Structural Properties and Catalytic Activity of Binary Poly (vinyl alcohol)/Al2O3 Nanocomposite Film for Synthesis of Thiazoles
Abstract
1. Introduction
2. Results and Discussion.
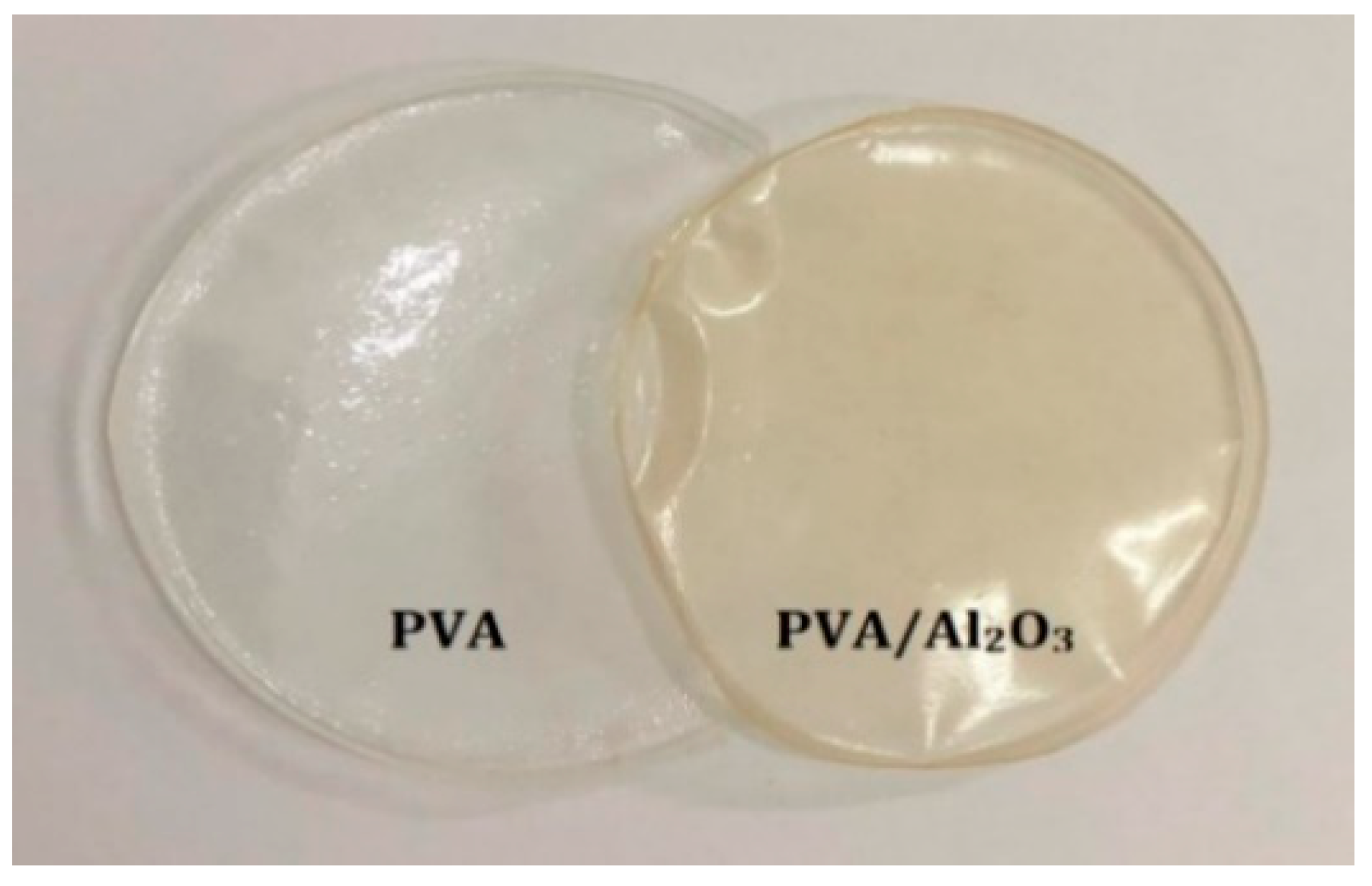
2.1. Characterization of PVA/Al2O3 Composite Film
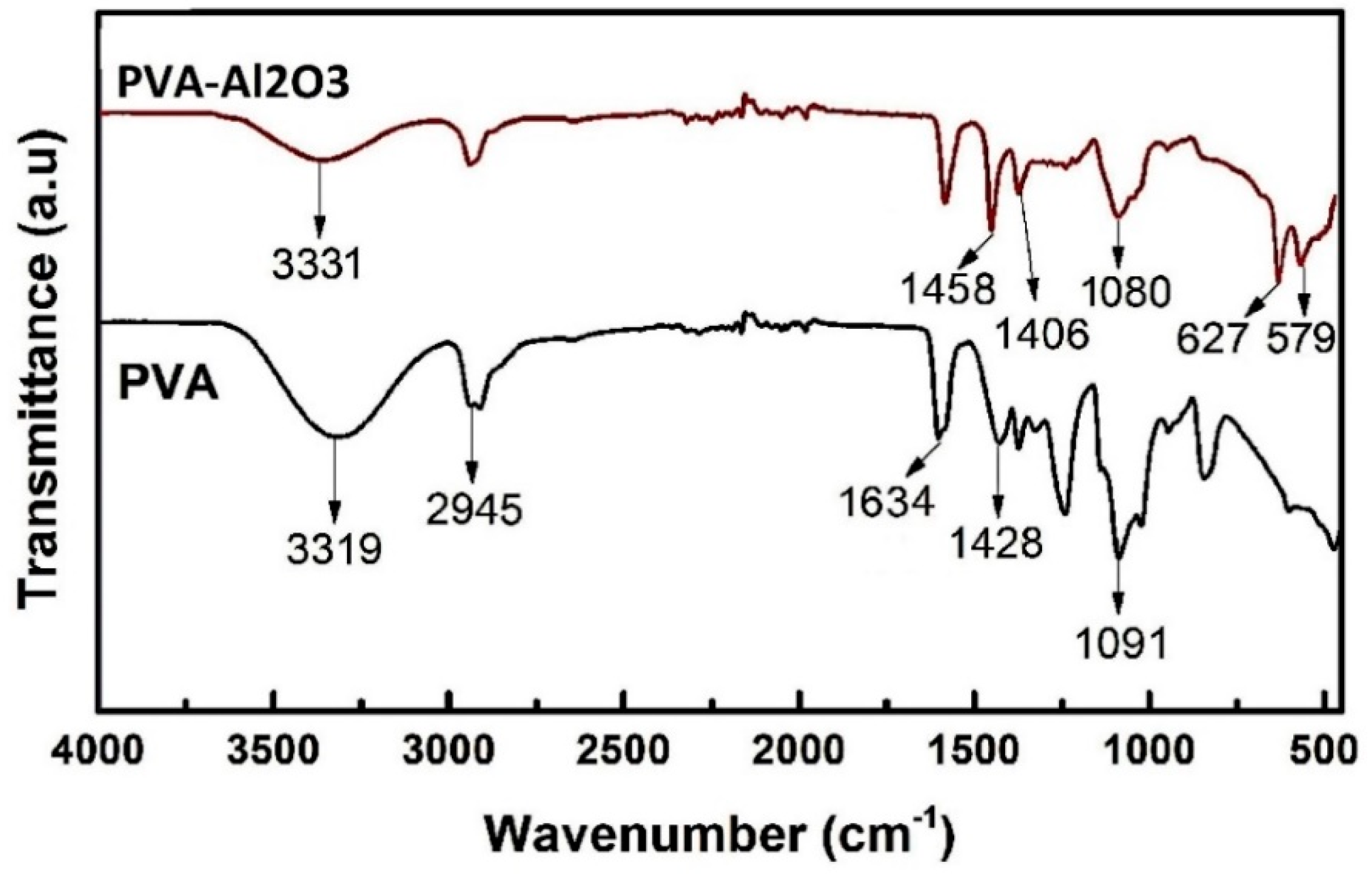
2.1.1. Fourier-Transform Infrared (FTIR) Spectra
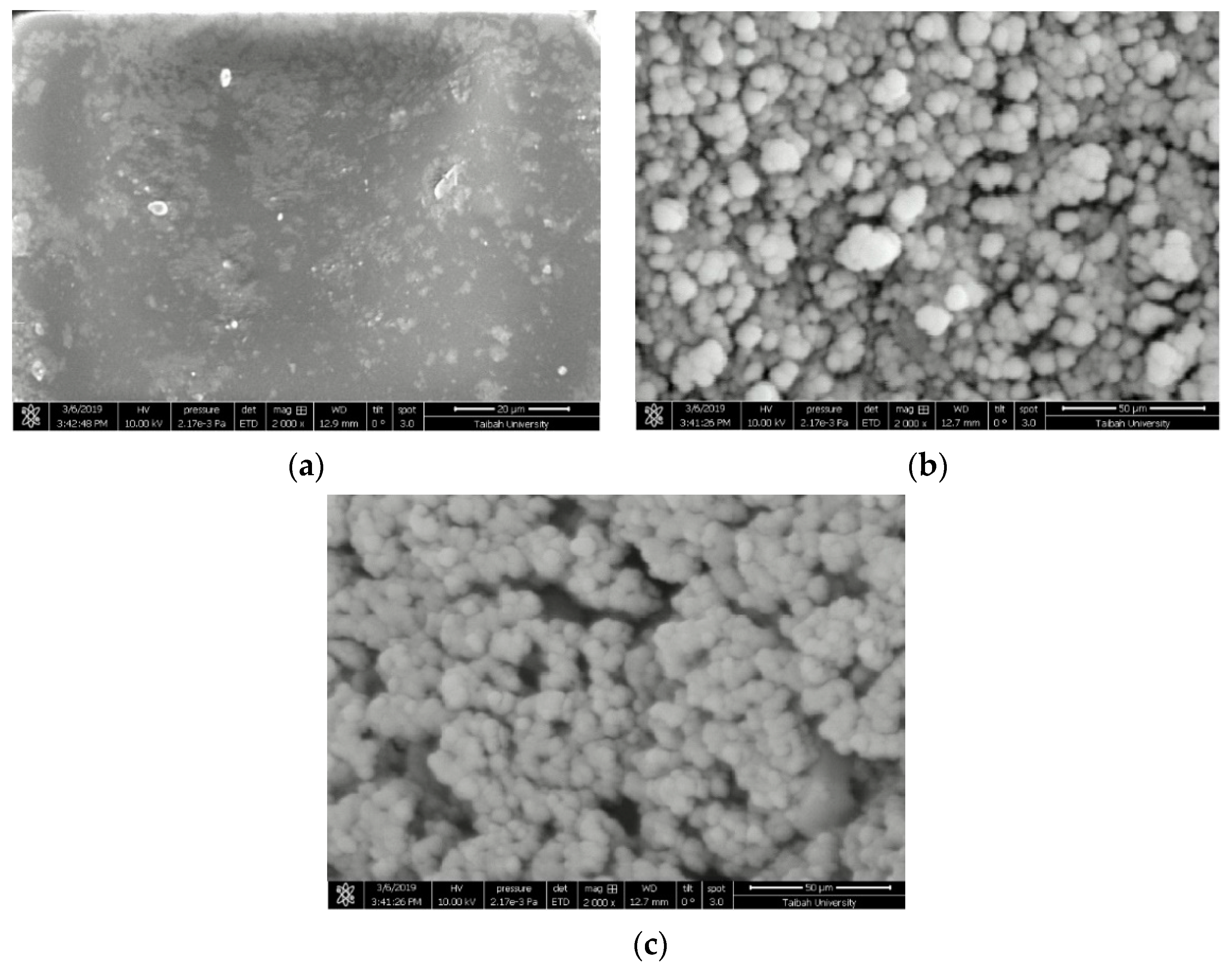
2.1.2. Field Emission Scanning Electron Microscope (FESEM) and Energy Dispersive X-ray (EDX) Analyses
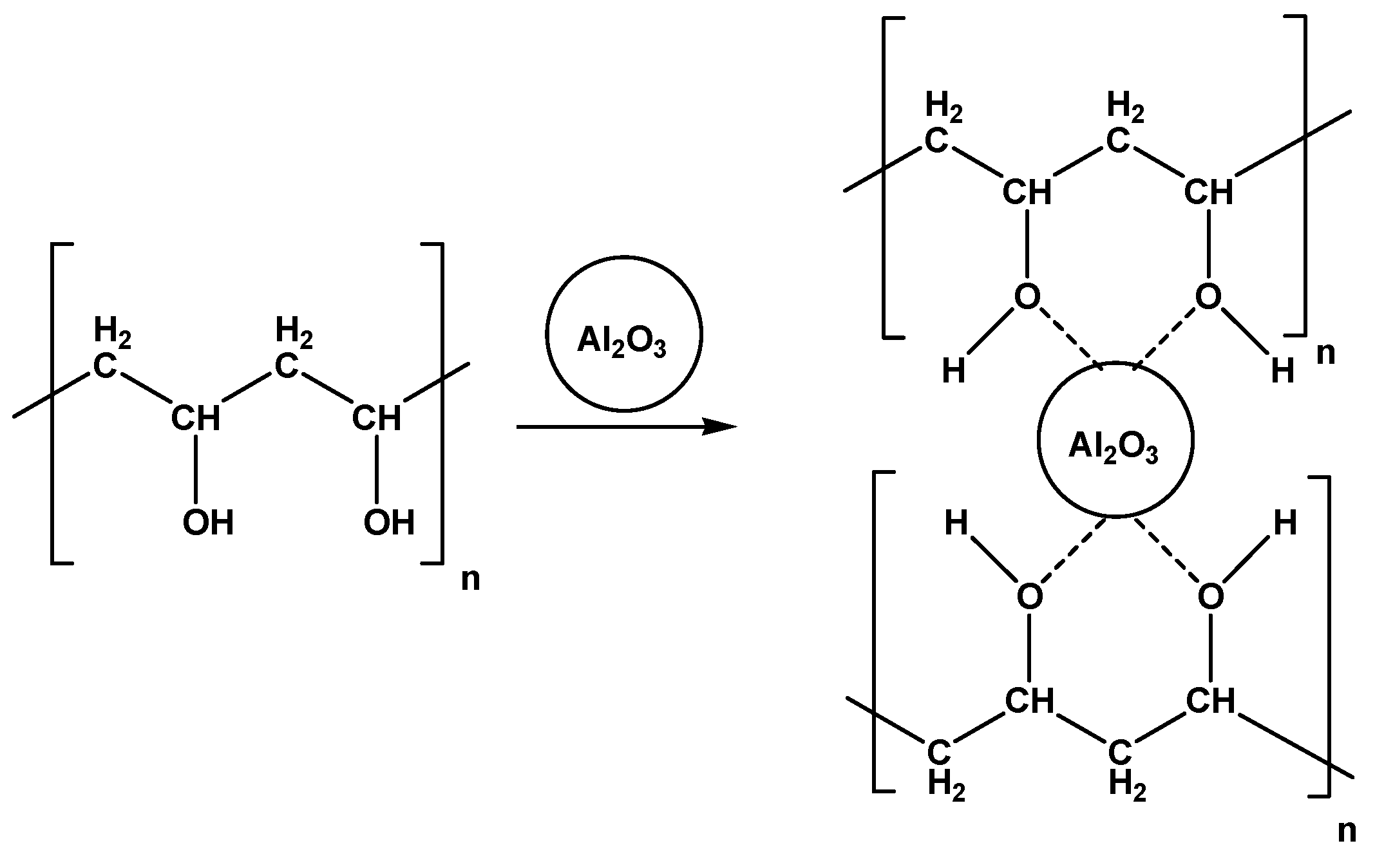
2.1.3. Structure Analysis
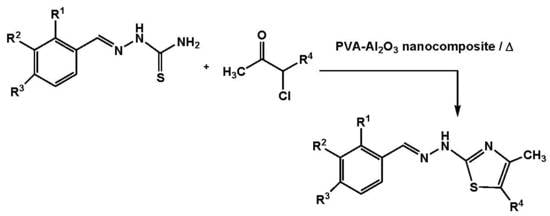
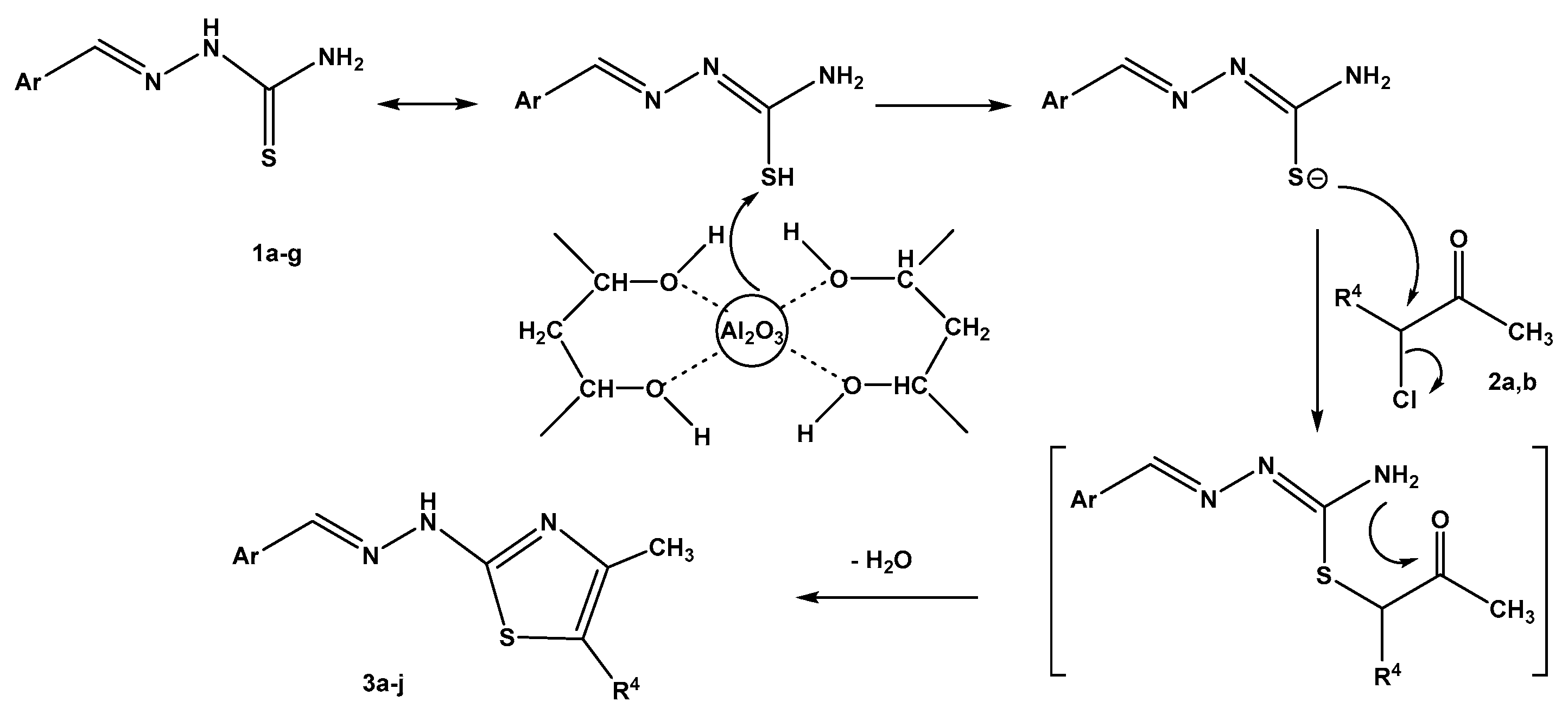
2.2. Synthesis of Thiazole Derivatives
3. Materials and Methods
3.1. Preparation of PVA-Al2O3 Composite Films
3.2. General Procedures for the Synthesis of 2- Hydrazonothiazoles 3a-j
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Puga, A.V.; Garcia-Valls, R.; Fernández-Prieto, S.; Smets, J.; York, D. Dual xanthan gum/poly (vinyl acetate) or alkyl-functionalized poly (vinyl alcohol) films as models for advanced coatings. J. Appl. Polym. Sci. 2014, 131, 40870–40880. [Google Scholar] [CrossRef]
- Li, M.; Zhang, W.; Zhou, S.; Zhao, Y. Preparation of poly (vinyl alcohol)/palygorskite-poly (ionic liquids) hybrid. Sep. Purif. Technol. 2020, 230, 115746. [Google Scholar] [CrossRef]
- Chakraborty, I.; Das, S.; Dubey, B.K.; Ghangrekar, M.M. Novel low cost proton exchange membrane made from sulphonated biochar for application in microbial fuel cells. Mater. Chem. Phys. 2020, 239, 122025. [Google Scholar] [CrossRef]
- Mandru, M.; Bercea, M.; Gradinaru, L.M.; Ciobanu, C.; Drobota, M.; Vlad, S.; Albulescu, R. Polyurethane/poly (vinyl alcohol) hydrogels: Preparation, characterization and drug delivery. Eur. Polym. J. 2019, 118, 137–145. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, M.; Liu, X. Monolayer assembly of oriented zeolite crystals on α-Al2O3 supported polymer thin films. Adv. Mater. 2008, 20, 2183–2189. [Google Scholar] [CrossRef]
- Prajapati, G.K.; Gupta, P.N. Comparative study of the electrical and dielectric properties of PVA-PEG-Al2O3-MI (M = Na, K, Ag) complex polymer electrolytes. Phys. B Condens. Matt. 2011, 406, 3108–3113. [Google Scholar] [CrossRef]
- Smith, S.R.; Rafati, R.; Haddad, A.S.; Cooper, A.; Hamidi, H. Application of aluminum oxide nanoparticles to enhance rheological and filtration properties of water based muds at HPHT conditions. Coll. Surf. A 2018, 537, 361–371. [Google Scholar] [CrossRef]
- Wei, N.; Hu, J.; Zhang, M.; He, J.; Ni, P. Cross-linked porous polymer separator using vinyl-modified aluminum oxide nanoparticles as cross-linker for lithium-ion batteries. Electrochim. Acta 2019, 307, 495–502. [Google Scholar] [CrossRef]
- Parvin, M.H.; Arjomandi, J.; Lee, J.Y. γ-Al2O3 nanoparticle catalyst mediated polyaniline gold electrode biosensor for vitamin E. Catal. Commun. 2018, 110, 59–63. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, A.; Sharma, K.V.; Gupta, R. Application of High Conductive Nanoparticles to Enhance the Thermal and Mechanical Properties of Wood Composite. Mater. Today Proc. 2018, 5, 3143–3149. [Google Scholar] [CrossRef]
- Zarnegar, Z.; Shokrani, Z.; Safari, J. Asparagine functionalized Al2O3 nanoparticle as a superior heterogeneous organocatalyst in the synthesis of 2-aminothiazoles. J. Mol. Struct. 2019, 1185, 143–152. [Google Scholar] [CrossRef]
- Goudarzi, M.; Ghanbari, D.; Salavati-Niasari, M.; Ahmadi, A. Synthesis and Characterization of Al(OH)3, Al2O3 Nanoparticles and Polymeric Nanocomposites. J. Clust. Sci. 2016, 27, 25–38. [Google Scholar] [CrossRef]
- Riyadh, S.M.; Khalil, K.D.; Aljuhani, A. Chitosan-MgO Nanocomposite: One Pot Preparation and Its Utility as an Ecofriendly Biocatalyst in the Synthesis of Thiazoles and [1,3,4] thiadiazoles. Nanomaterials 2018, 8, 928. [Google Scholar] [CrossRef]
- Khalil, K.D.; Riyadh, S.M.; Gomha, S.M.; Ali, I. Synthesis, characterization and application of copper oxide chitosan nanocomposite for green regioselective synthesis of [1,2,3] triazoles. Int. J. Biol. Macromol. 2019, 130, 928–937. [Google Scholar] [CrossRef]
- Mishra, R.; Rao, K.J. Thermal and morphological studies of binary and ternary composites of poly (vinylalcohol) with alumina and zirconia. Ceram. Int. 2000, 26, 371–378. [Google Scholar] [CrossRef]
- Sonmez, M.; Ficai, D.; Stan, A.; Bleotu, C.; Matei, L.; Ficai, A.; Andronescu, E. Synthesis and characterization of hybrid PVA/Al2O3 thin film. Mater. Lett. 2012, 74, 132–136. [Google Scholar] [CrossRef]
- Chandar, S.B.; Sathish, S.; Sathyamoorthy, R. Morphology, structure and conduction properties of PVA nano-scale films for field effect organic thin film transistor applications. Int. J. Nanotechnol. Appl. 2011, 5, 297–308. [Google Scholar]
- Qi, Y.N.; Xu, F.; Ma, H.J.; Sun, L.X.; Zhang, J.; Jiang, T. Thermal stability and glass transition behaviour of PANI/γ-Al2O3 composites. J. Therm. Anal. Calorim. 2008, 91, 219–223. [Google Scholar] [CrossRef]
- Sugumaran, S.; Bellan, C.S.; Nadimuthu, M. Characterization of composite PVA-Al2O3 thin films prepared by dip coating method. Iran. Polym. J. 2015, 24, 63–74. [Google Scholar] [CrossRef]
- Shakshooki, S.K.; Elejmi, A.A.; Hamassi, A.M.; El-Akari, F.A.; Masoud, N.A. Facile Synthesis of Poly (vinylalcohol)/Fibrous Cerium Phosphate Nanocomposite Membranes. Sci. Educ. 2014, 2, 1–6. [Google Scholar]
- Makam, P.; Kankanala, R.; Parkash, A.; Kannan, T. 2-(2-Hydrazinyl) thiazole derivatives: Design, synthesis and in vitro antimycobacterial studies. Eur. J. Med. Chem. 2013, 69, 564–576. [Google Scholar] [CrossRef]
- Grozav, A.; Gaina, L.I.; Pileczki, V.; Crisan, O.; Silaghi-Dumitrescu, L.; Therrien, B.; Zaharia, V.; Berindan-Neagoe, I. The Synthesis and Antiproliferative Activities of New Arylidene-Hydrazinyl-Thiazole Derivatives. Int. J. Mol. Sci. 2014, 15, 22059–22072. [Google Scholar] [CrossRef]
- Raghav, N.; Kaur, R. Synthesis and evaluation of some semicarbazone- and thiosemicarbazone-based cathepsin B inhibitors. Med. Chem. Res. 2014, 23, 4669–4679. [Google Scholar] [CrossRef]
- Bharti, S.K.; Singh, S.K. Design, synthesis and biological evaluation of some novel benzylidene-2-(4-phenylthiazol-2-yl) hydrazines as potential anti-inflammatory agents. Med. Chem. Res. 2014, 23, 1004–1015. [Google Scholar] [CrossRef]
- Alam, M.S.; Liu, L.; Lee, D.-U. Cytotoxicity of New 5-Phenyl-4,5-dihydro-1,3,4-thiadiazole Analogues. Chem. Pharm. Bull. 2011, 59, 1413–1416. [Google Scholar] [CrossRef]
- Alam, M.S.; Ahmed, J.U.; Lee, D.-U. Synthesis, Antibacterial, Antioxidant Activity and QSAR Studies of Novel 2-Arylidenehydrazinyl-4-arylthiazole Analogues. Chem. Pharm. Bull. 2014, 62, 1259–1268. [Google Scholar] [CrossRef]
- Gingras, B.A.; Hornal, R.W.; Bayley, C.H. The Preparation of Some Thiosemicarbazones and their Copper Complexes. Can. J. Chem. 1960, 38, 712–719. [Google Scholar] [CrossRef]
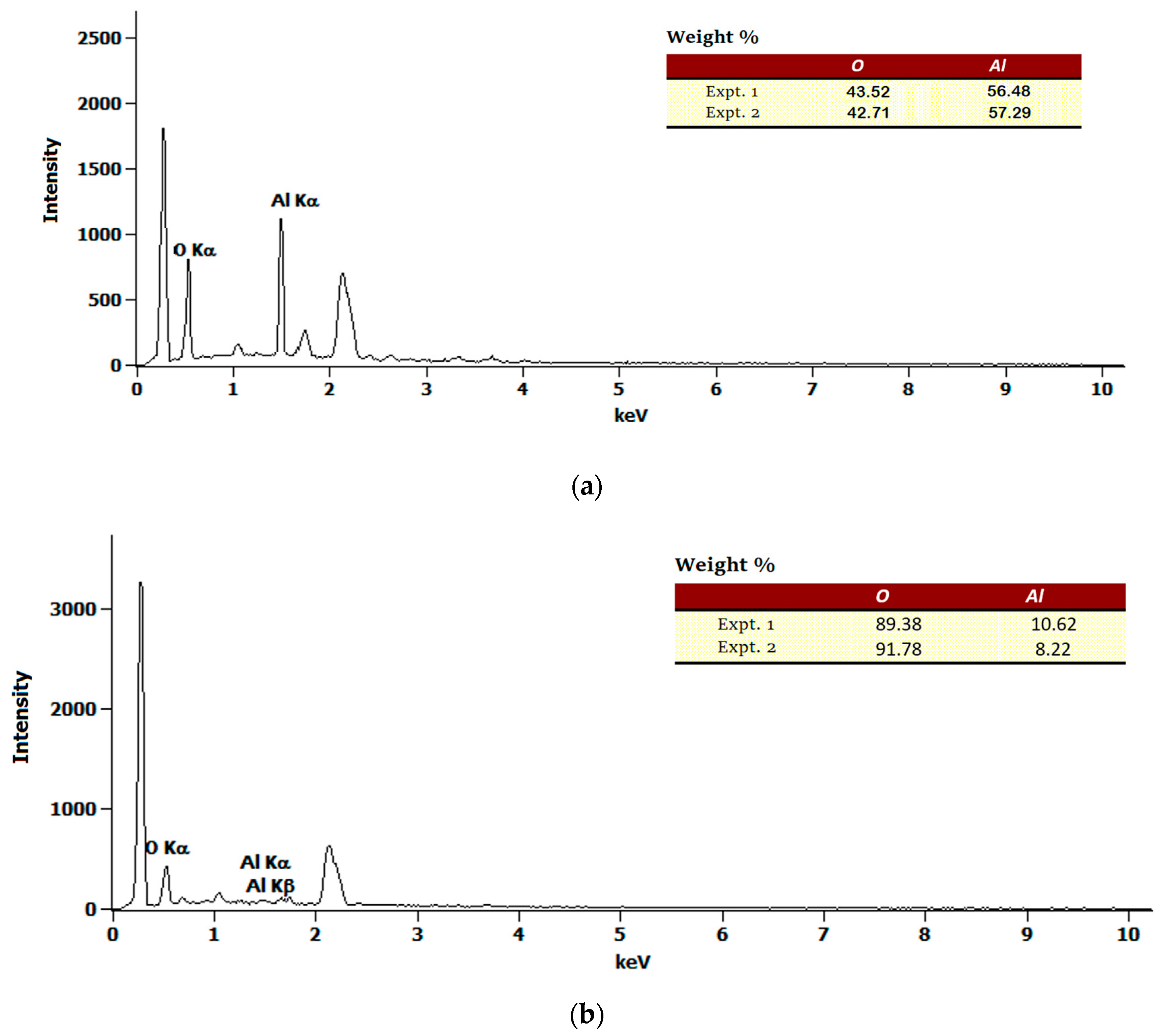
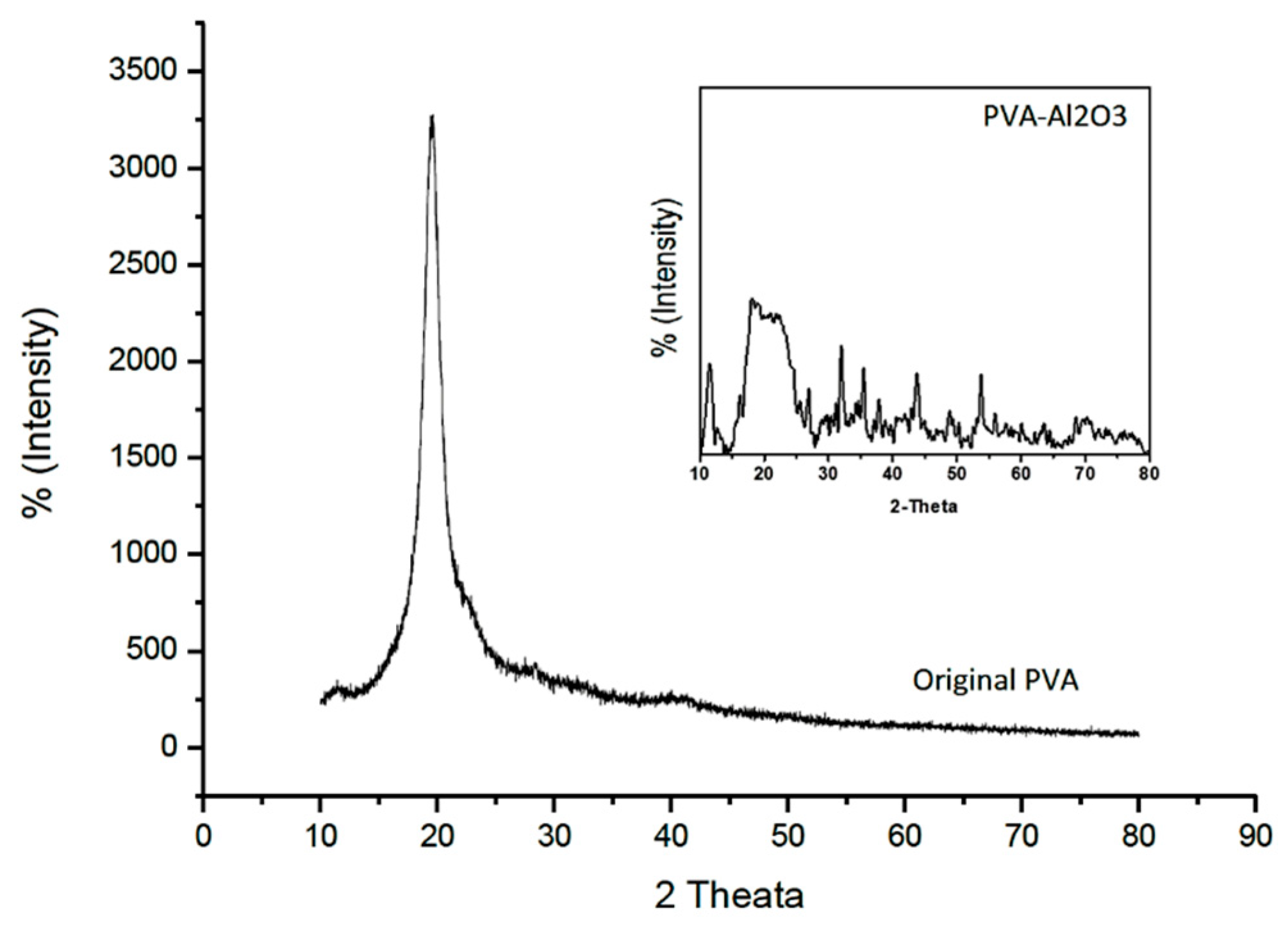
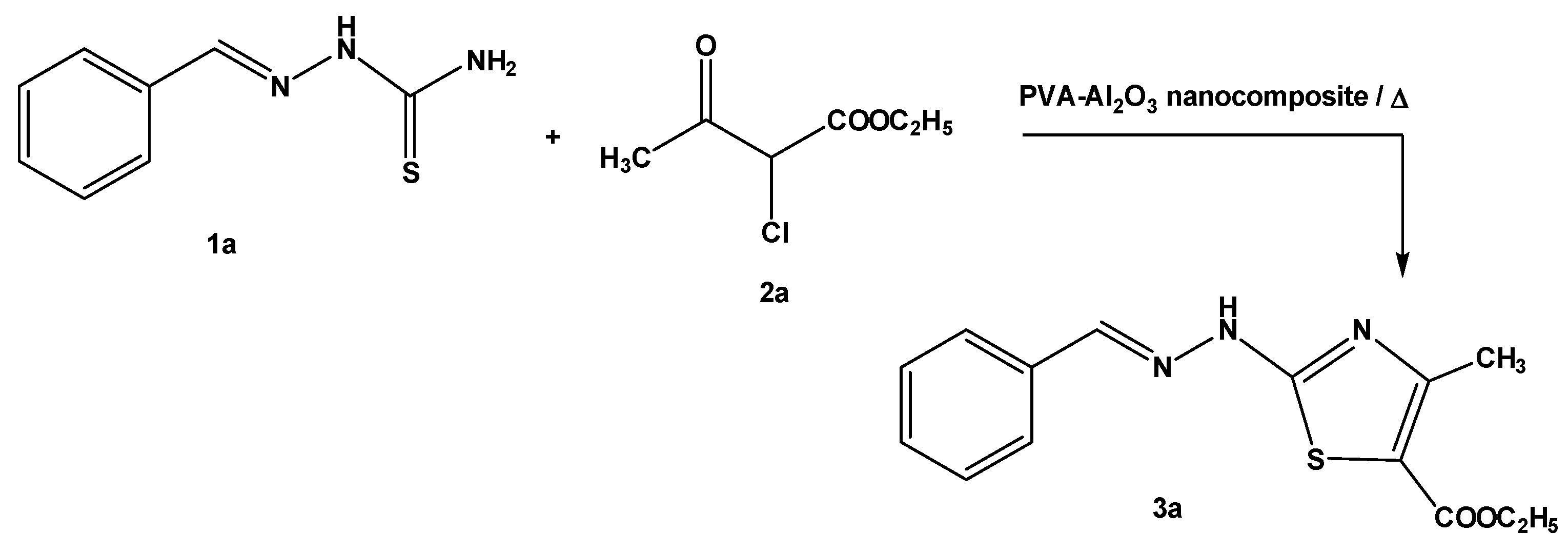
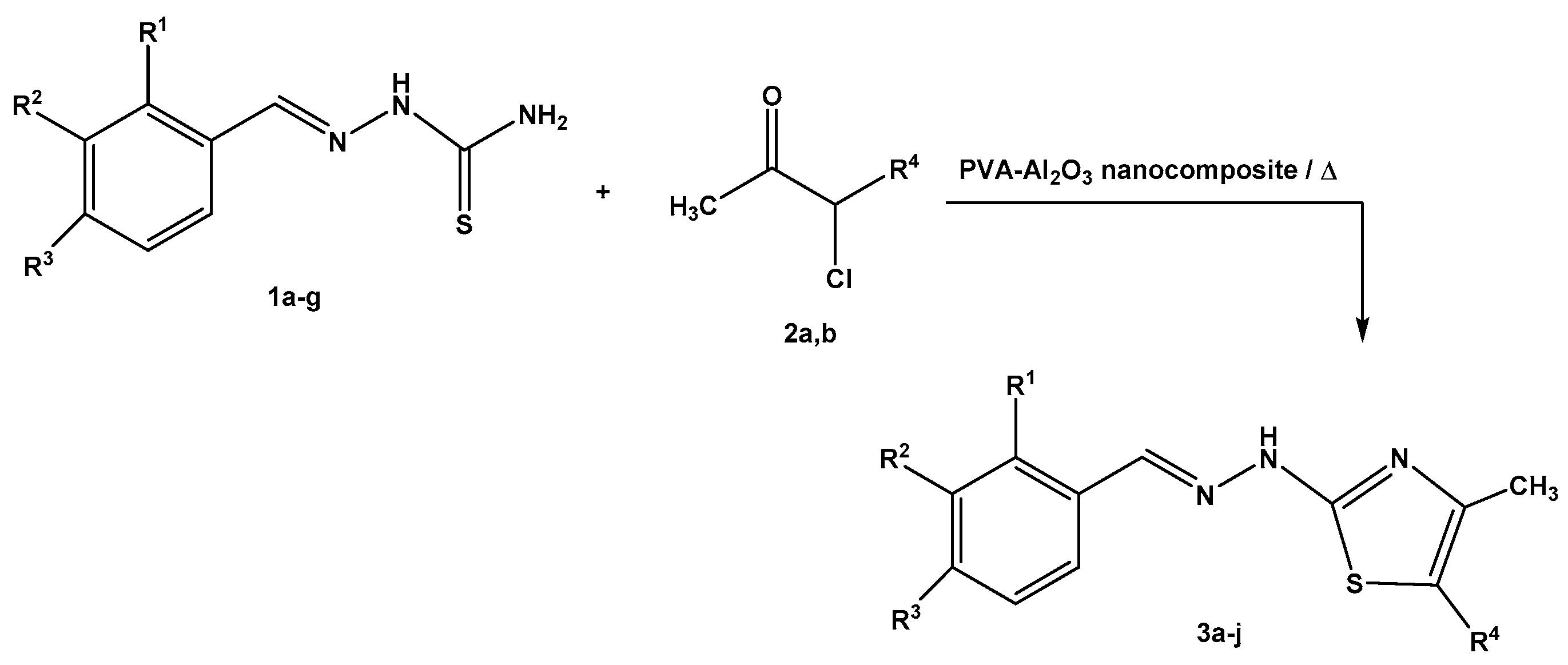
| Entry | Loading Catalyst (Wt%; *) | Time (min) | Yield (%) | Entry | Loading Catalyst (Wt%; *) | Time (min) | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 60 | 40 | 7 | 10 | 30 | 40 |
| 2 | 5 | 60 | 50 | 8 | 10 | 60 | 60 |
| 3 | 10 | 60 | 60 | 9 | 10 | 90 | 72 |
| 4 | 15 | 60 | 60 | 10 | 10 | 120 | 80 |
| 5 | 20 | 60 | 59 | 11 | 10 | 150 | 87 |
| 6 | 25 | 60 | 56 | 12 | 10 | 180 | 92 |
| State of Catalyst | Fresh Catalyst | Recycled (1) | Recycled (2) | Recycled (3) |
|---|---|---|---|---|
| Yield (%) of product 3a (*) | 92 | 91 | 90 | 90 |
| Compd. No. | R1 | R2 | R3 | R4 | Yield (%) | M.P. (°C) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| No Catalyst | Al2O3 (*) | PVA/Al2O3 (**) | |||||||
| 3a | H | H | H | COOEt | 78 | 90 | 92 | 195–197 | [21] |
| 3b | H | H | Cl | COOEt | 77 | 87 | 90 | 205–207 | [21] |
| 3c | H | H | NO2 | COOEt | - | - | 94 | 260–262 | [21] |
| 3d | H | H | N(CH3)2 | COOEt | - | - | 95 | 230–232 | [21] |
| 3e | H | H | OCH3 | COOEt | - | - | 95 | 180–182 | [21] |
| 3f | Cl | H | Cl | COOEt | - | - | 92 | 222–224 | [21] |
| 3g | H | OCH3 | OH | COOEt | - | - | 97 | 226–228 | [21] |
| 3h | H | H | H | COCH3 | - | - | 93 | 222–224 | [22] |
| 3i | H | H | OCH3 | COCH3 | - | - | 91 | 214–216 | [22] |
| 3j | Cl | H | Cl | COCH3 | - | - | 96 | 240–242 | [22] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riyadh, S.M.; Khalil, K.D.; Bashal, A.H. Structural Properties and Catalytic Activity of Binary Poly (vinyl alcohol)/Al2O3 Nanocomposite Film for Synthesis of Thiazoles. Catalysts 2020, 10, 100. https://doi.org/10.3390/catal10010100
Riyadh SM, Khalil KD, Bashal AH. Structural Properties and Catalytic Activity of Binary Poly (vinyl alcohol)/Al2O3 Nanocomposite Film for Synthesis of Thiazoles. Catalysts. 2020; 10(1):100. https://doi.org/10.3390/catal10010100
Chicago/Turabian StyleRiyadh, Sayed M., Khaled D. Khalil, and Ali H. Bashal. 2020. "Structural Properties and Catalytic Activity of Binary Poly (vinyl alcohol)/Al2O3 Nanocomposite Film for Synthesis of Thiazoles" Catalysts 10, no. 1: 100. https://doi.org/10.3390/catal10010100
APA StyleRiyadh, S. M., Khalil, K. D., & Bashal, A. H. (2020). Structural Properties and Catalytic Activity of Binary Poly (vinyl alcohol)/Al2O3 Nanocomposite Film for Synthesis of Thiazoles. Catalysts, 10(1), 100. https://doi.org/10.3390/catal10010100