Aqueous Miscible Organic LDH Derived Ni-Based Catalysts for Efficient CO2 Methanation
Abstract
1. Introduction
2. Results and Discussion
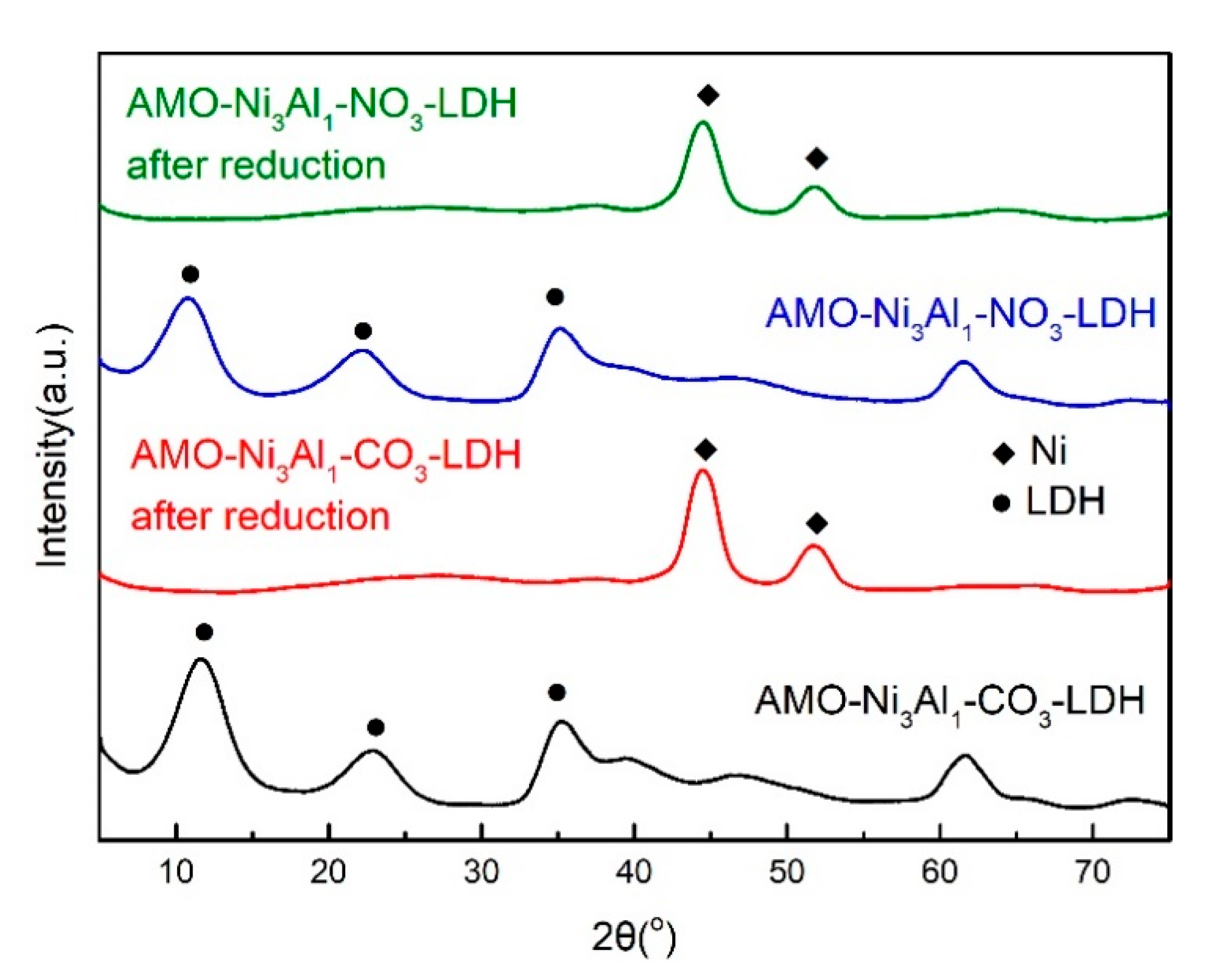
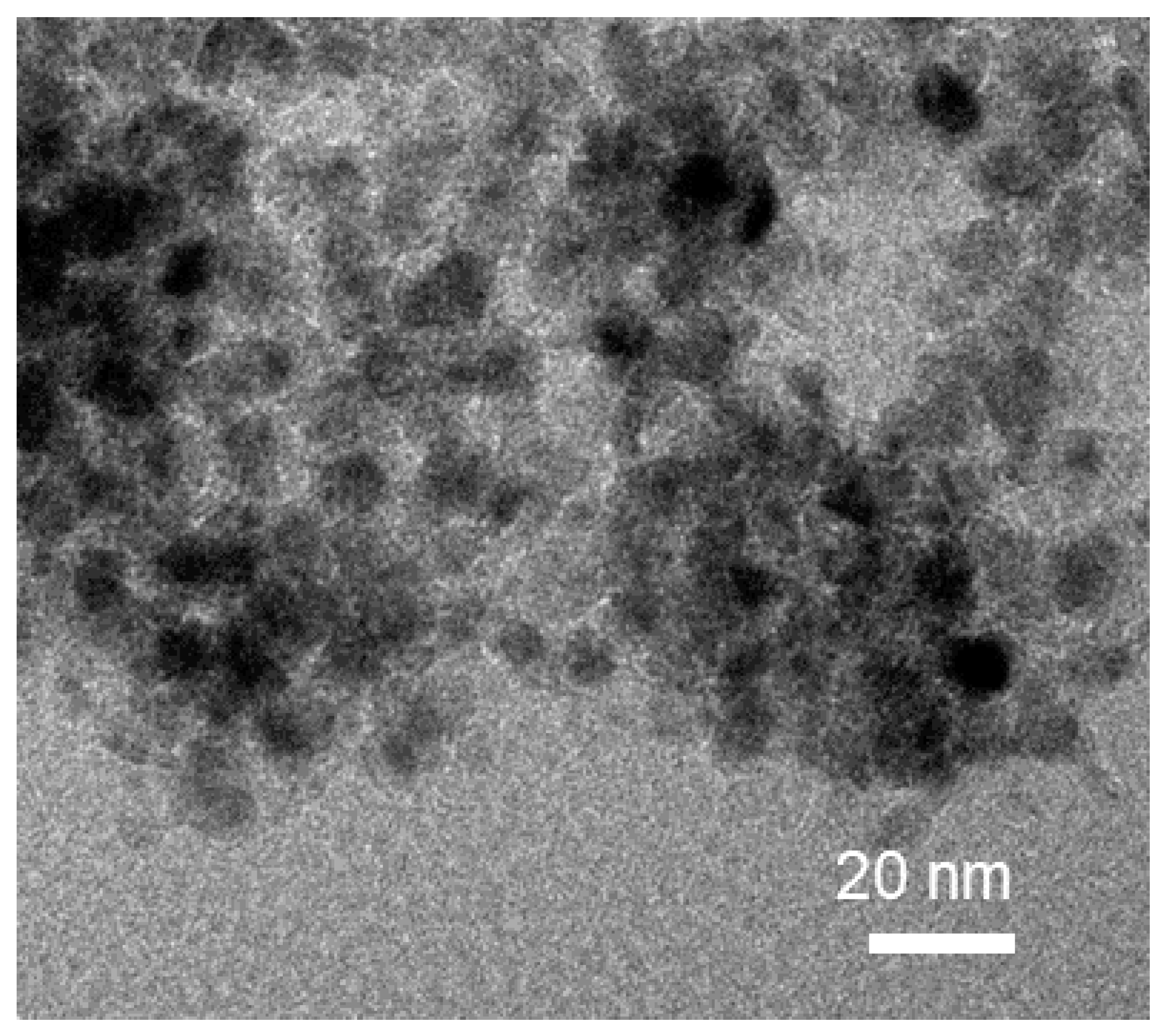
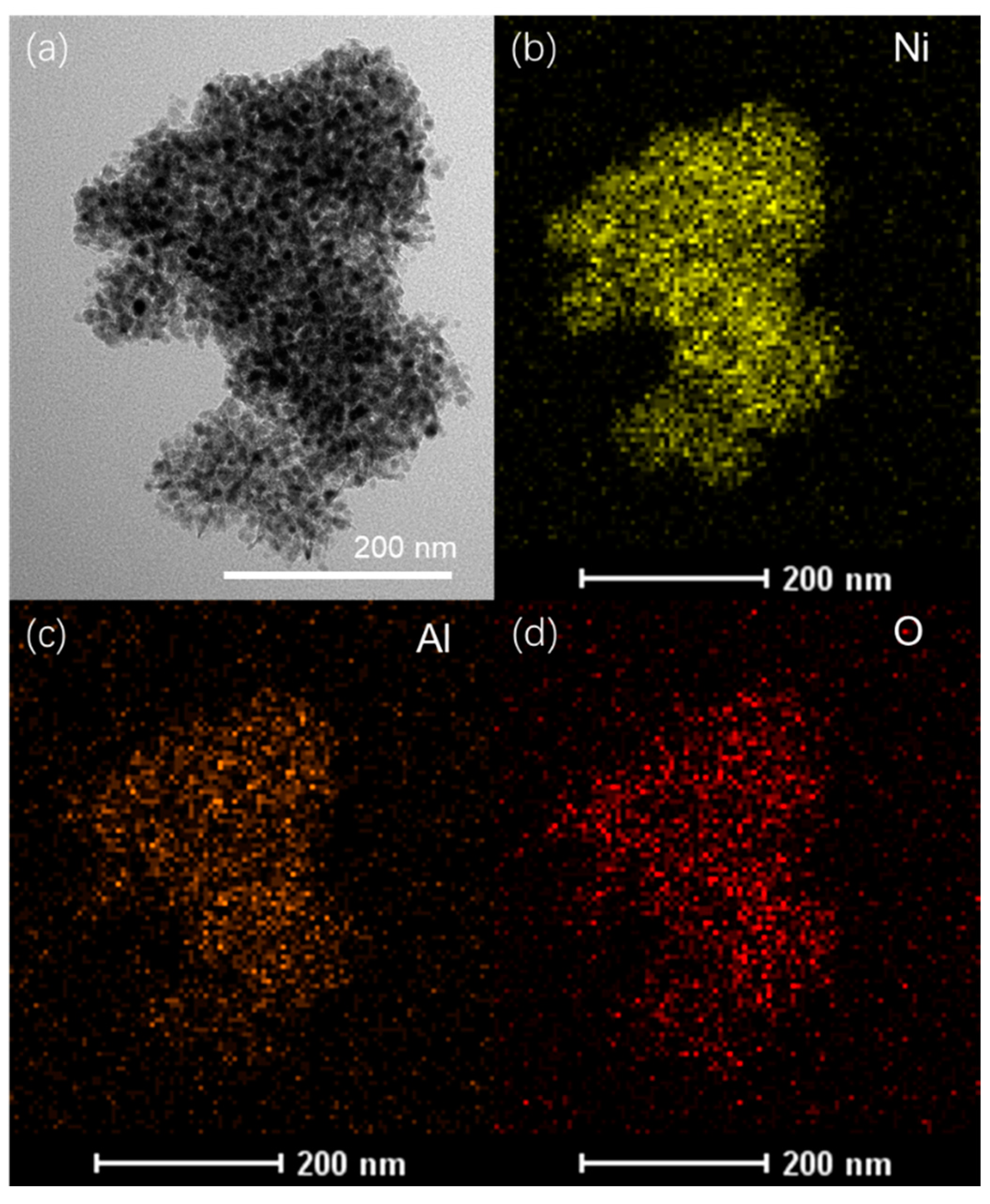
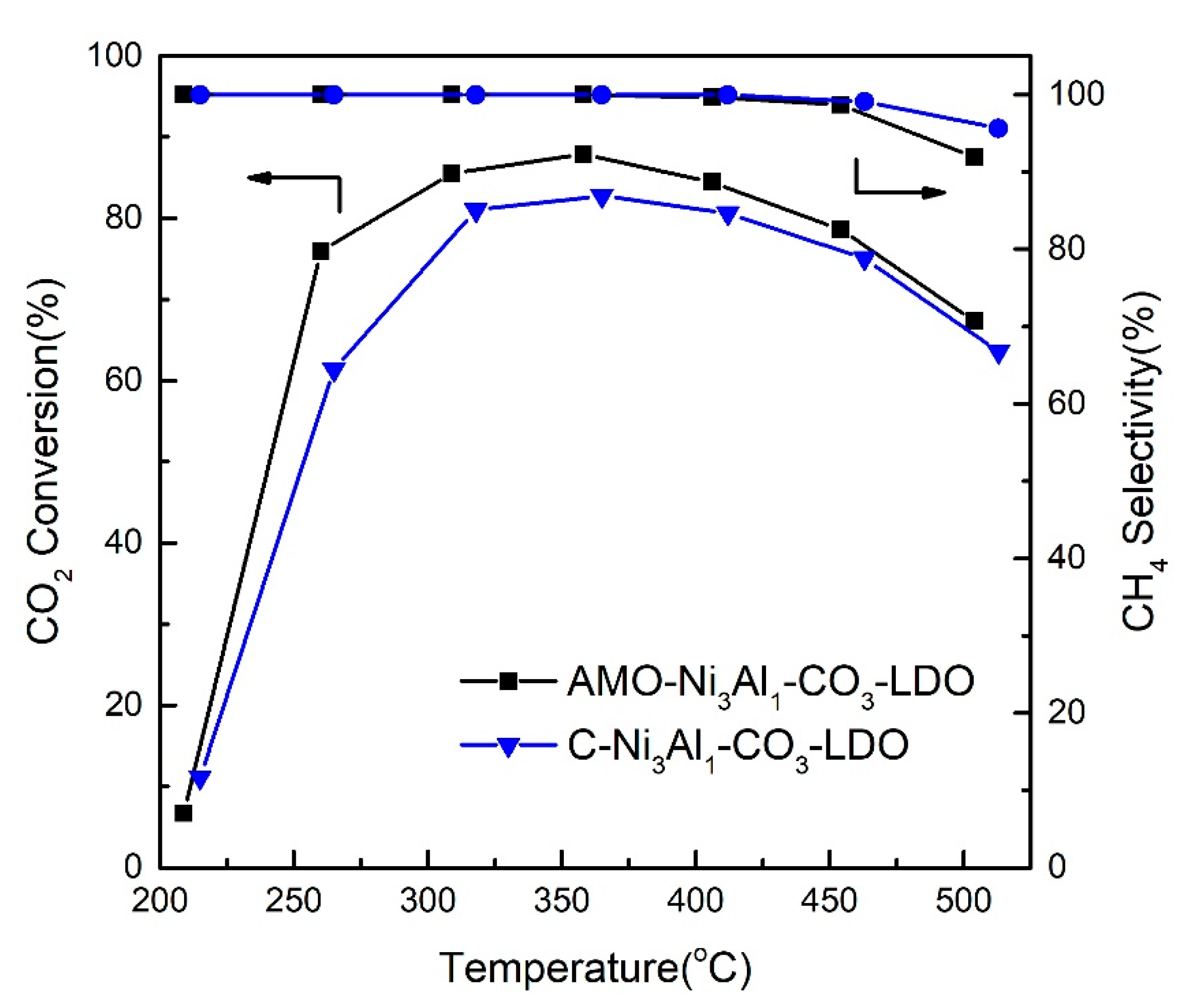
2.1. Influence of Interlayer Anions of AMO-Ni3Al1 LDHs on CO2 Methanation
2.2. The Influence of Catalysts Preparation Procedure on the CO2 Methanation
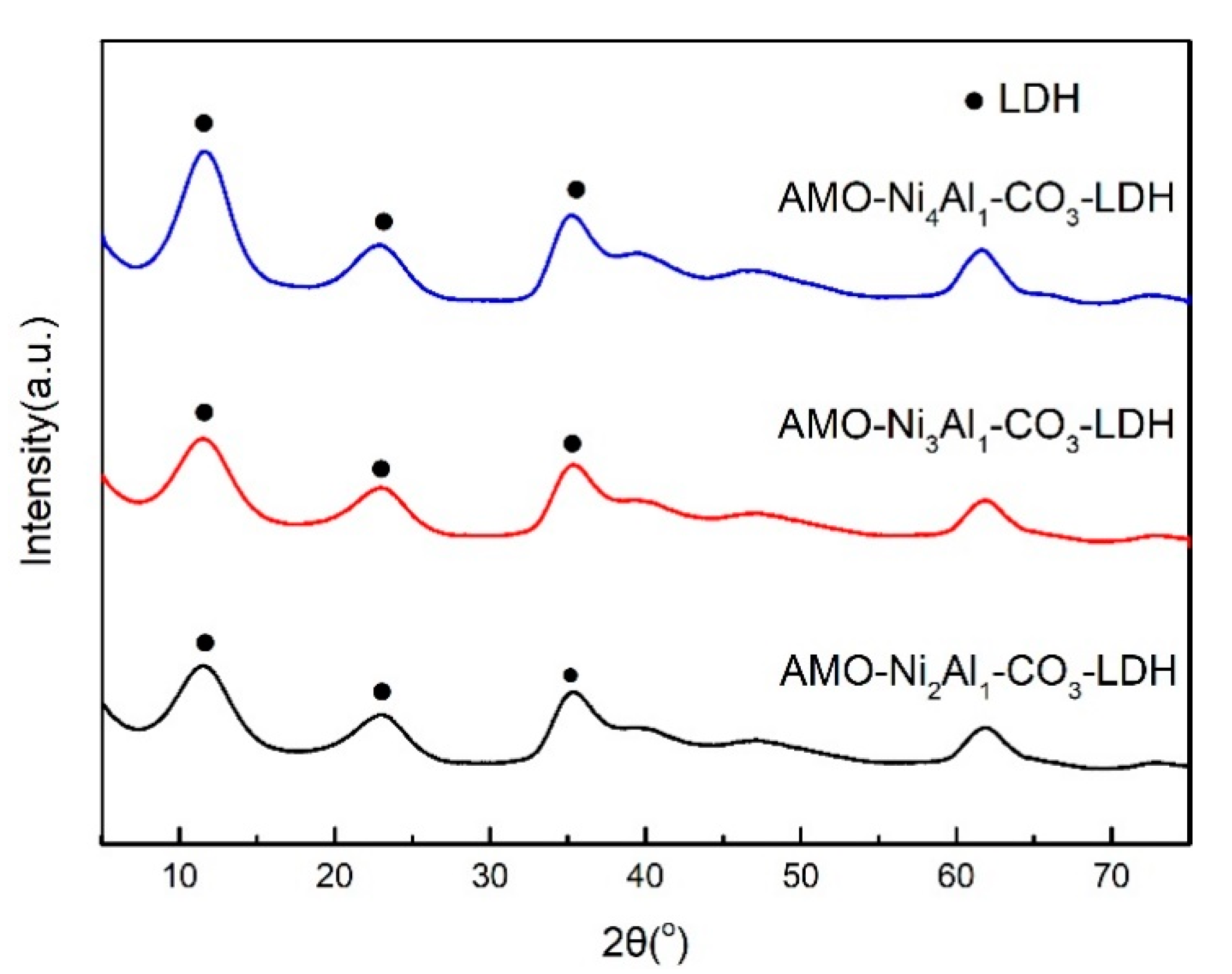
2.3. Influence of Ni/Al Ratio on Catalyst CO2 Methanation Activity
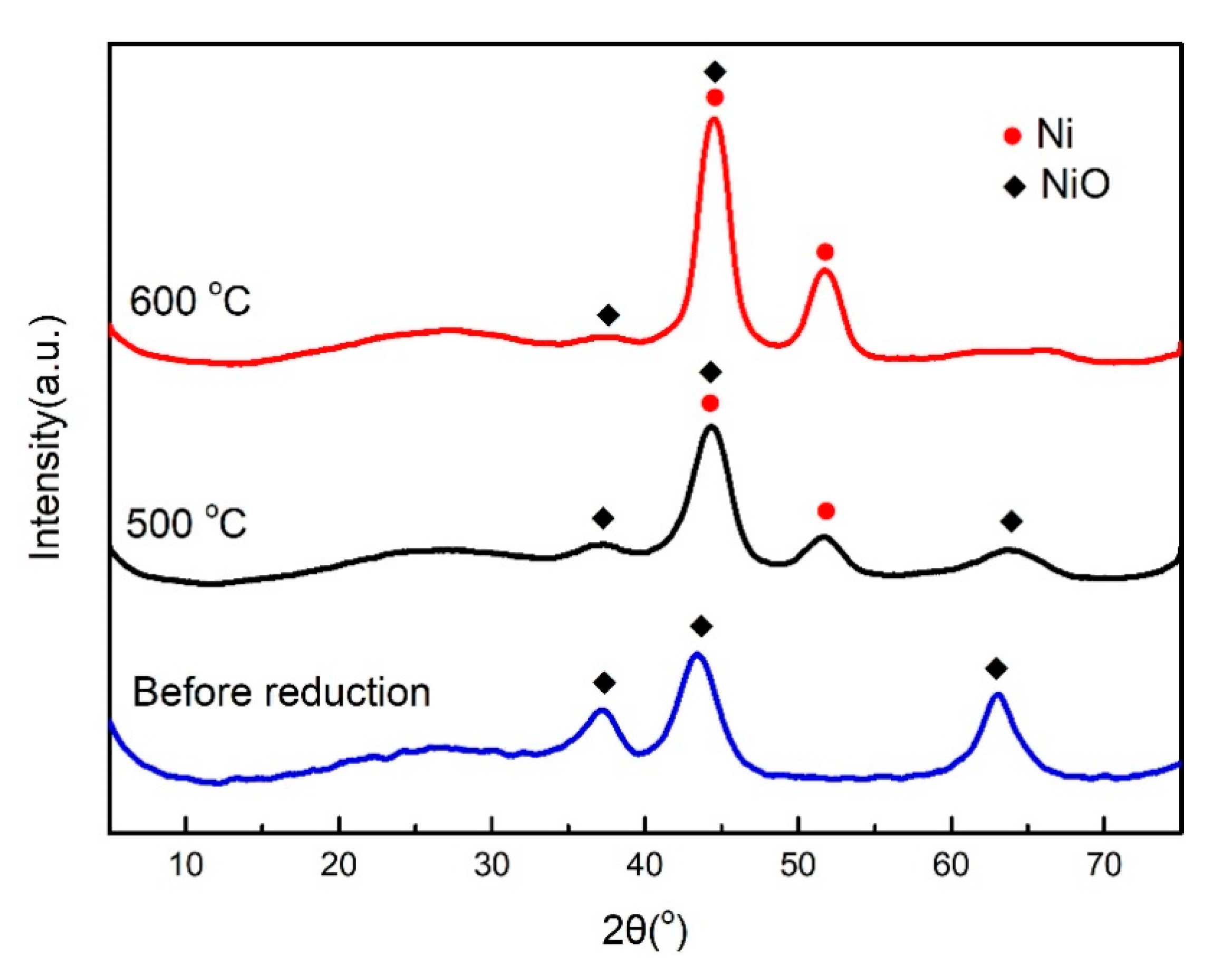
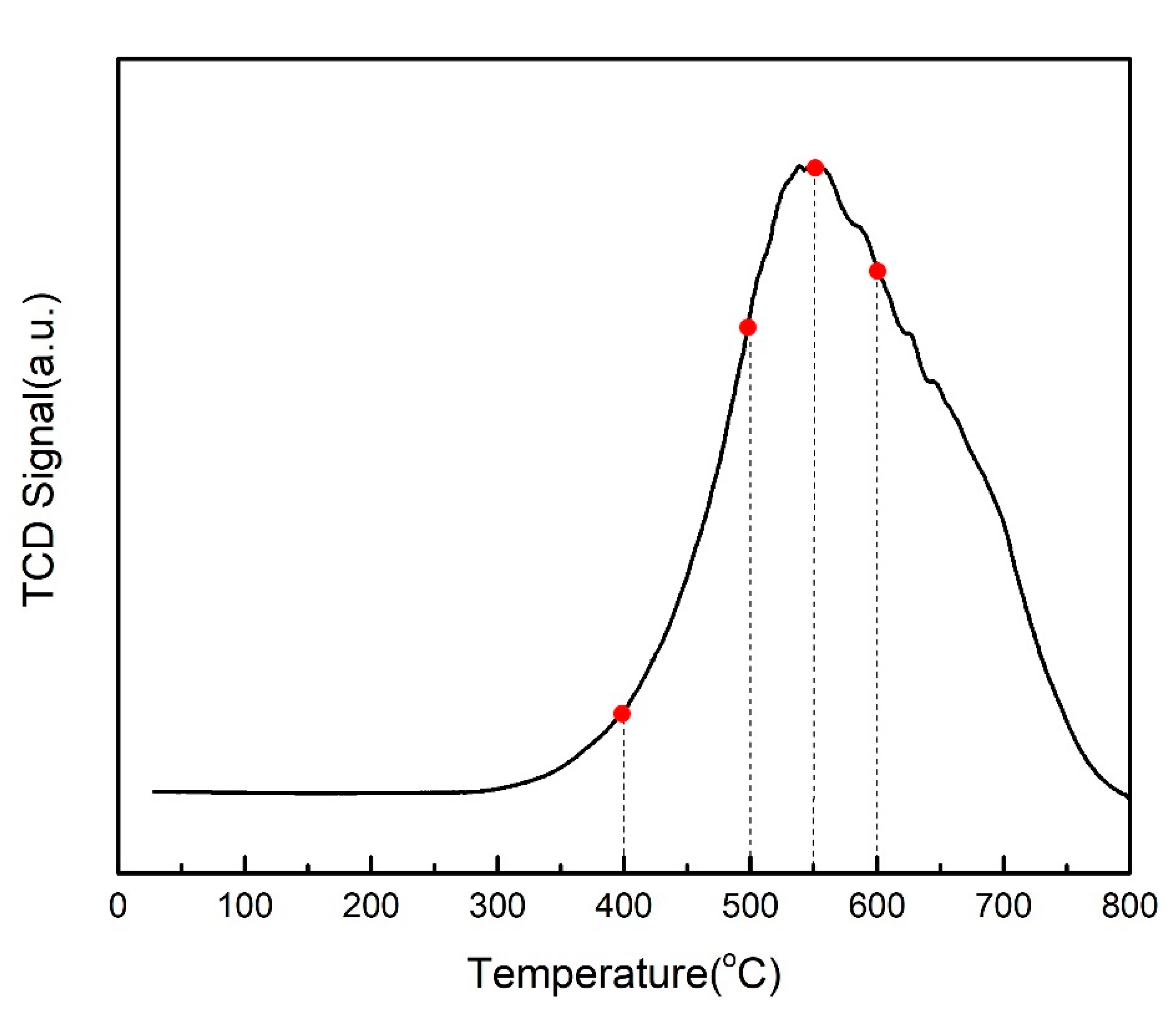
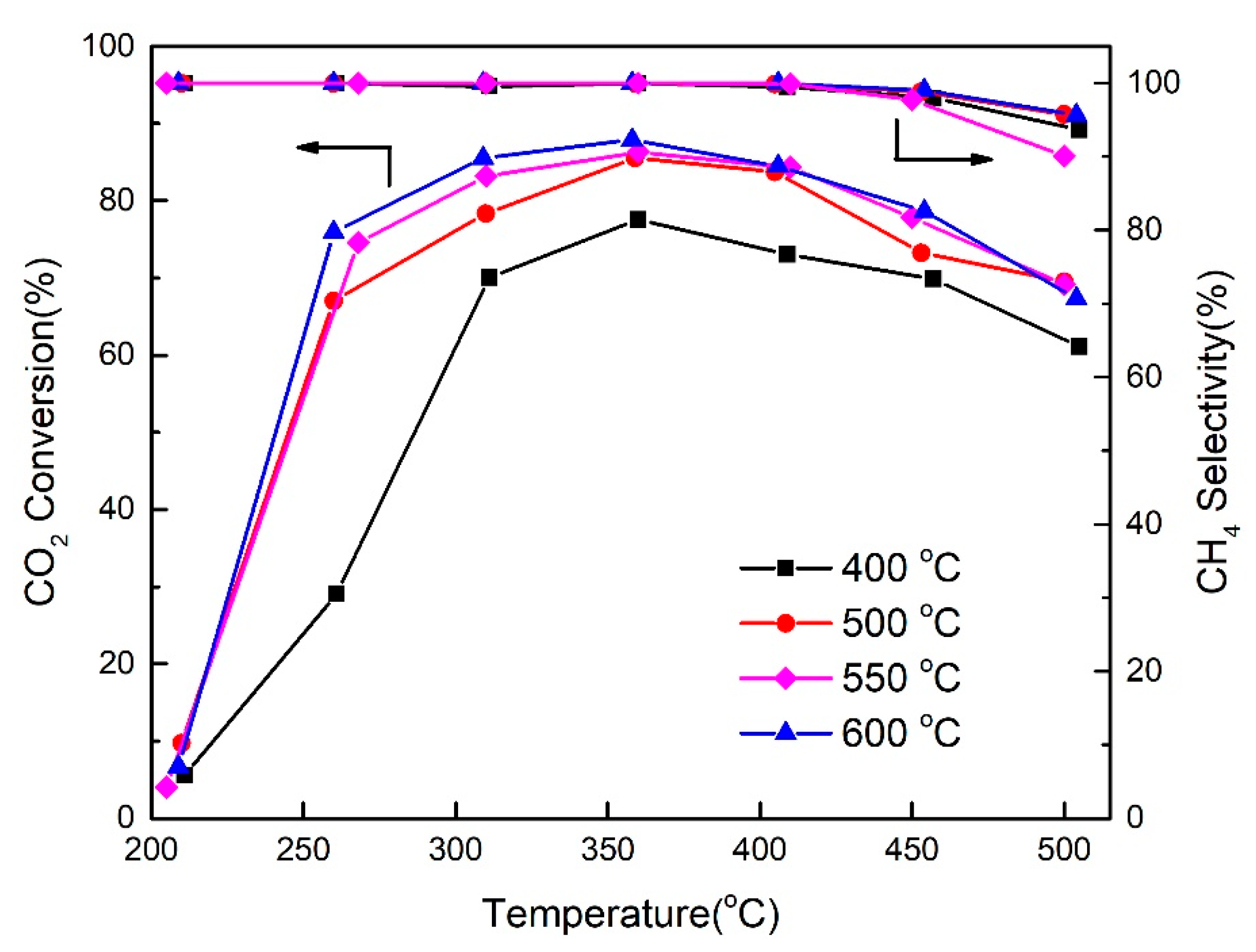
2.4. Influence of Reduction Temperature in Hydrogen on Catalyst CO2 Methanation Activity
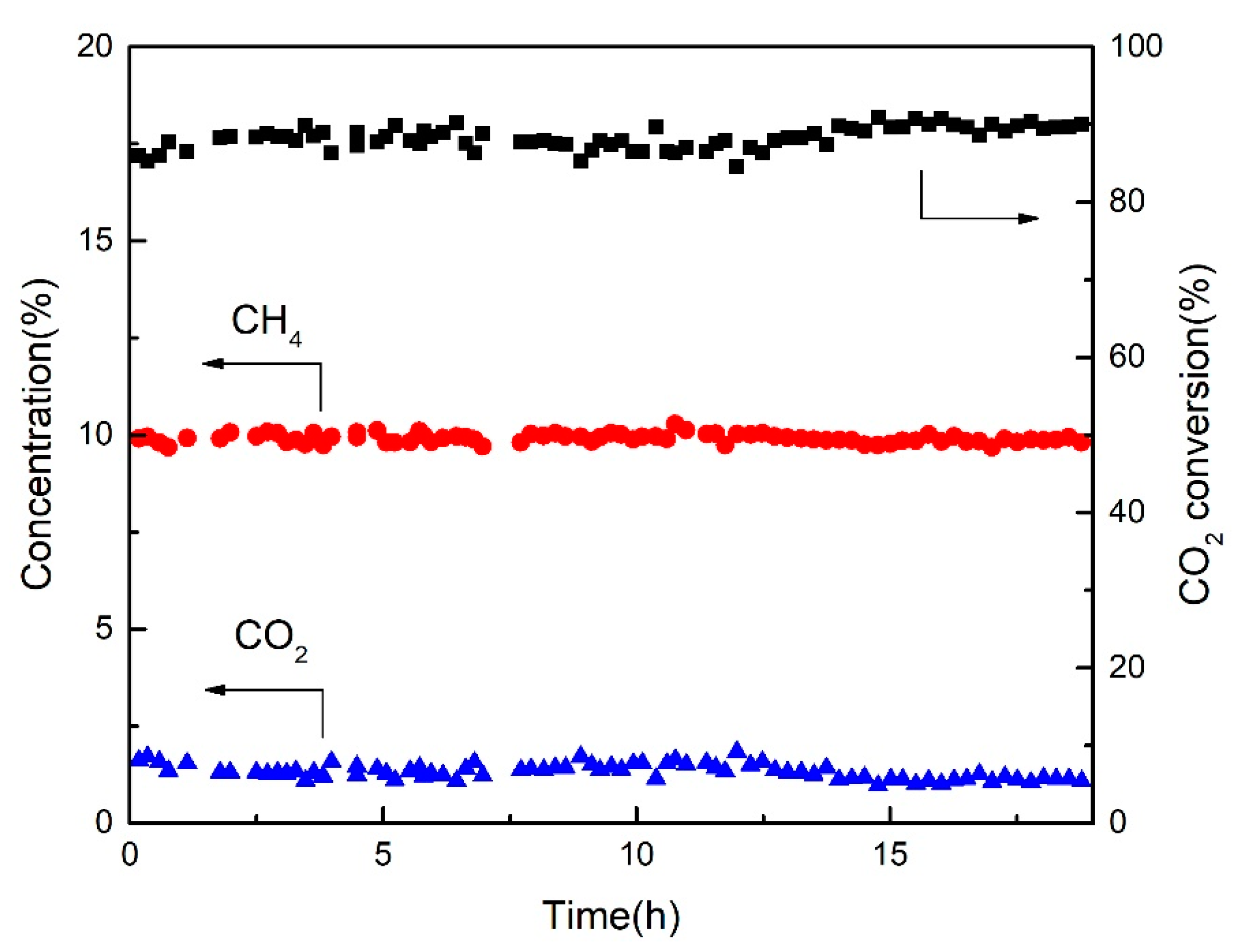
2.5. Stability Performance of AMO-Ni3Al1-CO3-LDO
3. Experimental
3.1. Preparation of Catalysts
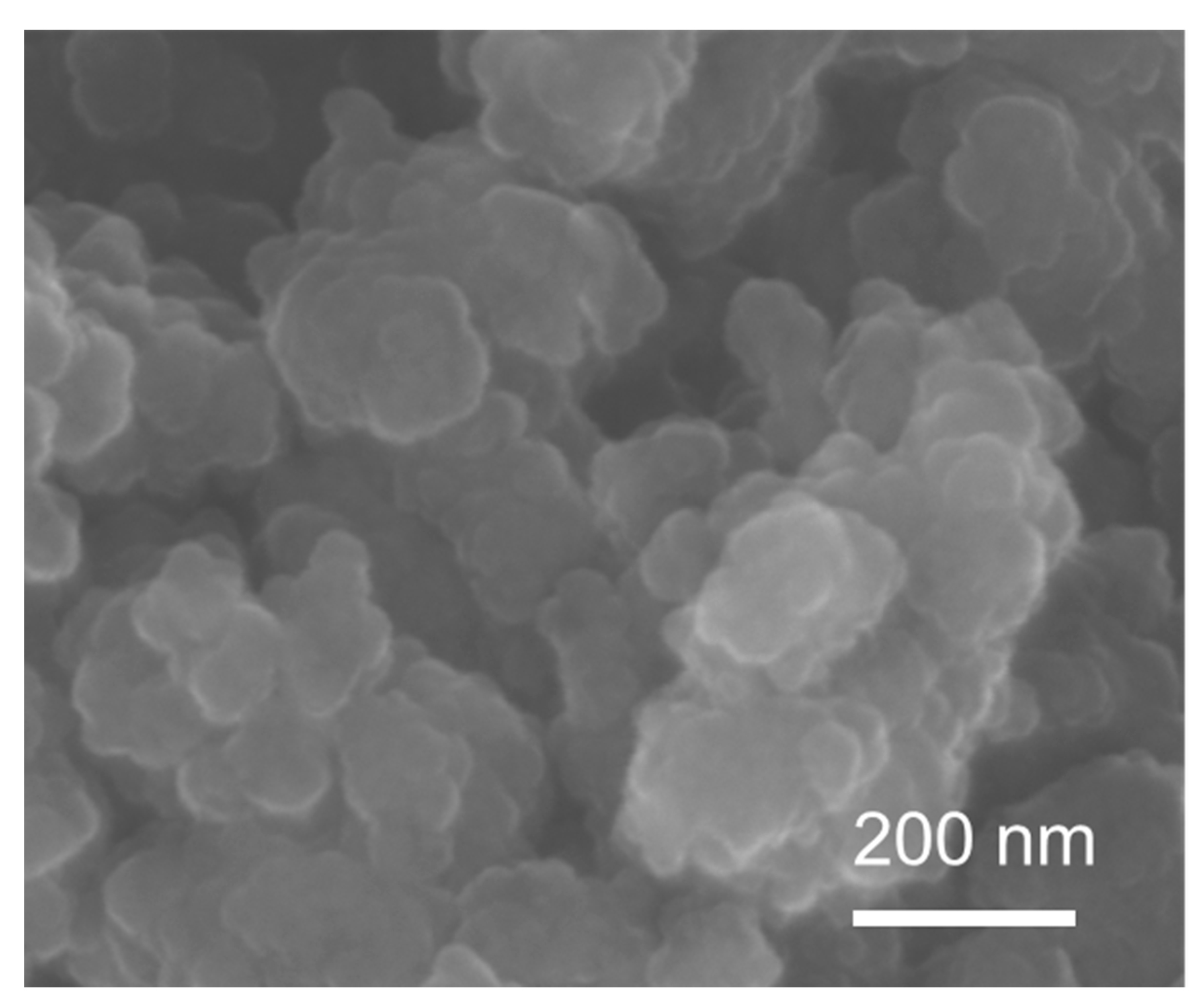
3.2. Catalysts Characterization
3.3. Catalytic Activity Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Smol, J.P. Climate Change: A planet in flux. Nature 2012, 483, S12–S15. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Altaf, N.; Huang, L.; Gao, Y.; Wang, Q. Electrolytic cell design for electrochemical CO2 reduction. J. CO2 Util. 2020, 35, 90–105. [Google Scholar] [CrossRef]
- Li, W.; Nie, X.; Jiang, X.; Zhang, A.; Ding, F.; Liu, M. ZrO2 Support Imparts Superior Activity and Stability of Co Catalysts for CO2 Methanation. Appl. Catal. B Environ. 2018, 220, 397–408. [Google Scholar] [CrossRef]
- Rahmani, S.; Rezaei, M.; Meshkani, F. Preparation of promoted nickel catalysts supported on mesoporous nanocrystalline gamma alumina for carbon dioxide methanation reaction. J. Ind. Eng. Chem. 2014, 20, 4176–4182. [Google Scholar] [CrossRef]
- Renda, S.; Ricca, A.; Palma, V. Study of the effect of noble metal promotion in Ni-based catalyst for the Sabatier reaction. Int. J. Hydrog. Energy 2020. [Google Scholar] [CrossRef]
- Branco, J.B.; Brito, P.E.; Ferreira, A.C. Methanation of CO2 over nickel-lanthanide bimetallic oxides supported on silica. Chem. Eng. J. 2020, 380, 122465. [Google Scholar] [CrossRef]
- García-Gutiérrez, P.; Cuéllar-Franca, R.M.; Reed, D.; Dowson, G.; Styring, P.; Azapagic, A. Environmental sustainability of cellulosesupported solid ionic liquids for CO2 capture. Green Chem. 2019, 21, 4100–4114. [Google Scholar] [CrossRef]
- Zhou, Z.; Sun, N.; Wang, B.; Han, Z.; Cao, S.; Hu, D.; Zhu, T.; Shen, Q.; Wei, W. 2D-Layered Ni-MgO-Al2O3 Nanosheets for Integrated Capture and Methanation of CO2. ChemSusChem 2019, 13, 360–368. [Google Scholar] [CrossRef]
- Dou, L.; Yan, C.; Zhong, L.; Zhang, D.; Zhang, J.; Li, X.; Xiao, L. Enhancing CO2 methanation over a metal foam structured catalyst by electric internal heating. Chem. Commun. 2020, 56, 205–208. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Patel, V.; Le Saché, E.; Reina, T.R. CO2 methanation in the presence of methane: Catalysts design and effect of methane concentration in the reaction mixture. J. Energy Inst. 2020, 93, 415–424. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Saché, E.L.; Jones, C.; Gu, S.; Arellano-Garcia, H.; Reina, T.R. Synthetic natural gas production from CO2 over Ni-x/CeO2-ZrO2 (x = Fe, Co) catalysts: Influence of promoters and space velocity. Catal. Today 2018, 317, 108–113. [Google Scholar] [CrossRef]
- Jarvis, S.M.; Samsatli, S. Technologies and infrastructures underpinning future CO2 value chains: A comprehensive review and comparative analysis. Renew. Sustain. Energy Rev. 2018, 85, 46–68. [Google Scholar] [CrossRef]
- Thema, M.; Bauer, F.; Sterner, M. Power-to-Gas: Electrolysis and methanation status review. Renew. Sustain. Energy Rev. 2019, 112, 775–787. [Google Scholar] [CrossRef]
- Dannesboe, C.; Hansen, J.B.; Johannsen, I. Catalytic methanation of CO2 in biogas: Experimental results from a reactor at full scale. React. Chem. Eng. 2020, 5, 183–189. [Google Scholar] [CrossRef]
- Beuls, A.; Swalus, C.; Jacquemin, M.; Heyen, G.; Karelovic, A.; Ruiz, P. Methanation of CO2: Further insight into the mechanism over Rh/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2012, 113-114, 2–10. [Google Scholar] [CrossRef]
- Wang, F.; He, S.; Chen, H.; Wang, B.; Zheng, L.; Wei, M.; Evans, D.G.; Duan, X. Active Site Dependent Reaction Mechanism over Ru/CeO2 Catalyst toward CO2 Methanation. J. Am. Chem. Soc. 2016, 138, 6298–6305. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Ahmad, A. CO2 methanation over heterogeneous catalysts: Recent progress and future prospects. Green Chem. 2015, 17, 2647–2663. [Google Scholar] [CrossRef]
- Sun, J.; Wang, Y.; Zou, H.; Guo, X.; Wang, Z.-J. Ni catalysts supported on nanosheet and nanoplate γ-Al2O3 for carbon dioxide methanation. J. Energy Chem. 2019, 29, 3–7. [Google Scholar] [CrossRef]
- Ab Halim, A.Z.; Ali, R.; Wan Abu Bakar, W.A. CO2/H2 methanation over M*/Mn/Fe-Al2O3 (M*: Pd, Rh, and Ru) catalysts in natural gas; optimization by response surface methodology-central composite design. Clean Technol. Environ. Policy 2014, 17, 627–636. [Google Scholar] [CrossRef]
- Zamani, A.H.; Ali, R.; Abu Bakar, W.A.W. Optimization of CO2 methanation reaction over M*/Mn/Cu–Al2O3 (M*: Pd, Rh and Ru) catalysts. J. Ind. Eng. Chem. 2015, 29, 238–248. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent trend in thermal catalytic low temperature CO2 methanation: A critical review. Catal. Today 2020. [Google Scholar] [CrossRef]
- Chein, R.-Y.; Wang, C.-C. Experimental Study on CO2 Methanation over Ni/Al2O3, Ru/Al2O3, and Ru-Ni/Al2O3 Catalysts. Catalysts 2020, 10, 1112. [Google Scholar] [CrossRef]
- Ghaib, K.; Ben-Fares, F.-Z. Power-to-Methane: A state-of-the-art review. Renew. Sustain. Energy Rev. 2018, 81, 433–446. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Zhang, S.; An, K.; Ma, Z.; Wang, L.; Liu, Y. Cerium-modified Ni-La2O3/ZrO2 for CO2 methanation. J. Energy Chem. 2020, 43, 155–164. [Google Scholar] [CrossRef]
- Solis-Garcia, A.; Fierro-Gonzalez, J.C. Mechanistic Insights into the CO2 Methanation Catalyzed by Supported Metals: A Review. J. Nanosci. Nanotechnol. 2019, 19, 3110–3123. [Google Scholar] [CrossRef] [PubMed]
- Frontera, P.; Macario, A.; Monforte, G.; Bonura, G.; Ferraro, M.; Dispenza, G.; Antonucci, V.; Aricò, A.S.; Antonucci, P.L. The role of Gadolinia Doped Ceria support on the promotion of CO2 methanation over Ni and Ni Fe catalysts. Int. J. Hydrog. Energy 2017, 42, 26828–26842. [Google Scholar] [CrossRef]
- Wang, J.; Mei, X.; Huang, L.; Zheng, Q.; Qiao, Y.; Zang, K.; Mao, S.; Yang, R.; Zhang, Z.; Gao, Y.; et al. Synthesis of layered double hydroxides/graphene oxide nanocomposite as a novel high-temperature CO2 adsorbent. J. Energy Chem. 2015, 24, 127–137. [Google Scholar] [CrossRef]
- Huang, L.; Wang, J.; Gao, Y.; Qiao, Y.; Zheng, Q.; Guo, Z.; Zhao, Y.; O’Hare, D.; Wang, Q. Synthesis of LiAl2-layered double hydroxides for CO2 capture over a wide temperature range. J. Mater. Chem. A 2014, 2, 18454–18462. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Large-scale synthesis of highly dispersed layered double hydroxide powders containing delaminated single layer nanosheets. Chem. Commun. 2013, 49, 6301. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S.; Epping, K.; Velty, A. Increasing the basicity and catalytic activity of hydrotalcites by different synthesis procedures. J. Catal. 2004, 225, 316–326. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Y.; Qiu, L.; Guo, Z.; O’Hare, D.; Wang, Q. Preparation of 4,4′-diaminostilbene-2,2′-disulfonic acid intercalated LDH/polypropylene nanocomposites with enhanced UV absorption property. Polym. Compos. 2017, 38, 1937–1947. [Google Scholar] [CrossRef]
- Bian, L.; Wang, W.; Xia, R.; Li, Z. Ni-based catalyst derived from Ni/Al hydrotalcite-like compounds by the urea hydrolysis method for CO methanation. RSC Adv. 2016, 6, 677–686. [Google Scholar] [CrossRef]
- Marocco, P.; Morosanu, E.A.; Giglio, E.; Ferrero, D.; Mebrahtu, C.; Lanzini, A.; Abate, S.; Bensaid, S.; Perathoner, S.; Santarelli, M.; et al. CO2 methanation over Ni/Al hydrotalcite-derived catalyst: Experimental characterization and kinetic study. Fuel 2018, 225, 230–242. [Google Scholar] [CrossRef]
- Abelló, S.; Berrueco, C.; Montané, D. High-loaded nickel-alumina catalyst for direct CO2 hydrogenation into synthetic natural gas (SNG). Fuel 2013, 113, 598–609. [Google Scholar] [CrossRef]
- Liu, J.; Bing, W.; Xue, X.; Wang, F.; Wang, B.; He, S.; Zhang, Y.; Wei, M. Alkaline-assisted Ni nanocatalysts with largely enhanced low-temperature activity toward CO2 methanation. Catal. Sci. Technol. 2016, 6, 3976–3983. [Google Scholar] [CrossRef]
- Chen, C.; Yang, M.; Wang, Q.; Buffet, J.-C.; O’Hare, D. Synthesis and characterisation of aqueous miscible organic-layered double hydroxides. J. Mater. Chem. A 2014, 2, 15102–15110. [Google Scholar] [CrossRef]
- Ruengkajorn, K.; Erastova, V.; Buffet, J.-C.; Greenwell, H.C.; O’Hare, D. Aqueous immiscible layered double hydroxides: Synthesis, characterisation and molecular dynamics simulation. Chem. Commun. 2018, 54, 4394–4397. [Google Scholar] [CrossRef]
- Chen, C.; Wangriya, A.; Buffet, J.-C.; O’Hare, D. Tuneable ultra high specific surface area Mg/Al-CO3 layered double hydroxides. Dalton Trans. 2015, 44, 16392–16398. [Google Scholar] [CrossRef]
- Cermelj, K.; Ruengkajorn, K.; Buffet, J.-C.; O’Hare, D. Layered double hydroxide nanosheets via solvothermal delamination. J. Energy Chem. 2019, 35, 88–94. [Google Scholar] [CrossRef]
- Guo, X.; He, H.; Traitangwong, A.; Gong, M.; Meeyoo, V.; Li, P.; Li, C.; Peng, Z.; Zhang, S. Ceria imparts superior low temperature activity to nickel catalysts for CO2 methanation. Catal. Sci. Technol. 2019, 9, 5636–5650. [Google Scholar] [CrossRef]
- Yan, Y.; Dai, Y.; He, H.; Yu, Y.; Yang, Y. A novel W-doped Ni-Mg mixed oxide catalyst for CO2 methanation. Appl. Catal. B Environ. 2016, 196, 108–116. [Google Scholar] [CrossRef]
- Yang, L.; Pastor-Pérez, L.; Gu, S.; Sepúlveda-Escribano, A.; Reina, T.R. Highly efficient Ni/CeO2-Al2O3 catalysts for CO2 upgrading via reverse water-gas shift: Effect of selected transition metal promoters. Appl. Catal. B Environ. 2018, 232, 464–471. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, Z.; Tay, H.H.; Chen, L.; Liu, Y.; Chang, J.; Zhong, Z.; Luo, J.; Borgna, A. High temperature adsorption of CO2 on Mg-Al hydrotalcite: Effect of the charge compensating anions and the synthesis pH. Catal. Today 2011, 164, 198–203. [Google Scholar] [CrossRef]
- Zhen, W.; Li, B.; Lu, G.; Ma, J. Enhancing catalytic activity and stability for CO2 methanation on Ni@MOF-5 via control of active species dispersion. Chem. Commun. 2015, 51, 1728–1731. [Google Scholar] [CrossRef]
- Mak Yu, T.; Caroline Reis Meira, A.; Cristina Kreutz, J.; Effting, L.; Mello Giona, R.; Gervasoni, R.; Amado de Moura, A.; Maestá Bezerra, F.; Bail, A. Exploring the surface reactivity of the magnetic layered double hydroxide lithium-aluminum: An alternative material for sorption and catalytic purposes. Appl. Surf. Sci. 2019, 467–468, 1195–1203. [Google Scholar] [CrossRef]
- Yan, Q.; Nie, Y.; Yang, R.; Cui, Y.; O’Hare, D.; Wang, Q. Highly dispersed CuyAlOx mixed oxides as superior low-temperature alkali metal and SO2 resistant NH3 -SCR catalysts. Appl. Catal. A Gen. 2017, 538, 37–50. [Google Scholar] [CrossRef]
- Wang, L.; Niu, X.; Chen, J. SiO2 supported Ni-In intermetallic compounds: Efficient for selective hydrogenation of fatty acid methyl esters to fatty alcohols. Appl. Catal. B Environ. 2020, 278, 119293. [Google Scholar] [CrossRef]
- Li, S.; Yang, Y.; Huang, S.; He, Z.; Li, C.; Li, D.; Ke, B.; Lai, C.; Peng, Q. Adsorption of humic acid from aqueous solution by magnetic Zn/Al calcined layered double hydroxides. Appl. Clay Sci. 2020, 188, 105414. [Google Scholar] [CrossRef]
- Ye, R.-P.; Gong, W.; Sun, Z.; Sheng, Q.; Shi, X.; Wang, T.; Yao, Y.; Razink, J.J.; Lin, L.; Zhou, Z.; et al. Enhanced stability of Ni/SiO2 catalyst for CO2 methanation: Derived from nickel phyllosilicate with strong metal-support interactions. Energy 2019, 188, 116059. [Google Scholar] [CrossRef]
- Xu, M.; He, S.; Chen, H.; Cui, G.; Zheng, L.; Wang, B.; Wei, M. TiO2-x-Modified Ni Nanocatalyst with Tunable Metal–Support Interaction for Water–Gas Shift Reaction. ACS Catal. 2017, 7, 7600–7609. [Google Scholar] [CrossRef]
- Mori, K.; Taga, T.; Yamashita, H. Isolated Single-Atomic Ru Catalyst Bound on a Layered Double Hydroxide for Hydrogenation of CO2 to Formic Acid. ACS Catal. 2017, 7, 3147–3151. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A. Development of stable bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2007, 249, 300–310. [Google Scholar] [CrossRef]
- Li, Z.; Yan, Q.; Jiang, Q.; Gao, Y.; Xue, T.; Li, R.; Liu, Y.; Wang, Q. Oxygen vacancy mediated CuyCo3-yFe1Ox mixed oxide as highly active and stable toluene oxidation catalyst by multiple phase interfaces formation and metal doping effect. Appl. Catal. B Environ. 2020, 269, 118827. [Google Scholar] [CrossRef]
- Damaskinos, C.M.; Vasiliades, M.A.; Efstathiou, A.M. The effect of Ti4+ dopant in the 5 wt% Ni/Ce1-xTixO2-δ catalyst on the carbon pathways of dry reforming of methane studied by various transient and isotopic techniques. Appl. Catal. A Gen. 2019, 579, 116–129. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Huang, L.; Reina, T.R.; Efstathiou, A.M.; Wang, Q. Aqueous Miscible Organic LDH Derived Ni-Based Catalysts for Efficient CO2 Methanation. Catalysts 2020, 10, 1168. https://doi.org/10.3390/catal10101168
Wang Z, Huang L, Reina TR, Efstathiou AM, Wang Q. Aqueous Miscible Organic LDH Derived Ni-Based Catalysts for Efficient CO2 Methanation. Catalysts. 2020; 10(10):1168. https://doi.org/10.3390/catal10101168
Chicago/Turabian StyleWang, Ziling, Liang Huang, Tomas Ramirez Reina, Angelos M. Efstathiou, and Qiang Wang. 2020. "Aqueous Miscible Organic LDH Derived Ni-Based Catalysts for Efficient CO2 Methanation" Catalysts 10, no. 10: 1168. https://doi.org/10.3390/catal10101168
APA StyleWang, Z., Huang, L., Reina, T. R., Efstathiou, A. M., & Wang, Q. (2020). Aqueous Miscible Organic LDH Derived Ni-Based Catalysts for Efficient CO2 Methanation. Catalysts, 10(10), 1168. https://doi.org/10.3390/catal10101168








