Enhancement of HDO Activity of MoP/SiO2 Catalyst in Physical Mixture with Alumina or Zeolites
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
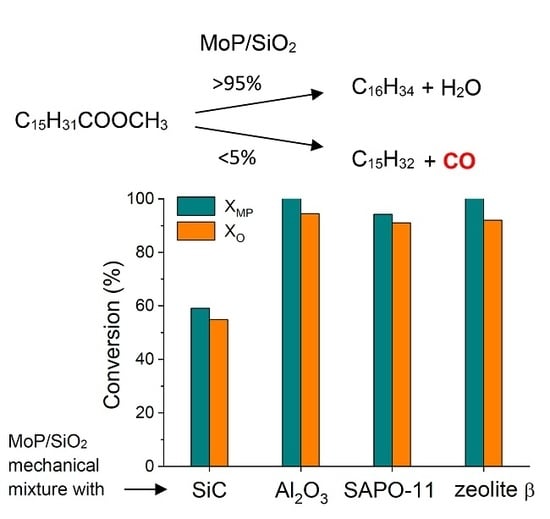
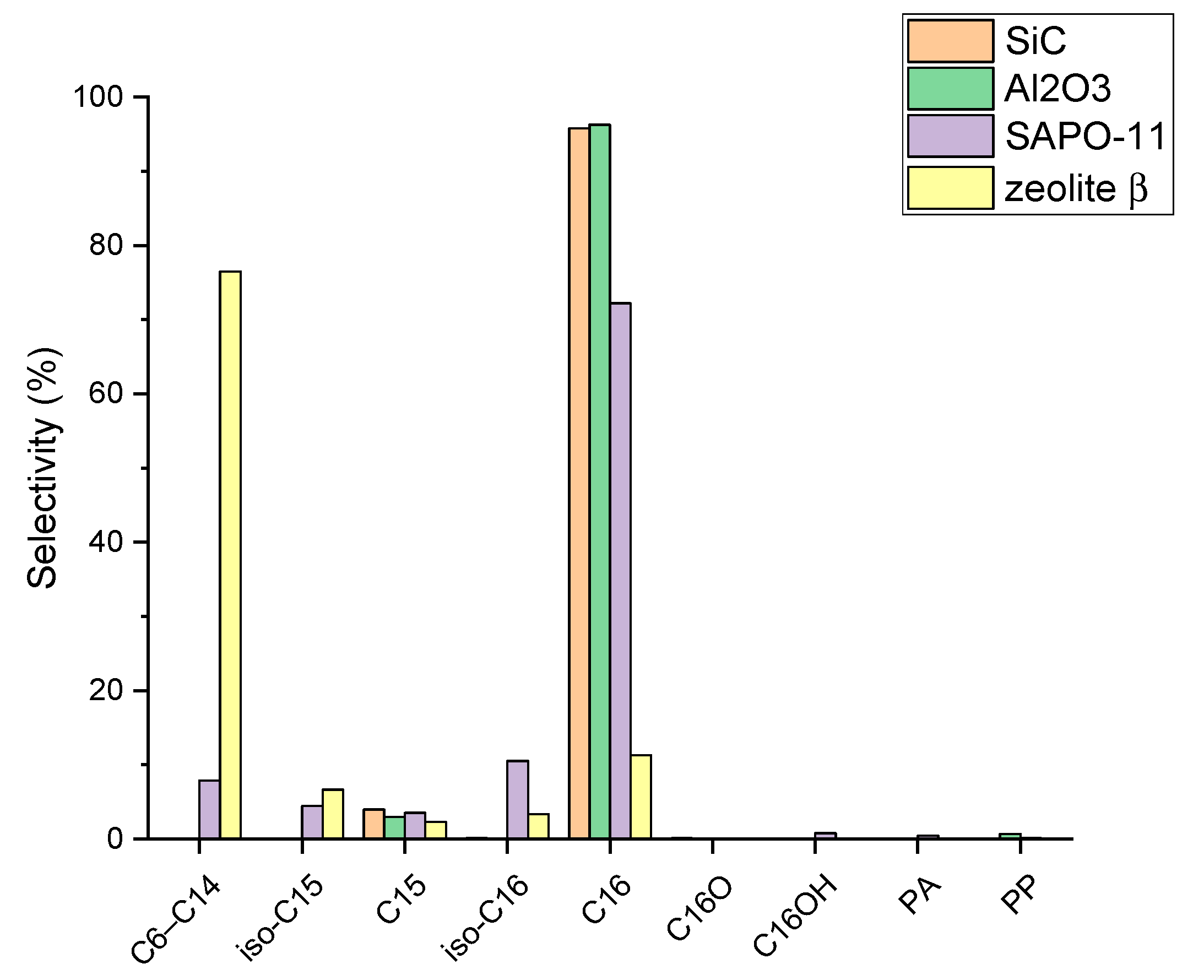
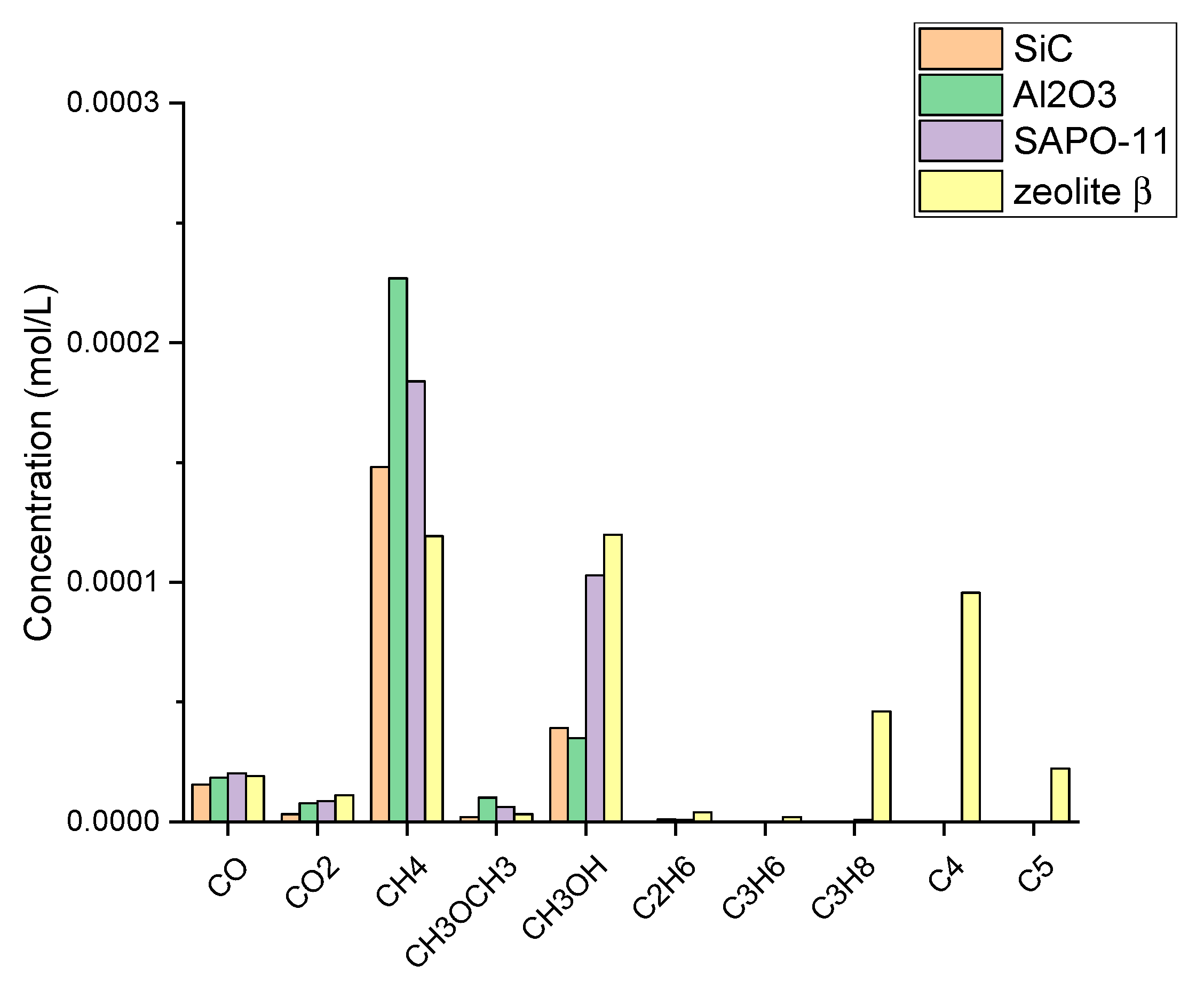
2.2. MP HDO Results
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.3. Experimental Setup and Procedure
3.4. Analysis of the Reaction Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Choudhary, T.V.; Phillips, C.B. Renewable fuels via catalytic hydrodeoxygenation. Appl. Catal. A Gen. 2011, 397, 1–12. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Ramos-Fernández, E.V.; Sepúlveda-Escribano, A. From biodiesel and bioethanol to liquid hydrocarbonfuels: New hydrotreating and advanced microbial technologies. Energy Environ. Sci. 2012, 5, 5638–5652. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Melero, J.A.; Iglesias, J.; Garcia, A. Biomass as renewable feedstock in standard refinery units. Feasibility, opportunities and challenges. Energy Environ. Sci. 2012, 5, 7393–7420. [Google Scholar] [CrossRef]
- Khan, S.; Kay Lup, A.N.; Qureshi, K.M.; Abnisa, F.; Wan Daud, W.M.A.; Patah, M.F.A. A review on deoxygenation of triglycerides for jet fuel range hydrocarbons. J. Anal. Appl. Pyrolysis 2019, 140, 1–24. [Google Scholar] [CrossRef]
- Why, E.S.K.; Ong, H.C.; Lee, H.V.; Gan, Y.Y.; Chen, W.-H.; Chong, C.T. Renewable aviation fuel by advanced hydroprocessing of biomass: Challenges and perspective. Energy Convers. Manag. 2019, 199, 112015. [Google Scholar] [CrossRef]
- Kubička, D.; Tukač, V. Chemical Engineering for Renewables Conversion. Advances in Chemical Engineering. In Advances in Chemical Engineering; Murzin, D.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 42, pp. 141–194. ISBN 9780123865052. [Google Scholar]
- Zharova, P.A.; Chistyakov, A.V.; Shapovalov, S.S.; Pasynskii, A.A.; Tsodikov, M.V. Original Pt-Sn/Al2O3 catalyst for selective hydrodeoxygenation of vegetable oils. Energy 2019, 172, 18–25. [Google Scholar] [CrossRef]
- Kukushkin, R.G.; Reshetnikov, S.I.; Zavarukhin, S.G.; Eletskii, P.M.; Yakovlev, V.A. Kinetics of the Hydrodeoxygenation of Ethyl Ester of Decanoic Acid over the Ni–Cu–Mo/ Al2O3 Catalyst. Catal. Ind. 2019, 11, 191–197. [Google Scholar] [CrossRef]
- Janampelli, S.; Darbha, S. Selective and reusable Pt-WOx/ Al2O3 catalyst for deoxygenation of fatty acids and their esters to diesel-range hydrocarbons. Catal. Today 2018, 309, 219–226. [Google Scholar] [CrossRef]
- Gosselink, R.W.; Stellwagen, D.R.; Bitter, J.H. Tungsten-Based Catalysts for Selective Deoxygenation. Angew. Chem. Int. Ed. 2013, 52, 5089–5092. [Google Scholar] [CrossRef]
- Wang, H.; Rogers, K.; Zhang, H.; Li, G.; Pu, J.; Zheng, H.; Lin, H.; Zheng, Y.; Ng, S. The Effects of Catalyst Support and Temperature on the Hydrotreating of Waste Cooking Oil (WCO) over CoMo Sulfided Catalysts. Catalysts 2019, 9, 689. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, L.; Cheng, S.; Julson, J. Review of Heterogeneous Catalysts for Catalytically Upgrading Vegetable Oils into Hydrocarbon Biofuels. Catalysts 2017, 7, 83. [Google Scholar] [CrossRef]
- Pinheiro, A.; Dupassieux, N.; Hudebine, D.; Geantet, C. Impact of the Presence of Carbon Monoxide and Carbon Dioxide on Gas Oil Hydrotreatment: Investigation on Liquids from Biomass Cotreatment with Petroleum Cuts. Energy Fuels 2011, 25, 804–812. [Google Scholar] [CrossRef]
- Philippe, M.; Richard, F.; Hudebine, D.; Brunet, S. Transformation of dibenzothiophenes model molecules over CoMoP/ Al2O3 catalyst in the presence of oxygenated compounds. Appl. Catal. B Environ. 2013, 132, 493–498. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, Q.; Guan, Q.; He, L.; Li, W. Bio-aviation fuel production from hydroprocessing castor oil promoted by the nickel-based bifunctional catalysts. Bioresour. Technol. 2015, 183, 93–100. [Google Scholar] [CrossRef]
- Li, X.; Luo, X. Preparation of mesoporous activated carbon supported Ni catalyst for deoxygenation of stearic acid into hydrocarbons. Environ. Prog. Sustain. Energy 2015, 34, 607–612. [Google Scholar] [CrossRef]
- Kukushkin, R.G.; Bulavchenko, O.A.; Kaichev, V.V.; Yakovlev, V.A. Influence of Mo on catalytic activity of Ni-based catalysts in hydrodeoxygenation of esters. Appl. Catal. B Environ. 2015, 163, 531–538. [Google Scholar] [CrossRef]
- Yakovlev, V.A.; Khromova, S.A.; Sherstyuk, O.V.; Dundich, V.O.; Ermakov, D.Y.; Novopashina, V.M.; Lebedev, M.Y.; Bulavchenko, O.; Parmon, V.N. Development of new catalytic systems for upgraded bio-fuels production from bio-crude-oil and biodiesel. Catal. Today 2009, 144, 362–366. [Google Scholar] [CrossRef]
- Zuo, H.; Liu, Q.; Wang, T.; Ma, L.; Zhang, Q.; Zhang, Q. Hydrodeoxygenation of Methyl Palmitate over Supported Ni Catalysts for Diesel-like Fuel Production. Energy Fuels 2012, 26, 3747–3755. [Google Scholar] [CrossRef]
- Ochoa-Hernández, C.; Yang, Y.; Pizarro, P.; de la Peña O’Shea, V.A.; Coronado, J.M.; Serrano, D.P. Hydrocarbons production through hydrotreating of methyl esters over Ni and Co supported on SBA-15 and Al-SBA-15. Catal. Today 2013, 210, 81–88. [Google Scholar] [CrossRef]
- Ki Kim, S.; Yoon, D.; Lee, S.-C.; Kim, J. Mo2C/Graphene Nanocomposite as a Hydrodeoxygenation Catalyst for the Production of Diesel Range Hydrocarbons. ACS Catal. 2015, 5, 3292–3303. [Google Scholar] [CrossRef]
- Hollak, S.A.W.; Gosselink, R.W.; van Es, D.S.; Bitter, J.H. Comparison of tungsten and molybdenum carbide catalysts for the hydrodeoxygenation of oleic acid. ACS Catal. 2013, 3, 2837–2844. [Google Scholar] [CrossRef]
- Wang, H.; Yan, S.; Salley, S.O.; Simon Ng, K.Y. Support effects on hydrotreating of soybean oil over NiMo carbide catalyst. Fuel 2013, 111, 81–87. [Google Scholar] [CrossRef]
- Monnier, J.; Sulimma, H.; Dalai, A.; Caravaggio, G. Hydrodeoxygenation of oleic acid and canola oil over alumina-supported metal nitrides. Appl. Catal. A Gen. 2010, 382, 176–180. [Google Scholar] [CrossRef]
- Chen, J.; Shi, H.; Li, L.; Li, K. Deoxygenation of methyl laurate as a model compound to hydrocarbons on transition metal phosphide catalysts. Appl. Catal. B Environ. 2014, 144, 870–884. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Y.; Shi, H.; Li, M.; Chu, Y.; Pan, Z.; Yu, X. Regulating product distribution in deoxygenation of methyl laurate on silica-supported Ni–Mo phosphides: Effect of Ni/Mo ratio. Fuel 2014, 129, 1–10. [Google Scholar] [CrossRef]
- Shi, H.; Chen, J.; Yang, Y.; Tian, S. Catalytic deoxygenation of methyl laurate as a model compound to hydrocarbons on nickel phosphide catalysts: Remarkable support effect. Fuel Process. Technol. 2013, 116, 161–170. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.; Shi, H. Deoxygenation of Methyl Laurate as a Model Compound to Hydrocarbons on Ni2P/SiO2, Ni2P/MCM-41, and Ni2P /SBA-15 catalysts with different dispersions. Energy Fuels 2013, 27, 3400–3409. [Google Scholar] [CrossRef]
- Yang, Y.; Ochoa-Hernández, C.; de la Peña O’Shea, V.A.; Coronado, J.M.; Serrano, D.P. Ni2P/SBA-15 As a Hydrodeoxygenation Catalyst with Enhanced Selectivity for the Conversion of Methyl Oleate Into n-Octadecane. ACS Catal. 2012, 2, 592–598. [Google Scholar] [CrossRef]
- Yang, Y.; Ochoa-Hernández, C.; Pizarro, P.; de la Peña O’Shea, V.A.; Coronado, J.M.; Serrano, D.P. Synthesis of Nickel Phosphide Nanorods as Catalyst for the Hydrotreating of Methyl Oleate. Top. Catal. 2012, 55, 991–998. [Google Scholar] [CrossRef]
- Yang, Y.; Ochoa-Hernández, C.; Pizarro, P.; de la Peña O’Shea, V.A.; Coronado, J.M.; Serrano, D.P. Influence of the Ni/P ratio and metal loading on the performance of NixPy/SBA-15 catalysts for the hydrodeoxygenation of methyl oleate. Fuel 2015, 144, 60–70. [Google Scholar] [CrossRef]
- Zarchin, R.; Rabaev, M.; Vidruk-Nehemya, R.; Landau, M.V.; Herskowitz, M. Hydroprocessing of soybean oil on nickel-phosphide supported catalysts. Fuel 2015, 139, 684–691. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, R.; Li, M.; Chu, Y.; Chen, J. Deoxygenation of methyl laurate to hydrocarbons on silica-supported Ni-Mo phosphides: Effect of calcination temperatures of precursor. J. Energy Chem. 2015, 24, 77–86. [Google Scholar] [CrossRef]
- Xue, Y.; Guan, Q.; Li, W. Synthesis of bulk and supported nickel phosphide using microwave radiation for hydrodeoxygenation of methyl palmitate. RSC Adv. 2015, 5, 53623–53628. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, M.-F.; Chu, Y.; Chen, J.-X. Influence of CS2 on performance of Ni2P/SiO2 for deoxygenation of methyl laurate as a model compound to hydrocarbons: Simultaneous investigation on catalyst deactivation. Fuel Process. Technol. 2015, 134, 259–269. [Google Scholar] [CrossRef]
- Lee, S.I.; Kim, D.W.; Jeon, H.J.; Ju, S.J.; Ryu, J.W.; Oh, S.H.; Koh, J.H. Metal Phosphorus Compound for Preparing Biodiesel and Method Preparing Biodiesel Using the Same. U.S. Patent US2014/0150332A1, 5 June 2014. [Google Scholar]
- Alvarez-Galvan, M.; Campos-Martin, J.; Fierro, J. Transition Metal Phosphides for the Catalytic Hydrodeoxygenation of Waste Oils into Green Diesel. Catalysts 2019, 9, 293. [Google Scholar] [CrossRef]
- Deliy, I.; Shamanaev, I.; Gerasimov, E.; Pakharukova, V.; Yakovlev, I.; Lapina, O.; Aleksandrov, P.; Bukhtiyarova, G. HDO of Methyl Palmitate over Silica-Supported Ni Phosphides: Insight into Ni/P Effect. Catalysts 2017, 7, 298. [Google Scholar] [CrossRef]
- Shamanaev, I.V.; Deliy, I.V.; Aleksandrov, P.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Kodenev, E.G.; Ayupov, A.B.; Andreev, A.S.; Lapina, O.B.; Bukhtiyarova, G.A. Effect of precursor on the catalytic properties of Ni2P/SiO2 in methyl palmitate hydrodeoxygenation. RSC Adv. 2016, 6, 30372–30383. [Google Scholar] [CrossRef]
- He, L.; Wu, C.; Cheng, H.; Yu, Y.; Zhao, F. Highly selective and efficient catalytic conversion of ethyl stearate into liquid hydrocarbons over a Ru/TiO2 catalyst under mild conditions. Catal. Sci. Technol. 2012, 2, 1328. [Google Scholar] [CrossRef]
- Shamanaev, I.; Aleksandrov, P.; Kodenev, E.; Pakharukova, V.; Gerasimov, E.; Deliy, I.; Bukhtiyarova, G. Synergetic Effect of Ni2P/SiO2 and γ-Al2O3 Physical Mixture in Hydrodeoxygenation of Methyl Palmitate. Catalysts 2017, 7, 329. [Google Scholar] [CrossRef]
- Deliy, I.; Shamanaev, I.; Aleksandrov, P.; Gerasimov, E.; Pakharukova, V.; Kodenev, E.; Yakovlev, I.; Lapina, O.; Bukhtiyarova, G. Support Effect on the Performance of Ni2P Catalysts in the Hydrodeoxygenation of Methyl Palmitate. Catalysts 2018, 8, 515. [Google Scholar] [CrossRef]
- Laurent, E.; Delmon, B. Influence of water in the deactivation of a sulfided NiMo?-Al2O3 catalyst during hydrodeoxygenation. J. Catal. 1994, 146, 281–291. [Google Scholar] [CrossRef]
- Şenol, O.İ.; Viljava, T.-R.; Krause, A.O.I. Hydrodeoxygenation of methyl esters on sulphided NiMo/γ- Al2O3 and CoMo/γ-Al2O3 catalysts. Catal. Today 2005, 100, 331–335. [Google Scholar] [CrossRef]
- Shamanaev, I.V.; Deliy, I.V.; Aleksandrov, P.V.; Reshetnikov, S.I.; Bukhtiyarova, G.A. Methyl palmitate hydrodeoxygenation over silica-supported nickel phosphide catalysts in flow reactor: Experimental and kinetic study. J. Chem. Technol. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Prins, R.; Bussell, M.E. Metal Phosphides: Preparation, Characterization and Catalytic Reactivity. Catal. Lett. 2012, 142, 1413–1436. [Google Scholar] [CrossRef]
- Liu, X.; Xu, L.; Zhang, B. Essential elucidation for preparation of supported nickel phosphide upon nickel phosphate precursor. J. Solid State Chem. 2014, 212, 13–22. [Google Scholar] [CrossRef]
- Peroni, M.; Mancino, G.; Baráth, E.; Gutiérrez, O.Y.; Lercher, J.A. Bulk and γ-Al2O3 supported Ni2P and MoP for hydrodeoxygenation of palmitic acid. Appl. Catal. B Environ. 2016, 180, 301–311. [Google Scholar] [CrossRef]
- Li, K.; Wang, R.; Chen, J. Hydrodeoxygenation of Anisole over Silica-Supported Ni2P, MoP, and NiMoP Catalysts. Energy Fuels 2011, 25, 854–863. [Google Scholar] [CrossRef]
- Bui, P.; Cecilia, J.A.; Oyama, S.T.; Takagaki, A.; Infantes-Molina, A.; Zhao, H.; Li, D.; Rodríguez-Castellón, E.; Jiménez López, A. Studies of the synthesis of transition metal phosphides and their activity in the hydrodeoxygenation of a biofuel model compound. J. Catal. 2012, 294, 184–198. [Google Scholar] [CrossRef]
- Montesinos-Castellanos, A.; Zepeda, T.A.; Pawelec, B.; Fierro, J.L.G.; De Los Reyes, J.A. Preparation, characterization, and performance of alumina-supported nanostructured Mo-phosphide systems. Chem. Mater. 2007, 19, 5627–5636. [Google Scholar] [CrossRef]
- Newsam, J.M.; Treacy, M.M.J.; Koetsier, W.T.; Gruyter, C.B.D. Structural Characterization of Zeolite Beta. Proc. R. Soc. A Math. Phys. Eng. Sci. 1988, 420, 375–405. [Google Scholar] [CrossRef]
- Flanigen, E.M.; Lok, B.M.; Patton, R.L.; Wilson, S.T. Aluminophosphate molecular sieves and the periodic table. Pure Appl. Chem. 1986, 58, 1351–1358. [Google Scholar] [CrossRef][Green Version]
- Peroni, M.; Lee, I.; Huang, X.; Baráth, E.; Gutiérrez, O.Y.; Lercher, J.A. Deoxygenation of Palmitic Acid on Unsupported Transition-Metal Phosphides. ACS Catal. 2017, 7, 6331–6341. [Google Scholar] [CrossRef]
- Gosselink, R.W.; Hollak, S.A.W.; Chang, S.W.; Van Haveren, J.; De Jong, K.P.; Bitter, J.H.; van Es, D.S. Reaction pathways for the deoxygenation of vegetable oils and related model compounds. ChemSusChem 2013, 6, 1576–1594. [Google Scholar] [CrossRef] [PubMed]
- File, P.D. JCPDS—International Centre for Diffraction Data; ICDD: Swarthmore, PA, USA, 1997. [Google Scholar]
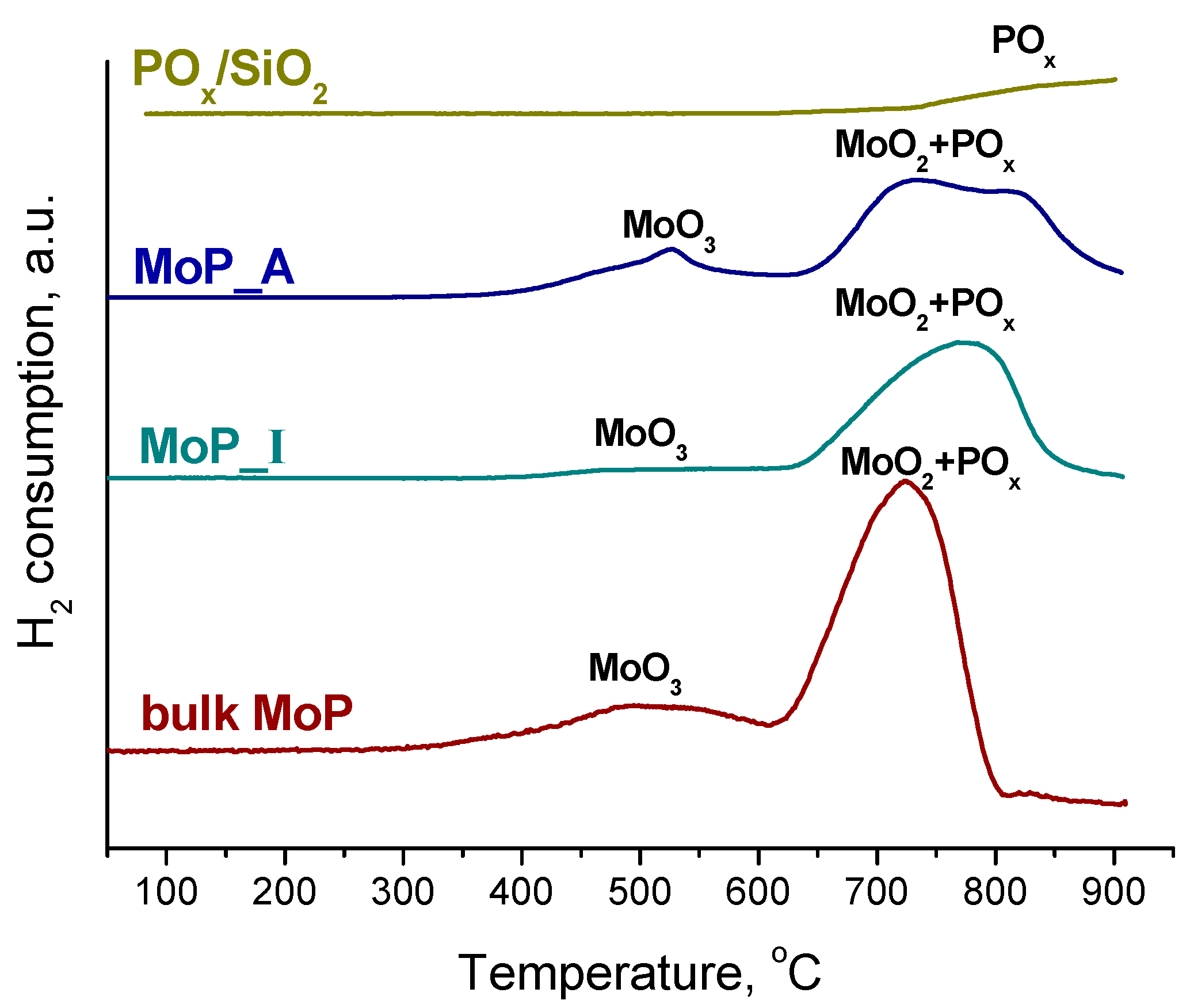
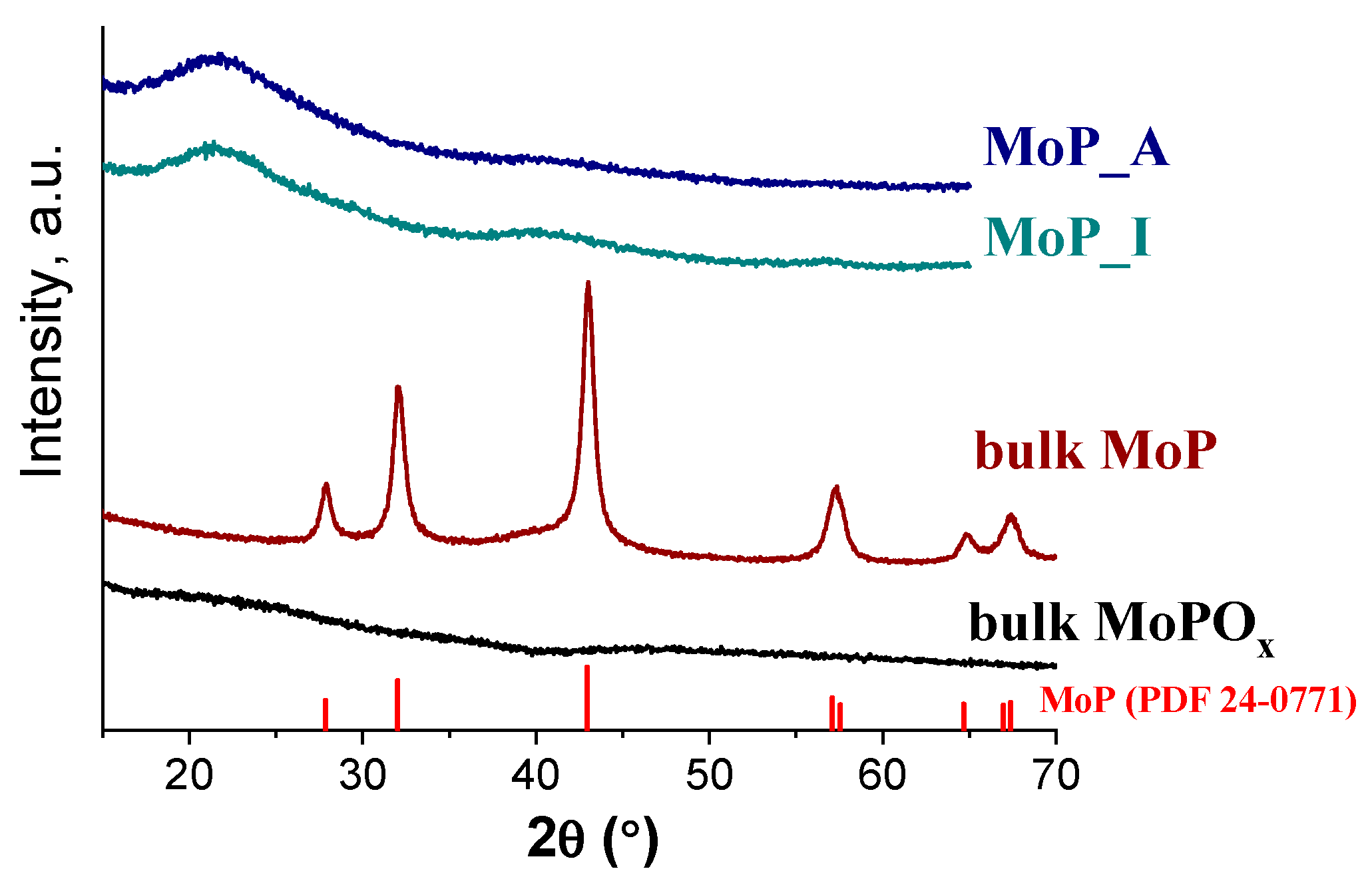

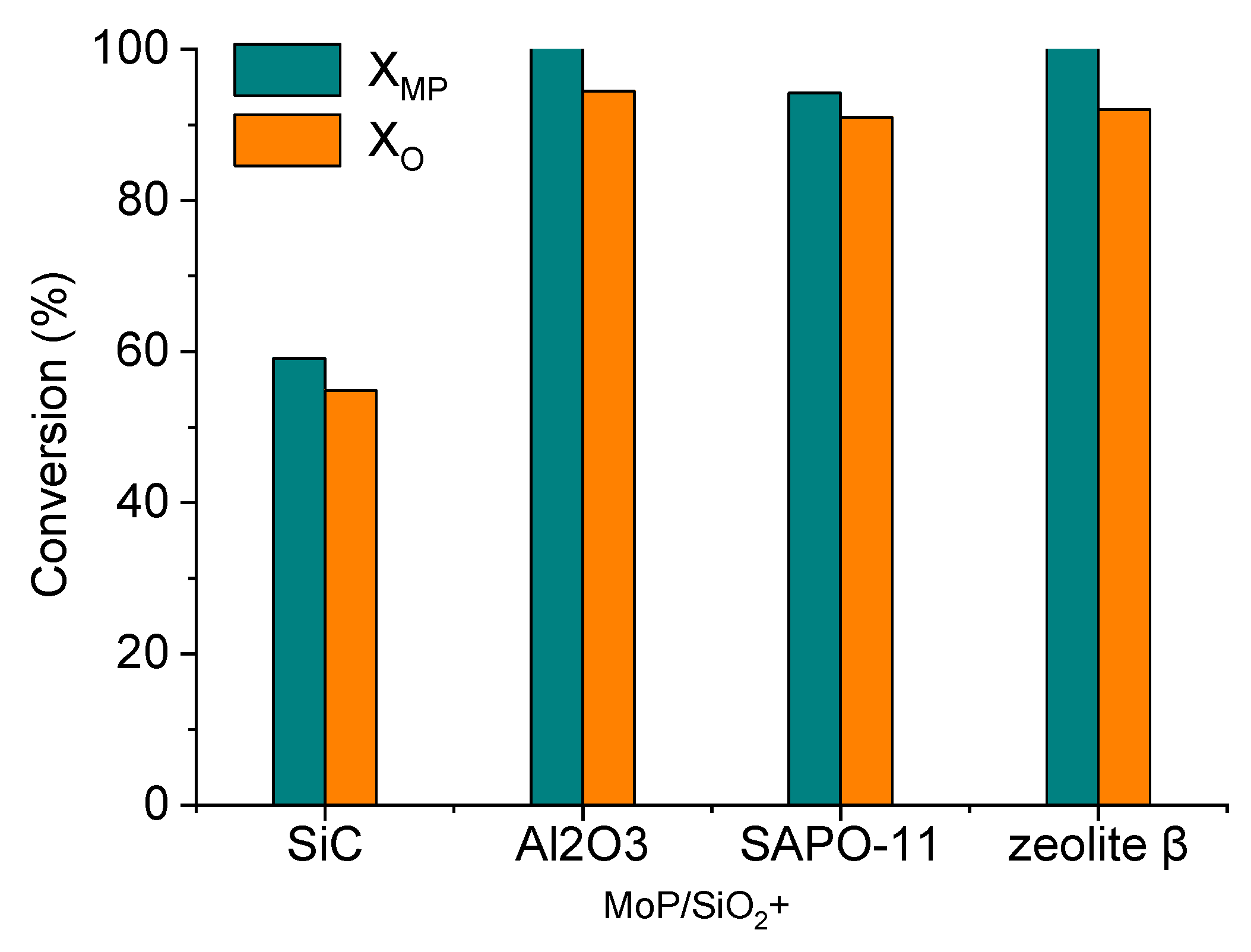
| Sample | Mo, wt% | P, wt% | Mo/P Molar Ratio in Reduced Catalyst | Mo/P EDX | SBET, m2/g | Dpore, nm | DTEM, nm | XRD Phase | DXRD, nm |
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | – | – | – | – | 300 | 10.6 | – | – | – |
| MoP_A | 11.5 | 4.0 | 0.91 | 1.02 | 178 | 10.4 | 1.4 | amorphous phase | – |
| MoP_I | 11.5 | 4.3 | 0.83 | 0.97 | 235 | 7.9 | 2.0 | amorphous phase | – |
| MoP | 51.7 | 18.4 | 0.91 | 1.17 | 211 | 2.0 | – | MoP | 12 |
| Diluter | SBET, m2/g | Vpore, cm3/g | Dpore, nm | NH3-TPD, 10−3 mol/g |
|---|---|---|---|---|
| SiC | 1 | – | – | – |
| γ-Al2O3 | 235 | 0.79 | 13.4 | 0.421 |
| SAPO-11 | 295 | 0.26 | 3.5 | 1.11 |
| zeolite β | 609 | 0.49 | 3.2 | 1.92 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamanaev, I.V.; Deliy, I.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Bukhtiyarova, G.A. Enhancement of HDO Activity of MoP/SiO2 Catalyst in Physical Mixture with Alumina or Zeolites. Catalysts 2020, 10, 45. https://doi.org/10.3390/catal10010045
Shamanaev IV, Deliy IV, Gerasimov EY, Pakharukova VP, Bukhtiyarova GA. Enhancement of HDO Activity of MoP/SiO2 Catalyst in Physical Mixture with Alumina or Zeolites. Catalysts. 2020; 10(1):45. https://doi.org/10.3390/catal10010045
Chicago/Turabian StyleShamanaev, Ivan V., Irina V. Deliy, Evgeny Yu. Gerasimov, Vera P. Pakharukova, and Galina A. Bukhtiyarova. 2020. "Enhancement of HDO Activity of MoP/SiO2 Catalyst in Physical Mixture with Alumina or Zeolites" Catalysts 10, no. 1: 45. https://doi.org/10.3390/catal10010045
APA StyleShamanaev, I. V., Deliy, I. V., Gerasimov, E. Y., Pakharukova, V. P., & Bukhtiyarova, G. A. (2020). Enhancement of HDO Activity of MoP/SiO2 Catalyst in Physical Mixture with Alumina or Zeolites. Catalysts, 10(1), 45. https://doi.org/10.3390/catal10010045