Interesterification of Egg-Yolk Phosphatidylcholine with p-Methoxycinnamic Acid Catalyzed by Immobilized Lipase B from Candida Antarctica
Abstract
:1. Introduction
2. Results and Discussion
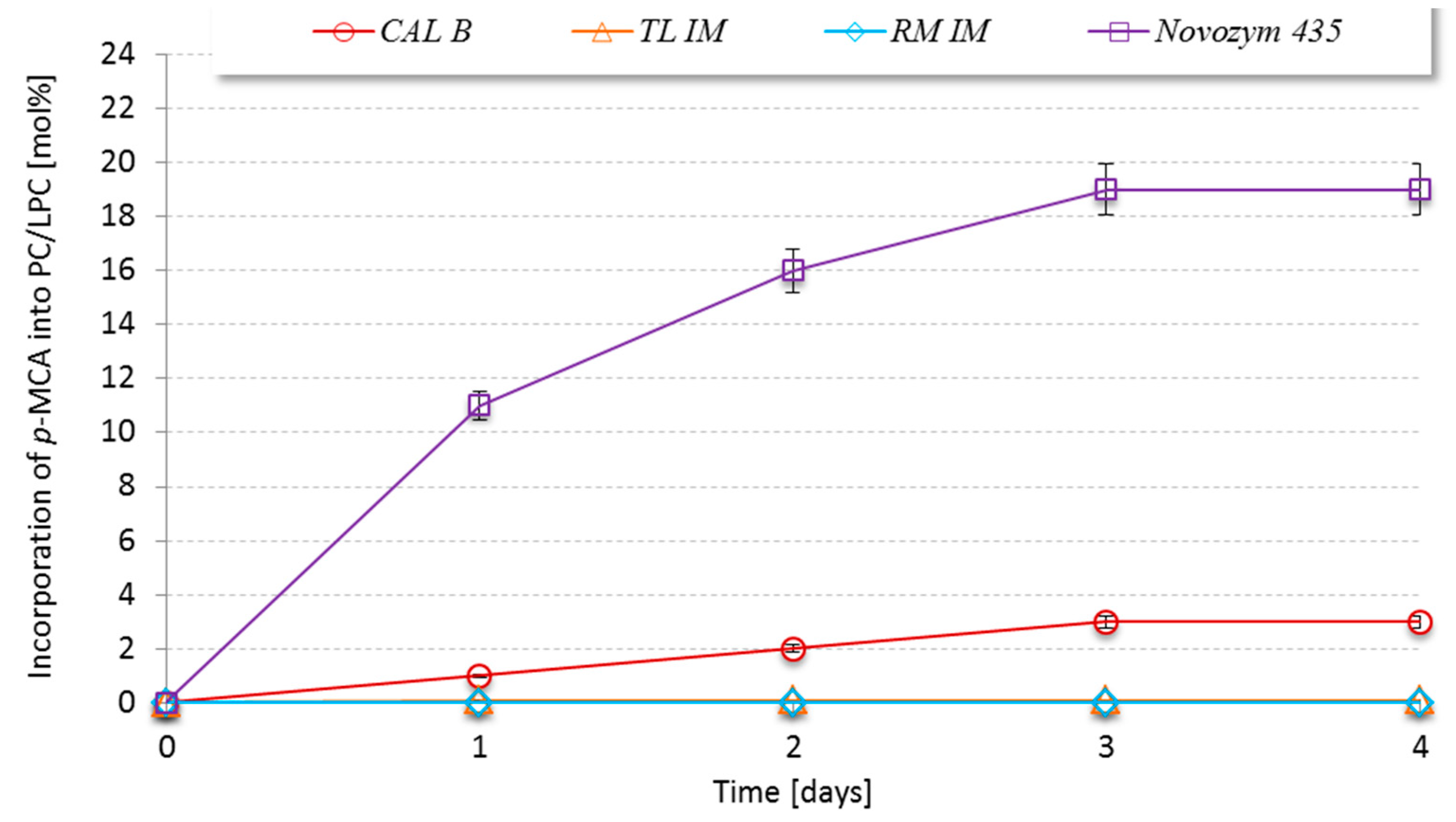
2.1. Screening of Lipase on the Interesterification Reaction
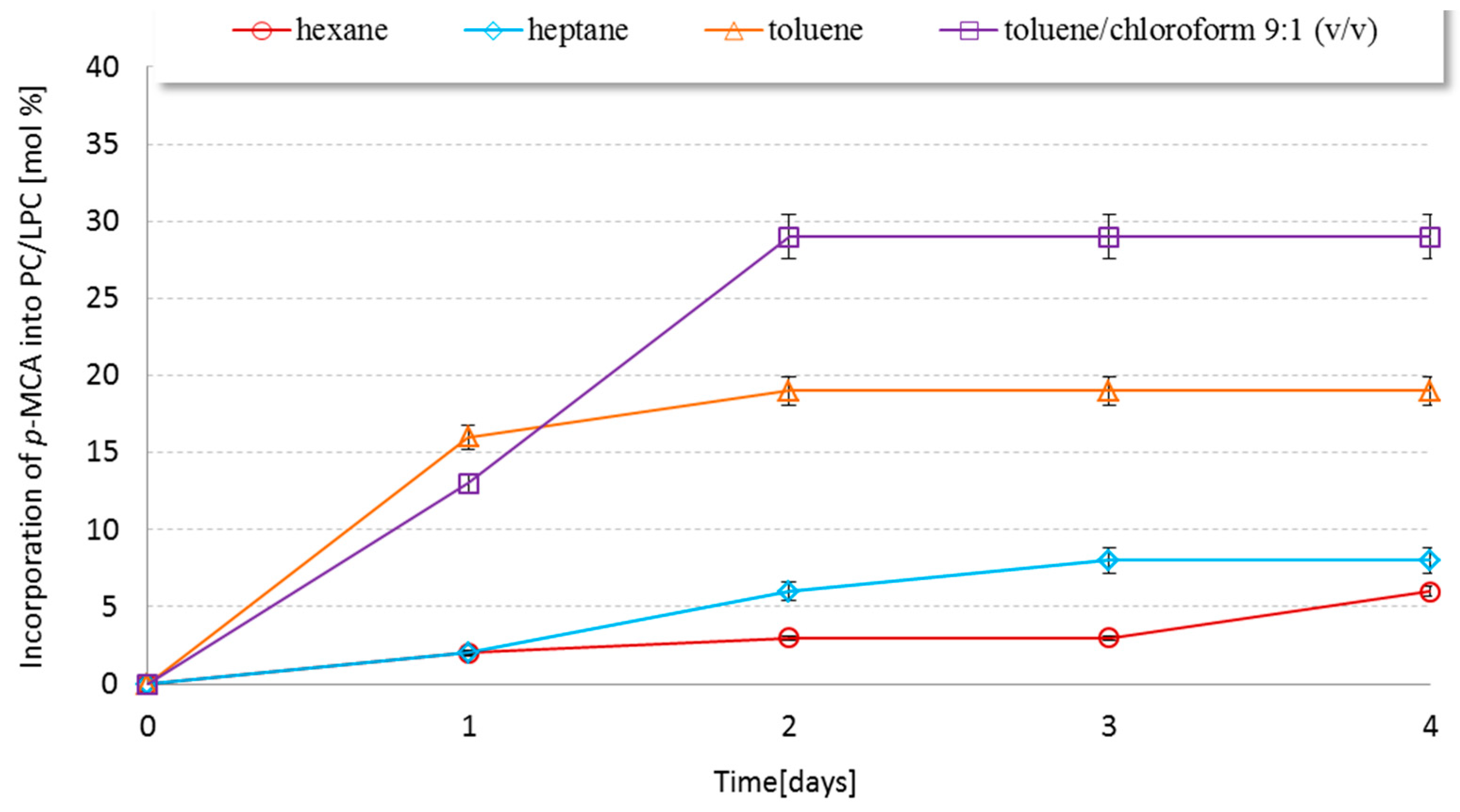
2.2. Effect of Reaction Medium on the Interesterification Reaction
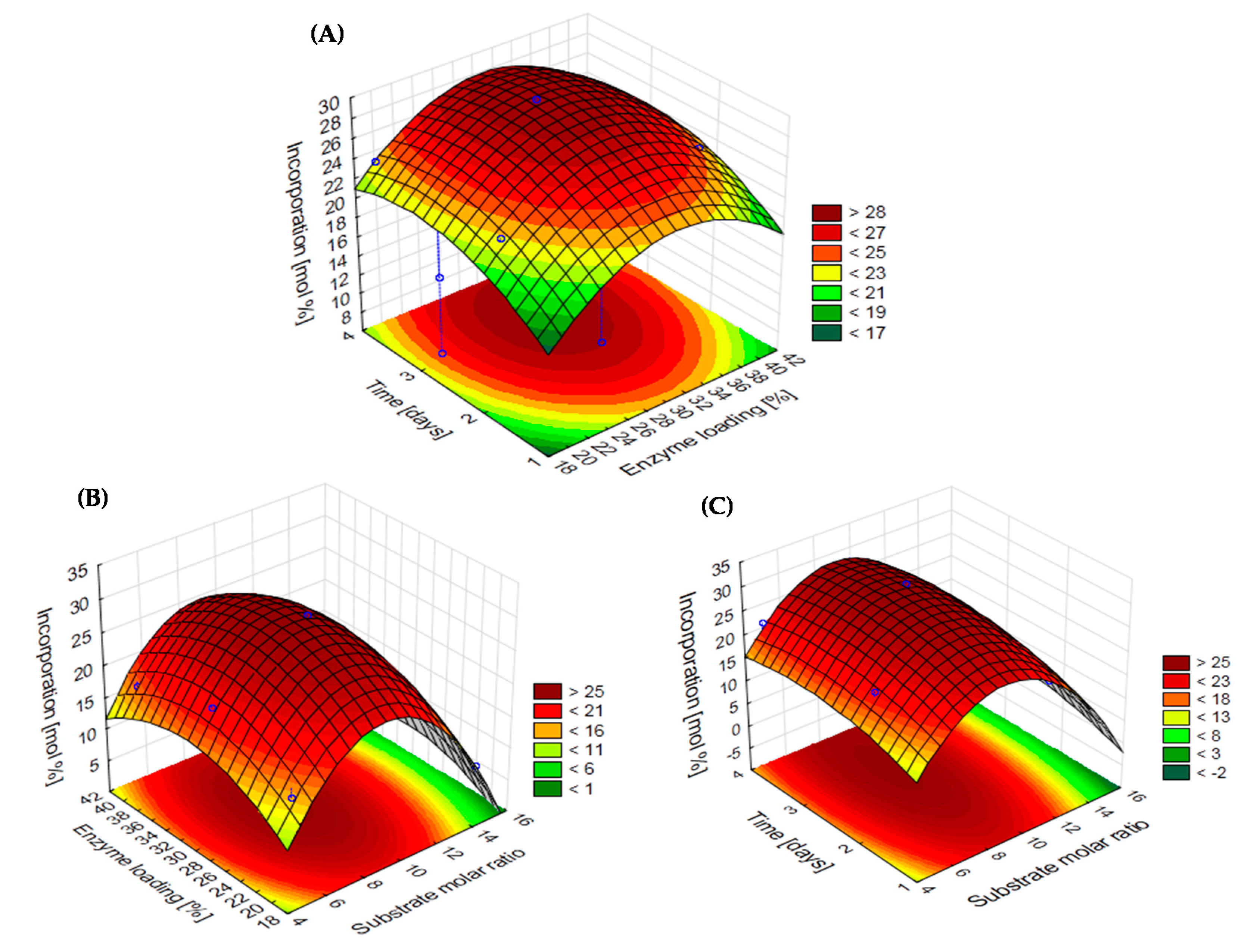
2.3. Development of Response Surface Methodology (RSM) Model
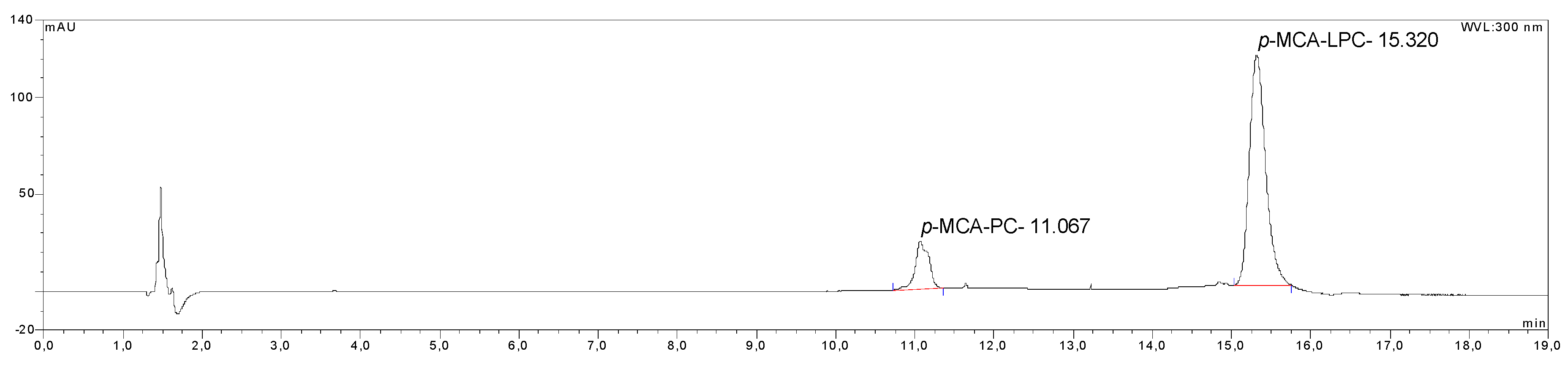
2.4. Identification of the Reaction Products
3. Materials and Methods
3.1. Substrates, Chemicals, and Enzymes
3.2. Enzymatic Interesterification of PC with Ep-MCA
3.3. Design of Experiment
3.4. Large-Scale Interesterification of PC with Ep-MCA Catalyzed by Novozym 435
3.5. Analytical Methods
3.5.1. Thin-Layer Chromatography (TLC)
3.5.2. Gas Chromatography (GC)
3.5.3. High-Performance Liquid Chromatography (HPLC)
3.5.4. Spectroscopic Spectra (NMR)
1-(4-methoxy) cinnamoyl-2-hydroxy-sn-glycero-3-phosphocholine (p-MCA-LPC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fernandez-Martinez, E.; Bobadilla, R.; Morales-Rios, M.; Muriel, P.; Perez-Alvarez, V. Trans-3-Phenyl-2-Propenoic Acid (Cinnamic Acid) Derivatives: Structure-Activity Relationship as Hepatoprotective Agents. Med. Chem. 2007, 3, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Sookkongwaree, K.; Roengsumran, S.; Petsom, A.; Ngamrojnavanich, N.; Chavasiri, W.; Deesamer, S.; Yibchok-anun, S. Structure-activity relationships of trans-cinnamic acid derivatives on α-glucosidase inhibition. Bioorg. Med. Chem. Lett. 2004, 14, 2893–2896. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Roengsamran, S.; Hsu, W.H.; Yibchok-Anun, S. Mechanisms of antihyperglycemic effect of p-methoxycinnamic acid in normal and streptozotocin-induced diabetic rats. Life Sci. 2005, 78, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Yibchok-Anun, S.; Adisakwattana, S.; Moonsan, P.; Hsu, W.H. Insulin-secretagogue activity of p-methoxycinnamic acid in rats, perfused rat pancreas and pancreatic β-cell line. Basic Clin. Pharmacol. Toxicol. 2008, 102, 476–482. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Moonsan, P.; Yibchok-Anun, S. Insulin-releasing properties of a series of cinnamic acid derivatives in vitro and in vivo. J. Agric. Food Chem. 2008, 56, 7838–7844. [Google Scholar] [CrossRef]
- Kim, S.R.; Kim, Y.C. Neuroprotective phenylpropanoid esters of rhamnose isolated from roots of Scrophularia buergeriana. Phytochemistry 2000, 54, 503–509. [Google Scholar] [CrossRef]
- Kim, S.R.; Sung, S.H.; Jang, Y.P.; Markelonis, G.J.; Oh, T.H.; Kim, Y.C. E-p-Methoxycinnamic acid protects cultured neuronal cells against neurotoxicity induced by glutamate. Br. J. Pharmacol. 2002, 135, 1281–1291. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.R.; Kang, S.Y.; Lee, K.Y.; Kim, S.H.; Markelonis, G.J.; Oh, T.H.; Kim, Y.C. Anti-amnestic activity of E-p-methoxycinnamic acid from Scrophularia buergeriana. Cogn. Brain Res. 2003, 17, 454–461. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Venkatachalam, K.; Namasivayam, N. Anti-inflammatory and anticancer effects of p-methoxycinnamic acid, an active phenylpropanoid, against 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Mol. Cell. Biochem. 2019, 451, 117–129. [Google Scholar] [CrossRef]
- Andrade, P.B.; Leitão, R.; Seabra, R.M.; Oliveira, M.B.; Ferreira, M.A. 3,4-Dimethoxycinnamic acid levels as a tool for differentiation of Coffea canephora var. robusta and Coffea arabica. Food Chem. 1998, 61, 511–514. [Google Scholar] [CrossRef]
- Sobolev, V.S.; Horn, B.W.; Potter, T.L.; Deyrup, S.T.; Gloer, J.B. Production of stilbenoids and phenolic acids by the peanut plant at early stages of growth. J. Agric. Food Chem. 2006, 54, 3505–3511. [Google Scholar] [CrossRef]
- Duarte-Almeida, J.M.; Negri, G.; Salatino, A.; de Carvalho, J.E.; Lajolo, F.M. Antiproliferative and antioxidant activities of a tricin acylated glycoside from sugarcane (Saccharum officinarum) juice. Phytochemistry 2007, 68, 1165–1171. [Google Scholar] [CrossRef]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.J.; Gescher, A.; Laboratory, J. Characterization of Potentially Chemopreventive Phenols in Extracts of Brown Rice That Inhibit the Growth of Human Breast and Colon Cancer Cells. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1163–1170. [Google Scholar]
- Sivagami, G.; Karthikkumar, V.; Balasubramanian, T.; Nalini, N. The modulatory influence of p-methoxycinnamic acid, an active rice bran phenolic acid, against 1,2-dimethylhydrazine-induced lipid peroxidation, antioxidant status and aberrant crypt foci in rat colon carcinogenesis. Chem. Biol. Interact. 2012, 196, 11–22. [Google Scholar] [CrossRef]
- Kumar, A. Phytochemistry, pharmacological activities and uses of traditional medicinal plant Kaempferia galanga L.—An overview. J. Ethnopharmacol. 2020, 253, 112667. [Google Scholar] [CrossRef]
- Rook, M. p-Methoxycinnamate and Its Metabolite. J. Pharm. Sci. 1968, 57, 6–9. [Google Scholar]
- Liu, B.; Liu, F.; Chen, C.; Gao, H. Supercritical carbon dioxide extraction of ethyl p-methoxycinnamate from Kaempferia galanga L. rhizome and its apoptotic induction in human HepG2 cells. Nat. Prod. Res. 2010, 24, 1927–1932. [Google Scholar] [CrossRef]
- Eun, J.L.; So, R.K.; Kim, J.; Young, C.K. Hepatoprotective phenylpropanoids from Scrophularia buergeriana roots against CCl4-induced toxicity: Action mechanism and structure-activity relationship. Planta Med. 2002, 68, 407–411. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Venkatachalam, K.; Namasivayam, N. P-Methoxycinnamic acid, an active phenylpropanoid induces mitochondrial mediated apoptosis in HCT-116 human colon adenocarcinoma cell line. Environ. Toxicol. Pharmacol. 2015, 40, 966–974. [Google Scholar] [CrossRef]
- Adisakwattana, S.; Hsu, W.H.; Yibchok-Anun, S. Mechanisms of p-Methoxycinnamic acid-induced increase in insulin secretion. Horm. Metab. Res. 2011, 43, 766–773. [Google Scholar] [CrossRef]
- Ambika, S.; Saravanan, R.; Thirumavalavan, K. Antidiabetic and antihyperlipidemic effect of p-hydroxycinnamic acid on streptozotocin-induced diabetic Wistar rats. Biomed. Aging Pathol. 2013, 3, 253–257. [Google Scholar] [CrossRef]
- Rijal, S.; Changdar, N.; Kinra, M.; Kumar, A.; Nampoothiri, M.; Arora, D.; Shenoy, R.R.; Ranganath Pai, K.S.; Joseph, A.; Mudgal, J. Neuromodulatory potential of phenylpropanoids; para-methoxycinnamic acid and ethyl-p-methoxycinnamate on aluminum-induced memory deficit in rats. Toxicol. Mech. Methods 2019, 29, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Santoso, B.S.A.; Khotib, J. Oil Fraction from Kaempferia galanga Alcoholic Extract Increases Apoptosis Activity in Mice Colon Cancer. In Proceedings of the Research and Application on Traditional Complementary and Alternative Medicine in Health Care (TCAM); Muhammadiyah University Press: Surakarta, Indonesia, 2012; pp. 66–72. [Google Scholar]
- Omar, M.N.; Hasali, N.H.M.; Yarmo, M.A. Cytotoxicity activity of biotransformed ethyl p-methoxycinnamate by Aspergillus niger. Orient. J. Chem. 2016, 32, 2731–2734. [Google Scholar] [CrossRef] [Green Version]
- Umar, M.I.; Iqbal, M.A.; Khadeer Ahamed, M.B.; Altaf, R.; Hassan, L.E.A.; Haque, R.A.; Abdul Majeed, A.M.S.; Asmawi, M.Z. Cytotoxic and Pro-Apoptotic Properties of Ethyl-p-Methoxycinnamate and Its Hydrophilic Derivative Potassium-p-Methoxycinnamate. Chem. Afr. 2018, 1, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Fennell, T.R.; Mathews, J.M.; Snyder, R.W.; Hong, Y.; Watson, S.L.; Black, S.R.; McIntyre, B.S.; Waidyanatha, S. Metabolism and disposition of 2-ethylhexyl-p-methoxycinnamate following oral gavage and dermal exposure in Harlan Sprague Dawley rats and B6C3F1/N mice and in hepatocytes in vitro. Xenobiotica 2018, 48, 1142–1156. [Google Scholar] [CrossRef]
- Kusumawati, I.; Yusuf, H. Phospholipid Complex As a Carrier of Kaempferia Galanga Rhizome Extract To Improve Its Analgesic Activity. Int. J. Pharm. Pharm. Sci. 2011, 3, 1–3. [Google Scholar]
- Zierenberg, O.; Grundf, S.M. Intestinal absorption of polyenephosphatidylcholine in man. J. Lipid Res. 1982, 23, 1136–1142. [Google Scholar]
- Irby, D.; Du, C.; Li, F. Lipid-Drug Conjugate for Enhancing Drug Delivery. Mol. Pharm. 2017, 14, 1325–1338. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Bolleddula, J.; Nichols, A.; Tang, L.; Zhao, Z.; Prakash, C. Metabolism of bioconjugate therapeutics: Why, when, and how? Drug Metab. Rev. 2020, 52, 66–124. [Google Scholar] [CrossRef]
- Jaeger, K.E.; Eggert, T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002, 13, 390–397. [Google Scholar] [CrossRef]
- Sakaki, K.; Aoyama, A.; Nakane, T.; Ikegami, T.; Negishi, H.; Watanabe, K.; Yanagishita, H. Enzymatic synthesis of sugar esters in organic solvent coupled with pervaporation. Desalination 2006, 193, 260–266. [Google Scholar] [CrossRef]
- Clapés, P.; Infante, M.R. Amino acid-based surfactants: Enzymatic synthesis, properties and potential applications. Biocatal. Biotransformation 2002, 20, 215–233. [Google Scholar] [CrossRef]
- Stamatis, H.; Sereti, V.; Kolisis, F.N. Enzymatic synthesis of hydrophilic and hydrophobic derivatives of natural phenolic acids in organic media. J. Mol. Catal. B Enzym. 2001, 11, 323–328. [Google Scholar] [CrossRef]
- Lee, G.S.; Widjaja, A.; Ju, Y.H. Enzymatic synthesis of cinnamic acid derivatives. Biotechnol. Lett. 2006, 28, 581–585. [Google Scholar] [CrossRef]
- Kumar, V.; Jahan, F.; Kameswaran, K.; Mahajan, R.V.; Saxena, R.K. Eco-friendly methodology for efficient synthesis and scale-up of 2-ethylhexyl-p-methoxycinnamate using Rhizopus oryzae lipase and its biological evaluation. J. Ind. Microbiol. Biotechnol. 2014, 41, 907–912. [Google Scholar] [CrossRef]
- Vosmann, K.; Weitkamp, P.; Weber, N. Erratum: Solvent-free lipase-catalyzed preparation of long-chain alkyl phenylpropanoates and phenylpropyl alkanoates. J. Agric. Food Chem. 2006, 54, 3764. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Zhu, S.; Bi, Y. Solvent-free enzymatic synthesis of feruloylated structured lipids by the transesterification of ethyl ferulate with castor oil. Food Chem. 2014, 158, 292–295. [Google Scholar] [CrossRef]
- Karboune, S.; St-Louis, R.; Kermasha, S. Enzymatic synthesis of structured phenolic lipids by acidolysis of flaxseed oil with selected phenolic acids. J. Mol. Catal. B Enzym. 2008, 52–53, 96–105. [Google Scholar] [CrossRef]
- Safari, M.; Safari, M.; Karboune, S.; St-louis, R. Enzymatic synthesis of structured phenolic lipids by incorporation of selected phenolic acids into triolein. Biocatal. Biotransformation 2009, 2422, 272–279. [Google Scholar] [CrossRef]
- Yang, H.; Mu, Y.; Chen, H.; Xiu, Z.; Yang, T. Enzymatic synthesis of feruloylated lysophospholipid in a selected organic solvent medium. Food Chem. 2013, 141, 3317–3322. [Google Scholar] [CrossRef]
- Balakrishna, M.; Kaki, S.S.; Karuna, M.S.L.; Sarada, S.; Kumar, C.G.; Prasad, R.B.N. Synthesis and in vitro antioxidant and antimicrobial studies of novel structured phosphatidylcholines with phenolic acids. Food Chem. 2016, 221, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, M.; Switalska, M.; Wietrzyk, J.; Maciejewska, G.; Gliszczyńska, A. Synthesis and biological evaluation of phosphatidylcholines with cinnamic and 3-methoxycinnamic acids with potent antiproliferative activity. RSC Adv. 2018, 8, 35744–35752. [Google Scholar] [CrossRef]
- Czarnecka, M.; Świtalska, M.; Wietrzyk, J.; Maciejewska, G.; Gliszczyńska, A. Synthesis, Characterization, and In Vitro Cancer Cell Growth Inhibition Evaluation of Novel Phosphatidylcholines with Anisic and Veratric Acids. Molecules 2018, 23, 2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rychlicka, M.; Maciejewska, G.; Niezgoda, N.; Gliszczyńska, A. Production of feruloylated lysophospholipids via a one-step enzymatic interesterification. Food Chem. 2020, 323, 126802. [Google Scholar] [CrossRef] [PubMed]
- Rychlicka, M.; Niezgoda, N.; Gliszczyńska, A. Development and Optimization of Lipase-Catalyzed Synthesis of Phospholipids Containing 3,4-Dimethoxycinnamic Acid by Response Surface Methodology. Catalysts 2020, 10, 588. [Google Scholar] [CrossRef]
- Okulus, M.; Gliszczy, A. Enzymatic Synthesis of O-Methylated Phenophospholipids by Lipase-Catalyzed Acidolysis of Egg-Yolk Phosphatidylcholine with Anisic and Veratric Acids. Catalysts 2020, 10, 538. [Google Scholar] [CrossRef]
- Drzazga, A.; Okulus, M.; Rychlicka, M.; Biegała, Ł.; Gliszczyńska, A.; Gendaszewska-Darmach, E. Lysophosphatidylcholine Containing Anisic Acid Is Able to Stimulate Insulin Secretion Targeting G Protein Coupled Receptors. Nutrients 2020, 12, 1173. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Virgen-ortíz, J.J.; José, C.S.; Berenguer-murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compton, D.L.; Goodell, J.R.; Berhow, M.A.; Kenar, J.A.; Cermak, S.C.; Evans, K.O. Feruloylated Products from Coconut Oil and Shea Butter. JAOCS J. Am. Oil Chem. Soc. 2017, 94, 397–411. [Google Scholar] [CrossRef]
- Laszlo, J.A.; Compton, D.L.; Eller, F.J.; Taylor, S.L.; Isbell, T.A. Packed-bed bioreactor synthesis of feruloylated monoacyl- and diacylglycerols: Clean production of a “green” sunscreen. Green Chem. 2003, 5, 382–386. [Google Scholar] [CrossRef]
- Patil, D.; Dev, B.; Nag, A. Lipase-catalyzed synthesis of 4-methoxy cinnamoyl glycerol. J. Mol. Catal. B Enzym. 2011, 73, 5–8. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Wu, X.M.; Branford-White, C.; Quan, J.; Zhu, L.M. Dual response surface-optimized process for feruloylated diacylglycerols by selective lipase-catalyzed transesterification in solvent free system. Bioresour. Technol. 2009, 100, 2896–2901. [Google Scholar] [CrossRef]
- Manohar, B.; Divakar, S. Applications of surface plots and statistical designs to selected lipase catalysed esterification reactions. Process Biochem. 2004, 39, 847–853. [Google Scholar] [CrossRef]
- Rychlicka, M.; Niezgoda, N.; Gliszczyńska, A. Lipase-Catalyzed Acidolysis of Egg-Yolk Phosphatidylcholine with Citronellic Acid. New Insight into Synthesis of Isoprenoid-Phospholipids. Molecules 2018, 23, 314. [Google Scholar] [CrossRef] [Green Version]
- Li, N.G.; Shi, Z.H.; Tang, Y.P.; Li, B.Q.; Duan, J.A. Highly efficient esterification of ferulic acid under microwave irradiation. Molecules 2009, 14, 2118–2126. [Google Scholar] [CrossRef] [Green Version]
- Stabile, R.G.; Dicks, A.P. Two-step semi-microscale preparation of a cinnamate ester sunscreen analog. J. Chem. Educ. 2004, 81, 1488–1491. [Google Scholar] [CrossRef]

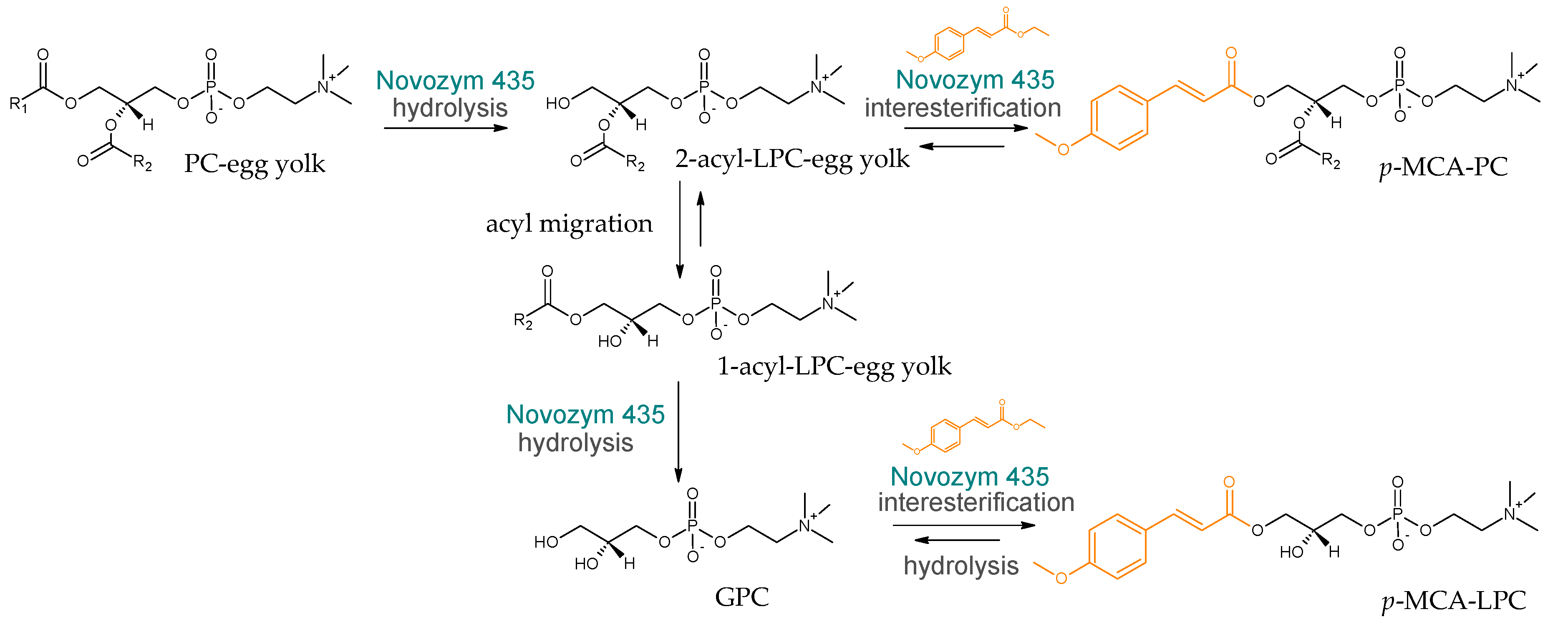
| Molecule | Research Model | Active Dose | Mechanism of Action | Ref. | |
|---|---|---|---|---|---|
| Antidiabetic and hepatoprotective activity | p-MCA | enzymes | 0.044 ± 0.006 (IC50) | α-glucosidase inhibition | [2] |
| p-MCA | * STPZ-induced rats | 40 mg/kg | insulin secretion, gluconeogenesis inhibition | [3] | |
| p-MCA | INS-1 cell line | 100 μM | insulin secretion | [5] | |
| p-MCA | Wistar rats | 5 mg/kg | insulin secretion | [5] | |
| p-MCA | * STPZ-induced rats | 40–100 mg/kg | insulin secretion | [4] | |
| p-MCA | INS-1 cell line | 100 μM | Ca2+ influx, insulin secretion | [20] | |
| p-MCA | * STPZ-induced rats | 10–40 mg/kg | insulin secretion | [21] | |
| p-MCA | CCl4 intoxicated rat hepatocytes | 1–5 μM | - | [18] | |
| p-MCA | CCl4-intoxicated rat | 50 mg/kg | - | [1] | |
| Neuroprotective activity | p-MCA | glutamate-insulted rat cortical cell | 1 μM | - | [6] |
| Ep-MCA | glutamate-insulted rat cortical cell | 0.01–1 μM | Ca2+ influx, glutamatergic antagonism | [7] | |
| Ep-MCA | ICR-mices | 0.01–2 mg/kg | - | [8] | |
| p-MCA, Ep-MCA | Wistar rats with cognitive dysfunction | 50–100 mg/kg | - | [22] | |
| Chemopreventive activity | p-MCA | HepG2 cell line | 27.1 μg/mL (IC50) | induction of apoptosis | [17] |
| p-MCA | Wistar rats | 40 mg/kg | - | [14] | |
| Ep-MCA | ** DMAB-induced mices | 23.4 mg/kg (oil fraction) | induction of apoptosis | [23] | |
| p-MCA | HCT-116 cell line | 10 μM (IC50) | induction of apoptosis | [19] | |
| Ep-MCA | MCF-7 cell line | 360 μg/mL (IC50) | - | [24] | |
| Ep-MCA | HCT-116 cell line | 42 μg/mL (IC50) | induction of apoptosis | [25] | |
| Ep-MCA | *** DMH-induced rats | 40 mg/kg | - | [9] |
| Run | Substrate Molar Ratio PC/Ep-MCA | Enzyme Load [%] | Reaction Time [days] | Incorporation of p-MCA into PC/LPC [mol%] a (Experimental) | Incorporation of p-MCA to PC/LPC [mol%] (Predicted) |
|---|---|---|---|---|---|
| 1 | 5 | 20 | 3 | 15 ± 0.2 | 16 |
| 2 | 15 | 20 | 3 | 7 ± 0.4 | 6 |
| 3 | 5 | 40 | 3 | 17 ± 0.3 | 18 |
| 4 | 15 | 40 | 3 | 9 ± 0.6 | 7 |
| 5 | 5 | 30 | 2 | 21± 0.8 | 20 |
| 6 | 15 | 30 | 2 | 8 ± 0.5 | 9 |
| 7 | 5 | 30 | 4 | 21 ± 0.7 | 20 |
| 8 | 15 | 30 | 4 | 9 ± 0.5 | 10 |
| 9 | 10 | 20 | 2 | 23 ± 0.3 | 23 |
| 10 | 10 | 40 | 2 | 24 ± 0.3 | 24 |
| 11 | 10 | 20 | 4 | 23 ± 0.1 | 23 |
| 12 | 10 | 40 | 4 | 24 ± 0.2 | 24 |
| 13 | 10 | 30 | 3 | 29 ± 0.5 | 29 |
| 14 | 10 | 30 | 3 | 29 ± 0.6 | 29 |
| 15 | 10 | 30 | 3 | 29 ± 0.4 | 29 |
| Evaluated Factors | Sum of Squares | Degrees of Freedom | Medium Square | F-Value | p-Value |
|---|---|---|---|---|---|
| (X1) Substrate molar ratio (L) | 210.1250 | 1 | 210.1250 | 97.7326 | 0.000181 |
| Substrate molar ratio (Q) | 612.0577 | 1 | 612.0577 | 284.6780 | 0.000013 |
| (X2) Enzyme load (L) | 4.5000 | 1 | 4.5000 | 2.0930 | 0.207617 |
| Enzyme load (Q) | 62.8269 | 1 | 62.8269 | 29.2218 | 0.002929 |
| (X3) Time of reaction (L) | 0.1250 | 1 | 0.1250 | 0.0581 | 0.819037 |
| Time of reaction (Q) | 6.9808 | 1 | 6.9808 | 3.2469 | 0.131434 |
| X1 by X2 | 0.0000 | 1 | 0.0000 | 0.00000 | 1.000000 |
| X1 by X3 | 0.2500 | 1 | 0.2500 | 0.1163 | 0.746968 |
| X2 by X3 | 0.0000 | 1 | 0.0000 | 0.0000 | 1.000000 |
| Error | 10.7500 | 5 | 2.1500 | ||
| Total error | 874.4000 | 14 | |||
| R2 = 0.98771 |
| Fatty and 4MCA Acids | Native PC | Modified Phospholipid Fraction PC/LPC |
|---|---|---|
| C16:0 (PA) | 34 ± 0.2 | 8 ± 0.6 |
| C16:1 (OPA) | 1 ± 0.7 | 1 ± 0.1 |
| C18:0 (SA) | 15 ± 0.9 | 8 ± 0.9 |
| C18:1 (OA) | 26 ± 0.7 | 23 ± 0.3 |
| C18:2 (LA) | 20 ± 0.3 | 22 ± 0.7 |
| C20:4 (AA) | 4 ± 0.2 | 9 ± 0.4 |
| p-MCA | - | 29 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rychlicka, M.; Gliszczyńska, A. Interesterification of Egg-Yolk Phosphatidylcholine with p-Methoxycinnamic Acid Catalyzed by Immobilized Lipase B from Candida Antarctica. Catalysts 2020, 10, 1181. https://doi.org/10.3390/catal10101181
Rychlicka M, Gliszczyńska A. Interesterification of Egg-Yolk Phosphatidylcholine with p-Methoxycinnamic Acid Catalyzed by Immobilized Lipase B from Candida Antarctica. Catalysts. 2020; 10(10):1181. https://doi.org/10.3390/catal10101181
Chicago/Turabian StyleRychlicka, Magdalena, and Anna Gliszczyńska. 2020. "Interesterification of Egg-Yolk Phosphatidylcholine with p-Methoxycinnamic Acid Catalyzed by Immobilized Lipase B from Candida Antarctica" Catalysts 10, no. 10: 1181. https://doi.org/10.3390/catal10101181
APA StyleRychlicka, M., & Gliszczyńska, A. (2020). Interesterification of Egg-Yolk Phosphatidylcholine with p-Methoxycinnamic Acid Catalyzed by Immobilized Lipase B from Candida Antarctica. Catalysts, 10(10), 1181. https://doi.org/10.3390/catal10101181






