohexane. Energy Fuels 2011, 25, 4217–4234. [Google Scholar] [CrossRef]
- Biniwale, R.B.; Kariya, N.; Ichikawa, M. Dehydrogenation of Cyclohexane Over Ni Based Catalysts Supported on Activated Carbon using Spray-pulsed Reactor and Enhancement in Activity by Addition of a Small Amount of Pt. Catal. Lett. 2005, 105, 83–87. [Google Scholar] [CrossRef]
- Hodoshima, S.; Nagata, H.; Saito, Y. Efficient hydrogen supply from tetralin with superheated liquid-film-type catalysis for operating fuel cells. Appl. Catal. A 2005, 292, 90–96. [Google Scholar] [CrossRef]
- Xia, Z.; Lu, H.; Liu, H.; Zhang, Z.; Chen, Y. Cyclohexane dehydrogenation over Ni-Cu/SiO2 catalyst: Effect of copper addition. Catal. Commun. 2017, 90, 39–42. [Google Scholar] [CrossRef]
- Desai, P.H.; Richardson, J.T. Crystallite size effects in nickel catalysts: Cyclohexane dehydrogenation and hydrogenolysis. J. Catal. 1986, 98, 392–400. [Google Scholar] [CrossRef]
- Sinfelt, J.H.; Carter, J.L.; Yates, D.J.C. Catalytic hydrogenolysis and dehydrogenation over copper-nickel alloys. J. Catal. 1972, 24, 283–296. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, H.; Lu, H.; Zhang, Z.; Chen, Y. Study on catalytic properties and carbon deposition of Ni-Cu/SBA-15 for cyclohexane dehydrogenation. Appl. Surf. Sci. 2017, 422, 905–912. [Google Scholar] [CrossRef]
- Preuster, P.; Alekseev, A.; Wasserscheid, P. Hydrogen Storage Technologies for Future Energy Systems. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 445–471. [Google Scholar] [CrossRef] [PubMed]
- Al-ShaikhAli, A.H.; Jedidi, A.; Anjum, D.H.; Cavallo, L.; Takanabe, K. Kinetics on NiZn Bimetallic Catalysts for Hydrogen Evolution via Selective Dehydrogenation of Methylcyclohexane to Toluene. ACS Catal. 2017, 7, 1592–1600. [Google Scholar] [CrossRef]
- Al-ShaikhAli, A.H.; Jedidi, A.; Cavallo, L.; Takanabe, K. Non-precious bimetallic catalysts for selective dehydrogenation of an organic chemical hydride system. Chem. Commun. 2015, 51, 12931–12934. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Pande, J.V.; Biniwale, R.B. Non-noble Ni–Cu/ACC bimetallic catalyst for dehydrogenation of liquid organic hydrides for hydrogen storage. Int. J. Hydrogen Energy 2013, 38, 15233–15241. [Google Scholar] [CrossRef]
- Lang, C.; Jia, Y.; Yao, X. Recent advances in liquid-phase chemical hydrogen storage. Energy Storage Mater. 2020, 26, 290–312. [Google Scholar] [CrossRef]
- Ermakov, D.Y.; Bykova, M.V.; Selisheva, S.A.; Khromova, S.A.; Yakovlev, V.A. Method of Preparing hydrotreatment Catalyst. Patent RU2496580C1, 8 October 2012. [Google Scholar]
- Yakovlev, V.A.; Khromova, S.A.; Sherstyuk, O.V.; Dundich, V.O.; Ermakov, D.Y.; Novopashina, V.M.; Lebedev, M.Y.; Bulavchenko, O.; Parmon, V.N. Development of new catalytic systems for upgraded bio-fuels production from bio-crude-oil and biodiesel. Catal. Today 2009, 144, 362–366. [Google Scholar] [CrossRef]
- Ardiyanti, A.R.; Bykova, M.V.; Khromova, S.A.; Yin, W.; Venderbosch, R.H.; Yakovlev, V.A.; Heeres, H.J. Ni-Based Catalysts for the Hydrotreatment of Fast Pyrolysis Oil. Energy Fuels 2016, 30, 1544–1554. [Google Scholar] [CrossRef]
- Bykova, M.V.; Ermakov, D.Y.; Kaichev, V.V.; Bulavchenko, O.A.; Saraev, A.A.; Lebedev, M.Y.; Yakovlev, V.A. Ni-based sol–gel catalysts as promising systems for crude bio-oil upgrading: Guaiacol hydrodeoxygenation study. Appl. Catal. B 2012, 113–114, 296–307. [Google Scholar] [CrossRef]
- Yin, W.; Kloekhorst, A.; Venderbosch, R.H.; Bykova, M.V.; Khromova, S.A.; Yakovlev, V.A.; Heeres, H.J. Catalytic hydrotreatment of fast pyrolysis liquids in batch and continuous set-ups using a bimetallic Ni-Cu catalyst with a high metal content. Catal. Sci. Technol. 2016, 6, 5899–5915. [Google Scholar] [CrossRef]
- Asedegbega-Nieto, E.; Guerrero-Ruíz, A.; Rodríguez-Ramos, I. Study of CO chemisorption on graphite-supported Ru–Cu and Ni–Cu bimetallic catalysts. Thermochim. Acta 2005, 434, 113–118. [Google Scholar] [CrossRef]
- Crisafulli, C.; Galvagno, S.; Maggiore, R.; Scirè, S.; Saeli, A. Performance of supported Ru-Cu bimetallic catalysts prepared from nitrate precursors. Catal. Lett. 1990, 6, 77–83. [Google Scholar] [CrossRef]
- Alekseeva, M.V.; Otyuskaya, D.S.; Rekhtina, M.A.; Bulavchenko, O.A.; Stonkus, O.A.; Kaichev, V.V.; Zavarukhin, S.G.; Thybaut, J.W.; Alexiadis, V.; Venderbosch, R.H.; et al. NiCuMo-SiO2 catalyst for pyrolysis oil upgrading: Model acidic treatment study. Appl. Catal. A 2019, 573, 1–12. [Google Scholar] [CrossRef]
- Mile, B.; Stirling, D.; Zammitt, M.A.; Lovell, A.; Webb, M. The Location of Nickel Oxide and Nickel in Silica-Supported Catalysts: Two Forms of “NiO” and the Assignment of Temperature-Programmed Reduction Profiles. J. Catal. 1988, 114, 217–229. [Google Scholar] [CrossRef]
- Tsay, M.-T.; Chang, F.-W. Characterization of rice husk ash-supported nickel catalysts prepared by ion exchange. Appl. Catal. A 2000, 203, 15–22. [Google Scholar] [CrossRef]
- Ashok, J.; Reddy, P.S.; Raju, G.; Subrahmanyam, M.; Venugopal, A. Catalytic Decomposition of Methane to Hydrogen and Carbon Nanofibers over Ni-Cu-SiO2 Catalysts. Energy Fuels 2009, 23, 5–13. [Google Scholar] [CrossRef]
- Chen, L.-C.; Lin, S.D. The ethanol steam reforming over Cu-Ni/SiO2 catalysts: Effect of Cu/Ni ratio. Appl. Catal. B 2011, 106, 639–649. [Google Scholar] [CrossRef]
- Bykova, M.V.; Ermakov, D.Y.; Khromova, S.A.; Smirnov, A.A.; Lebedev, M.Y.; Yakovlev, V.A. Stabilized Ni-based catalysts for bio-oil hydrotreatment: Reactivity studies using guaiacol. Catal. Today 2014, 220–222, 21–31. [Google Scholar] [CrossRef]
- Srivastava, S.; Jadeja, G.C.; Parikh, J. Synergism studies on alumina-supported copper-nickel catalysts towards furfural and 5-hydroxymethylfurfural hydrogenation. J. Mol. Catal. A: Chem. 2017, 426, 244–256. [Google Scholar] [CrossRef]
- Naghash, A.R.; Etsell, T.H.; Xu, S. XRD and XPS Study of Cu-Ni Interactions on Reduced Copper-Nickel-Aluminum Oxide Solid Solution Catalysts. Chem. Mater. 2006, 18, 2480–2488. [Google Scholar] [CrossRef]
- Fedorov, A.V.; Kukushkin, R.G.; Yeletsky, P.M.; Bulavchenko, O.A.; Chesalov, Y.A.; Yakovlev, V.A. Temperature-programmed reduction of model CuO, NiO and mixed CuO–NiO catalysts with hydrogen. J. Alloys Compd. 2020, 844, 156135. [Google Scholar] [CrossRef]
- Wu, Q.; Duchstein, L.D.L.; Chiarello, G.L.; Christensen, J.M.; Damsgaard, C.D.; Elkjær, C.F.; Wagner, J.B.; Temel, B.; Grunwaldt, J.-D.; Jensen, A.D. In Situ Observation of Cu–Ni Alloy Nanoparticle Formation by X-ray Diffraction, X-ray Absorption Spectroscopy, and Transmission Electron Microscopy: Influence of Cu/Ni Ratio. ChemCatChem 2014, 6, 301–310. [Google Scholar] [CrossRef]
- Ermakova, M.A.; Ermakov, D.Y. High-loaded nickel–silica catalysts for hydrogenation, prepared by sol–gel Route: Structure and catalytic behavior. Appl. Catal. A 2003, 245, 277–288. [Google Scholar] [CrossRef]
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
| Sample | Metal Loading, wt% | ABET1, m2/g | ABET2, m2/g | µmol CO/g | |
|---|---|---|---|---|---|
| Ni | Cu | ||||
| SG_Ni-SiO2 | 62.3 | - | 315 | 294 | 555 |
| SG_Ni95Cu5-SiO2 | 59.3 | 3.0 | 312 | 163 | 449 |
| SG_Ni90Cu10-SiO2 | 56.1 | 6.3 | 296 | 293 | 421 |
| SG_Ni80Cu20-SiO2 | 49.9 | 12.5 | 264 | 259 | 385 |
| pCu_Ni95Cu5-SiO2 | 59.9 | 3.3 | 301 | 234 | 384 |
| pCu_Ni90Cu10-SiO2 | 57.4 | 6.4 | 287 | 262 | 358 |
| pCu_Ni80Cu20-SiO2 | 52.2 | 13.0 | 225 | 235 | 266 |
| pCu_Ni70Cu30-SiO2 | 46.7 | 19.9 | 161 | 157 | 213 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulyaeva, Y.K.; Alekseeva, M.V.; Ermakov, D.Y.; Bulavchenko, O.A.; Zaikina, O.O.; Yakovlev, V.A. High-Loaded Nickel Based Sol–Gel Catalysts for Methylcyclohexane Dehydrogenation. Catalysts 2020, 10, 1198. https://doi.org/10.3390/catal10101198
Gulyaeva YK, Alekseeva MV, Ermakov DY, Bulavchenko OA, Zaikina OO, Yakovlev VA. High-Loaded Nickel Based Sol–Gel Catalysts for Methylcyclohexane Dehydrogenation. Catalysts. 2020; 10(10):1198. https://doi.org/10.3390/catal10101198
Chicago/Turabian StyleGulyaeva, Yuliya K., Maria V. Alekseeva (Bykova), Dmitry Yu. Ermakov, Olga A. Bulavchenko, Olesya O. Zaikina, and Vadim A. Yakovlev. 2020. "High-Loaded Nickel Based Sol–Gel Catalysts for Methylcyclohexane Dehydrogenation" Catalysts 10, no. 10: 1198. https://doi.org/10.3390/catal10101198
APA StyleGulyaeva, Y. K., Alekseeva, M. V., Ermakov, D. Y., Bulavchenko, O. A., Zaikina, O. O., & Yakovlev, V. A. (2020). High-Loaded Nickel Based Sol–Gel Catalysts for Methylcyclohexane Dehydrogenation. Catalysts, 10(10), 1198. https://doi.org/10.3390/catal10101198