Synthesis and Photocatalytic Activity of β-Ga2O3 Nanostructures for Decomposition of Formaldehyde under Deep Ultraviolet Irradiation
Abstract
:1. Introduction
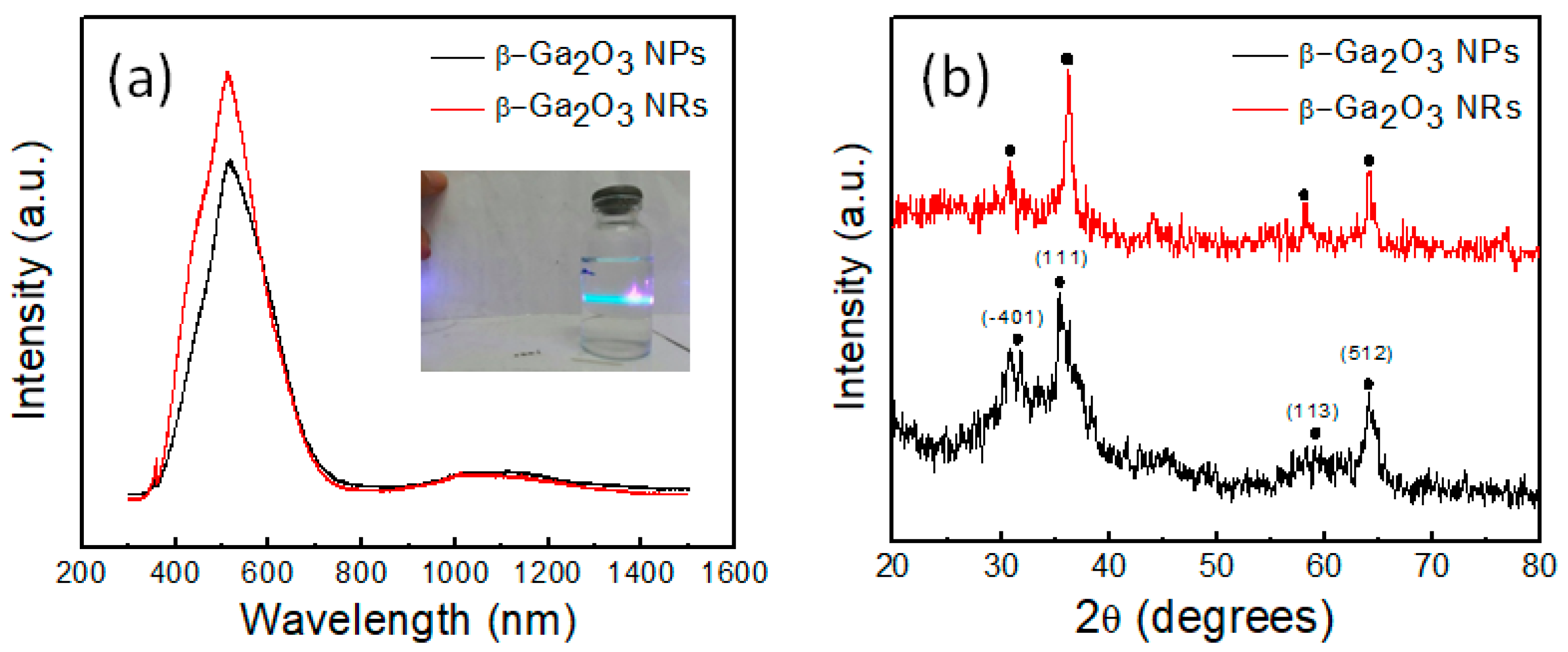
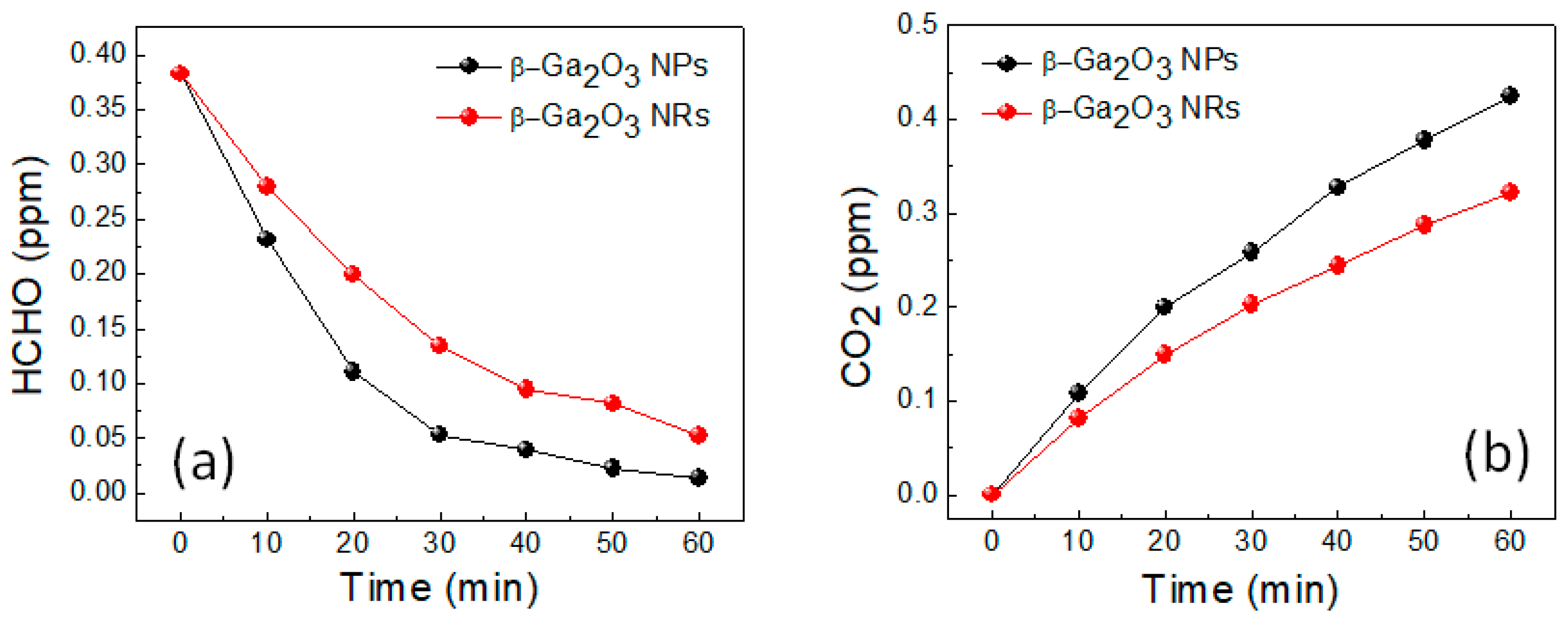
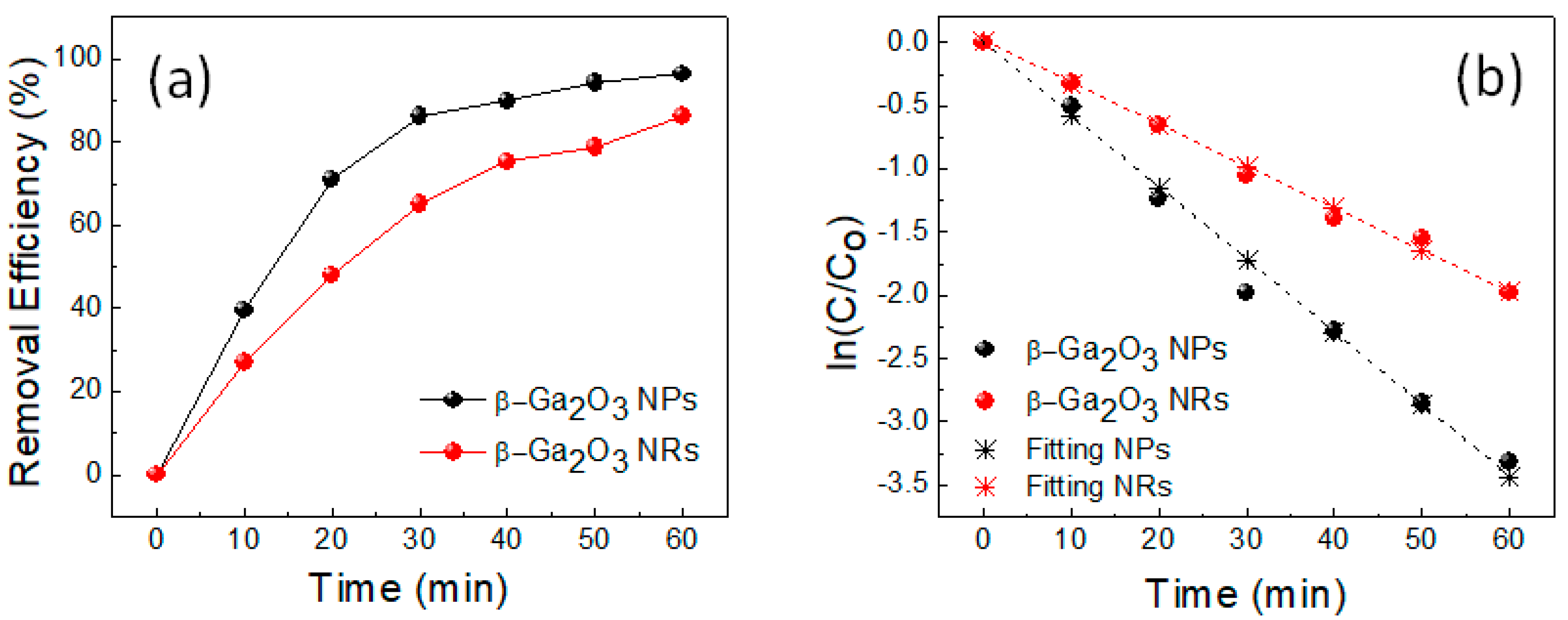
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of β-Ga2O3 NRs
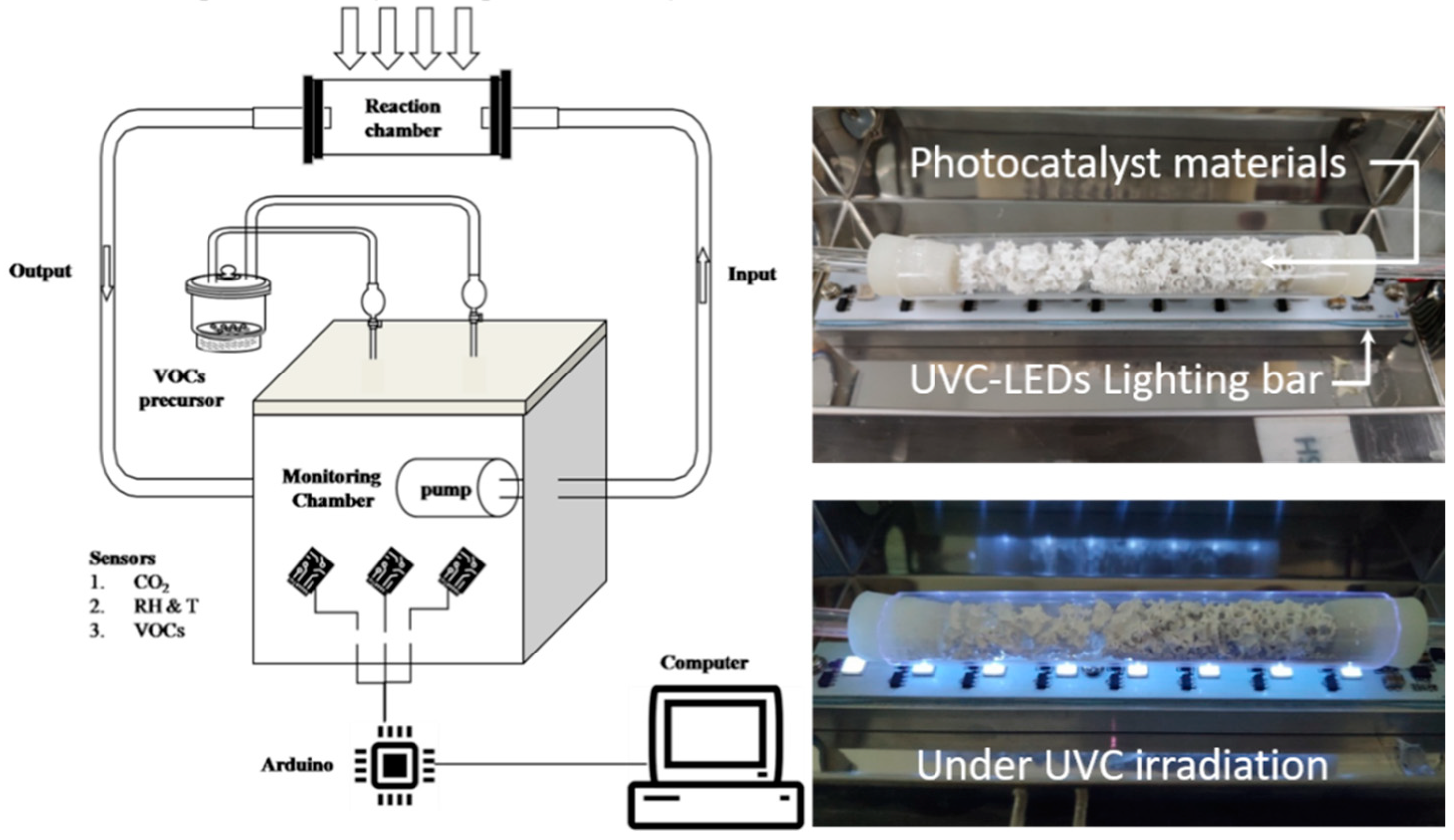
3.2. Photocatalytic Activity Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fukumura, T.; Sambandan, E.; Yamashita, H. Synthesis and VOC degradation ability of a CeO2/WO3 thin-layer visible-light photocatalyst. Mater. Res. Bull. 2017, 94, 493–499. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, S.S.H.; Niu, R.; Xu, L.; Lu, Y.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tseng, C.; Hsieh, C.; Chen, S. The removal of indoor formaldehyde by various air cleaners. In Proceedings of the Air & Waste Management Association´s 98th Annual Conference, Minneapolis, MN, USA, 21–24 June 2005; p. 457. [Google Scholar]
- Keller, N.; Ducamp, M.N.; Robert, D.; Keller, V. Ethylene removal and fresh product storage: A challenge at the frontiers of chemistry. Toward an approach by photocatalytic oxidation. Chem. Rev. 2013, 113, 5029–5070. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Tsukamoto, T.; Mitsutsuka, Y.; Takamura, T. Ga2O3-Photoassisted and Photolytic Degradation of the Flubendiamide Agrochemical in Aqueous Media under Irradiation. J. Water Environ. Technol. 2019, 17, 345–358. [Google Scholar] [CrossRef] [Green Version]
- Hidaka, H.; Tsukamoto, T.; Mitsutsuka, Y.; Oyama, T.; Serpone, N. Enhanced Ga2O3-photocatalyzed and photochemical degradation of the Fipronil insecticide by UVC irradiation in mixed aqueous/organic media under an inert atmosphere. Photochem. Photobiol. Sci. 2015, 14, 919–928. [Google Scholar] [CrossRef]
- Yoo, T.H.; Ryou, H.; Lee, I.G.; Cho, J.; Cho, B.J.; Hwang, W.S. Comparison of Ga2O3 and TiO2 Nanostructures for Photocatalytic Degradation of Volatile Organic Compounds. Catalysts 2020, 10, 545. [Google Scholar] [CrossRef]
- Binet, L.; Gourier, D. Origin of the blue luminescence of β−Ga2O3. J. Phys. Chem. Solids 1998, 59, 1241–1249. [Google Scholar] [CrossRef]
- Li, X.; Mo, X.; Li, K. Nanorod β−Ga2O3 semiconductor modified activated carbon as catalyst for improving power generation of microbial fuel cell. J. Solid State Electr. 2019, 23, 2843–2852. [Google Scholar] [CrossRef]
- Xiang, M.; Song, X.; Jia, M.; Fang, Y.; Lu, C.; Huang, Y. Hydro-thermal synthesis and photocatalytic reaction of Ga2O3 with different surfactants. Chin. J. Environ. Eng. 2016, 10, 2361–2366. [Google Scholar]
- Gai, L.; Jiang, H.; Tian, Y.; Cui, D.; Wang, Q. Low-temperature synthesis of β−Ga2O3 nanorods on SBA-15 microparticles by solvothermal method. Nanotechnology 2006, 17, 5858. [Google Scholar] [CrossRef]
- Han, W.Q.; Kohler-Redlich, P.; Ernst, F.; Ruhle, M. Growth and microstructure of Ga2O3 nanorods. Solid State Commun. 2005, 115, 527. [Google Scholar] [CrossRef]
- Tas, A.C.; Majewski, P.J.; Aldinger, F. Synthesis of Gallium Oxide Hydroxide Crystals in Aqueous Solutions with or without Urea and Their Calcination Behavior. J. Am. Ceram. Soc. 2002, 85, 1421–1429. [Google Scholar] [CrossRef] [Green Version]
- Manna, L.; Scher, E.C.; Alivisatos, A.P. Synthesis of soluble and processable rod-, arrow-, teardrop-, and tetrapod-shaped CdSe nanocrystals. J. Am. Chem. Soc. 2000, 122, 12700–12706. [Google Scholar] [CrossRef]
- Fujihara, S.; Shibata, Y.; Hosono, E. Chemical deposition of rodlike GaOOH and β-Ga2O3 films using simple aqueous solutions. J. Electrochem. Soc. 2005, 152, 764–768. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Duan, P.; Sun, X.; Chu, B.; He, Q. Properties and photocatalytic activity of β-Ga2O3 nanorods under simulated solar irradiation. J. Nanomater. 2015, 2015, 191793. [Google Scholar]
- Zhou, S.; Feng, G.; Wu, B.; Jiang, N.; Xu, S.; Qiu, J. Intense infrared luminescence in transparent glass-ceramics containing β-Ga2O3:Ni2+ Nanocrystals. J. Phys. Chem. C 2007, 111, 7335–7338. [Google Scholar] [CrossRef]
- López, I.; Utrilla, A.D.; Nogales, E.; Méndez, B.; Piqueras, J.; Peche, A.; Ramírez-Castellanos, J.; González-Calbet, J.M. In-doped gallium oxide micro- and nanostructures: Morphology, structure, and luminescence properties. J. Phys. Chem. C 2012, 116, 3935–3943. [Google Scholar] [CrossRef]
- Dohy, D.; Lucazeau, G.; Revcolevschi, A. Raman spectra and valence force field of single-crystalline β-Ga2O3. J. Solid State Chem. 1982, 45, 180–192. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, S. Fabrication and photoluminescence of β- Ga2O3 nanorods. Superlattices Microstruct. 2008, 44, 715–720. [Google Scholar] [CrossRef]
- Kumar, A.; Raizada, P.; Singh, P.; Saini, R.; Saini, A.; Hosseini-Bandegharaei, A. Perspective and status of polymeric graphitic carbon nitride based Z-scheme photocatalytic systems for sustainable photocatalytic water purification. Chem. Eng. J. 2019, 391, 123496. [Google Scholar] [CrossRef]
- Huang, C.-W.; Nguyen, V.-H.; Zhou, S.-R.; Hsu, S.-Y.; Tan, J.-X.; Wu, K.C.-W. Metal-organic frameworks: Preparation and applications in highly efficient heterogeneous photocatalysis. Sustain. Energy Fuels 2020, 4, 504–521. [Google Scholar] [CrossRef]
- Cano-Lerida, L.; Rose, M.; Walton, P. Polycyclic Aromatic Hydrocarbons Dalam Bioactive Compounds in Food; Blackwell Publishing: Oxford, UK, 2008; pp. 378–399. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Doan, T.A.; Park, Y.J.; Hoa, H.T.M.; Phuong, P.H.; Le, D.T.; Hung, N.H.; Tran, Q.T.; Lee, H.-S.; Ryu, J.H.; et al. Synthesis and Photocatalytic Activity of β-Ga2O3 Nanostructures for Decomposition of Formaldehyde under Deep Ultraviolet Irradiation. Catalysts 2020, 10, 1105. https://doi.org/10.3390/catal10101105
Lee J-H, Doan TA, Park YJ, Hoa HTM, Phuong PH, Le DT, Hung NH, Tran QT, Lee H-S, Ryu JH, et al. Synthesis and Photocatalytic Activity of β-Ga2O3 Nanostructures for Decomposition of Formaldehyde under Deep Ultraviolet Irradiation. Catalysts. 2020; 10(10):1105. https://doi.org/10.3390/catal10101105
Chicago/Turabian StyleLee, Jin-Hwan, Tuan Anh Doan, Young Jae Park, Huynh Tran My Hoa, Pham Hoai Phuong, Dung Tien Le, Nguyen Hoang Hung, Quang Trung Tran, Hong-Shik Lee, Jae Hyoung Ryu, and et al. 2020. "Synthesis and Photocatalytic Activity of β-Ga2O3 Nanostructures for Decomposition of Formaldehyde under Deep Ultraviolet Irradiation" Catalysts 10, no. 10: 1105. https://doi.org/10.3390/catal10101105




