Recent Advances in the Electroreduction of CO2 over Heteroatom-Doped Carbon Materials
Abstract
:1. Introduction
2. CO2 Electroreduction over Heteroatom-Doped Carbon Materials
2.1. Nitrogen-Doped Carbon Materials
2.1.1. Synthesis Methods
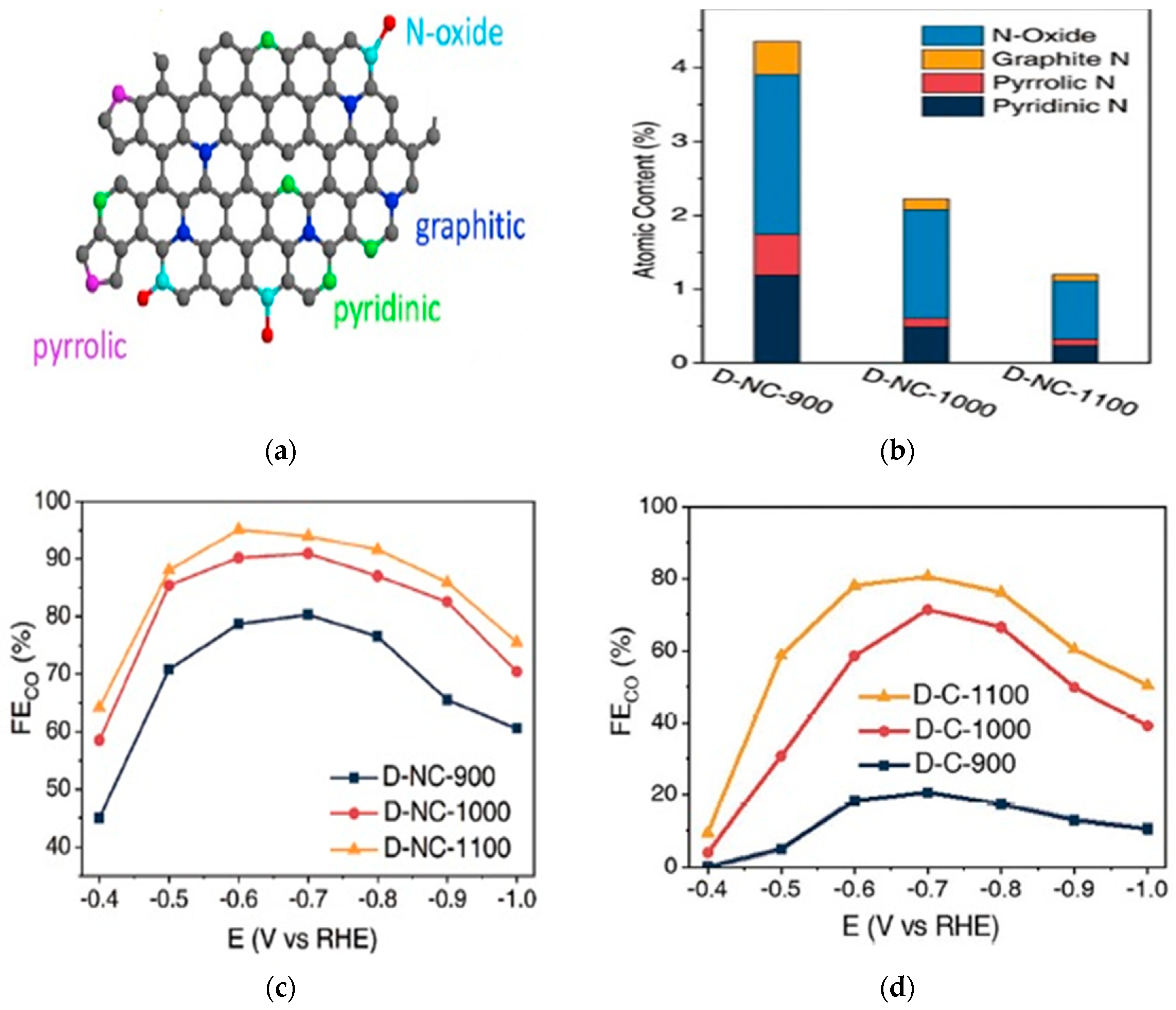
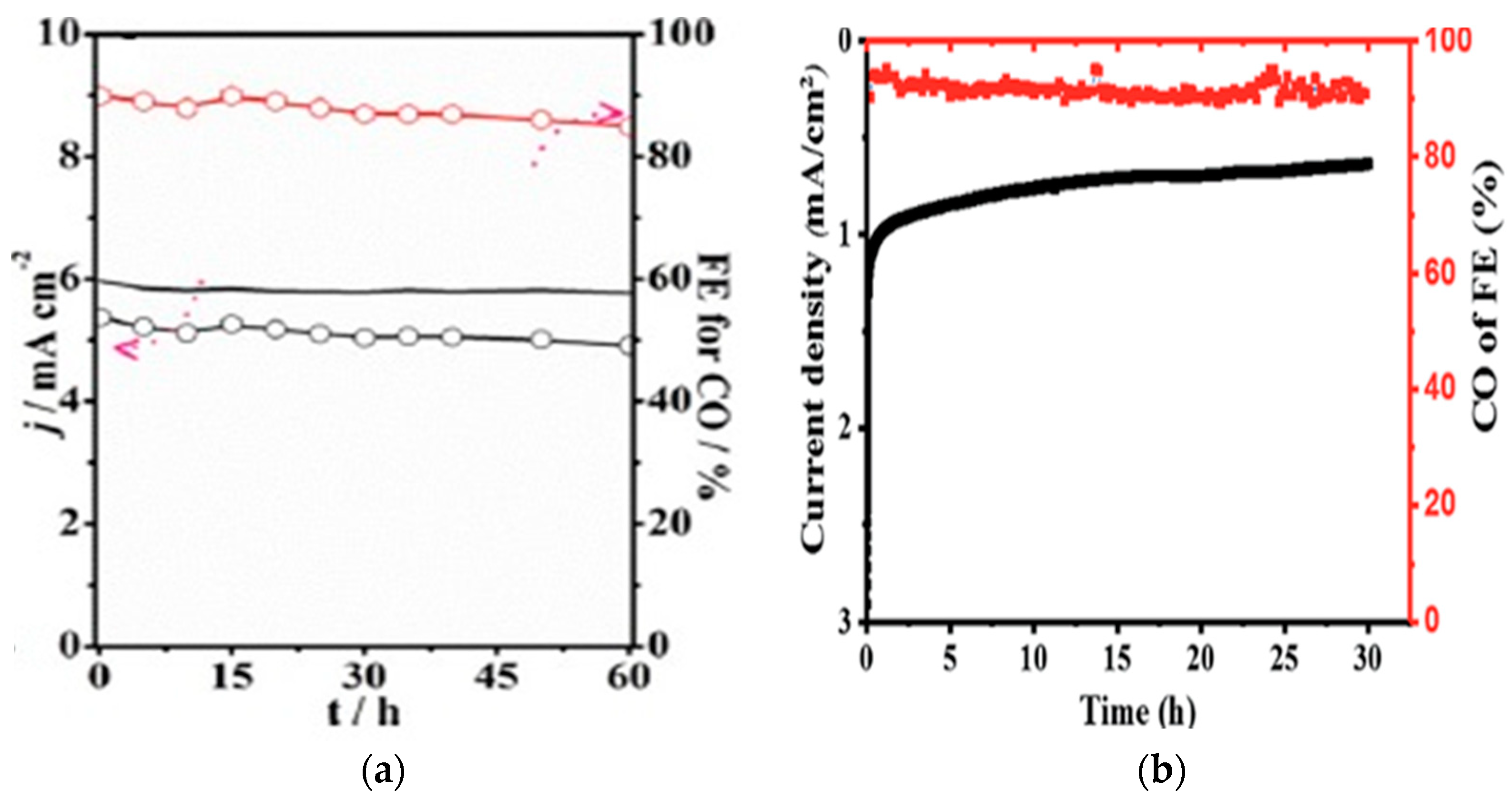
2.1.2. CO2 Electroreduction Performance
2.2. Boron-Doped Carbon Materials
2.2.1. Synthesis Methods
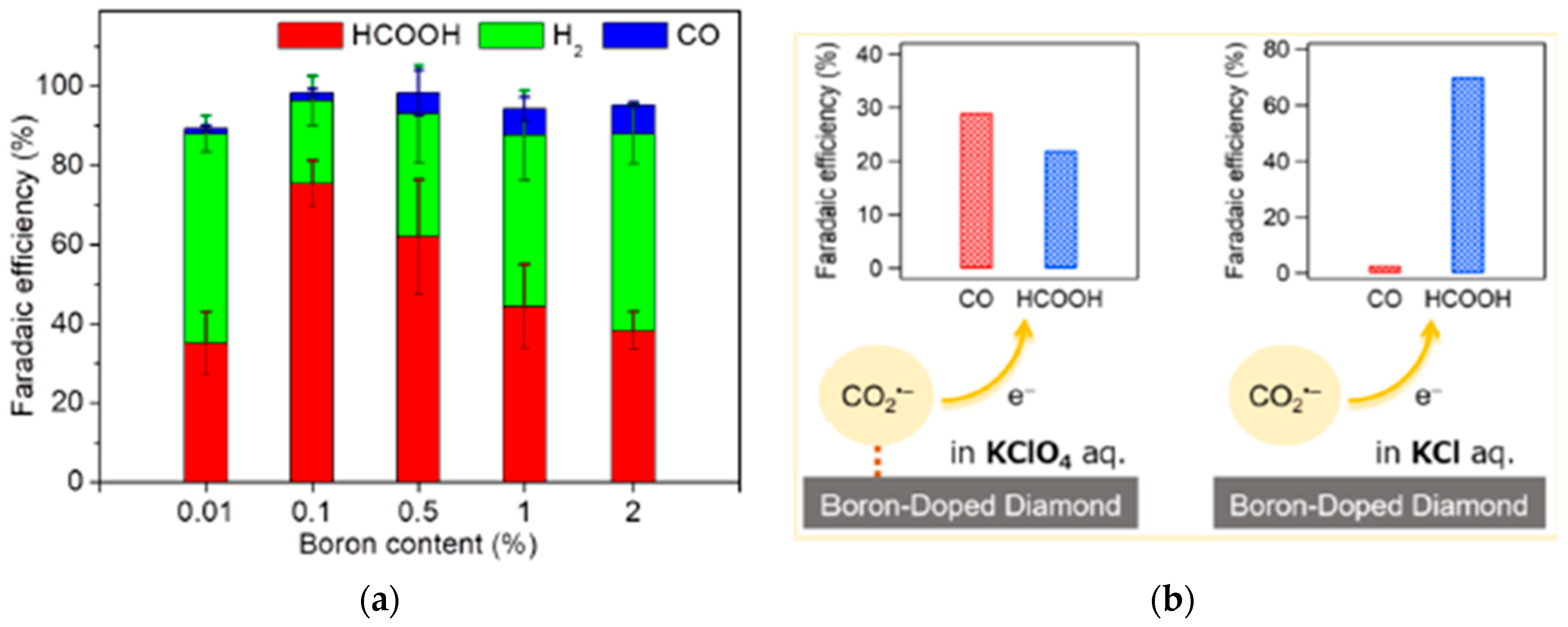
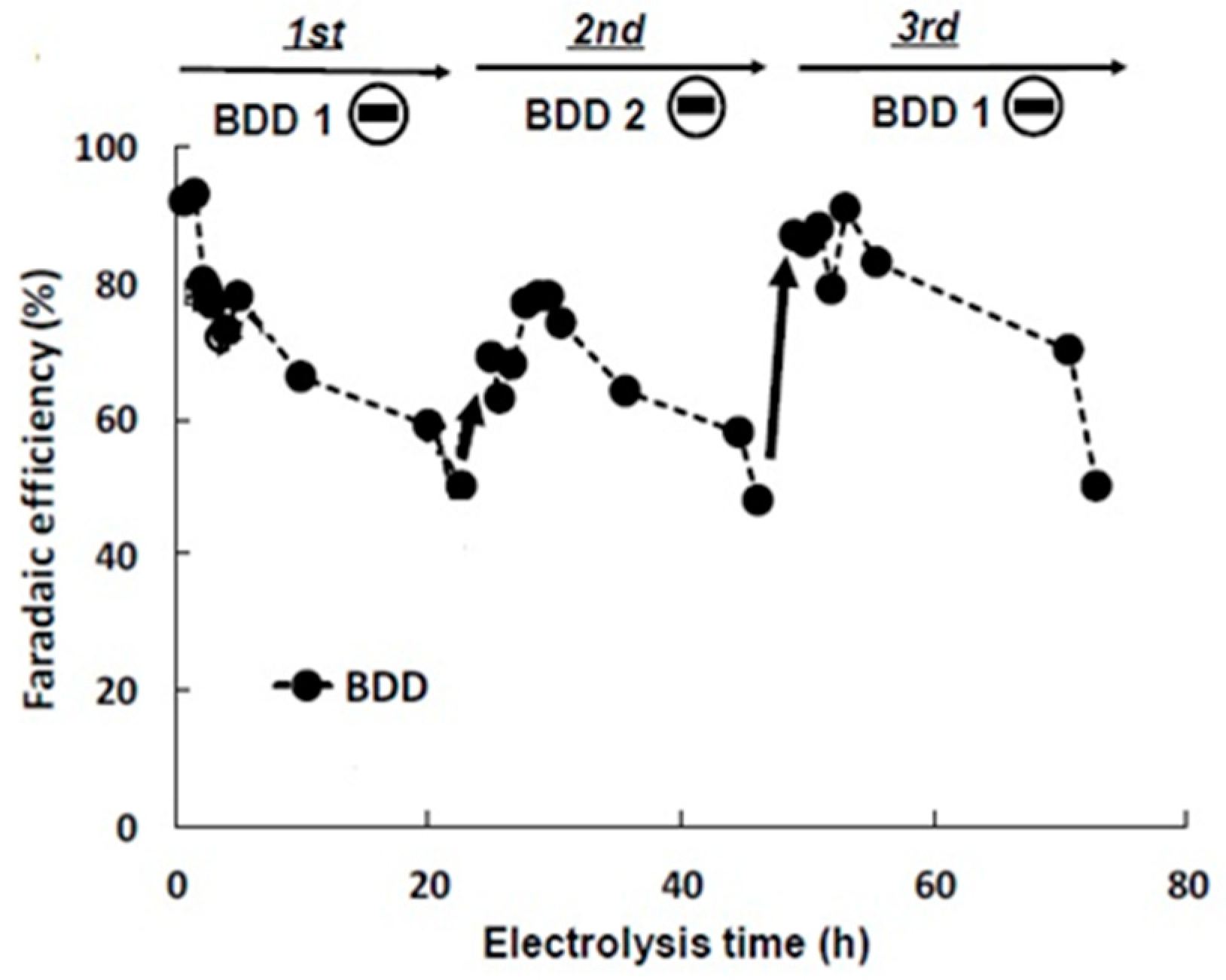
2.2.2. CO2 Electroreduction Performance
2.3. Sulfur-Doped Carbon Materials
2.3.1. Synthesis Methods
2.3.2. CO2 Electroreduction Performance
2.4. Fluorine-Doped Carbon Materials
2.4.1. Synthesis Methods
2.4.2. CO2 Electroreduction Performance
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- WMO (World Meteorological Organization). WMO Greenhouse Gas Bulletin. The State of Greenhouse Gases in the Atmosphere Based on Global Observations through 2018; WMO: Geneva, Switzerland, 2019. [Google Scholar]
- Petroleum British. BP Statistical Review of World Energy; Petroleum British: London, UK, 2019. [Google Scholar]
- WMO (World Meteorological Organization). The Global Climate 2015–2019; WMO: Geneva, Switzerland, 2019. [Google Scholar]
- Hoegh-Guldberg, O.; Jacob, M.D.; Taylor, M.; Bindi, S.; Brown, I.; Camilloni, A.; Diedhiou, R.; Djalante, K.L.; Ebi, F.; Engelbrecht, J.; et al. Impacts of 1.5 °C of Global Warming on Natural and Human Systems. In Global Warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change; V. Masson-Delmotte: Geneva, Switzerland, 2018; pp. 175–311. [Google Scholar]
- Abdmouleh, Z.; Gastli, A.; Ben-Brahim, L.; Haouari, M.; Al-Emadi, N.A. Review of optimization techniques applied for the integration of distributed generation from renewable energy sources. Renew. Energy 2017, 113, 266–280. [Google Scholar] [CrossRef]
- Li, L.; Wong-Ng, W.; Huang, K.; Cook, L.P. Materials and Processes for CO2 Capture, Conversion, and Sequestratin, 1st ed.; The American Ceramic Society, Wiley: Hoboken, NJ, USA, 2018; ISBN 9781119231066. [Google Scholar]
- Chen, C.; Kotyk, J.F.K.; Sheehan, S.W. Progress toward Commercial Application of Electrochemical Carbon Dioxide Reduction. Chem 2018, 4, 2571–2586. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y. On the Selective Heterogeneous CO2 Electroreduction to Methanol. ACS Catal. 2015, 5, 965–971. [Google Scholar] [CrossRef]
- Zhou, Z.Y.; Sun, S.G. A breakthrough in electrocatalysis of CO2 conversion. Natl. Sci. Rev. 2017, 4, 155–156. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Cai, F.; Wang, G.; Bao, X. Nanostructured heterogeneous catalysts for electrochemical reduction of CO2. Curr. Opin. Green Sustain. Chem. 2017, 3, 39–44. [Google Scholar] [CrossRef]
- Hashiba, H.; Weng, L.C.; Chen, Y.; Sato, H.K.; Yotsuhashi, S.; Xiang, C.; Weber, A.Z. Effects of electrolyte buffer capacity on surface reactant species and the reaction rate of CO2 in Electrochemical CO2 reduction. J. Phys. Chem. C 2018, 122, 3719–3726. [Google Scholar] [CrossRef] [Green Version]
- Pupo, M.M.d.P.; Kortlever, R. Electrolyte Effects on the Electrochemical Reduction of CO2. ChemPhysChem 2019, 20, 2926–2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, D.; Hahn, C.; Xiang, C.; Jaramillo, T.F.; Weber, A.Z. Gas-Diffusion Electrodes for Carbon Dioxide Reduction: A New Paradigm. ACS Energy Lett. 2019, 4, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Weng, L.C.; Bell, A.T.; Weber, A.Z. Modeling gas-diffusion electrodes for CO2 reduction. Phys. Chem. Chem. Phys. 2018, 20, 16973–16984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royer, M. Réduction de l’acide carbonique en acide formique. Compt. Rend. 1870, 70, 731–732. [Google Scholar]
- Hori, Y.; Suzuki, S. Electrolytic Reduction of Carbon Dioxide at Mercury Electrode in Aqueous Solution. Bull. Chem. Soc. Jpn. 1982, 55, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Hori, Y.; Suzuki, S. Cathodic Reduction of Carbon Dioxide for Energy Storage. J. Res. Inst. Catal. Hokkaido Univ. 1983, 30, 81–82. [Google Scholar]
- Hori, Y.; Kikuchi, K.; Suzuki, S. Production of CO and CH4 in Electrochemical Reduction of CO2 at metal electrodes in aqueos Hydrogen Carbonate Solution. Chem. Lett. 1985, 14, 1695–1698. [Google Scholar] [CrossRef]
- Hori, Y.; Kikuchi, K.; Murata, A.; Suzuki, S. Production of Methane and Ethylene in Electrochemical Reduction of Carbon Dioxide At Copper Electrode in Aqueous Hydrogencarbonate Solution. Chem. Lett. 1986, 15, 897–898. [Google Scholar] [CrossRef] [Green Version]
- She, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A Review of Catalysts for the Electroreduction of Carbon Dioxide to Produce Low-Carbon Fuels. Chem. Soc. Rev. 2014, 43, 8190600893. [Google Scholar] [CrossRef]
- Hossain, M.N.; Liu, Z.; Wen, J.; Chen, A. Enhanced catalytic activity of nanoporous Au for the efficient electrochemical reduction of carbon dioxide. Appl. Catal. B Environ. 2018, 236, 483–489. [Google Scholar] [CrossRef]
- Fan, T.; Wu, Q.; Yang, Z.; Song, Y.; Zhang, J.; Huang, P.; Chen, Z.; Dong, Y.; Fang, W.; Yi, X. Electrochemically Driven Formation of Sponge-Like Porous Silver Nanocubes Toward Efficient CO2 Electroreduction to CO. ChemSusChem 2020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, X.; Qiu, Y.; Su, P.; Xu, W.; Zhong, H.; Zhang, H. Multilayered Zn nanosheets as an electrocatalyst for efficient electrochemical reduction of CO2. J. Catal. 2018, 357, 154–162. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, L.; Yang, P.; Hu, C.; Luo, Z.; Chang, X.; Zhao, Z.J.; Gong, J. Low-Coordinated Edge Sites on Ultrathin Palladium Nanosheets Boost Carbon Dioxide Electroreduction Performance. Angew. Chem. 2018, 130, 11718–11722. [Google Scholar] [CrossRef]
- Lai, Q.; Yuan, W.; Huang, W.; Yuan, G. Sn/SnOx electrode catalyst with mesoporous structure for efficient electroreduction of CO2 to formate. Appl. Surf. Sci. 2020, 508, 145221. [Google Scholar] [CrossRef]
- Fan, M.; Garbarino, S.; Botton, G.A.; Tavares, A.C.; Guay, D. Selective electroreduction of CO2 to formate on 3D [100] Pb dendrites with nanometer-sized needle-like tips. J. Mater. Chem. A 2017, 5, 20747–20756. [Google Scholar] [CrossRef]
- Shao, L.; Lv, W.; Zhang, R.; Kong, F.; Cheng, L.; Wang, W. A highly efficient bi-based electrocatalyst for the reduction of CO2 to formate. Int. J. Electrochem. Sci. 2019, 14, 114–125. [Google Scholar] [CrossRef]
- Luo, W.; Xie, W.; Li, M.; Zhang, J.; Züttel, A. 3D hierarchical porous indium catalyst for highly efficient electroreduction of CO2. J. Mater. Chem. A 2019, 7, 4505–4515. [Google Scholar] [CrossRef]
- Larrazábal, G.O.; Martín, A.J.; Pérez-Ramírez, J. Building Blocks for High Performance in Electrocatalytic CO2 Reduction: Materials, Optimization Strategies, and Device Engineering. J. Phys. Chem. Lett. 2017, 8, 3933–3944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagger, A.; Ju, W.; Varela, A.S.; Strasser, P.; Rossmeisl, J. Electrochemical CO2 Reduction: A Classification Problem. ChemPhysChem 2017, 18, 3266–3273. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Huang, Y.; Ye, W.; Li, Y. CO2 Reduction: From the Electrochemical to Photochemical Approach. Adv. Sci. 2017, 4, 1700194. [Google Scholar] [CrossRef]
- Long, C.; Li, X.; Guo, J.; Shi, Y.; Liu, S.; Tang, Z. Electrochemical Reduction of CO2 over Heterogeneous Catalysts in Aqueous Solution: Recent Progress and Perspectives. Small Methods 2018, 3, 1800369. [Google Scholar] [CrossRef]
- Gao, D.; Zegkinoglou, I.; Divins, N.J.; Scholten, F.; Sinev, I.; Grosse, P.; Cuenya, B.R. Plasma-Activated Copper Nanocube Catalysts for Efficient Carbon Dioxide Electroreduction to Hydrocarbons and Alcohols. ACS Nano 2017, 11, 4825–4831. [Google Scholar] [CrossRef]
- Grosse, P.; Gao, D.; Scholten, F.; Sinev, I.; Mistry, H.; Cuenya, B.R. Dynamic Changes in the Structure, Chemical State and Catalytic Selectivity of Cu Nanocubes during CO2 Electroreduction: Size and Support Effects. Angew. Chem. Int. Ed. 2018, 57, 6192–6197. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Huang, S. Size effects and active sites of Cu nanoparticle catalysts for CO2 electroreduction. Appl. Surf. Sci. 2019, 475, 20–27. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Albo, J.; Irabien, A. Tailoring gas-phase CO2 electroreduction selectivity to hydrocarbons at Cu nanoparticles. Nanotechnology 2017, 29, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vennekoetter, J.B.; Sengpiel, R.; Wessling, M. Beyond the catalyst: How electrode and reactor design determine the product spectrum during electrochemical CO2 reduction. Chem. Eng. J. 2019, 364, 89–101. [Google Scholar] [CrossRef]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Irabien, A. Continuous Electrochemical Reduction of CO2 to Formate: Comparative Study of the Influence of the Electrode Configuration with Sn and Bi-Based Electrocatalysts. Molecules 2020, 25, 4457. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Zhang, J.; Li, M.; Züttel, A. Boosting CO Production in Electrocatalytic CO2 Reduction on Highly Porous Zn Catalysts. ACS Catal. 2019, 9, 3783–3791. [Google Scholar] [CrossRef] [Green Version]
- Duan, X.; Xu, J.; Wei, Z.; Ma, J.; Guo, S.; Wang, S.; Liu, H.; Dou, S. Metal-Free Carbon Materials for CO2 Electrochemical Reduction. Adv. Mater. 2017, 29, 1701784. [Google Scholar] [CrossRef]
- Cui, H.; Guo, Y.; Guo, L.; Wang, L.; Zhou, Z.; Peng, Z. Heteroatom-doped carbon materials and their composites as electrocatalysts for CO2 reduction. J. Mater. Chem. A 2018, 6, 18782–18793. [Google Scholar] [CrossRef]
- Liu, X.; Dai, L. Carbon-based metal-free catalysts. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
- Sangiorgi, N.; Tuci, G.; Sanson, A.; Peruzzini, M.; Giambastiani, G. Metal-free carbon-based materials for electrocatalytic and photo-electrocatalytic CO2 reduction. Rend. Lincei 2019, 30, 497–513. [Google Scholar] [CrossRef]
- Li, L.; Huang, Y.; Li, Y. Carbonaceous materials for electrochemical CO2 reduction. EnergyChem 2020, 2, 100024. [Google Scholar] [CrossRef]
- Wu, J.; Sharifi, T.; Gao, Y.; Zhang, T.; Ajayan, P.M. Emerging Carbon-Based Heterogeneous Catalysts for Electrochemical Reduction of Carbon Dioxide into Value-Added Chemicals. Adv. Mater. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Dastafkan, K.; Ren, W.; Yang, W.; Zhao, C. Carbon-based catalysts for electrochemical CO2 reduction. Sustain. Energy Fuels 2019. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.; Su, X.; Ding, J.; Mao, Q.; Huang, Y.; Zhang, T.; Liu, B. Rational design of carbon-based metal-free catalysts for electrochemical carbon dioxide reduction: A review. J. Energy Chem. 2019. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, D.M.; Peixoto, A.F.; Freire, C. Nitrogen-doped metal-free carbon catalysts for (electro)chemical CO2 conversion and valorisation. Dalton Trans. 2019, 48, 13508–13528. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Hou, X.; Ma, C.; Tan, T. Nitrogen-doped graphenes as efficient electrocatalysts for the selective reduction of carbon dioxide to formate in aqueous solution. Green Chem. 2016, 18, 3250–3256. [Google Scholar] [CrossRef]
- Sun, X.; Kang, X.; Zhu, Q.; Ma, J.; Yang, G.; Liu, Z.; Han, B. Very highly efficient reduction of CO2 to CH4 using metal-free N-doped carbon electrodes. Chem. Sci. 2016, 7, 2883–2887. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Zhi, W.Y.; Liu, L.; Yang, M.P.; Wang, H.; Lu, J.X. Electrochemical reduction of CO2 at metal-free N-functionalized graphene oxide electrodes. Electrochim. Acta 2018, 282, 694–701. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.; Huang, X.; Liu, L.; Cai, W.; Gao, J.; Li, X.; Zhang, T.; Huang, Y.; Liu, B. Identifying Active Sites of Nitrogen-Doped Carbon Materials for the CO2 Reduction Reaction. Adv. Funct. Mater. 2018, 28, 1800499. [Google Scholar] [CrossRef]
- Wu, J.; Ma, S.; Sun, J.; Gold, J.I.; Tiwary, C.; Kim, B.; Zhu, L.; Chopra, N.; Odeh, I.N.; Vajtai, R.; et al. A metal-free electrocatalyst for carbon dioxide reduction to multi-carbon hydrocarbons and oxygenates. Nat. Commun. 2016, 7, 13869. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Nwabara, U.O.; Kenis, P.J.A. Carbon-Based Electrodes and Catalysts for the Electroreduction of Carbon Dioxide (CO2) to Value-Added Chemicals; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 9783319929170. [Google Scholar]
- Wu, J.; Yadav, R.M.; Liu, M.; Sharma, P.P.; Tiwary, C.S.; Ma, L.; Zou, X.; Zhou, X.; Yakobson, B.I.; Lou, J.; et al. Achieving Highly Efficient, Selective, and Stable CO2 Reduction on Nitrogen-Doped Carbon Nanotubes. ACS Nano 2015, 9, 5364–5371. [Google Scholar] [CrossRef]
- Xu, J.; Kan, Y.; Huang, R.; Zhang, B.; Wang, B.; Wu, K.H.; Lin, Y.; Sun, X.; Li, Q.; Centi, G.; et al. Revealing the Origin of Activity in Nitrogen-Doped Nanocarbons towards Electrocatalytic Reduction of Carbon Dioxide. ChemSusChem 2016, 9, 1085–1089. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.; Hou, P.; Wang, X.; Wang, Z.; Li, W.; Kang, P. Carbon nanotubes with rich pyridinic nitrogen for gas phase CO2 electroreduction. Appl. Catal. B Environ. 2019, 250, 347–354. [Google Scholar] [CrossRef]
- Zhou, W.; Shen, H.; Wang, Q.; Onoe, J.; Kawazoe, Y.; Jena, P. N-doped peanut-shaped carbon nanotubes for efficient CO2 electrocatalytic reduction. Carbon N. Y. 2019, 152, 241–246. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, P.; Xu, J.; Han, J.; Wang, D.; Hao, C.; Alanagh, H.R.; Long, C.; Shi, X.; Tang, Z. MOF-derived nitrogen-doped nanoporous carbon for electroreduction of CO2 to CO: The calcining temperature effect and the mechanism. Nanoscale 2019, 11, 4911–4917. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zhong, H.; Jin, F. Zeolitic imidazolate frameworks derived nitrogen doped porous carbon for electrochemical reduction of CO2. IOP Conf. Ser. Earth Environ. Sci. 2020, 450. [Google Scholar] [CrossRef]
- Chen, Z.; Mou, K.; Yao, S.; Liu, L. Highly selective electrochemical reduction of CO2 to formate on metal-free nitrogen-doped PC61BM. J. Mater. Chem. A 2018, 6, 11236–11243. [Google Scholar] [CrossRef]
- Sun, Z.; Li, K.; Koh, S.W.; Jiao, L. Low-Cost and Scalable Fabrication of Hierarchically Porous N-Doped Carbon for Energy Storage and Conversion Application. ChemistrySelect 2020, 5, 533–537. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Quan, X.; Yu, H. Efficient Electrochemical Reduction of Carbon Dioxide to Acetate on Nitrogen-Doped Nanodiamond. J. Am. Chem. Soc. 2015, 137, 11631–11636. [Google Scholar] [CrossRef]
- Salinas-Torres, D.; Navlani-García, M.; Mori, K.; Kuwahara, Y.; Yamashita, H. Nitrogen-doped carbon materials as a promising platform toward the efficient catalysis for hydrogen generation. Appl. Catal. A Gen. 2019, 571, 25–41. [Google Scholar] [CrossRef]
- Liu, T.; Ali, S.; Lian, Z.; Li, B.; Su, D.S. CO2 electoreduction reaction on heteroatom-doped carbon cathode materials. J. Mater. Chem. A 2017, 5, 21596–21603. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Xiao, N.; Li, H.; Ji, Y.; Guo, Z.; Liu, C.; Qiu, J. Nitrogen-doped porous carbon from coal for high efficiency CO2 electrocatalytic reduction. Carbon N. Y. 2019, 151, 46–52. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, B.; Wang, B.; Wu, K.H.; Peng, Z.; Li, Q.; Centi, G.; Su, D.S. Decisive Intermediates Responsible for the Carbonaceous Products of CO2 Electro-reduction on Nitrogen-Doped sp2 Nanocarbon Catalysts in NaHCO3 Aqueous Electrolyte. ChemElectroChem 2017, 4, 1274–1278. [Google Scholar] [CrossRef]
- Li, H.; Xiao, N.; Wang, Y.; Li, C.; Ye, X.; Guo, Z.; Pan, X.; Liu, C.; Bai, J.; Xiao, J.; et al. Nitrogen-doped tubular carbon foam electrodes for efficient electroreduction of CO2 to syngas with potential-independent CO/H2 ratios. J. Mater. Chem. A 2019, 7, 18852–18860. [Google Scholar] [CrossRef]
- Kuang, M.; Guan, A.; Gu, Z.; Han, P.; Qian, L.; Zheng, G. Enhanced N-doping in mesoporous carbon for efficient electrocatalytic CO2 conversion. Nano Res. 2019, 12, 2324–2329. [Google Scholar] [CrossRef]
- Wang, H.; Jia, J.; Song, P.; Wang, Q.; Li, D.; Min, S.; Qian, C.; Wang, L.; Li, Y.F.; Ma, C.; et al. Efficient Electrocatalytic Reduction of CO2 by Nitrogen-Doped Nanoporous Carbon/Carbon Nanotube Membranes: A Step Towards the Electrochemical CO2 Refinery. Angew. Chem. Int. Ed. 2017, 56, 7847–7852. [Google Scholar] [CrossRef] [Green Version]
- Saravanan, K.; Gottlieb, E.; Keith, J.A. Nitrogen-doped nanocarbon materials under electroreduction operating conditions and implications for electrocatalysis of CO2. Carbon N. Y. 2017, 111, 859–866. [Google Scholar] [CrossRef]
- Siahrostami, S.; Jiang, K.; Karamad, M.; Chan, K.; Wang, H.; Nørskov, J. Theoretical Investigations into Defected Graphene for Electrochemical Reduction of CO2. ACS Sustain. Chem. Eng. 2017, 5, 11080–11085. [Google Scholar] [CrossRef]
- Silva, W.O.; Silva, G.C.; Webster, R.F.; Benedetti, T.M.; Tilley, R.D.; Ticianelli, E.A. Electrochemical Reduction of CO2 on Nitrogen-Doped Carbon Catalysts With and Without Iron. ChemElectroChem 2019, 6, 4626–4636. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, J.; Cai, Q. Pyrrolic-nitrogen doped graphene: A metal-free electrocatalyst with high efficiency and selectivity for the reduction of carbon dioxide to formic acid: A computational study. Phys. Chem. Chem. Phys. 2016, 18, 5491–5498. [Google Scholar] [CrossRef]
- Ma, T.; Fan, Q.; Tao, H.; Han, Z.; Jia, M.; Gao, Y.; Ma, W.; Sun, Z. Heterogeneous electrochemical CO2 reduction using nonmetallic carbon-based catalysts: Current status and future challenges. Nanotechnology 2017, 28, 472001. [Google Scholar] [CrossRef]
- Song, Y.; Chen, W.; Zhao, C.; Li, S.; Wei, W.; Sun, Y. Metal-Free Nitrogen-Doped Mesoporous Carbon for Electroreduction of CO2 to Ethanol. Angew. Chem. Int. Ed. 2017, 56, 10840–10844. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shang, L.; Chang, G.; Yan, C.; Shi, R.; Zhao, Y.; Waterhouse, G.I.N.; Yang, D.; Zhang, T. Intrinsic Carbon-Defect-Driven Electrocatalytic Reduction of Carbon Dioxide. Adv. Mater. 2019, 31, 1808276. [Google Scholar] [CrossRef] [PubMed]
- Wanninayake, N.; Ai, Q.; Zhou, R.; Hoque, M.A.; Herrell, S.; Guzman, M.I.; Risko, C.; Kim, D.Y. Understanding the effect of host structure of nitrogen doped ultrananocrystalline diamond electrode on electrochemical carbon dioxide reduction. Carbon N. Y. 2020, 157, 408–419. [Google Scholar] [CrossRef]
- Hursán, D.; Samu, A.A.; Janovák, L.; Artyushkova, K.; Asset, T.; Atanassov, P.; Janáky, C. Morphological Attributes Govern Carbon Dioxide Reduction on N-Doped Carbon Electrodes. Joule 2019, 3, 1719–1733. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Min, S.; Wang, F.; Zhang, Z.; Kong, C. Efficient electrocatalytic CO2 reduction to CO with high selectivity using a N-doped carbonized wood membrane. New J. Chem. 2020. [Google Scholar] [CrossRef]
- Zhu, Y.; Lv, K.; Wang, X.; Yang, H.; Xiao, G.; Zhu, Y. 1D/2D nitrogen-doped carbon nanorod arrays/ultrathin carbon nanosheets: Outstanding catalysts for the highly efficient electroreduction of CO2 to CO. J. Mater. Chem. A 2019, 7, 14895–14903. [Google Scholar] [CrossRef]
- Chen, C.; Sun, X.; Yan, X.; Wu, Y.; Liu, H.; Zhu, Q.; Bediako, B.B.A.; Han, B. Boosting CO2 electroreduction on N, P-co-doped carbon aerogels. Angew. Chem. 2020. [Google Scholar] [CrossRef]
- Ikemiya, N.; Natsui, K.; Nakata, K.; Einaga, Y. Effect of alkali-metal cations on the electrochemical reduction of carbon dioxide to formic acid using boron-doped diamond electrodes. RSC Adv. 2017, 7, 22510–22514. [Google Scholar] [CrossRef] [Green Version]
- Ikemiya, N.; Natsui, K.; Nakata, K.; Einaga, Y. Long-Term Continuous Conversion of CO2 to Formic Acid Using Boron-Doped Diamond Electrodes. ACS Sustain. Chem. Eng. 2018, 6, 8108–8112. [Google Scholar] [CrossRef]
- Tomisaki, M.; Natsui, K.; Ikemiya, N.; Nakata, K.; Einaga, Y. Influence of Electrolyte on the Electrochemical Reduction of Carbon Dioxide Using Boron-Doped Diamond Electrodes. ChemistrySelect 2018, 3, 10209–10213. [Google Scholar] [CrossRef]
- Jiwanti, P.K.; Natsui, K.; Nakata, K.; Einaga, Y. Selective production of methanol by the electrochemical reduction of CO2 on boron-doped diamond electrodes in aqueous ammonia solution. RSC Adv. 2016, 6, 102214–102217. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Cheng, K.; Quan, X.; Fan, X.; Su, Y.; Chen, S.; Zhao, H.; Zhang, Y.; Yu, H.; et al. Selective Electrochemical Reduction of Carbon Dioxide to Ethanol on a Boron- and Nitrogen-Co-doped Nanodiamond. Angew. Chem. Int. Ed. 2017, 56, 15607–15611. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Ren, W.; Chen, X.; Yang, W.; Zhao, C. (N, B) Dual Heteroatom-Doped Hierarchical Porous Carbon Framework for Efficient Electroreduction of Carbon Dioxide. ACS Sustain. Chem. Eng. 2020, 8, 6003–6010. [Google Scholar] [CrossRef]
- Sreekanth, N.; Nazrulla, M.A.; Vineesh, T.V.; Sailaja, K.; Phani, K.L. Metal-free boron-doped graphene for selective electroreduction of carbon dioxide to formic acid/formate. Chem. Commun. 2015, 51, 16061–16064. [Google Scholar] [CrossRef] [PubMed]
- Kumik, A.; Ivandini, T.A.; Wibowo, R. Modification of boron-doped diamond with gold-palladium nanoparticles for CO2 electroreduction. IOP Conf. Ser. Mater. Sci. Eng. 2020, 763. [Google Scholar] [CrossRef]
- Saprudin, M.H.; Atriardi, S.R.; Gunlazuardi, J.; Einaga, Y.; Ivandini, T.A. Electroreduction of carbon dioxide using platinum-iridium modified boron-doped diamond (BDD) with various platinum-iridium ratios. IOP Conf. Ser. Mater. Sci. Eng. 2020, 763, 012051. [Google Scholar] [CrossRef]
- Denala, D.; Khalil, M.; Ivandini, T.A. Preparation of boron doped diamond modified with cuprous oxide as working electrode for electroreduction of CO2. AIP Conf. Proc. 2019, 2168, 020057. [Google Scholar]
- Dewandaru, R.K.P.; Gunlazuardi, J.; Ivandini, T.A. Preparation of iridium-modified boron-doped diamond (BDD) electrodes for electroreduction of CO2. AIP Conf. Proc. 2018, 2023, 020098. [Google Scholar] [CrossRef]
- Ivandini, T.A. Electrochemical conversion of CO2 at metal-modified boron-doped diamond electrodes. AIP Conf. Proc. 2018, 2023, 020102. [Google Scholar] [CrossRef]
- Jasril Gunlazuardi, J.; Ivandini, T.A. Preparation of platinum-modified boron-doped diamond for electroreduction of CO2. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012037. [Google Scholar] [CrossRef] [Green Version]
- Jiwanti, P.K.; Ichzan, A.M.; Dewandaru, R.K.P.; Atriardi, S.R.; Einaga, Y.; Ivandini, T.A. Improving the CO2 electrochemical reduction to formic acid using iridium-oxide-modified boron-doped diamond electrodes. Diam. Relat. Mater. 2020, 106, 107874. [Google Scholar] [CrossRef]
- Jiwanti, P.K.; Aritonang, R.P.; Abdullah, I.; Einaga, Y.; Ivandini, T.A. Copper-nickel-modified Boron-doped Diamond Electrode for CO2 Electrochemical Reduction Application: A Preliminary Study. IOP Conf. Ser. Mater. Sci. Eng. 2019, 23, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Ichzan, A.M.; Gunlazuardi, J.; Ivandini, T.A. Preparation of boron doped diamond modified by iridium for electroreduction of carbon dioxide (CO2). IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012030. [Google Scholar] [CrossRef] [Green Version]
- Yetri, N.Y.; Ivandini, T.A.; Gunlazuardi, J. Preparation of copper oxide modified boron-doped diamond electrodes and its preliminary study for CO2 reduction. IOP Conf. Ser. Mater. Sci. Eng. 2017, 188, 012011. [Google Scholar] [CrossRef] [Green Version]
- Jiwanti, P.K.; Natsui, K.; Einaga, Y. The Utilization of Boron-doped Diamond Electrodes for the Electrochemical Reduction of CO2: Toward the production compounds with a high number of carbon atoms. Electrochemistry 2019, 87, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Natsui, K.; Iwakawa, H.; Ikemiya, N.; Nakata, K.; Einaga, Y. Stable and Highly Efficient Electrochemical Production of Formic Acid from Carbon Dioxide Using Diamond Electrodes. Angew. Chem. Int. Ed. 2018, 57, 2639–2643. [Google Scholar] [CrossRef]
- Xu, J.; Natsui, K.; Naoi, S.; Nakata, K.; Einaga, Y. Effect of doping level on the electrochemical reduction of CO2 on boron-doped diamond electrodes. Diam. Relat. Mater. 2018, 86, 167–172. [Google Scholar] [CrossRef]
- Tomisaki, M.; Kasahara, S.; Natsui, K.; Ikemiya, N.; Einaga, Y. Switchable Product Selectivity in the Electrochemical Reduction of Carbon Dioxide Using Boron-Doped Diamond Electrodes. J. Am. Chem. Soc. 2019, 141, 7414–7420. [Google Scholar] [CrossRef]
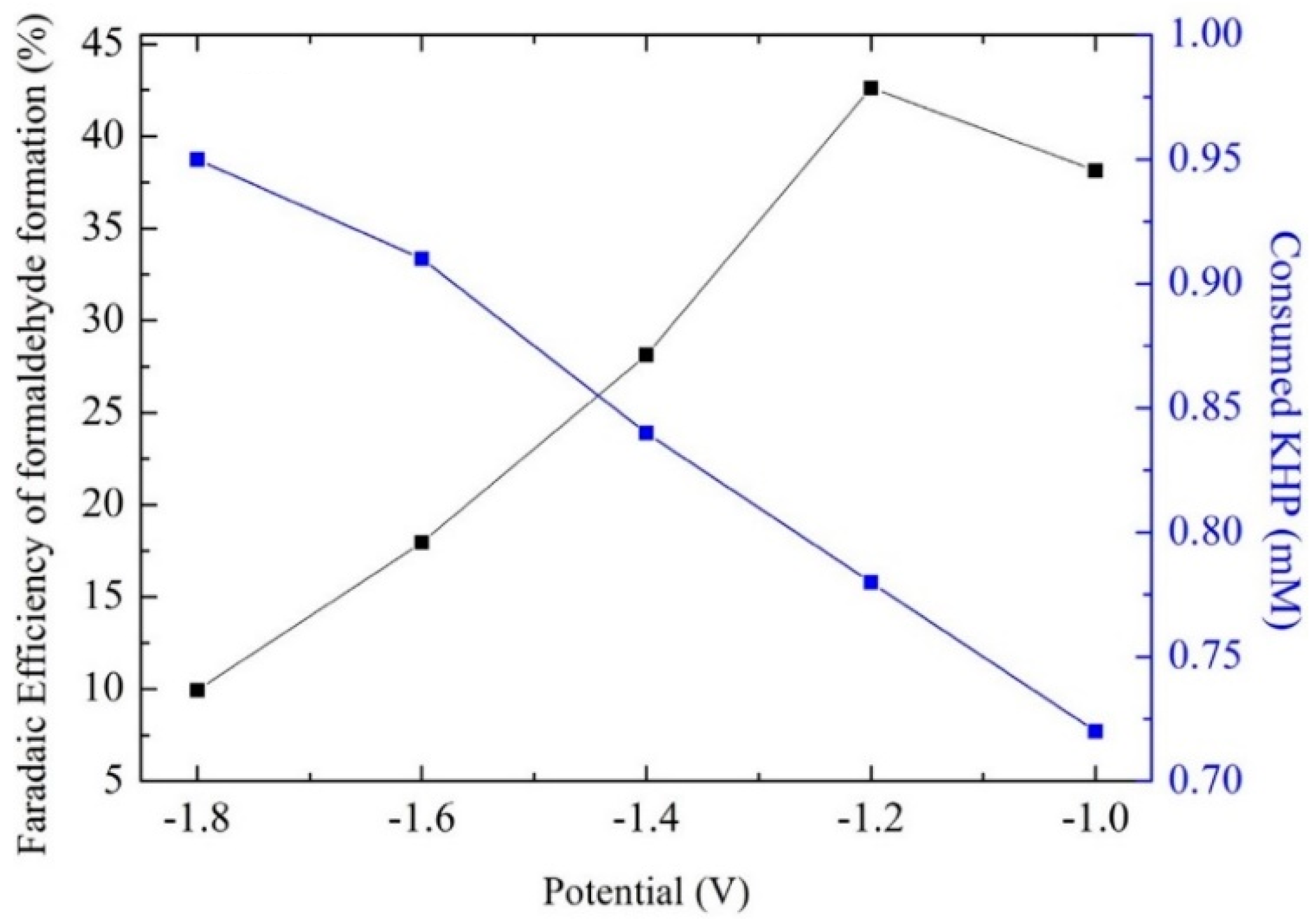
- Luo, D.; Liu, S.; Nakata, K.; Fujishima, A. Electrochemical reduction of CO2 and degradation of KHP on boron-doped diamond electrodes in a simultaneous and enhanced process. Chin. Chem. Lett. 2019, 30, 509–512. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Z.; Zhao, J. Metal-free graphdiyne doped with sp-hybridized boron and nitrogen atoms at acetylenic sites for high-efficiency electroreduction of CO2 to CH4 and C2H4. J. Mater. Chem. A 2019, 7, 4026–4035. [Google Scholar] [CrossRef]
- Mou, S.; Wu, T.; Xie, J.; Zhang, Y.; Ji, L.; Huang, H.; Wang, T.; Luo, Y.; Xiong, X.; Tang, B.; et al. Boron Phosphide Nanoparticles: A Nonmetal Catalyst for High-Selectivity Electrochemical Reduction of CO2 to CH3OH. Adv. Mater. 2019, 31, 1903499. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Song, S.; Li, K.; Li, J.; Pan, J.; Yao, S.; Ge, X.; Feng, J.; Wang, X.; Zhang, H. A “Solid Dual-Ions-Transformation” Route to S,N Co-Doped Carbon Nanotubes as Highly Efficient “Metal-Free” Catalysts for Organic Reactions. Adv. Mater. 2016, 28, 10679–10683. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Seredych, M.; Rodríguez-Castellón, E.; Bandosz, T.J. Metal-free Nanoporous Carbon as a Catalyst for Electrochemical Reduction of CO2 to CO and CH4. ChemSusChem 2016, 9, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Li, B.; Deng, W.; Du, Z.; Gang, Y.; Wang, G.; Li, Y. Promoting electrocatalytic CO2 reduction on nitrogen-doped carbon with sulfur addition. Appl. Catal. B Environ. 2019, 252, 240–249. [Google Scholar] [CrossRef]
- Yang, H.; Wu, Y.; Lin, Q.; Fan, L.; Chai, X.; Zhang, Q.; Liu, J.; He, C.; Lin, Z. Composition Tailoring via N and S Co-doping and Structure Tuning by Constructing Hierarchical Pores: Metal-Free Catalysts for High-Performance Electrochemical Reduction of CO2. Angew. Chem. 2018, 130, 15702–15706. [Google Scholar] [CrossRef]
- Li, G.; Qin, Y.; Wu, Y.; Pei, L.; Hu, Q.; Yang, H.; Zhang, Q.; Liu, J.; He, C. Nitrogen and sulfur dual-doped high-surface-area hollow carbon nanospheres for efficient CO2 reduction. Chin. J. Catal. 2020, 41, 830–838. [Google Scholar] [CrossRef]
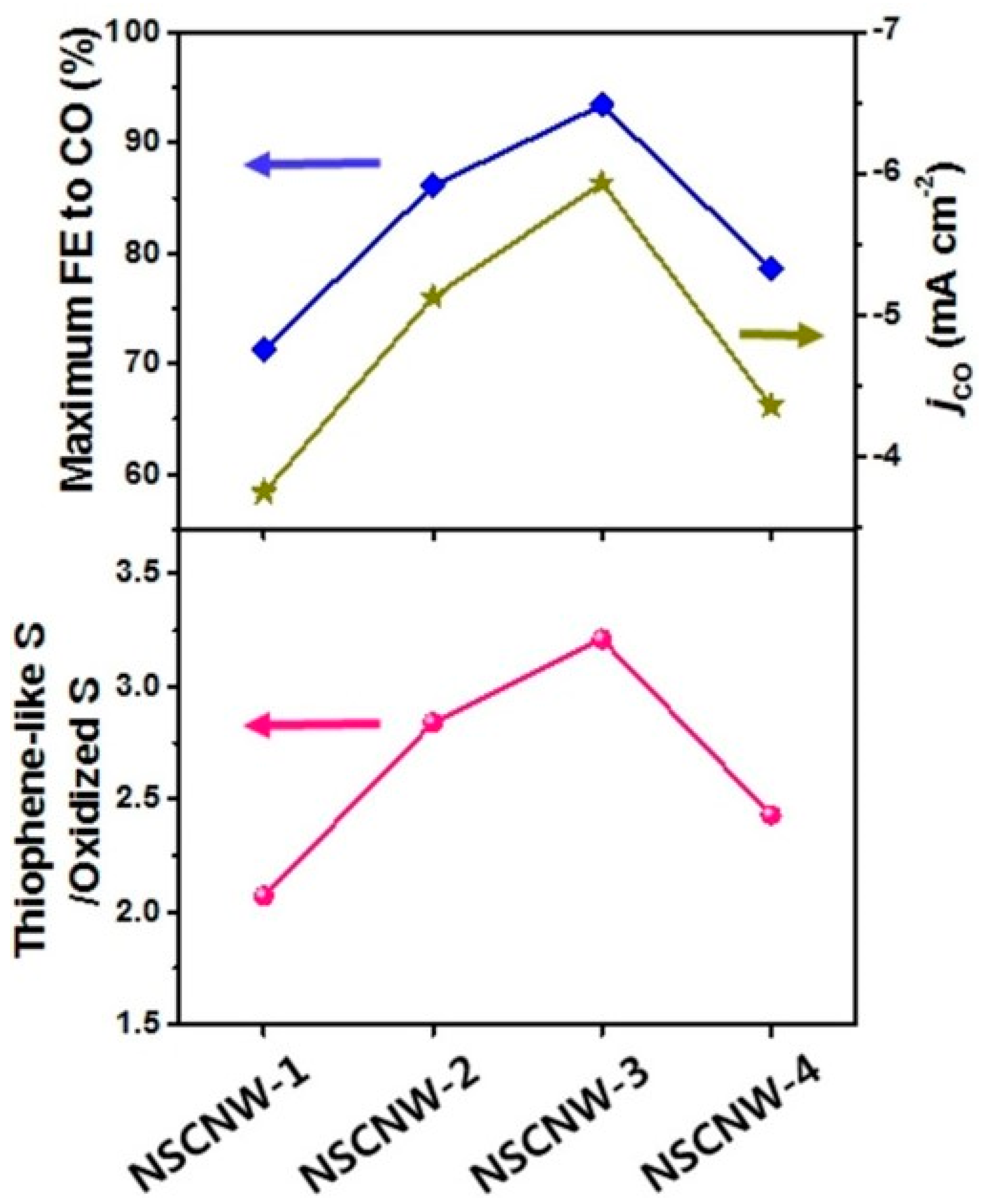
- Han, H.; Park, S.; Jang, D.; Lee, S.; Kim, W.B. Electrochemical Reduction of CO2 to CO by N,S Dual-Doped Carbon Nanoweb Catalysts. ChemSusChem 2020, 13, 539–547. [Google Scholar] [CrossRef]
- Wang, G.; Liu, M.; Jia, J.; Xu, H.; Zhao, B.; Lai, K.; Tu, C.; Wen, Z. Nitrogen and Sulfur Co-doped Carbon Nanosheets for Electrochemical Reduction of CO2. ChemCatChem 2020, 12, 2203–2208. [Google Scholar] [CrossRef]
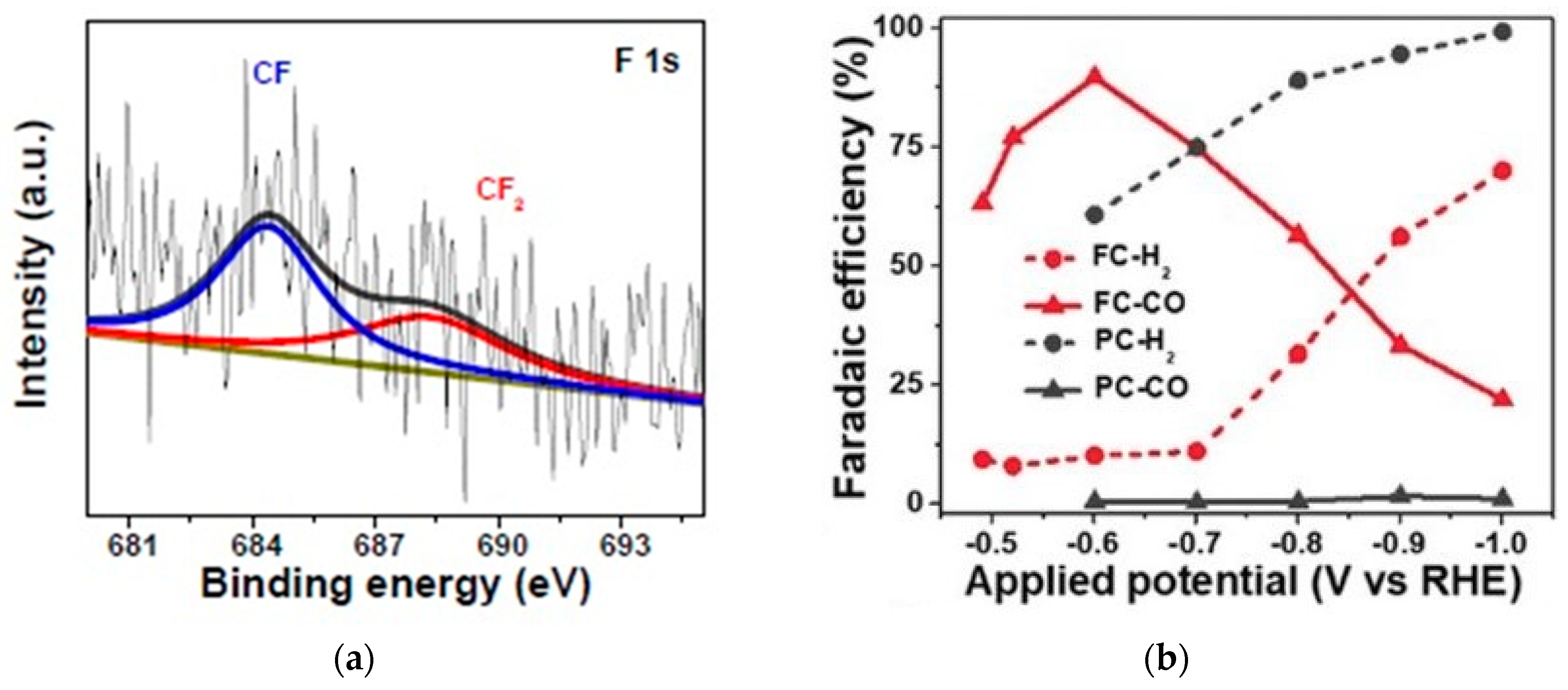
- Xie, J.; Zhao, X.; Wu, M.; Li, Q.; Wang, Y.; Yao, J. Metal-Free Fluorine-Doped Carbon Electrocatalyst for CO 2 Reduction Outcompeting Hydrogen Evolution. Angew. Chem. 2018, 130, 9788–9792. [Google Scholar] [CrossRef]
- Ni, W.; Xue, Y.; Zang, X.; Li, C.; Wang, H.; Yang, Z.; Yan, Y.M. Fluorine Doped Cagelike Carbon Electrocatalyst: An Insight into the Structure-Enhanced CO Selectivity for CO2 Reduction at High Overpotential. ACS Nano 2020, 14, 2014–2023. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Simple one-step synthesis of fluorine-doped carbon nanoparticles as potential alternative metal-free electrocatalysts for oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 9972–9981. [Google Scholar] [CrossRef]
- Zaderko, A.N.; Horodetska, D.S.; Grishchenko, L.M.; Diyuk, V.E.; Boldyrieva, O.Y.; Lisnyak, V.V.; Novychenko, N.S.; Skryshevsky, V.A.; Khomenko, V.G. Fluororganic Groups Grafted on Carbon Microspheres. In Proceedings of the 2019 IEEE 9th International Conference on Nanomaterials: Applications and Properties, NAP 2019, Odesa, Ukraine, 15–20 September 2019; pp. 15–20. [Google Scholar]
- Pu, L.; Ma, Y.; Zhang, W.; Hu, H.; Zhou, Y.; Wang, Q.; Pei, C. Simple method for the fluorinated functionalization of graphene oxide. RSC Adv. 2013, 3, 3881–3884. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, J.; Wang, G.; Li, Y.; Wen, Z. Perfluorinated Covalent Triazine Framework Derived Hybrids for the Highly Selective Electroconversion of Carbon Dioxide into Methane. Angew. Chem. Int. Ed. 2018, 57, 13120–13124. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Li, B.; Xiang, X.; Wang, G.; Li, Y. Efficient CO2 Electroreduction by Highly Dense and Active Pyridinic Nitrogen on Holey Carbon Layers with Fluorine Engineering. ACS Catal. 2019, 9, 2124–2133. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Sequera, A.C.; Díaz-Pérez, M.A.; Serrano-Ruiz, J.C. Recent Advances in the Electroreduction of CO2 over Heteroatom-Doped Carbon Materials. Catalysts 2020, 10, 1179. https://doi.org/10.3390/catal10101179
Pérez-Sequera AC, Díaz-Pérez MA, Serrano-Ruiz JC. Recent Advances in the Electroreduction of CO2 over Heteroatom-Doped Carbon Materials. Catalysts. 2020; 10(10):1179. https://doi.org/10.3390/catal10101179
Chicago/Turabian StylePérez-Sequera, Ana Cristina, Manuel Antonio Díaz-Pérez, and Juan Carlos Serrano-Ruiz. 2020. "Recent Advances in the Electroreduction of CO2 over Heteroatom-Doped Carbon Materials" Catalysts 10, no. 10: 1179. https://doi.org/10.3390/catal10101179
APA StylePérez-Sequera, A. C., Díaz-Pérez, M. A., & Serrano-Ruiz, J. C. (2020). Recent Advances in the Electroreduction of CO2 over Heteroatom-Doped Carbon Materials. Catalysts, 10(10), 1179. https://doi.org/10.3390/catal10101179






