Towards the Green Synthesis of Furfuryl Alcohol in A One-Pot System from Xylose: A Review
Abstract
:1. Introduction
2. Scope of the Review
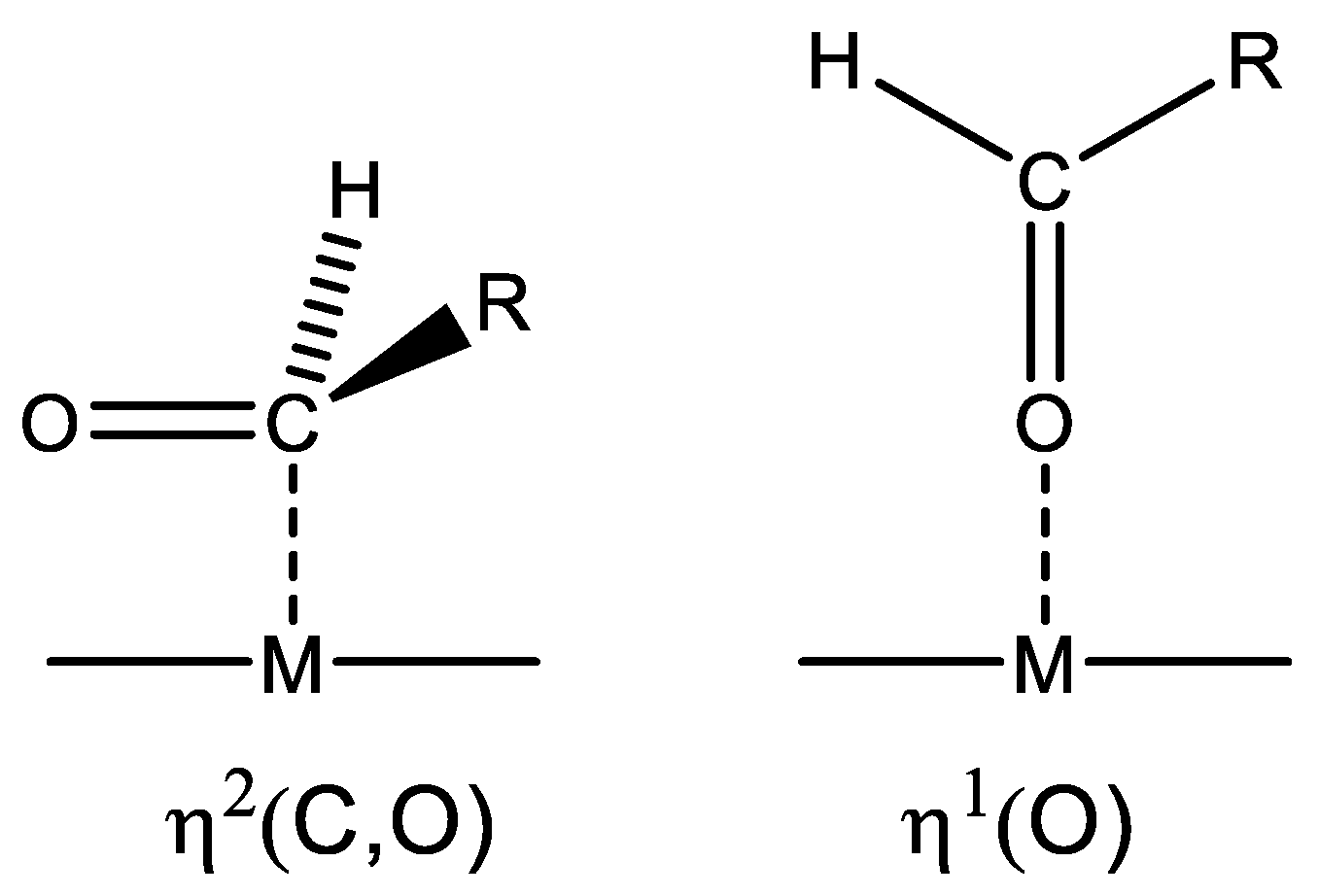
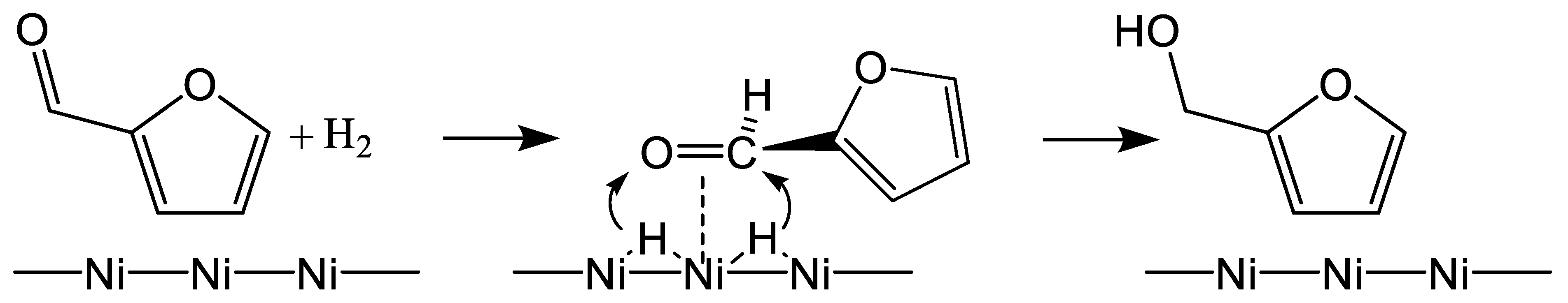
3. Reaction Mechanisms
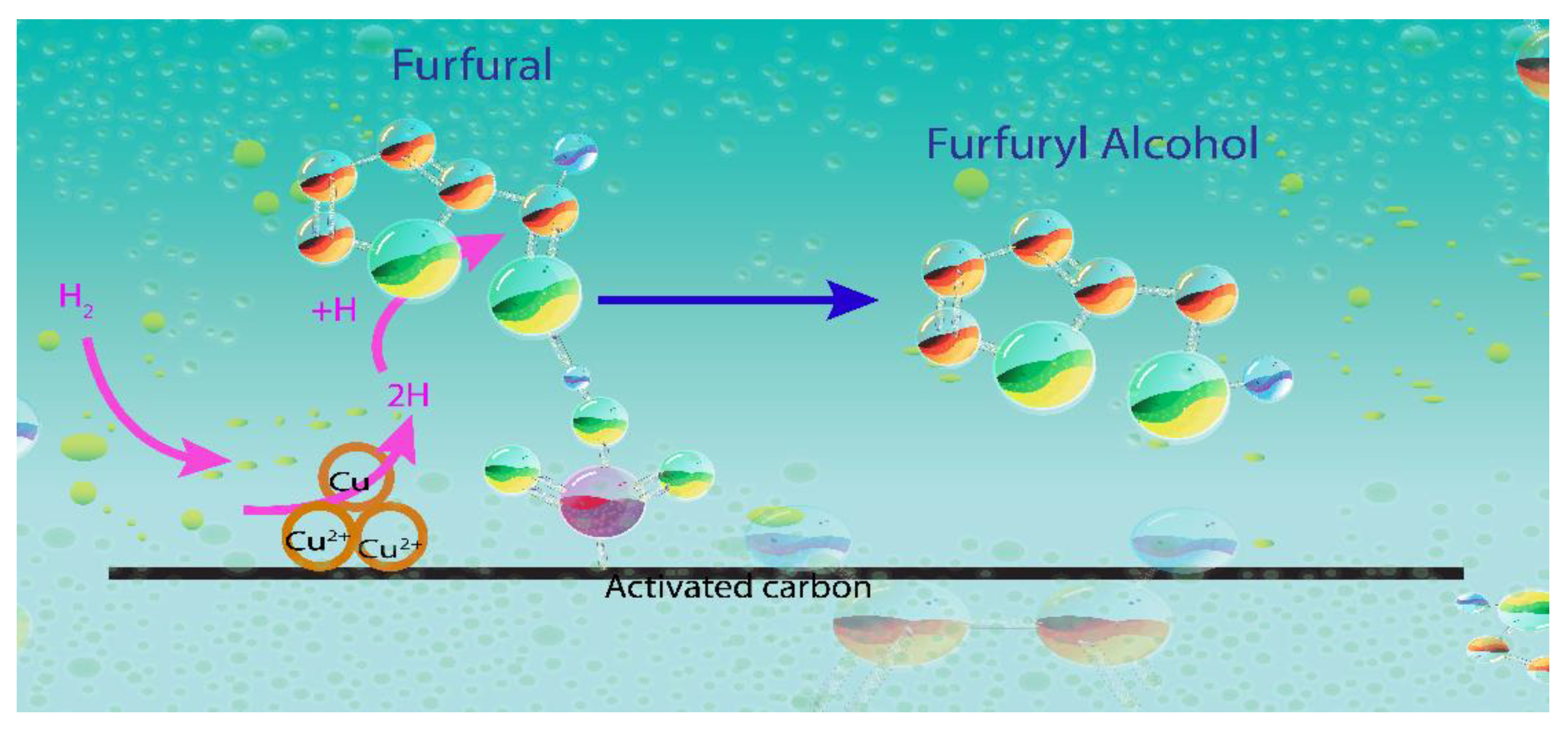
- FUR can be adsorbed through the carbonyl group over both Lewis acid sites (Cu(I)) and Brønsted acids sites (–SO3H group).
- The metallic Cu(0) provides the sites for H2 dissociation, whereas the Cu(I) species can adsorb and activate the –C=O bond of FUR molecules.
- The reduction reaction takes place when the active hydrogen atom attacks the bound aldehyde group on the active surface and FUR is converted into FuOH.
- FuOH is desorbed from the surface of the catalyst with the aid of the solvent (2-propanol) and the stirring effect.
4. Biochemical Conversion of FUR to FuOH
5. Patents on Furfuryl Alcohol Formation
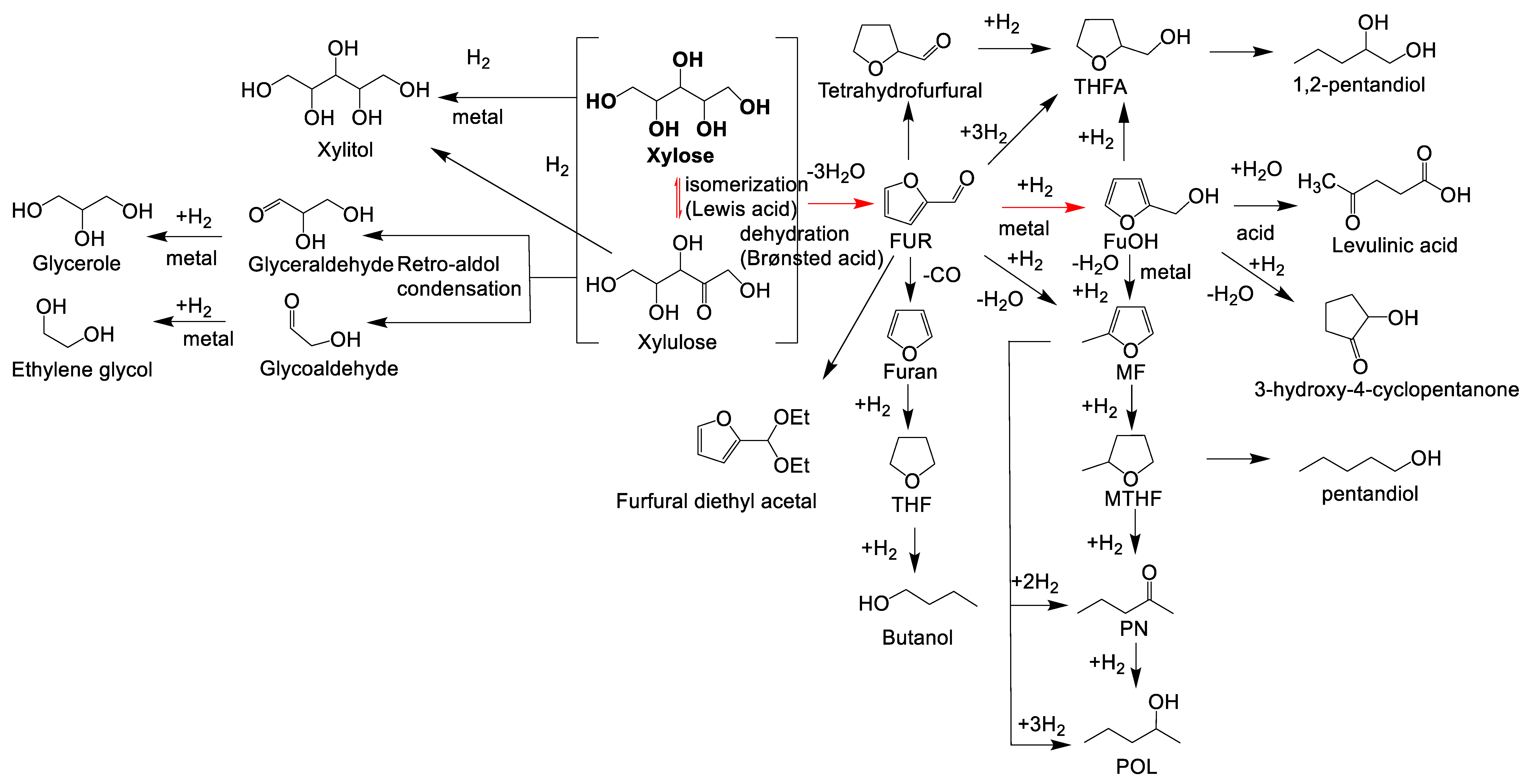
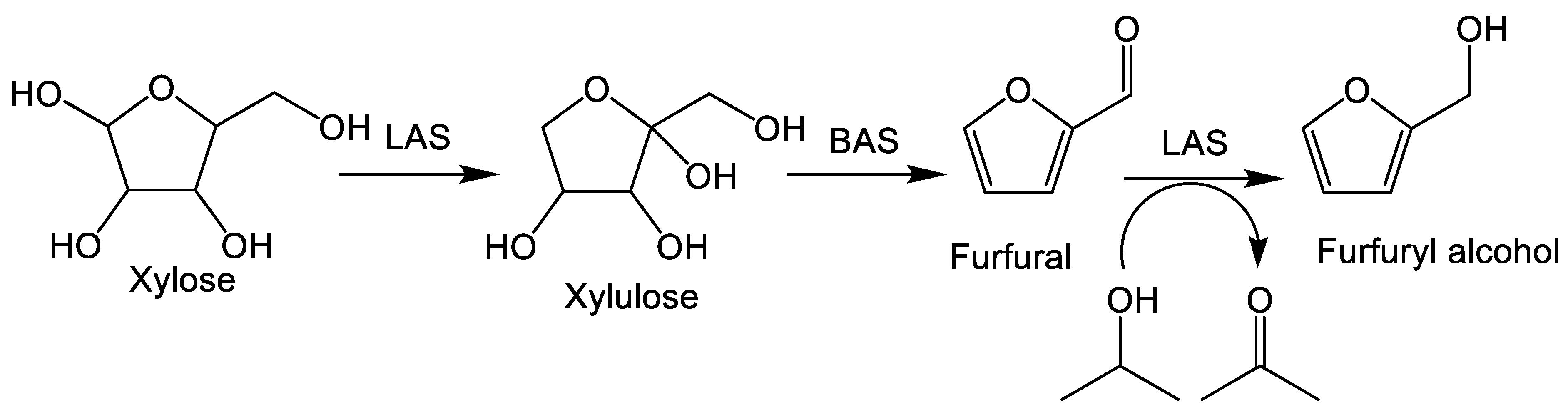
6. Formation of Furfuryl Alcohol from Xylose in One-Pot Reactions
7. Formation of Furfuryl Alcohol from Biomass-Derived Xylose in One-Pot Reactions
8. Effect of Solvents in the Formation of Furfuryl Alcohol
9. Economic Aspects
10. Summary and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Serrano-Ruiz, J.; Luque, R.; Campelo, J.M.; Romero, A.A. Continuous-Flow Processes in Heterogeneously Catalyzed Transformations of Biomass Derivatives into Fuels and Chemicals. Challenges 2012, 3, 114–132. [Google Scholar] [CrossRef]
- Lê, H.Q. Wood Biorefinery Concept Based on γ-Valerolactone/Water Fractionation. Ph.D. Thesis, Aalto University, Espoo, Finland, 2018. [Google Scholar]
- Hashmi, S.F.; Meriö-Talvio, H.; Hakonen, K.J.; Ruuttunen, K.; Sixta, H. Hydrothermolysis of organosolv lignin for the production of bio-oil rich in monoaromatic phenolic compounds. Fuel Process. Technol. 2017, 168, 74–83. [Google Scholar] [CrossRef]
- Venkateswar Rao, L.; Goli, J.K.; Gentela, J.; Koti, S. Bioconversion of lignocellulosic biomass to xylitol: An overview. Bioresour. Technol. 2016, 213, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Gómez Millán, G.; Hellsten, S.; Llorca, J.; Luque, R.; Sixta, H.; Balu, A.M. Recent Advances in the Catalytic Production of Platform Chemicals from Holocellulosic Biomass. ChemCatChem 2019, 11, 2022–2042. [Google Scholar] [CrossRef]
- Ståhlberg, T.; Fu, W.; Woodley, J.M.; Riisager, A. Synthesis of 5-(Hydroxymethyl)furfural in Ionic Liquids: Paving the Way to Renewable Chemicals. ChemSusChem 2011, 4, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Abdelaziz, O.Y.; Hulteberg, C.P.; Riisager, A. New synthetic approaches to biofuels from lignocellulosic biomass. Curr. Opin. Green Sustain. Chem. 2020, 21, 16–21. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; US Department of Energy (US): Oak Ridge, TN, USA, 2004.
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates- the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 4, 539–554. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Dumesic, J.A. Catalytic upgrading of lactic acid to fuels and chemicals by dehydration/hydrogenation and C-C coupling reactions. Green Chem. 2009, 11, 1101–1104. [Google Scholar] [CrossRef]
- Chheda, J.N.; Huber, G.W.; Dumesic, J.A. Liquid-Phase Catalytic Processing of Biomass-Derived Oxygenated Hydrocarbons to Fuels and Chemicals. Angew. Chem. Int. Ed. 2007, 46, 7164–7183. [Google Scholar] [CrossRef]
- Weingarten, R.; Cho, J.; Conner, W.C.; Huber, G.W. Kinetics of furfural production by dehydration of xylose in a biphasic reactor with microwave heating. Green Chem. 2010, 12, 1423–1429. [Google Scholar] [CrossRef] [Green Version]
- Wettstein, S.G.; Alonso, D.M.; Gürbüz, E.I.; Dumesic, J.A. A roadmap for conversion of lignocellulosic biomass to chemicals and fuels. Curr. Opin. Chem. Eng. 2012, 1, 218–224. [Google Scholar] [CrossRef]
- Heng, Z.; Grinstaff, M.W. Recent Advances in Glycerol Polymers: Chemistry and Biomedical Applications. Macromol. Rapid Commun. 2014, 35, 1906–1924. [Google Scholar]
- Adkins, H.; Connor, R. The catalytic hydrogenation of organic compounds over copper chromite. J. Am. Chem. Soc. 1931, 53, 1091–1095. [Google Scholar] [CrossRef]
- Nagaraja, B.M.; Padmasri, A.H.; David Raju, B.; Rama Rao, K.S. Vapor phase selective hydrogenation of furfural to furfuryl alcohol over Cu–MgO coprecipitated catalysts. J. Mol. Catal. A Chem. 2007, 265, 90–97. [Google Scholar] [CrossRef]
- Seo, G.; Chon, H. Hydrogenation of furfural over copper-containing catalysts. J. Catal. 1981, 67, 424–429. [Google Scholar] [CrossRef]
- Rao, R.; Dandekar, A.; Baker, R.T.K.; Vannice, M.A. Properties of Copper Chromite Catalysts in Hydrogenation Reactions. J. Catal. 1997, 171, 406–419. [Google Scholar] [CrossRef]
- Zhang, H.; Lei, Y.; Kropf, A.J.; Zhang, G.; Elam, J.W.; Miller, J.T.; Sollberger, F.G.; Ribeiro, F.H.; Akatay, M.C.; Stach, E.A.; et al. Enhancing the stability of copper chromite catalysts for the selective hydrogenation of furfural using ALD overcoating. J. Catal. 2014, 317, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Zemlyanov, D.; Wu, T.; Lobolapidus, R.J.; Dumesic, J.A.; Miller, J.T.; Marshall, C.L. Deactivation mechanistic studies of copper chromite catalyst for selective hydrogenation of 2-furfuraldehyde. J. Catal. 2013, 299, 336–345. [Google Scholar] [CrossRef]
- NIIR Board of Consultants and Engineers. Synthetic Resins Technology Handbook; ASIA PACIFIC BUSINESS PRESS Inc.: Kamla Nagar, Delhi, India, 2005; p. 588. [Google Scholar]
- Erdmann, E. Zur Charakteristik des Furfuralkohols. Ber. Dtsch. Chem. Ges. 1902, 35, 1855–1862. [Google Scholar] [CrossRef] [Green Version]
- Industrial Development of Furfuryl Alcohol. Available online: http://www.furan.com/furfuryl_alcohol_historical_overview.html (accessed on 23 May 2020).
- Wojcik, B.H. Catalytic Hydrogenation of Furan Compounds. Ind. Eng. Chem. 1948, 40, 210–216. [Google Scholar] [CrossRef]
- Tamura, M.; Tokonami, K.; Nakagawa, Y.; Tomishige, K. Rapid synthesis of unsaturated alcohols under mild conditions by highly selective hydrogenation. Chem. Commun. 2013, 49, 7034–7036. [Google Scholar] [CrossRef] [PubMed]
- Kijeński, J.; Winiarek, P.; Paryjczak, T.; Lewicki, A.; Mikołajska, A. Platinum deposited on monolayer supports in selective hydrogenation of furfural to furfuryl alcohol. Appl. Catal. A 2002, 233, 171–182. [Google Scholar] [CrossRef]
- Pang, S.H.; Medlin, J.W. Adsorption and Reaction of Furfural and Furfuryl Alcohol on Pd(111): Unique Reaction Pathways for Multifunctional Reagents. ACS Catal. 2011, 1, 1272–1283. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, D.; van Haandel, L.; Ye, F.; Xue, T.; Hensen, E.J.M.; Guan, Y. Selective liquid phase hydrogenation of furfural to furfuryl alcohol by Ru/Zr-MOFs. J. Mol. Catal. A Chem. 2015, 406, 58–64. [Google Scholar] [CrossRef]
- Yu, W.; Tang, Y.; Mo, L.; Chen, P.; Lou, H.; Zheng, X. Bifunctional Pd/Al-SBA-15 catalyzed one-step hydrogenation–esterification of furfural and acetic acid: A model reaction for catalytic upgrading of bio-oil. Catal. Commun. 2011, 13, 35–39. [Google Scholar] [CrossRef]
- Gong, W.; Chen, C.; Zhang, Y.; Zhou, H.; Wang, H.; Zhang, H.; Zhang, Y.; Wang, G.; Zhao, H. Efficient Synthesis of Furfuryl Alcohol from H2-Hydrogenation/Transfer Hydrogenation of Furfural Using Sulfonate Group Modified Cu Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2172–2180. [Google Scholar] [CrossRef]
- Sharma, R.V.; Das, U.; Sammynaiken, R.; Dalai, A.K. Liquid phase chemo-selective catalytic hydrogenation of furfural to furfuryl alcohol. Appl. Catal. A 2013, 454, 127–136. [Google Scholar] [CrossRef]
- Sitthisa, S.; Sooknoi, T.; Ma, Y.; Balbuena, P.B.; Resasco, D.E. Kinetics and mechanism of hydrogenation of furfural on Cu/SiO2 catalysts. J. Catal. 2011, 277, 1–13. [Google Scholar] [CrossRef]
- Sitthisa, S.; Resasco, D.E. Hydrodeoxygenation of Furfural over Supported Metal Catalysts: A Comparative Study of Cu, Pd and Ni. Catal. Lett. 2011, 141, 784–791. [Google Scholar] [CrossRef]
- Merlo, A.B.; Vetere, V.; Ruggera, J.F.; Casella, M.L. Bimetallic PtSn catalyst for the selective hydrogenation of furfural to furfuryl alcohol in liquid-phase. Catal. Commun. 2009, 10, 1665–1669. [Google Scholar] [CrossRef]
- Li, H.; Luo, H.; Zhuang, L.; Dai, W.; Qiao, M. Liquid phase hydrogenation of furfural to furfuryl alcohol over the Fe-promoted Ni-B amorphous alloy catalysts. J. Mol. Catal. A Chem. 2003, 203, 267–275. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Luo, H.; Qiao, M. Liquid phase hydrogenation of furfural to furfuryl alcohol over Mo-doped Co-B amorphous alloy catalysts. Appl. Catal. A 2002, 233, 13–20. [Google Scholar] [CrossRef]
- Fulajtárova, K.; Soták, T.; Hronec, M.; Vávra, I.; Dobročka, E.; Omastová, M. Aqueous phase hydrogenation of furfural to furfuryl alcohol over Pd–Cu catalysts. Appl. Catal. A 2015, 502, 78–85. [Google Scholar] [CrossRef]
- Baijun, L.; Lianhai, L.; Bingchun, W.; Tianxi, C.; Iwatani, K. Liquid phase selective hydrogenation of furfural on Raney nickel modified by impregnation of salts of heteropolyacids. Appl. Catal. A 1998, 171, 117–122. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic Reduction of Biomass-Derived Furanic Compounds with Hydrogen. ACS Catal. 2013, 3, 2655–2668. [Google Scholar] [CrossRef]
- Du, H.; Ma, X.; Jiang, M.; Yan, P.; Zhao, Y.; Zhang, Z.C. Efficient Ni/SiO2 catalyst derived from nickel phyllosilicate for xylose hydrogenation to xylitol. Catal. Today 2020, in press. [Google Scholar] [CrossRef]
- Bachosz, K.; Synoradzki, K.; Staszak, M.; Pinelo, M.; Meyer, A.S.; Zdarta, J.; Jesionowski, T. Bioconversion of xylose to xylonic acid via co-immobilized dehydrogenases for conjunct cofactor regeneration. Bioorg. Chem. 2019, 93, 102747. [Google Scholar] [CrossRef]
- (Chamnankid, B.; Ratanatawanate, C.; Faungnawakij, K. Conversion of xylose to levulinic acid over modified acid functions of alkaline-treated zeolite Y in hot-compressed water. Chem. Eng. J. 2014, 258, 341–347. [Google Scholar] [CrossRef]
- Rao, R.S.; Baker, R.T.; Vannice, M.A. Furfural hydrogenation over carbon-supported copper. Catal. Lett. 1999, 60, 51–57. [Google Scholar] [CrossRef]
- O’Driscoll, Á.; Leahy, J.J.; Curtin, T. The influence of metal selection on catalyst activity for the liquid phase hydrogenation of furfural to furfuryl alcohol. Catal. Today 2017, 279, 194–201. [Google Scholar] [CrossRef]
- Taylor, M.J.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.A.; Wilson, K.; Lee, A.F.; Kyriakou, G. Highly selective hydrogenation of furfural over supported Pt nanoparticles under mild conditions. Appl. Catal. B 2016, 180, 580–585. [Google Scholar] [CrossRef]
- Lee, J.; Burt, S.P.; Carrero, C.A.; Albarubio, A.C.; Ro, I.; Oneill, B.J.; Kim, H.J.; Jackson, D.H.K.; Kuech, T.F.; Hermans, I.; et al. Stabilizing cobalt catalysts for aqueous-phase reactions by strong metal-support interaction. J. Catal. 2015, 330, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Mironenko, R.M.; Belskaya, O.B.; Gulyaeva, T.I.; Nizovskii, A.I.; Kalinkin, A.V.; Bukhtiyarov, V.I.; Lavrenov, A.V.; Likholobov, V.A. Effect of the nature of carbon support on the formation of active sites in Pd/C and Ru/C catalysts for hydrogenation of furfural. Catal. Today 2015, 249, 145–152. [Google Scholar] [CrossRef]
- Liu, L.; Lou, H.; Chen, M. Selective hydrogenation of furfural over Pt based and Pd based bimetallic catalysts supported on modified multiwalled carbon nanotubes (MWNT). Appl. Catal. A 2018, 550, 1–10. [Google Scholar] [CrossRef]
- Claus, P. Selective hydrogenation of α,β-unsaturated aldehydes and other C=O and C=C bonds containing compounds. Top. Catal. 1998, 5, 51–62. [Google Scholar] [CrossRef]
- Deng, T.; Xu, G.; Fu, Y. One-pot cascade conversion of xylose to furfuryl alcohol over a bifunctional Cu/SBA-15-SO3H catalyst. Chin. J. Catal. 2020, 41, 404–414. [Google Scholar] [CrossRef]
- Jouve, A.; Cattaneo, S.; Delgado, D.; Scotti, N.; Evangelisti, C.; Nieto, J.M.L.; Prati, L. Furfural Hydrogenation on Modified Niobia. Appl. Sci. 2019, 9, 2287. [Google Scholar] [CrossRef] [Green Version]
- Jaatinen, S.K.; Karinen, R.S.; Lehtonen, J.S. Liquid Phase Furfural Hydrotreatment to 2-Methylfuran on Carbon Supported Nickel Catalyst - Effect of Process Conditions. ChemistrySelect 2016, 1, 5363–5373. [Google Scholar] [CrossRef]
- Zeitsch, K.J. (Ed.) Furfuryl alcohol. In The Chemistry and Technology of Furfural and Its Many by-Products; Elsevier: Amsterdam, The Netherlands, 2000; pp. 150–155. [Google Scholar]
- Lange, J.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A Promising Platform for Lignocellulosic Biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef]
- Moreau, C.; Belgacem, M.N.; Gandini, A. Recent Catalytic Advances in the Chemistry of Substituted Furans from Carbohydrates and in the Ensuing Polymers. Top. Catal. 2004, 27, 11–30. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.; Muralidhara, A.; El Ouardi, K.; Marlair, G.; Len, C. Hydrolysis of hemicellulose and derivatives—A review of recent advances in the production of furfural. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, Z.; Li, X.; Liu, X.; Fan, J.; Clark, J.H.; Hu, C. The production of furfural directly from hemicellulose in lignocellulosic biomass: A review. Catal. Today 2019, 319, 14–24. [Google Scholar] [CrossRef]
- Agirrezabal-Telleria, I.; Gandarias, I.; Arias, P.L. Heterogeneous acid-catalysts for the production of furan-derived compounds (furfural and hydroxymethylfurfural) from renewable carbohydrates: A review. Catal. Today 2014, 234, 42–58. [Google Scholar] [CrossRef]
- Romo, J.E.; Bollar, N.V.; Zimmermann, C.J.; Wettstein, S.G. Conversion of Sugars and Biomass to Furans Using Heterogeneous Catalysts in Biphasic Solvent Systems. ChemCatChem 2018, 10, 4805–4816. [Google Scholar] [CrossRef] [Green Version]
- Iroegbu, A.O.; Hlangothi, S.P. Furfuryl Alcohol a Versatile, Eco-Sustainable Compound in Perspective. Chem. Afr. 2019, 2, 223–239. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhao, D.; Rodrí guez-Padrón, D.; Len, C. Recent Advances in Catalytic Hydrogenation of Furfural. Catalysts 2019, 9, 796. [Google Scholar] [CrossRef] [Green Version]
- Gómez Millán, G.; Hellsten, S.; King, A.W.T.; Pokki, J.; Llorca, J.; Sixta, H. A comparative study of water-immiscible organic solvents in the production of furfural from xylose and birch hydrolysate. J. Ind. Eng. Chem. 2019, 72, 354–363. [Google Scholar] [CrossRef] [Green Version]
- Domínguez de Marí a, P.; Guajardo, N. Biocatalytic Valorization of Furans: Opportunities for Inherently Unstable Substrates. ChemSusChem 2017, 10, 4123–4134. [Google Scholar] [CrossRef]
- Mavrikakis, M.; Barteau, M.A. Oxygenate reaction pathways on transition metal surfaces. J. Mol. Catal. A Chem. 1998, 131, 135–147. [Google Scholar] [CrossRef]
- Baker, L.R.; Kennedy, G.; van Spronsen, M.A.; Hervier, A.; Cai, X.; Chen, S.; Wang, L.; Somorjai, G.A. Furfuraldehyde Hydrogenation on Titanium Oxide-Supported Platinum Nanoparticles Studied by Sum Frequency Generation Vibrational Spectroscopy: Acid-Base Catalysis Explains the Molecular Origin of Strong Metal-Support Interactions. J. Am. Chem. Soc. 2012, 134, 14208–14216. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.; Laliberté, M.; Lavoie, S.; Castonguay, M.; McBreen, P.H. Adsorption states of acetaldehyde and butane-2,3-dione on Ni(111). Surf. Sci. 2005, 578, 43–56. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakazawa, H.; Watanabe, H.; Tomishige, K. Total Hydrogenation of Furfural over a Silica-Supported Nickel Catalyst Prepared by the Reduction of a Nickel Nitrate Precursor. ChemCatChem 2012, 4, 1791–1797. [Google Scholar] [CrossRef]
- Christmann, K.; Behm, R.J.; Ertl, G.; Van Hove, M.A.; Weinberg, W.H. Chemisorption geometry of hydrogen on Ni(111): Order and disorder. J. Chem. Phys. 1979, 70, 4168–4184. [Google Scholar] [CrossRef]
- Vicente, A.; Lafaye, G.; Especel, C.; Marécot, P.; Williams, C.T. The relationship between the structural properties of bimetallic Pd–Sn/SiO2 catalysts and their performance for selective citral hydrogenation. J. Catal. 2011, 283, 133–142. [Google Scholar] [CrossRef]
- Gallezot, P.; Richard, D. Selective Hydrogenation of α,β-Unsaturated Aldehydes. Catal. Rev. 1998, 40, 81–126. [Google Scholar] [CrossRef]
- Liberková, K.; Touroude, R. Performance of Pt/SnO2 catalyst in the gas phase hydrogenation of crotonaldehyde. J. Mol. Catal. A Chem. 2002, 180, 221–230. [Google Scholar] [CrossRef]
- Ammari, F.; Milone, C.; Touroude, R. Selective hydrogenation of crotonaldehyde on Pt/ZnCl2/SiO2 catalysts. J. Catal. 2005, 235, 1–9. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, J.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A.; Arcoya, A. Selective hydrogenation of citral over Pt/KL type catalysts doped with Sr, La, Nd and Sm. Appl. Catal. A 2011, 401, 56–64. [Google Scholar] [CrossRef]
- López-Asensio, R.; Jiménez Gómez, C.P.; Garcí a Sancho, C.; Moreno-Tost, R.; Cecilia, J.A.; Maireles-Torres, P. Influence of Structure-modifying Agents in the Synthesis of Zr-doped SBA-15 Silica and Their Use as Catalysts in the Furfural Hydrogenation to Obtain High Value-added Products through the Meerwein-Ponndorf-Verley Reduction. Int. J. Mol. Sci. 2019, 20, 828. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, J.; Melero, J.A.; Morales, G.; Moreno, J.; Segura, Y.; Paniagua, M.; Cambra, A.; Hernández, B. Zr-SBA-15 Lewis Acid Catalyst: Activity in Meerwein Ponndorf Verley Reduction. Catalysts 2015, 5, 1911–1927. [Google Scholar] [CrossRef] [Green Version]
- Knauer, B.; Krohn, K. A reinvestigation of the Meerwein-Ponndorf-Verley reduction A highly efficient variation using zirconium catalysts. Liebigs Ann. Recl. 1995, 1995, 677–683. [Google Scholar] [CrossRef]
- De bruyn, M.; Limbourg, M.; Denayer, J.; Baron, G.V.; Parvulescu, V.; Grobet, P.J.; De Vos, D.E.; Jacobs, P.A. Mesoporous Zr and Hf catalysts for chemoselective MPV reductions of unsaturated ketones. Appl. Catal. A 2003, 254, 189–201. [Google Scholar] [CrossRef]
- Corma, A.; Domine, M.E.; Valencia, S. Water-resistant solid Lewis acid catalysts: Meerwein–Ponndorf–Verley and Oppenauer reactions catalyzed by tin-beta zeolite. J. Catal. 2003, 215, 294–304. [Google Scholar] [CrossRef]
- Lanzafame, P.; Temi, D.M.; Perathoner, S.; Centi, G.; Macario, A.; Aloise, A.; Giordano, G. Etherification of 5-hydroxymethyl-2-furfural (HMF) with ethanol to biodiesel components using mesoporous solid acidic catalysts. Catal. Today 2011, 175, 435–441. [Google Scholar] [CrossRef]
- Luo, J.; Yu, J.; Gorte, R.J.; Mahmoud, E.; Vlachos, D.G.; Smith, M.A. The effect of oxide acidity on HMF etherification. Catal. Sci. Technol. 2014, 4, 3074–3081. [Google Scholar] [CrossRef]
- Paulino, P.N.; Perez, R.F.; Figueiredo, N.G.; Fraga, M.A. Tandem dehydration-transfer hydrogenation reactions of xylose to furfuryl alcohol over zeolite catalysts. Green Chem. 2017, 19, 3759–3763. [Google Scholar] [CrossRef]
- Perez, R.F.; Albuquerque, E.M.; Borges, L.E.P.; Hardacre, C.; Fraga, M.A. Aqueous-phase tandem catalytic conversion of xylose to furfuryl alcohol over [Al]-SBA-15 molecular sieves. Catal. Sci. Technol. 2019, 9, 5350–5358. [Google Scholar] [CrossRef]
- Moreno-Marrodan, C.; Barbaro, P.; Caporali, S.; Bossola, F. Low-Temperature Continuous-Flow Dehydration of Xylose Over Water-Tolerant Niobia-Titania Heterogeneous Catalysts. ChemSusChem 2018, 11, 3649–3660. [Google Scholar] [CrossRef]
- Choudhary, V.; Sandler, S.I.; Vlachos, D.G. Conversion of Xylose to Furfural Using Lewis and Brönsted Acid Catalysts in Aqueous Media. ACS Catal. 2012, 2, 2022–2028. [Google Scholar] [CrossRef]
- Choudhary, V.; Pinar, A.B.; Sandler, S.I.; Vlachos, D.G.; Lobo, R.F. Xylose Isomerization to Xylulose and its Dehydration to Furfural in Aqueous Media. ACS Catal. 2011, 1, 1724–1728. [Google Scholar] [CrossRef]
- Hernández, B.; Iglesias, J.; Morales, G.; Paniagua, M.; López-Aguado, C.; García Fierro, J.L.; Wolf, P.; Hermans, I.; Melero, J.A. One-pot cascade transformation of xylose into y-valerolactone (GVL) over bifunctional Brönsted-Lewis Zr-Al-beta zeolite. Green Chem. 2016, 18, 5777–5781. [Google Scholar] [CrossRef]
- Corma, A.; Domine, M.E.; Nemeth, L.; Valencia, S. Al-Free Sn-Beta Zeolite as a Catalyst for the Selective Reduction of Carbonyl Compounds (Meerwein-Ponndorf-Verley Reaction). J. Am. Chem. Soc. 2002, 124, 3194–3195. [Google Scholar] [CrossRef] [PubMed]
- Gonell, F.; Boronat, M.; Corma, A. Structure-reactivity relationship in isolated Zr sites present in Zr-zeolite and ZrO2 for the Meerwein-Ponndorf-Verley reaction. Catal. Sci. Technol. 2017, 7, 2865–2873. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Bu, C.; He, Q.; Zheng, Z.; Ouyang, J. Efficient bioconversion of furfural to furfuryl alcohol by Bacillus coagulans NL01. RSC Adv. 2018, 8, 26720–26727. [Google Scholar] [CrossRef] [Green Version]
- Belay, N.; Boopathy, R.; Voskuilen, G. Anaerobic Transformation of Furfural by Methanococcus deltae (Delta)LH. Appl. Environ. Microbiol. 1997, 63, 2092–2094. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.L.; Slininger, P.J.; Gorsich, S.W. Enhanced Biotransformation of Furfural and Hydroxymethylfurfural by Newly Developed Ethanologenic Yeast Strains. Appl. Biochem. Biotechnol. 2005, 121, 451–460. [Google Scholar] [CrossRef]
- Liu, Z.L.; Slininger, P.J.; Dien, B.S.; Berhow, M.A.; Kurtzman, C.P.; Gorsich, S.W. Adaptive response of yeasts to furfural and 5-hydroxymethylfurfural and new chemical evidence for HMF conversion to 2,5-bis-hydroxymethylfuran. J. Ind. Microbiol. Biotechnol. 2004, 31, 345–352. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ding, Y.; Ma, C.; Di, J.; Jiang, C.; Li, A. One-pot conversion of biomass-derived xylose to furfuralcohol by a chemo-enzymatic sequential acid-catalyzed dehydration and bioreduction. Green Chem. 2017, 19, 3844–3850. [Google Scholar] [CrossRef]
- Gutiérrez, T.; Ingram, L.O.; Preston, J.F. Purification and characterization of a furfural reductase (FFR) from Escherichia coli strain LYO1—An enzyme important in the detoxification of furfural during ethanol production. J. Biotechnol. 2006, 121, 154–164. [Google Scholar] [CrossRef]
- Jiang, T.; Qiao, H.; Zheng, Z.; Chu, Q.; Li, X.; Yong, Q.; Ouyang, J. Lactic Acid Production from Pretreated Hydrolysates of Corn Stover by a Newly Developed Bacillus coagulans Strain. PLoS ONE 2016, 11, e0149101. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jiang, C.; Chong, G.; Di, J.; Wu, Y.; Wang, B.; Xue, X.X.; Ma, C.L. Chemical-enzymatic conversion of corncob-derived xylose to furfuralcohol by the tandem catalysis with SO42−/SnO2-kaoline and E. coli CCZU-T15 cells in toluene–water media. Bioresour. Technol. 2017, 245, 841–849. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jiang, C.; Jiang, J.; Di, J.; Liu, F.; Ding, Y.; Qing, Q.; Ma, C. One-pot chemo-enzymatic synthesis of furfuralcohol from xylose. Bioresour. Technol. 2017, 238, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A. Binder Composition for Mold Making Purposes. Patent No. WO/2012/081577, 21 June 2012. [Google Scholar]
- Kanekawa, H.; Kawakatsu, Y.; Sakai, M. Binder for Casting Mold. Patent No. JP22385883A, 21 June 1983. [Google Scholar]
- Smith, S.B. Method of Making a Low-Griability, Thermosetting Foam. Patent US23846781A, 21 September 1982. [Google Scholar]
- Hamermesh, C.L.; Hogenson, P.A.; Tung, C.M. Intumescent Flame-Resistant Coating. U.S. Patent US19690580A, 1 December 1980. [Google Scholar]
- Katada, H.; Kobayashi, S.; Ogasawara, S.; Tsujimoto, N. Method for Producing Furfuryl Alcohol-Formaldehyde Copolymer. Patent No. JP2012238393A, 6 December 2012. [Google Scholar]
- Matsumoto, Y.; Murai, Y. Method for Producing Spherical Furfuryl Alcohol Resin Particle, Spherical Furfuryl Alcohol Resin Particle Produced Thereby, Spherical Carbon Particle, and Spherical Activated Carbon Particle. Patent No. JP2011059103A, 11 October 2011. [Google Scholar]
- Chen, M.C.; Everett, G.S.; Maclennan, G.R. Resinous Binder Compositions. Patent CN94191466A, 27 March 1996. [Google Scholar]
- Kozinski, A.A. Furfuryl Alcohol Production Process. U.S. Patent US4185022A, 22 January 1980. [Google Scholar]
- Swadesh, S. Catalytic Production of Furfuryl Alcohol and Catalyst Therefor. U.S. Patent US2754304A, 10 July 1956. [Google Scholar]
- Lillwitz, L.D. Manufacture of Furfuryl Alcohol. U.S. Patent US4089871A, 16 May 1978. [Google Scholar]
- Kangyi, L. Method for Preparing Furfuryl Alcohol by Catalyzing Furfural. Patent No. CN105418551A, 23 March 2016. [Google Scholar]
- Zhao, H.; Liu, C.; Jing, M.; Yin, C.; Chai, Y.; Zhao, R.; Zhang, K.; Liu, Y. Method for Preparing Furfuryl Alcohol through Furfural Liquid-Phase Catalytic Hydrogenation. Patent No. CN102603681A, 25 July 2012. [Google Scholar]
- Liu, C.; Liu, X.; Wang, H.; Xia, Y.; Xie, Y.; Xu, G. Preparation Method of 2-Zirconium Hydroxyphosphinyl Acetate and Application of 2-Zironiumhydroxyphosphinyl Acetate in Synthesis of Furfuryl Alcohol. Patent No. CN201910077968A, 16 April 2019. [Google Scholar]
- Liu, Y.; Qi, Z.; Zhang, J. Method for Preparing Furfuryl Alcohol through Transfer Hydrogenation of Furfural and Low-Grade Alkanol. Patent No. CN201910664083A, 12 November 2019. [Google Scholar]
- André Fraga, M.; Farias Perez, R. Process for Obtaining Furfuryl Alcohol by Multifunctional Catalysts. Patent No. BR102013025330A2, 18 August 2015. [Google Scholar]
- Perez, R.F.; Fraga, M.A. Hemicellulose-derived chemicals: One-step production of furfuryl alcohol from xylose. Green Chem. 2014, 16, 3942–3950. [Google Scholar] [CrossRef]
- Perez, R.F.; Canhaci, S.J.; Borges, L.E.P.; Fraga, M.A. One-step conversion of xylose to furfuryl alcohol on sulfated zirconia-supported Pt catalyst—Balance between acid and metal sites. Catal. Today 2017, 289, 273–279. [Google Scholar] [CrossRef]
- Perez, R.F.; Soares, O.S.G.P.; de Farias, A.M.D.; Pereira, M.F.R.; Fraga, M.A. Conversion of hemicellulose-derived pentoses over noble metal supported on 1D multiwalled carbon nanotubes. Appl. Catal. B 2018, 232, 101–107. [Google Scholar] [CrossRef]
- Canhaci, S.J.; Perez, R.F.; Borges, L.E.P.; Fraga, M.A. Direct conversion of xylose to furfuryl alcohol on single organic–inorganic hybrid mesoporous silica-supported catalysts. Appl. Catal. B 2017, 207, 279–285. [Google Scholar] [CrossRef]
- Cui, J.; Tan, J.; Cui, X.; Zhu, Y.; Deng, T.; Ding, G.; Li, Y. Conversion of Xylose to Furfuryl Alcohol and 2-Methylfuran in a Continuous Fixed-Bed Reactor. ChemSusChem 2016, 9, 1259–1262. [Google Scholar] [CrossRef]
- Zheng, H.; Zhu, Y.; Teng, B.; Bai, Z.; Zhang, C.; Xiang, H.; Li, Y. Towards understanding the reaction pathway in vapour phase hydrogenation of furfural to 2-methylfuran. J. Mol. Catal. A Chem. 2006, 246, 18–23. [Google Scholar] [CrossRef]
- Zheng, H.; Zhu, Y.; Bai, Z.; Huang, L.; Xiang, H.; Li, Y. An environmentally benign process for the efficient synthesis of cyclohexanone and 2-methylfuran. Green Chem. 2006, 8, 107–109. [Google Scholar] [CrossRef]
- Xu, L.; Nie, R.; Xu, H.; Chen, X.; Li, Y.; Lu, X. One-Pot Tandem Dehydration-Hydrogenation of Xylose with Formic Acid over Co Catalysts. Ind. Eng. Chem. Res. 2020, 59, 2754–2760. [Google Scholar] [CrossRef]
- Ordomsky, V.V.; Schouten, J.C.; van der Schaaf, J.; Nijhuis, T.A. Biphasic single-reactor process for dehydration of xylose and hydrogenation of produced furfural. Appl. Catal. A 2013, 451, 6–13. [Google Scholar] [CrossRef]
- Gómez Millán, G. Valorization of Low Concentration Sugar Side-Stream from Dissolving Pulp Production. Ph.D. Thesis, Aalto University, Espoo, Finland, 2019. [Google Scholar]
- Gómez Millán, G.; Phiri, J.; Makela, M.; Maloney, T.; Balu, A.M.; Pineda, A.; Llorca, J.; Sixta, H. Furfural production in a biphasic system using a carbonaceous solid acid catalyst. Appl. Catal. A 2019, 585, 117180. [Google Scholar] [CrossRef] [Green Version]
- Deng, A.; Lin, Q.; Yan, Y.; Li, H.; Ren, J.; Liu, C.; Sun, R. A feasible process for furfural production from the pre-hydrolysis liquor of corncob via biochar catalysts in a new biphasic system. Bioresour. Technol. 2016, 216, 754–760. [Google Scholar] [CrossRef]
- Li, H.; Deng, A.; Ren, J.; Liu, C.; Wang, W.; Peng, F.; Sun, R. A modified biphasic system for the dehydration of d-xylose into furfural using SO42−/TiO2-ZrO2/La3+ as a solid catalyst. Catal. Today 2014, 234, 251–256. [Google Scholar] [CrossRef]
- Singh, U.K.; Vannice, M.A. Kinetics of liquid-phase hydrogenation reactions over supported metal catalysts—A review. Appl. Catal. A 2001, 213, 1–24. [Google Scholar] [CrossRef]
- Bonita, Y.; Jain, V.; Geng, F.; O’Connell, T.P.; Wilson, W.N.; Rai, N.; Hicks, J.C. Direct synthesis of furfuryl alcohol from furfural: Catalytic performance of monometallic and bimetallic Mo and Ru phosphides. Catal. Sci. Technol. 2019, 9, 3656–3668. [Google Scholar] [CrossRef]
- López-Asensio, R.; Cecilia, J.A.; Jiménez-Gómez, C.P.; García-Sancho, C.; Moreno-Tost, R.; Maireles-Torres, P. Selective production of furfuryl alcohol from furfural by catalytic transfer hydrogenation over commercial aluminas. Appl. Catal. A 2018, 556, 1–9. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, A.; Zhao, Z.; Wang, X.; Jiang, G.; Liu, J.; Wang, C.; Jin, M. Synthesis, characterization and catalytic performance of meso-microporous material Beta-SBA-15-supported NiMo catalysts for hydrodesulfurization of dibenzothiophene. Catal. Today 2011, 175, 477–484. [Google Scholar] [CrossRef]
- Da Silva Carolina, X.A.; Gonçalves, V.L.C.; Mota, C.J.A. Water-tolerant zeolite catalyst for the acetalisation of glycerol. Green Chem. 2009, 11, 38–41. [Google Scholar] [CrossRef]
- Santos, K.M.A.; Albuquerque, E.M.; Innocenti, G.; Borges, L.E.P.; Sievers, C.; Fraga, M.A. The Role of Brönsted and Water-Tolerant Lewis Acid Sites in the Cascade Aqueous-Phase Reaction of Triose to Lactic Acid. ChemCatChem 2019, 11, 3054–3063. [Google Scholar] [CrossRef]
- Furfural and Furfuryl Alcohol—A Global Market Overview. Available online: http://industry-experts.com/verticals/files/articles/cp071-furfural-and-furfuryl-alcohol-a-global-market-overview.pdf (accessed on 5 June 2020).
- Global Furfuryl Alcohol Market Expected to Reach US$ 1493.7 Mn By 2028, Driven by Growth in the Foundry Industry: Future Market Insights. Available online: https://www.nsenergybusiness.com/pressreleases/companies/future-market-insights/global-furfuryl-alcohol-market-expected-to-reach-us-1493-7-mn-by-2028-driven-by-growth-in-the-foundry-industry-future-market-insights/ (accessed on 12 June 2020).
- Global Furfuryl Alcohol Market Status (2015–2019) and Forecast (2020–2024) by Region, Product Type & End Use. Available online: https://www.marketintellica.com/report/MI92760-global-furfuryl-alcohol-market-status-2015 (accessed on 12 June 2020).
- Dalvand, K.; Rubin, J.; Gunukula, S.; Clayton Wheeler, M.; Hunt, G. Economics of biofuels: Market potential of furfural and its derivatives. Biomass Bioenergy 2018, 115, 56–63. [Google Scholar] [CrossRef]
- Furfural Market by Raw Material (Sugarcane Bagasse, Corncob, Rice Husk and Others), Application (Derivatives (Furfural Alcohol and Other Derivatives), solvent) and Region (Asia-Pacific, Americas, Europe, Middle East and Africa)—Global Forecast to 2024. Available online: https://www.marketsandmarkets.com/Market-Reports/furfural-market-101056456.html?gclid=CjwKCAiA3abwBRBqEiwAKwICA36nDx4JefULK0Qjh9p7BuGbaaJhSNTnwj6NxD1sARXKMnvriydCtBoC1AIQAvD_BwE (accessed on 20 June 2020).
- Tseng, Y.; Wang, W.; Ward, J.D.; Lee, H. Design and control of a process to produce furfuryl alcohol. J. Taiwan Inst. Chem. Eng. 2015, 51, 44–52. [Google Scholar] [CrossRef]
- Oanda. Available online: https://www1.oanda.com/currency/converter/ (accessed on 30 December 2019).

| Strain | Reaction Conditions Furfural Concentration [mM] | FUR Conversion (%) | FuOH Yield (%) | Reference |
|---|---|---|---|---|
| B. coagulans NL01 | 42 mM FUR, 15–20 g/L glucose, 9 mg/mL, 50 °C, 6 h | 97 | 87 a | [90] |
| M. deltae ΔLH | 10 mM FUR, H2-CO2 as substrate, 48 h | 100 | 100 | [91] |
| E. coli CCZU-A13 | 300 mM FUR, 1 mM glucose/mM FUR, 0.1 g wet cells/mL, pH = 6.5, 30 °C, 12 h | 100 | 100 | [94] |
| E. coli CCZU-T15 | 50.5 mM FUR, 12.5 mM OP-10, 1.6 mM glucose/mM FUR, 1:3 toluene water (v/v), pH = 6.5, 30 °C, 2 h | 100 | 100 | [97] |
| E. coli CCZU-K14 | 200 mM FUR, 1.5 mol glucose/mol furfural, pH = 6.5, 30 °C, 24 h | 100 | 100 | [98] |
| Catalyst | Substrate | Pore Width (nm) | Surface Area (m2 g−1) | Metal Loading (wt%) | Total Acidity (µmol g−1) | Temperature (°C) | Time (min) | Pressure (MPa) | Solvent | Xylose Conversion (%) | FuOH Yield (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu/SBA-15-SO3H | Xylose | 4.1 | 433.2 | 18.3 | 980 | 140 | 360 | 4 | water/n-butanol | 94 | 63 | [51] |
| Zeolite Beta | Xylose | N/R | 551 | 11 b | 1516 | 130 | 60 | 3 | water/isopropanol | N/R | 75 | [82] |
| [Al]-SBA-15 | Xylose | 6.3 | 894 | 2.1 | 390 | 130 | 240 | 3 e | water/2-propanol | 13 f | 11 f | [83] |
| SO42−/SnO2-MMT + E. coli CCZU-K14 | Xylose | 5.6 | 120.35 | N/R | N/R | 170 | 20 | N/R | water | 100 | 93.4 | [98] |
| 30 | 1440 | |||||||||||
| Pt/SiO2 + ZrO2-SO4 | Xylose | 6.8–4.7 | 207–137 | 0.8 | 317 | 130 | 360 | 3 | 2-propanol | 65 | 51 a | [114] |
| Pt/ZrO2-SO4 | Xylose | N/R | 137 | Pt = 1, S = 2.9 | 293 | 130 | 60 | 3 | water/isopropanol | 32 | 27 a | [115] |
| Pd/MWCNT | Xylose | N/R | 439 d | N/R | N/R | 130 | 360 | 3 | water/2-propanol | 66 | 12 | [116] |
| Pt/SBA-15-SO3H | Xylose | N/R | N/R | N/R | N/R | 130 | 360 | 3 | water/isopropanol | 65 | 83 a | [117] |
| Hβ + Cy/ZnO/Al2 O3 | Xylose | N/R | N/R | N/R | 361 | 150 | 600 c | 0.1 | water/γ-butyrolactone | 100 | 87.2 | [118] |
| Formic acid + Co-N-C | Xylose | N/R | N/R | N/R | N/R | 160 | 300 | 0.5 | water/1,4-dioxane | 100 | 69.5 | [121] |
| Catalyst | Substrate | Pore Width (nm) | Surface Area (m2 g−1) | Metal Loading (wt%) | Total Acidity (µmol g−1) | Temperature (°C) | Time (min) | Pressure (MPa) | Solvent | Xylose Conversion (%) | FuOH Yield (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SO42−/SnO2-APG + E. coli CCZU-A13 | Corncob-derived xylose | 7.1 | 128 | 3.6 | N/R | 170 | 20 | N/R | water | 100 | 44 | [94] |
| 30 | 180 | |||||||||||
| SO42−/SnO2-KL+ E. coli CCTU-T15 | Corncob-derived xylose | 6.3 | 40 | N/R | N/R | 170 | 30 | N/R | toluene-H2O | 100 | 74.3 | [97] |
| 30 | 360 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Millán, G.; Sixta, H. Towards the Green Synthesis of Furfuryl Alcohol in A One-Pot System from Xylose: A Review. Catalysts 2020, 10, 1101. https://doi.org/10.3390/catal10101101
Gómez Millán G, Sixta H. Towards the Green Synthesis of Furfuryl Alcohol in A One-Pot System from Xylose: A Review. Catalysts. 2020; 10(10):1101. https://doi.org/10.3390/catal10101101
Chicago/Turabian StyleGómez Millán, Gerardo, and Herbert Sixta. 2020. "Towards the Green Synthesis of Furfuryl Alcohol in A One-Pot System from Xylose: A Review" Catalysts 10, no. 10: 1101. https://doi.org/10.3390/catal10101101
APA StyleGómez Millán, G., & Sixta, H. (2020). Towards the Green Synthesis of Furfuryl Alcohol in A One-Pot System from Xylose: A Review. Catalysts, 10(10), 1101. https://doi.org/10.3390/catal10101101