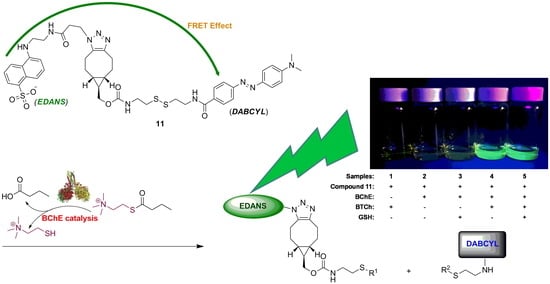
A Bioorthogonally Synthesized and Disulfide-Containing Fluorescence Turn-On Chemical Probe for Measurements of Butyrylcholinesterase Activity and Inhibition in the Presence of Physiological Glutathione
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis of the FRET Chemical Probe 11
2.2. FRET and Fluorogenic Properties of the FRET Chemical Probe 11
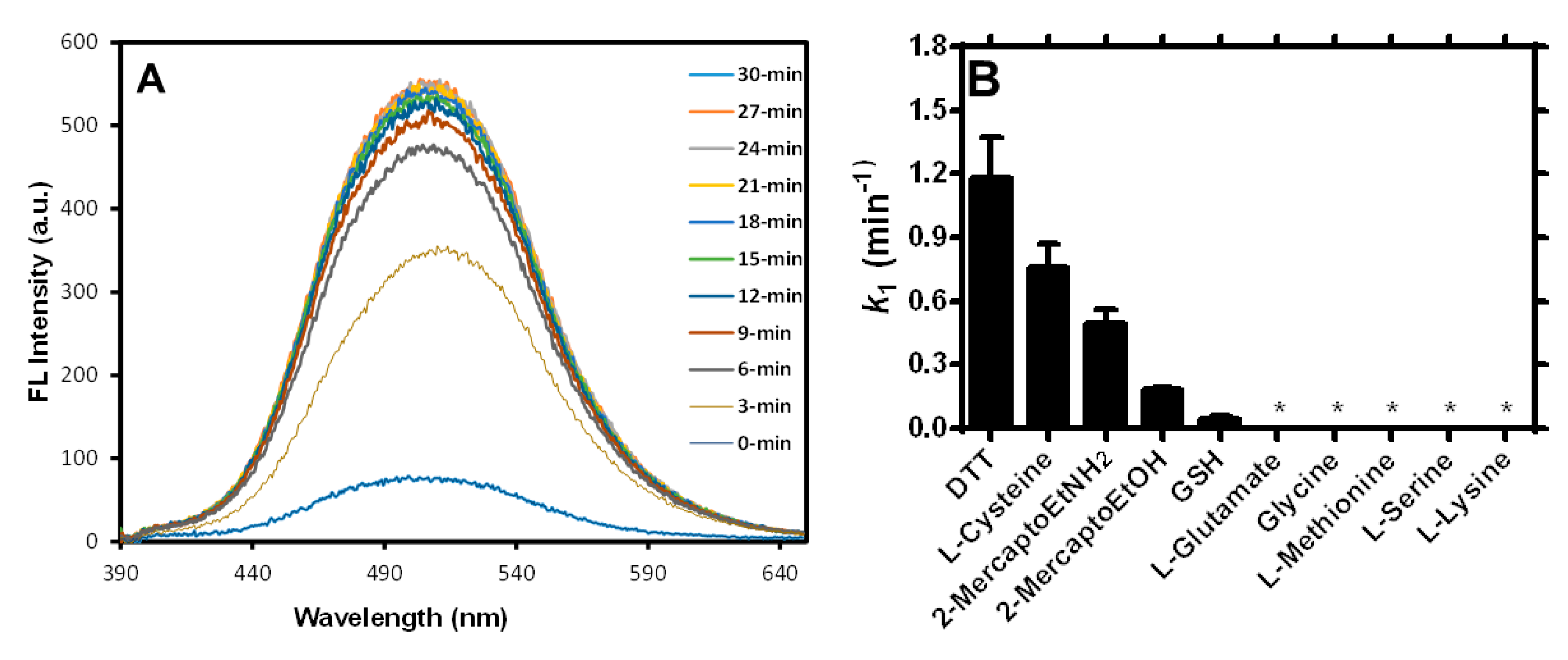
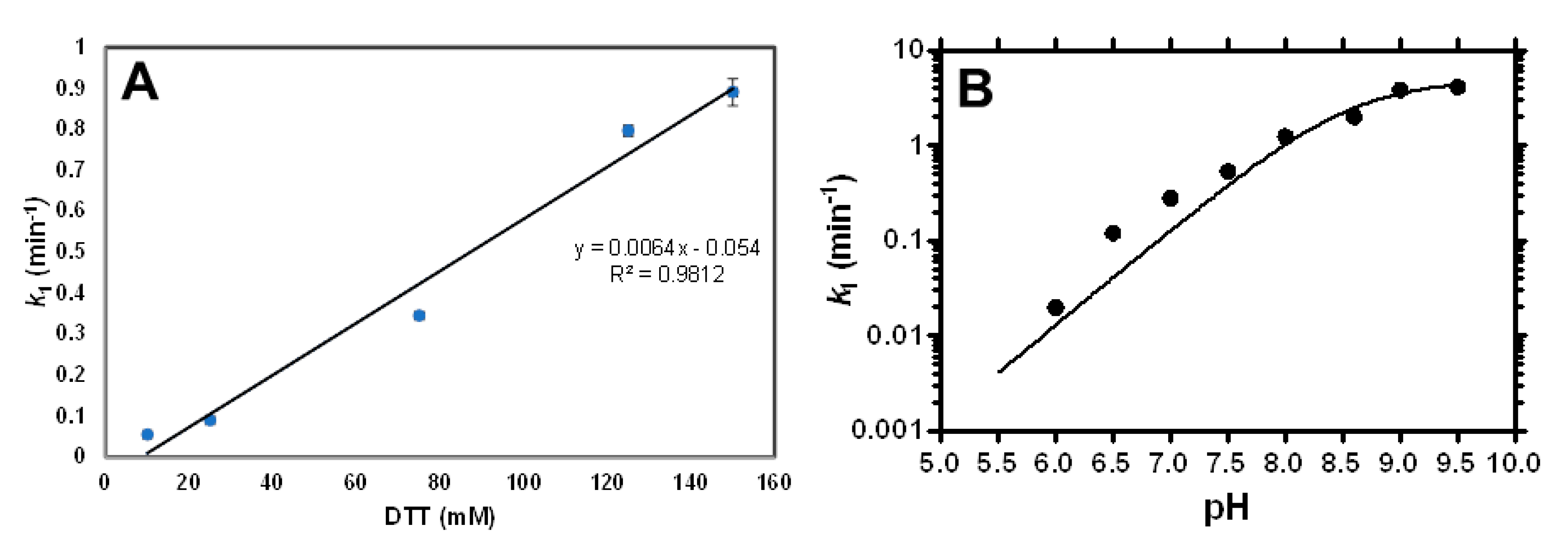
2.3. Study of the Fluorogenic Reaction Mechanism of 11 with DTT
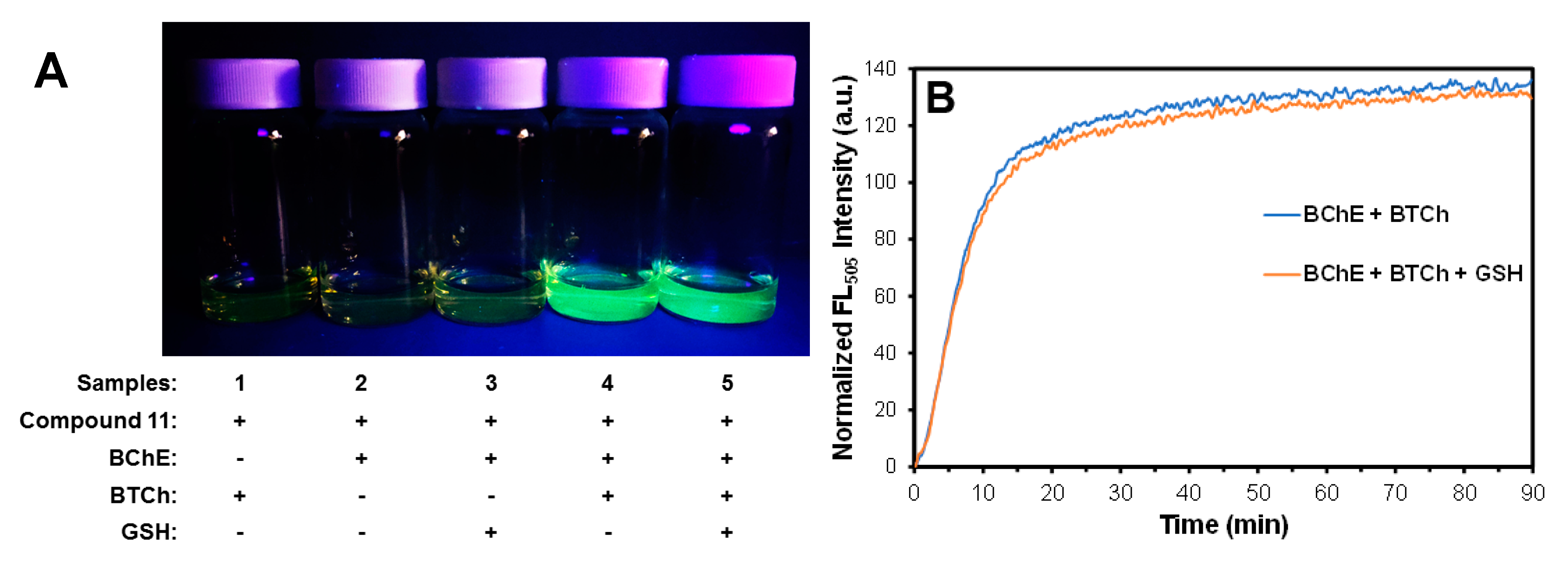
2.4. Deciphering the Ability of 11 to Discriminate Against Reactions with GSH
2.5. GSH not a Source of Interference in the 11-Based Fluorescence Turn-On Assay for Measuring BChE Activity
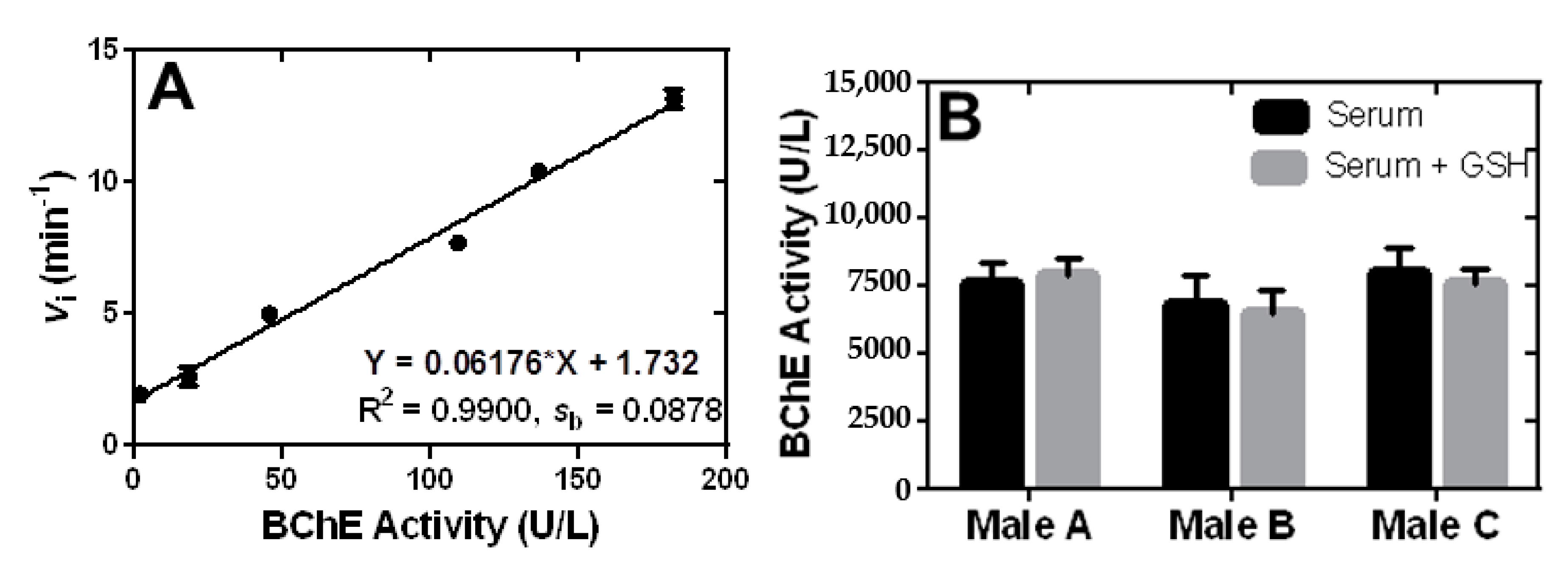
2.6. Direct and Accurate Determination of BChE Activity in Human Serum by the Fluorescence Turn-On Assay Based on 11
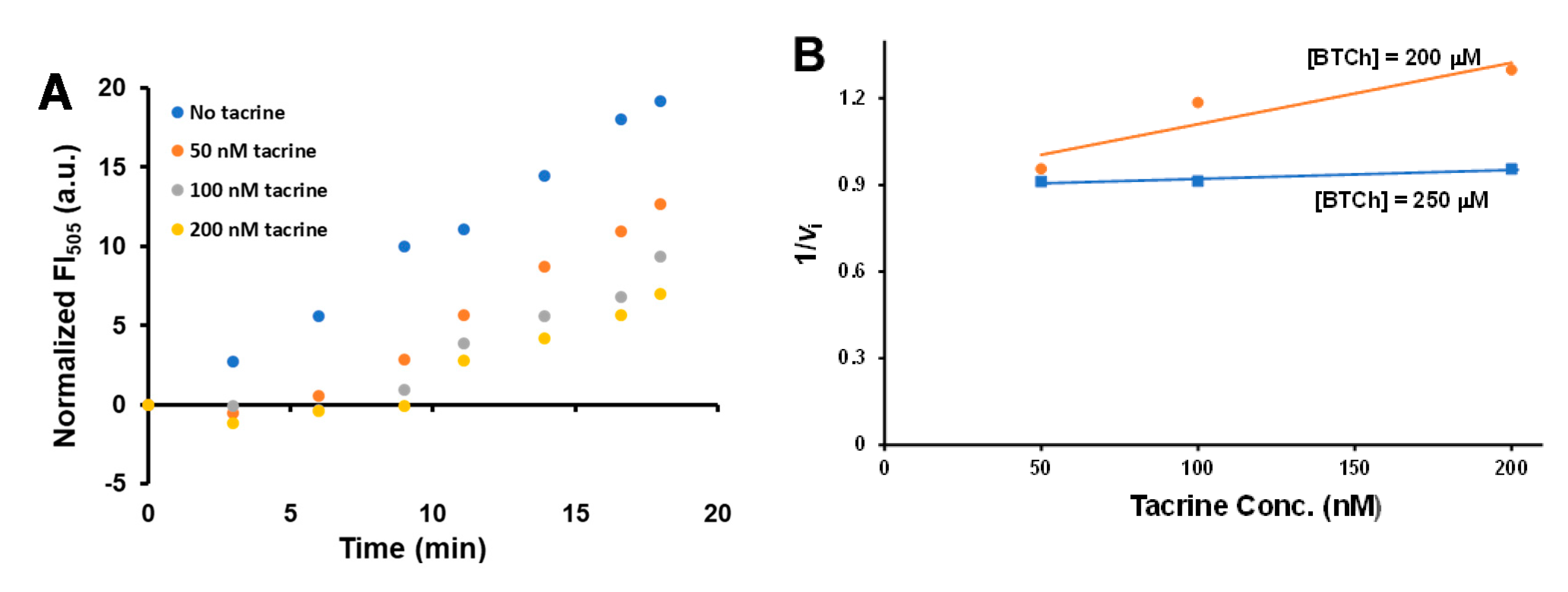
2.7. The 11-Based Fluorescence Assay for Kinetic Analysis of BChE Inhibition
3. Materials and Methods
3.1. Synthesis of the Key Bicyclononyne Derivatives (6)
3.1.1. (1. R,8S,9r,Z)-Ethyl Bicyclo [6.1.0]non-4-ene-9-Carboxylate (exo-2) and (1R,8S,9s,Z)-Ethyl Bicyclo[6.1.0]non-4-ene-9-Carboxylate (endo-2)
3.1.2. [(1. R,4Z,8S,9r)-Bicyclo[6.1.0]non-4-en-9-yl]Methanol (exo-3), [(1R,4Z,8S,9s)-Bicyclo[6.1.0]non-4-en-9-yl]Methanol (endo-3), (1R,8S,9r)-Bicyclo[6.1.0]non-4-yn-9-Ylmethanol (exo-4), (1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-Ylmethanol (endo-4), (1R,8S,9r)-Bicyclo[6.1.0]non-4-yn-9-Ylmethyl (4-nitrophenyl) Carbonate (exo-5), and (1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-Ylmethyl (4-Nitrophenyl) Carbonate (endo-5)
3.1.3. [(1. R,8S,9r)-Bicyclo[6.1.0]non-4-yn-9-yl]Methyl {2-[(2-Aminoethyl)Disulfanyl]ethyl}Carbamate (exo-6)
3.1.4. [(1. R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-yl]Methyl {2-[(2-Aminoethyl)Disulfanyl]ethyl}Carbamate (endo-6)
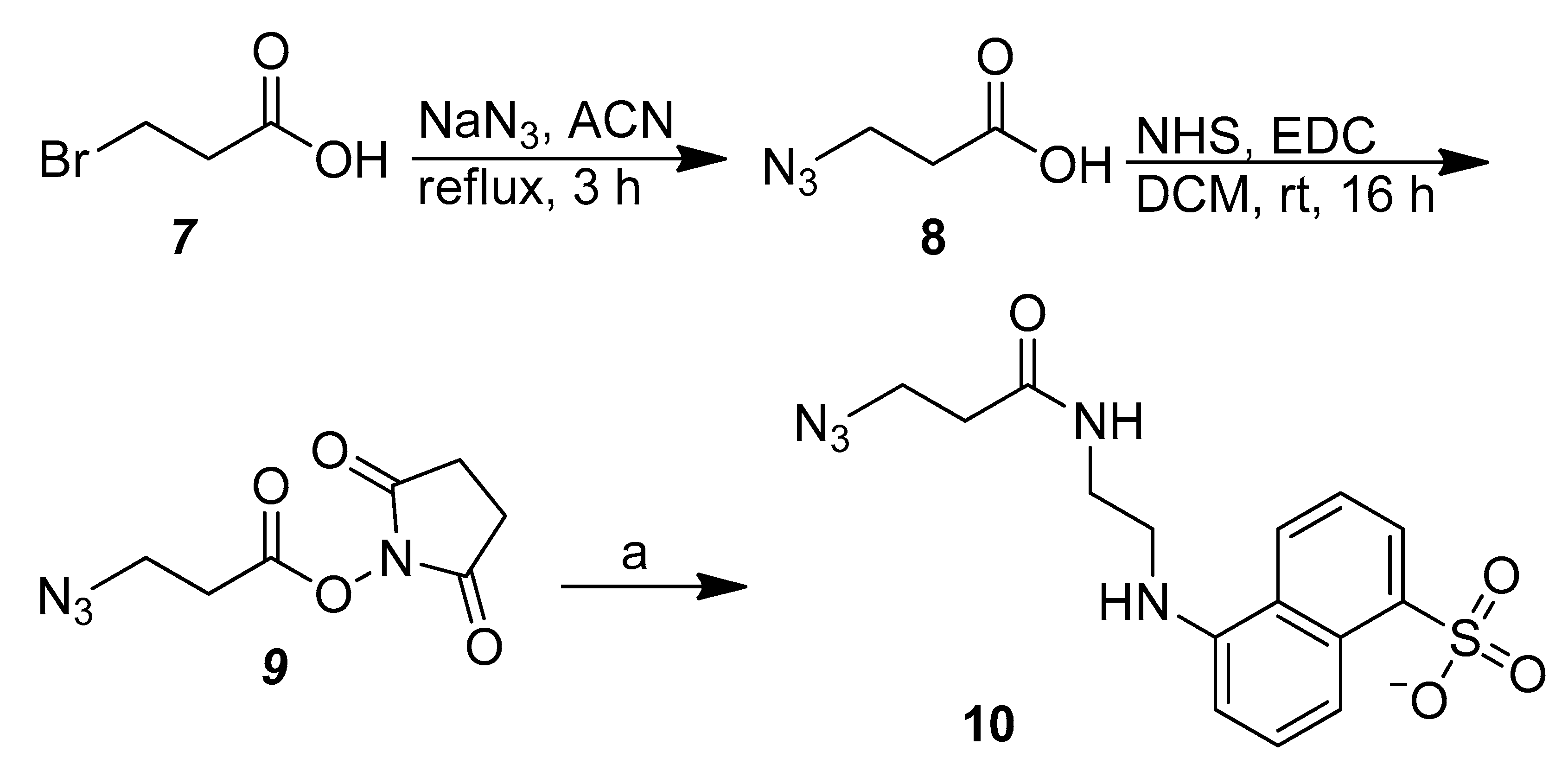
3.2. Synthesis of the Azido EDANS Derivative (10)
3.2.1. 3-Azidopropanoic acid (8) and 1-[(3-Azidopropanoyl)oxy]Pyrrolidine-2,5-Dione (9)
3.2.2. 5-{[2-(3-Azidopropanamido)Ethyl]Amino}Naphthalene-1-Sulfonate (10)
3.3. Synthesis of the EDANS–DABCYL-Paired FRET Chemical Probe (11)
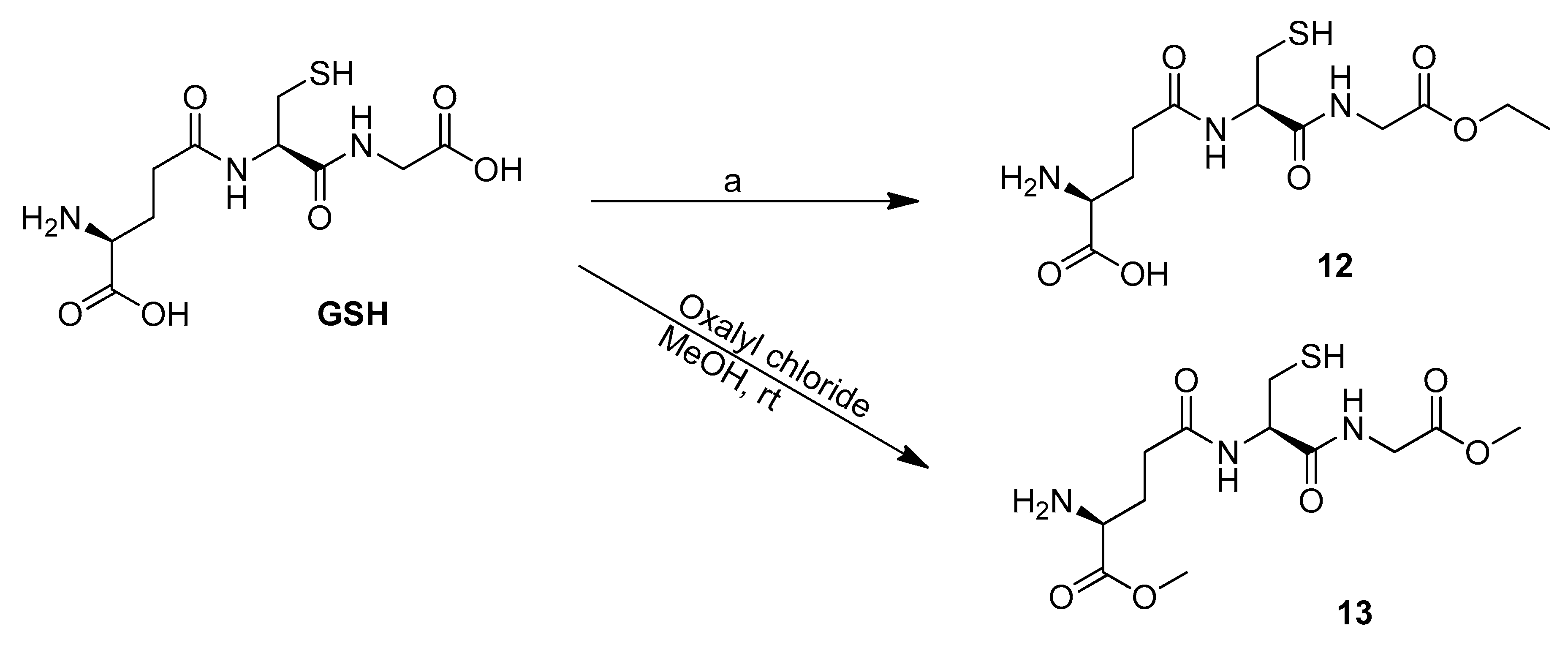
3.4. Synthesis of the GSH Derivatives, 12 and 13
3.4.1. Methyl (2S)-2-Amino-5-({(2R)-1-[(2-Ethoxy-2-Oxoethyl)Amino]-1-oxo-3-Sulfanylpropan-2-yl}Amino)-5-Oxopentanoic Acid (12)
3.4.2. (2. S)-2-Amino-5-({(2R)-1-[(2-Methoxy-2-Oxoethyl)Amino]-1-oxo-3-Sulfanylpropan-2-yl}Amino)-5-Oxopentanoate (13)
3.5. Spectroscopic Measurements
3.6. Specificity and Mechanism of the FRET Chemical Probe 11 Reactions with Thiols
3.7. The 11-Based Assay for Fluorescence Turn-On Analysis of BChE Activity in Serum and in the Presence of a BChE Inhibitor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase |
| BChE | butyrylcholinesterase |
| BTCh | S-butyrylthiocholine iodide |
| ChE | cholinesterase |
| CHES | 2-(cyclohexylamino)ethanesulfonic acid |
| DABCYL | 4-[4-(dimethylamino)phenylazo]benzoic acid |
| DABCYL NHS ester | N-succinimidyl 4-[4-(dimethylamino)phenylazo]benzoate |
| DCM | dichloromethane |
| DIPEA | N,N-diisopropylethylamine |
| DMF | dimethylformamide |
| DTNB | 5′-dithiobis(2-nitrobenzoic acid) |
| DTT | DL-dithiothreitol |
| EDANS | 5-(2-aminoethylamino)-1-naphthalenesulfonic acid |
| EPPS | 4-(2-hydroxyethyl)-1-piperazinepropanesulfonic acid |
| Et3N | trimethylamine |
| Et2O | diethyl ether |
| EtOH | ethanol |
| FRET | fluorescence resonance energy transfer |
| FT-MS | Fourier-transfer mass spectrometry |
| GSH | glutathione |
| LOD | limit of detection |
| MeOH | methanol |
| MES | 2-(N-morpholino)ethanesulfonic acid |
| PB | phosphate buffer |
| PBS | phosphate buffered saline |
| PIPES | 1,4-piperazinediethanesulfonic acid |
| SPAAC | strained-promoted azide-alkyne cycloaddition |
| TCh | thiocholine |
References
- Lockridge, O. Review of human butyrylcholinesterase structure, function, genetic variants, history of use in the clinic, and potential therapeutic uses. Pharmacol. Ther. 2015, 148, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Pope, C.N.; Brimijoin, S. Cholinesterases and the fine line between poison and remedy. Biochem. Pharmacol. 2018, 153, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.V.; Gao, Y.; Geng, L.; Brimijoin, S. Butyrylcholinesterase gene transfer in obese mice prevents postdieting body weight rebound by suppressing ghrelin signaling. Proc. Natl. Acad. Sci. USA 2017, 114, 10960–10965. [Google Scholar] [CrossRef]
- Poetsch, N.; Sturdza, A.; Aust, S.; Polterauer, S.; Grimm, C.; Schwameis, R.; Pötter, R.; Koelbl, H.; Reinthaller, A.; Seebacher, V. The value of pretreatment serum butyrylcholinesterase level as a novel prognostic biomarker in patients with cervical cancer treated with primary (chemo-)radiation therapy. Strahlenther. Onkol. 2019, 195, 430–440. [Google Scholar] [CrossRef]
- Zivkovic, A.R.; Schmidt, K.; Stein, T.; Münzberg, M.; Brenner, T.; Weigand, M.A.; Kleinschmidt, S.; Hofer, S. Bedside-measurement of serum cholinesterase activity predicts patient morbidity and length of the intensive care unit stay following major traumatic injury. Sci. Rep. 2019, 9, 10437. [Google Scholar] [CrossRef]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef]
- Santarpia, L.; Grandone, I.; Contaldo, F.; Pasanisi, F. Butyrylcholinesterase as a prognostic marker: A review of the literature. J. Cachex Sarcopenia Muscle 2012, 4, 31–39. [Google Scholar] [CrossRef]
- Klocker, E.V.; Barth, D.A.; Riedl, J.M.; Prinz, F.; Szkandera, J.; Schlick, K.; Kornprat, P.; Lackner, C.; Lindenmann, J.; Stoeger, H.; et al. Decreased Activity of Circulating Butyrylcholinesterase in Blood Is an Independent Prognostic Marker in Pancreatic Cancer Patients. Cancers 2020, 12, 1154. [Google Scholar] [CrossRef]
- Chen, G.; Feng, H.; Jiang, X.; Xu, J.; Pan, S.; Qian, Z. Redox-Controlled Fluorescent Nanoswitch Based on Reversible Disulfide and Its Application in Butyrylcholinesterase Activity Assay. Anal. Chem. 2018, 90, 1643–1651. [Google Scholar] [CrossRef]
- Meden, A.; Knez, D.; Jukič, M.; Brazzolotto, X.; Gršič, M.; Pišlar, A.; Zahirović, A.; Kos, J.; Nachon, F.; Svete, J.; et al. Tryptophan-derived butyrylcholinesterase inhibitors as promising leads against Alzheimer’s disease. Chem. Commun. 2019, 55, 3765–3768. [Google Scholar] [CrossRef]
- Zhou, S.; Yuan, Y.; Zheng, F.; Zhan, C.-G. Structure-based virtual screening leading to discovery of highly selective butyrylcholinesterase inhibitors with solanaceous alkaloid scaffolds. Chem. Interact. 2019, 308, 372–376. [Google Scholar] [CrossRef]
- Jing, L.; Wu, G.; Kang, D.; Zhou, Z.; Song, Y.; Liu, X.; Zhan, P. Contemporary medicinal-chemistry strategies for the discovery of selective butyrylcholinesterase inhibitors. Drug Discov. Today 2019, 24, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, H.; Chen, Y.; Sun, H. Recent progress in the identification of selective butyrylcholinesterase inhibitors for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 132, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Pajk, S.; Knez, D.; Košak, U.; Zorović, M.; Brazzolotto, X.; Coquelle, N.; Nachon, F.; Colletier, J.-P.; Živin, M.; Stojan, J.; et al. Development of potent reversible selective inhibitors of butyrylcholinesterase as fluorescent probes. J. Enzym. Inhib. Med. Chem. 2020, 35, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xiong, H.; Yang, J.-Q.; Yang, S.-H.; Li, Y.; Yang, W.; Yang, G.-F. Discovery of Butyrylcholinesterase-Activated Near-Infrared Fluorogenic Probe for Live-Cell and In Vivo Imaging. ACS Sens. 2018, 3, 2118–2128. [Google Scholar] [CrossRef]
- Seo, M.; Yamada, T.; Tamaki, S.; Hikoso, S.; Yasumura, Y.; Higuchi, Y.; Nakagawa, Y.; Uematsu, M.; Abe, H.; Fuji, H.; et al. Prognostic Significance of Serum Cholinesterase Level in Patients With Acute Decompensated Heart Failure With Preserved Ejection Fraction: Insights From the PURSUIT-HFpEF Registry. J. Am. Hear. Assoc. 2020, 9, 014100. [Google Scholar] [CrossRef] [PubMed]
- Cerasoli, D.M.; Armstrong, S.J.; Reeves, T.E.; Hodgins, S.M.; Kasten, S.A.; Lee-Stubbs, R.B.; Cadieux, C.L.; Otto, T.C.; Capacio, B.R.; Lenz, D.E. Butyrylcholinesterase, a stereospecific in vivo bioscavenger against nerve agent intoxication. Biochem. Pharmacol. 2019, 171, 113670. [Google Scholar] [CrossRef]
- Vallianou, N.G.; Evangelopoulos, A.; Bountziouka, V.; Bonou, M.; Katsagoni, C.; Vogiatzakis, E.D.; Avgerinos, P.C.; Barbetseas, J.; Panagiotakos, D.B.; Katsagoni, C. Association of butyrylcholinesterase with cardiometabolic risk factors among apparently healthy adults. J. Cardiovasc. Med. 2014, 15, 377–383. [Google Scholar] [CrossRef]
- Adam, E.H.; Haas, V.; Lindau, S.; Zacharowski, K.; Scheller, B. Cholinesterase alterations in delirium after cardiosurgery: A German monocentric prospective study. BMJ Open 2020, 10, e031212. [Google Scholar] [CrossRef]
- Pohanka, M. Butyrylcholinesterase as a biochemical marker. Bratisl. Lek. List. 2013, 114, 726–734. [Google Scholar] [CrossRef]
- Reed, B.A.; Sabourin, C.L.; Lenz, D.E. Human butyrylcholinesterase efficacy against nerve agent exposure. J. Biochem. Mol. Toxicol. 2017, 31, e21886. [Google Scholar] [CrossRef] [PubMed]
- Lockridge, O.; Norgren, R.B., Jr.; Johnson, R.C.; Blake, T.A. Naturally Occurring Genetic Variants of Human Acetylcholinesterase and Butyrylcholinesterase and Their Potential Impact on the Risk of Toxicity from Cholinesterase Inhibitors. Chem. Res. Toxicol. 2016, 29, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ni, Y.; Tian, X. Low Plasma Cholinesterase Activity is Associated with Postoperative Delirium After Noncardiac Surgery in Elderly Patients: A Prospective Observational Study. J. Psychosom. Res. 2019, 60, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Biswas, S.; Rahman, M.; Rahman, A.; Siddique, A.E.; Karim, Y.; Aktar, S.; Nikkon, F.; Haque, A.; Himeno, S.; et al. Butyrylcholinesterase—A potential plasma biomarker in manganese-induced neurobehavioral changes. Environ. Sci. Pollut. Res. 2019, 26, 6378–6387. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; He, N.; Zhu, J.-J. History and New Developments of Assays for Cholinesterase Activity and Inhibition. Chem. Rev. 2010, 110, 5216–5234. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. Voltammetric assay of butyrylcholinesterase in plasma samples and its comparison to the standard spectrophotometric test. Talanta 2014, 119, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Qin, W. Potentiometric sensing of butyrylcholinesterase based on in situ generation and detection of substrates. Chem. Commun. 2009, 971–973. [Google Scholar] [CrossRef]
- El-Rahman, M.K.A.; Eid, S.M.; Elghobashy, M.R.; Kelani, K.M. Inline potentiometric monitoring of Butyrylcholinesterase activity based on metabolism of bambuterol at the point of care. Sens. Actuators B Chem. 2019, 285, 216–223. [Google Scholar] [CrossRef]
- Wang, L.; Du, D.; Lu, D.; Lin, C.-T.; Smith, J.N.; Timchalk, C.; Liu, F.; Wang, J.; Lin, Y. Enzyme-linked immunosorbent assay for detection of organophosphorylated butyrylcholinesterase: A biomarker of exposure to organophosphate agents. Anal. Chim. Acta 2011, 693, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, H.; Chen, Z.; Wu, T.; Jiang, Z.; Tong, L.; Tang, B. A Simple 3D-Printed Enzyme Reactor Paper Spray Mass Spectrometry Platform for Detecting BuChE Activity in Human Serum. Anal. Chem. 2019, 91, 12874–12881. [Google Scholar] [CrossRef] [PubMed]
- Jońca, J.; Żuk, M.; Wasąg, B.; Janaszak-Jasiecka, A.; Lewandowski, K.; Wielgomas, B.; Waleron, K.; Jasiecki, J. New Insights into Butyrylcholinesterase Activity Assay: Serum Dilution Factor as a Crucial Parameter. PLoS ONE 2015, 10, e0139480. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Jones, D.P.; Carlson, J.L.; Samiec, P.S.; Sternberg, P.; Mody, V.C.; Reed, R.L.; Brown, L.A.S. Glutathione measurement in human plasma. Clin. Chim. Acta 1998, 275, 175–184. [Google Scholar] [CrossRef]
- Pohanka, M.; Drtinova, L. Spectrophotometric methods based on 2,6-dichloroindophenol acetate and indoxylacetate for butyrylcholinesterase activity assay in plasma. Talanta 2013, 106, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Cinti, S.; Caratelli, V.; Massoud, R.; Saraji, M.; Moscone, D.; Arduini, F. A 96-well wax printed Prussian Blue paper for the visual determination of cholinesterase activity in human serum. Biosens. Bioelectron. 2019, 134, 97–102. [Google Scholar] [CrossRef]
- Lu, L.; Xia, Y. Enzymatic Reaction Modulated Gold Nanorod End-to-End Self-Assembly for Ultrahigh Sensitively Colorimetric Sensing of Cholinesterase and Organophosphate Pesticides in Human Blood. Anal. Chem. 2015, 87, 8584–8591. [Google Scholar] [CrossRef]
- Li, P.Y.; Chen, Y.; Chen, C.H.; Liu, Y. Multi-charged bis(p-calixarene)/pillararene functionalized gold nanoparticles for ultra-sensitive sensing of butyrylcholinesterase. Soft Matter 2019, 15, 8197–8200. [Google Scholar] [CrossRef]
- Guilbault, G.G.; Kramer, D.N. Resorufin Butyrate and Indoxyl Acetate as Fluorogenic Substrates for Cholinesterase. Anal. Chem. 1965, 37, 120–123. [Google Scholar] [CrossRef]
- Kang, S.; Yang, W.; Oh, J.; Lee, S.; Seo, J. A direct assay of butyrylcholinesterase activity using a fluorescent substrate. Org. Biomol. Chem. 2016, 14, 8815–8820. [Google Scholar] [CrossRef]
- Yang, S.-H.; Sun, Q.; Xiong, H.; Liu, S.; Moosavi, B.; Yang, W.-C.; Yang, G.-F. Discovery of a butyrylcholinesterase-specific probe via a structure-based design strategy. Chem. Commun. 2017, 53, 3952–3955. [Google Scholar] [CrossRef]
- Yoo, S.; Oh, J. A fluorescent probe for butyrylcholinesterase activity in human serum based on a fluorophore with specific binding affinity for human serum albumin. Chem. Commun. 2019, 55, 14574–14577. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Feng, H.; Xi, W.; Xu, J.; Pan, S.; Qian, Z. Thiol–ene click reaction-induced fluorescence enhancement by altering the radiative rate for assaying butyrylcholinesterase activity. Analyst 2019, 144, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Chen, S.; Li, S.; Liu, Q.; Kou, M.; Xu, T.; Luo, H.; Huang, K.; Zhang, M. Enzymatic reaction modulation of G-quadruplex formation for the sensitive homogeneous fluorescence sensing of cholinesterase and organophosphate pesticides. Anal. Methods 2019, 11, 980–988. [Google Scholar] [CrossRef]
- Deng, W.; Chen, P.; Hu, P.; He, Z.; Zhang, M.; Yuan, X.; Huang, K. Enzymatic reaction modulated synthesis of quantum dots for visual detection of cholinesterase activity and inhibitor. Sens. Actuators B Chem. 2019, 292, 180–186. [Google Scholar] [CrossRef]
- Xu, X.; Cen, Y.; Xu, G.; Wei, F.; Shi, M.; Hu, Q. A ratiometric fluorescence probe based on carbon dots for discriminative and highly sensitive detection of acetylcholinesterase and butyrylcholinesterase in human whole blood. Biosens. Bioelectron. 2019, 131, 232–236. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Zhao, C.-X.; Shu, Y.; Wang, J. Gold Nanoclusters/Iron Oxyhydroxide Platform for Ultrasensitive Detection of Butyrylcholinesterase. Anal. Chem. 2019, 91, 15866–15872. [Google Scholar] [CrossRef]
- Li, T.; Gao, Y.; Li, H.; Zhang, C.; Xing, Y.; Jiao, M.; Shi, Y.-E.; Li, W.; Zhai, Y.; Wang, Z.; et al. Ultrasensitive detection of butyrylcholinesterase activity based on the inner filter effect of MnO2 nanosheets on sulfur nanodots. Analyst 2020, 145, 5206–5212. [Google Scholar] [CrossRef]
- Dommerholt, J.; Schmidt, S.; Temming, R.; Hendriks, L.J.A.; Rutjes, F.P.J.T.; Van Hest, J.C.M.; Lefeber, D.J.; Friedl, P.; Van Delft, F.L. Readily Accessible Bicyclononynes for Bioorthogonal Labeling and Three-Dimensional Imaging of Living Cells. Angew. Chem. Int. Ed. 2010, 49, 9422–9425. [Google Scholar] [CrossRef]
- Clegg, R.M.; Murchie, A.I.H.; Zechel, A.; Carlberg, C.; Diekmann, S.; Lilley, D.M.J. Fluorescence resonance energy transfer analysis of the structure of the four-way DNA junction. Biochemistry 1992, 31, 4846–4856. [Google Scholar] [CrossRef]
- Piggott, A.M.; Karuso, P. Fluorometric Assay for the Determination of Glutathione Reductase Activity. Anal. Chem. 2007, 79, 8769–8773. [Google Scholar] [CrossRef]
- Su, Y.-C.; Lo, Y.-L.; Hwang, C.-C.; Wang, L.-F.; Wu, M.H.; Wang, E.-C.; Wang, Y.-M.; Wang, T.-P. Azide–alkyne cycloaddition for universal post-synthetic modifications of nucleic acids and effective synthesis of bioactive nucleic acid conjugates. Org. Biomol. Chem. 2014, 12, 6624–6633. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-C.; Chen, H.-Y.; Ko, N.C.; Hwang, C.-C.; Wu, M.H.; Wang, L.-F.; Wang, Y.-M.; Chang, S.-N.; Wang, E.-C.; Wang, T.-P. Effective and site-specific phosphoramidation reaction for universally labeling nucleic acids. Anal. Biochem. 2014, 449, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Lukesh, I.J.C.; Wallin, K.K.; Raines, R.T. Pyrazine-derived disulfide-reducing agent for chemical biology. Chem. Commun. 2014, 50, 9591–9594. [Google Scholar] [CrossRef]
- MirzaHosseini, A.; Somlyay, M.; Noszál, B. The comprehensive acid–base characterization of glutathione. Chem. Phys. Lett. 2015, 622, 50–56. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, M.-M.; Dai, C.-Y.; Severance, S.; Hwang, C.-C.; Fang, B.-K.; Lin, H.-B.; Huang, C.-H.; Ong, C.-W.; Wang, J.-J.; Lee, P.-L.; et al. A Bioorthogonally Synthesized and Disulfide-Containing Fluorescence Turn-On Chemical Probe for Measurements of Butyrylcholinesterase Activity and Inhibition in the Presence of Physiological Glutathione. Catalysts 2020, 10, 1169. https://doi.org/10.3390/catal10101169
Gong M-M, Dai C-Y, Severance S, Hwang C-C, Fang B-K, Lin H-B, Huang C-H, Ong C-W, Wang J-J, Lee P-L, et al. A Bioorthogonally Synthesized and Disulfide-Containing Fluorescence Turn-On Chemical Probe for Measurements of Butyrylcholinesterase Activity and Inhibition in the Presence of Physiological Glutathione. Catalysts. 2020; 10(10):1169. https://doi.org/10.3390/catal10101169
Chicago/Turabian StyleGong, Ming-Mao, Chia-Yen Dai, Scott Severance, Chi-Ching Hwang, Bo-Kai Fang, Heng-Bo Lin, Chien-Hui Huang, Chi-Wi Ong, Jeh-Jeng Wang, Pei-Lun Lee, and et al. 2020. "A Bioorthogonally Synthesized and Disulfide-Containing Fluorescence Turn-On Chemical Probe for Measurements of Butyrylcholinesterase Activity and Inhibition in the Presence of Physiological Glutathione" Catalysts 10, no. 10: 1169. https://doi.org/10.3390/catal10101169
APA StyleGong, M.-M., Dai, C.-Y., Severance, S., Hwang, C.-C., Fang, B.-K., Lin, H.-B., Huang, C.-H., Ong, C.-W., Wang, J.-J., Lee, P.-L., & Wang, T.-P. (2020). A Bioorthogonally Synthesized and Disulfide-Containing Fluorescence Turn-On Chemical Probe for Measurements of Butyrylcholinesterase Activity and Inhibition in the Presence of Physiological Glutathione. Catalysts, 10(10), 1169. https://doi.org/10.3390/catal10101169