The Latest Diagnostic Imaging Technologies and AI: Applications for Melanoma Surveillance Toward Precision Oncology
Abstract
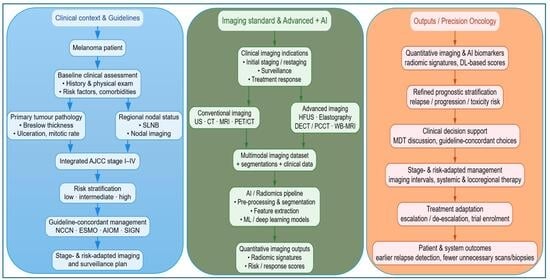
1. Introduction
2. Melanoma Management Guidelines
3. Imaging in Melanoma Management
Diagnostic Imaging in Melanoma: An Overview of Ultrasound (US), CT, MRI, and PET/CT
4. Emerging Radiological Technologies in Melanoma Diagnosis and Management: Current Applications and Future Perspectives
4.1. High Frequency Ultrasonography (HFUS)
4.2. Elastography
4.3. Photon Counting CT (PCCT) and Dual Energy CT (DECT)
- Spatial Resolution: PCCT excels in visualizing peripheral trabecular and pulmonary structures, whereas DECT is particularly effective in detecting metastases through spectral imaging of iodinated contrast.
- Dose Reduction: Both techniques contribute to radiation dose reduction, with DECT specifically optimizing dose by separating tissues based on their chemical composition.
- Spectral Imaging: PCCT provides superior energy separation with minimal spectral overlap, while DECT employs advanced algorithms to enhance the diagnostic quality of spectral images.
- Clinical Applications: PCCT is ideal for structural assessment, such as bone density evaluation or infiltration analysis, whereas DECT is particularly suited for oncologic imaging, including metastasis staging and monitoring.
4.4. Whole-Body Magnetic Resonance Imaging (WB-MRI)
4.5. Clinical Development Directions
5. Artificial Intelligence as a Decision Support Tool for Melanoma: Comparative Analysis of Imaging Modalities
5.1. Ethical Implications, Data Privacy and Regulatory Perspectives in Medical AI
Ethical Considerations Specific to Melanoma Surveillance
6. Conclusions
- Use stage-adapted imaging—ultrasound for nodal basins, brain MRI for stage III–IV disease, and selective use of DECT or whole-body MRI—while balancing diagnostic yield against radiation exposure and cost.
- Deploy AI where evidence is strongest: CT for early outcome and response assessment, PET/CT for lesion-level risk stratification, and brain MRI for prognostic evaluation. Recognize current evidence gaps for ultrasound, whole-body MRI, and photon-counting CT.
- Ensure external validation, robustness testing, and transparent reporting before routine clinical implementation, and integrate AI models into traceable, clinician-in-the-loop workflows.
- Protect vulnerable groups by adhering to DRLs and ALARA principles, prioritizing non-ionizing modalities when feasible, and auditing both dose and cumulative exposure.
- Invest in standardization, shared datasets, and prospective trials to demonstrate clinical utility and cost-effectiveness.
7. Methods
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AI Act | Artificial Intelligence Act (European Union Regulation) |
| AIOM | Italian Association of Medical Oncology |
| AJCC | American Joint Committee on Cancer |
| CAD | Computer-Aided Diagnosis |
| CNN | Convolutional Neural Network |
| CT | Computed Tomography |
| DECT | Dual-Energy Computed Tomography |
| DWI | Diffusion-Weighted Imaging |
| ESMO | European Society for Medical Oncology |
| GDPR | General Data Protection Regulation |
| HFUS | High-Frequency Ultrasound |
| HIQA | Health Information and Quality Authority |
| MRI | Magnetic Resonance Imaging |
| NCCN | National Comprehensive Cancer Network |
| PCCT | Photon-Counting Computed Tomography |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| SIGN | Scottish Intercollegiate Guidelines Network |
| STIR | Short Tau Inversion Recovery |
| SWE | Shear-Wave Elastography |
| TLA | Three-Letter Acronym |
| US | Ultrasound |
| WB-MRI | Whole-Body Magnetic Resonance Imaging |
References
- Valenti, F.; Falcone, I.; Ungania, S.; Desiderio, F.; Giacomini, P.; Bazzichetto, C.; Conciatori, F.; Gallo, E.; Cognetti, F.; Ciliberto, G.; et al. Precision Medicine and Melanoma: Multi-Omics Approaches to Monitoring the Immunotherapy Response. Int. J. Mol. Sci. 2021, 22, 3837. [Google Scholar] [CrossRef]
- Valenti, A.; Falcone, I.; Valenti, F.; Ricciardi, E.; Di Martino, S.; Maccallini, M.T.; Cerro, M.; Desiderio, F.; Miseo, L.; Russillo, M.; et al. Biobanks as an Indispensable Tool in the “Era” of Precision Medicine: Key Role in the Management of Complex Diseases, Such as Melanoma. J. Pers. Med. 2024, 14, 731. [Google Scholar] [CrossRef] [PubMed]
- Falcone, I.; Conciatori, F.; Bazzichetto, C.; Ferretti, G.; Cognetti, F.; Ciuffreda, L.; Milella, M. Tumor Microenvironment: Implications in Melanoma Resistance to Targeted Therapy and Immunotherapy. Cancers 2020, 12, 2870. [Google Scholar] [CrossRef]
- Richard, V.; Kumar, T.R.S.; Pillai, R.M. Transitional dynamics of cancer stem cells in invasion and metastasis. Transl. Oncol. 2021, 14, 100909. [Google Scholar] [CrossRef]
- Grzywa, T.M.; Paskal, W.; Wlodarski, P.K. Intratumor and Intertumor Heterogeneity in Melanoma. Transl. Oncol. 2017, 10, 956–975. [Google Scholar] [CrossRef]
- Kurtansky, N.R.; Primiero, C.A.; Betz-Stablein, B.; Combalia, M.; Guitera, P.; Halpern, A.; Kentley, J.; Kittler, H.; Liopyris, K.; Malvehy, J.; et al. Effect of patient-contextual skin images in human- and artificial intelligence-based diagnosis of melanoma: Results from the 2020 SIIM-ISIC melanoma classification challenge. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Guerrisi, A.; Falcone, I.; Valenti, F.; Rao, M.; Gallo, E.; Ungania, S.; Maccallini, M.T.; Fanciulli, M.; Frascione, P.; Morrone, A.; et al. Artificial Intelligence and Advanced Melanoma: Treatment Management Implications. Cells 2022, 11, 3965. [Google Scholar] [CrossRef]
- Keung, E.Z.; Gershenwald, J.E. The eighth edition American Joint Committee on Cancer (AJCC) melanoma staging system: Implications for melanoma treatment and care. Expert. Rev. Anticancer. Ther. 2018, 18, 775–784. [Google Scholar] [CrossRef] [PubMed]
- AIOM. Homepage. Available online: https://www.iss.it/documents/20126/8403839/LG+127_Melanoma_agg-ago2023_rev-nov.pdf (accessed on 14 November 2025).
- SIGN. Homepage. Available online: https://www.sign.ac.uk/media/2108/sign-146-cutaneous-melanoma-2023.pdf (accessed on 14 November 2025).
- Amaral, T.; Ottaviano, M.; Arance, A.; Blank, C.; Chiarion-Sileni, V.; Donia, M.; Dummer, R.; Garbe, C.; Gershenwald, J.E.; Gogas, H.; et al. Cutaneous melanoma: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2025, 36, 10–30. [Google Scholar] [CrossRef]
- Swetter, S.M.; Johnson, D.; Albertini, M.R.; Barker, C.A.; Bateni, S.; Baumgartner, J.; Bhatia, S.; Bichakjian, C.; Boland, G.; Chandra, S.; et al. NCCN Guidelines(R) Insights: Melanoma: Cutaneous, Version 2.2024. J. Natl. Compr. Cancer Netw. 2024, 22, 290–298. [Google Scholar] [CrossRef]
- Liu, Z.F.; Sylivris, A.; Wu, J.; Tan, D.; Hong, S.; Lin, L.; Wang, M.; Chew, C. Ultrasound Surveillance in Melanoma Management: Bridging Diagnostic Promise with Real-World Adherence: A Systematic Review and Meta-Analysis. Am. J. Clin. Dermatol. 2024, 25, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Sellyn, G.E.; Lopez, A.A.; Ghosh, S.; Topf, M.C.; Chen, H.; Tkaczyk, E.; Powers, J.G. High-frequency ultrasound accuracy in preoperative cutaneous melanoma assessment: A meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Bronstein, Y.; Ross, M.I.; Askew, R.L.; Lee, J.E.; Gershenwald, J.E.; Royal, R.; Cormier, J.N. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: A meta-analysis. J. Natl. Cancer Inst. 2011, 103, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Reinert, C.P.; Liang, C.; Weissinger, M.; Vogel, J.; Forschner, A.; Nikolaou, K.; la Fougere, C.; Seith, F. Whole-Body Magnetic Resonance Imaging (MRI) for Staging Melanoma Patients in Direct Comparison to Computed Tomography (CT): Results from a Prospective Positron Emission Tomography (PET)/CT and PET/MRI Study. Diagnostics 2023, 13, 1963. [Google Scholar] [CrossRef]
- Strobel, K.; Dummer, R.; Husarik, D.B.; Perez Lago, M.; Hany, T.F.; Steinert, H.C. High-risk melanoma: Accuracy of FDG PET/CT with added CT morphologic information for detection of metastases. Radiology 2007, 244, 566–574. [Google Scholar] [CrossRef]
- Zamani-Siahkali, N.; Mirshahvalad, S.A.; Pirich, C.; Beheshti, M. Diagnostic Performance of [(18)F]F-FDG Positron Emission Tomography (PET) in Non-Ophthalmic Malignant Melanoma: A Systematic Review and Meta-Analysis of More than 10,000 Melanoma Patients. Cancers 2024, 16, 215. [Google Scholar] [CrossRef]
- Ungureanu, L.; Botar Jid, C.; Candrea, E.; Cosgarea, R.; Senila, S.C. The role of lymph node ultrasound evaluation in melanoma—Review of the literature. Med. Ultrason. 2016, 18, 224–230. [Google Scholar] [CrossRef]
- Horton, L.; Fakhoury, J.W.; Manwar, R.; Rajabi-Estarabadi, A.; Turk, D.; O’Leary, S.; Fotouhi, A.; Daveluy, S.; Jain, M.; Nouri, K.; et al. Review of Non-Invasive Imaging Technologies for Cutaneous Melanoma. Biosensors 2025, 15, 297. [Google Scholar] [CrossRef]
- Howard, M.D. Melanoma Radiological Surveillance: A Review of Current Evidence and Clinical Challenges. Yale J. Biol. Med. 2020, 93, 207–213. [Google Scholar]
- Vetto, J.T. Clinical and Imaging Follow-Up for High-Risk Cutaneous Melanoma: Current Evidence and Guidelines. Cancers 2024, 16, 2572. [Google Scholar] [CrossRef]
- Papageorgiou, C.; Apalla, Z.; Manoli, S.M.; Lallas, K.; Vakirlis, E.; Lallas, A. Melanoma: Staging and Follow-Up. Dermatol. Pract. Concept. 2021, 11, e2021162S. [Google Scholar] [CrossRef]
- Deike-Hofmann, K.; Thunemann, D.; Breckwoldt, M.O.; Schwarz, D.; Radbruch, A.; Enk, A.; Bendszus, M.; Hassel, J.; Schlemmer, H.P.; Baumer, P. Sensitivity of different MRI sequences in the early detection of melanoma brain metastases. PLoS ONE 2018, 13, e0193946. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, R.; Tiwari, T.; Goyal, S. Anorectal malignant melanoma: MRI findings with pathological correlation. BMJ Case Rep. 2021, 14, e247421. [Google Scholar] [CrossRef]
- Vulasala, S.S.; Virarkar, M.; Karbasian, N.; Calimano-Ramirez, L.F.; Daoud, T.; Amini, B.; Bhosale, P.; Javadi, S. Whole-body MRI in oncology: A comprehensive review. Clin. Imaging 2024, 108, 110099. [Google Scholar] [CrossRef] [PubMed]
- Mena, E.; Sanli, Y.; Marcus, C.; Subramaniam, R.M. Precision Medicine and PET/Computed Tomography in Melanoma. PET Clin. 2017, 12, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.L.; Miller, E.D.; Contreras, C.; Knopp, M.V. Precision Nuclear Medicine: The Evolving Role of PET in Melanoma. Radiol. Clin. N. Am. 2021, 59, 755–772. [Google Scholar] [CrossRef] [PubMed]
- Bronstein, Y.; Ng, C.S.; Rohren, E.; Ross, M.I.; Lee, J.E.; Cormier, J.; Johnson, V.E.; Hwu, W.J. PET/CT in the management of patients with stage IIIC and IV metastatic melanoma considered candidates for surgery: Evaluation of the additive value after conventional imaging. AJR Am. J. Roentgenol. 2012, 198, 902–908. [Google Scholar] [CrossRef]
- Tan, A.C.; Emmett, L.; Lo, S.; Liu, V.; Kapoor, R.; Carlino, M.S.; Guminski, A.D.; Long, G.V.; Menzies, A.M. FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann. Oncol. 2018, 29, 2115–2120. [Google Scholar] [CrossRef]
- Borm, F.J.; Smit, J.; Oprea-Lager, D.E.; Wondergem, M.; Haanen, J.; Smit, E.F.; de Langen, A.J. Response Prediction and Evaluation Using PET in Patients with Solid Tumors Treated with Immunotherapy. Cancers 2021, 13, 3083. [Google Scholar] [CrossRef]
- Kong, B.Y.; Menzies, A.M.; Saunders, C.A.; Liniker, E.; Ramanujam, S.; Guminski, A.; Kefford, R.F.; Long, G.V.; Carlino, M.S. Residual FDG-PET metabolic activity in metastatic melanoma patients with prolonged response to anti-PD-1 therapy. Pigment. Cell Melanoma Res. 2016, 29, 572–577. [Google Scholar] [CrossRef]
- Reginelli, A.; Belfiore, M.P.; Russo, A.; Turriziani, F.; Moscarella, E.; Troiani, T.; Brancaccio, G.; Ronchi, A.; Giunta, E.; Sica, A.; et al. A Preliminary Study for Quantitative Assessment with HFUS (High-Frequency Ultrasound) of Nodular Skin Melanoma Breslow Thickness in Adults Before Surgery: Interdisciplinary Team Experience. Curr. Radiopharm. 2020, 13, 48–55. [Google Scholar] [CrossRef]
- Bard, R.L. High-Frequency Ultrasound Examination in the Diagnosis of Skin Cancer. Dermatol. Clin. 2017, 35, 505–511. [Google Scholar] [CrossRef]
- Izzetti, R.; Oranges, T.; Janowska, A.; Gabriele, M.; Graziani, F.; Romanelli, M. The Application of Ultra-High-Frequency Ultrasound in Dermatology and Wound Management. Int. J. Low. Extrem. Wounds 2020, 19, 334–340. [Google Scholar] [CrossRef]
- Samimi, M.; Perrinaud, A.; Naouri, M.; Maruani, A.; Perrodeau, E.; Vaillant, L.; Machet, L. High-resolution ultrasonography assists the differential diagnosis of blue naevi and cutaneous metastases of melanoma. Br. J. Dermatol. 2010, 163, 550–556. [Google Scholar] [CrossRef]
- Heibel, H.D.; Hooey, L.; Cockerell, C.J. A Review of Noninvasive Techniques for Skin Cancer Detection in Dermatology. Am. J. Clin. Dermatol. 2020, 21, 513–524. [Google Scholar] [CrossRef]
- Solivetti, F.M.; Desiderio, F.; Guerrisi, A.; Bonadies, A.; Maini, C.L.; Di Filippo, S.; D’Orazi, V.; Sperduti, I.; Di Carlo, A. HF ultrasound vs PET-CT and telethermography in the diagnosis of In-transit metastases from melanoma: A prospective study and review of the literature. J. Exp. Clin. Cancer Res. 2014, 33, 96. [Google Scholar] [CrossRef]
- Voit, C.; Mayer, T.; Kron, M.; Schoengen, A.; Sterry, W.; Weber, L.; Proebstle, T.M. Efficacy of ultrasound B-scan compared with physical examination in follow-up of melanoma patients. Cancer 2001, 91, 2409–2416. [Google Scholar] [CrossRef] [PubMed]
- Corvino, A.; Corvino, F.; Catalano, O.; Sandomenico, F.; Petrillo, A. The Tail and the String Sign: New Sonographic Features of Subcutaneous Melanoma Metastasis. Ultrasound Med. Biol. 2017, 43, 370–374. [Google Scholar] [CrossRef]
- Chen, I.; Yu, S. Enhancing HFUS accuracy in melanoma assessment: The role of artificial intelligence and statistical precision. J. Eur. Acad. Dermatol. Venereol. 2025, 39, e460–e461. [Google Scholar] [CrossRef] [PubMed]
- Ogata, D.; Uematsu, T.; Yoshikawa, S.; Kiyohara, Y. Accuracy of real-time ultrasound elastography in the differential diagnosis of lymph nodes in cutaneous malignant melanoma (CMM): A pilot study. Int. J. Clin. Oncol. 2014, 19, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, W.; Li, D.; Wang, Z.; Zhao, Q.; Li, Y.; Cui, R.; Shen, L. Value of the strain ratio in the differential diagnosis of intraocular tumors by elastosonography: A retrospective case-control study. Indian J. Ophthalmol. 2023, 71, 983–988. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Barr, R.G. Breast elastography: How does it works, and for what purposes? Eur. Radiol. 2024, 34, 928–929. [Google Scholar] [CrossRef]
- Idrees, A.; Shahzad, R.; Fatima, I.; Shahid, A. Strain Elastography for Differentiation Between Benign and Malignant Thyroid Nodules. J. Coll. Physicians Surg. Pak. 2020, 30, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, Y.; Togawa, Y.; Yamamoto, Y.; Wakabayashi, S.; Matsue, H.; Inafuku, K. Usefulness of 2-D shear wave elastography for the diagnosis of inguinal lymph node metastasis of malignant melanoma and squamous cell carcinoma. J. Dermatol. 2020, 47, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Botar Jid, C.; Bolboaca, S.D.; Cosgarea, R.; Senila, S.; Rogojan, L.; Lenghel, M.; Vasilescu, D.; Dudea, S.M. Doppler ultrasound and strain elastography in the assessment of cutaneous melanoma: Preliminary results. Med. Ultrason. 2015, 17, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Naito, K.; Horiguchi, J.; Fukuda, H.; Tachikake, T.; Ito, K. Accuracy of sonographic elastography in the differential diagnosis of enlarged cervical lymph nodes: Comparison with conventional B-mode sonography. AJR Am. J. Roentgenol. 2008, 191, 604–610. [Google Scholar] [CrossRef]
- Choi, J.J.; Kang, B.J.; Kim, S.H.; Lee, J.H.; Jeong, S.H.; Yim, H.W.; Song, B.J.; Jung, S.S. Role of sonographic elastography in the differential diagnosis of axillary lymph nodes in breast cancer. J. Ultrasound Med. 2011, 30, 429–436. [Google Scholar] [CrossRef]
- Hinz, T.; Hoeller, T.; Wenzel, J.; Bieber, T.; Schmid-Wendtner, M.H. Real-time tissue elastography as promising diagnostic tool for diagnosis of lymph node metastases in patients with malignant melanoma: A prospective single-center experience. Dermatology 2013, 226, 81–90. [Google Scholar] [CrossRef]
- Hagen, F.; Walder, L.; Fritz, J.; Gutjahr, R.; Schmidt, B.; Faby, S.; Bamberg, F.; Schoenberg, S.; Nikolaou, K.; Horger, M. Image Quality and Radiation Dose of Contrast-Enhanced Chest-CT Acquired on a Clinical Photon-Counting Detector CT vs. Second-Generation Dual-Source CT in an Oncologic Cohort: Preliminary Results. Tomography 2022, 8, 1466–1476. [Google Scholar] [CrossRef]
- Willemink, M.J.; Persson, M.; Pourmorteza, A.; Pelc, N.J.; Fleischmann, D. Photon-counting CT: Technical Principles and Clinical Prospects. Radiology 2018, 289, 293–312. [Google Scholar] [CrossRef]
- Hagen, F.; Soschynski, M.; Weis, M.; Hagar, M.T.; Krumm, P.; Ayx, I.; Taron, J.; Krauss, T.; Hein, M.; Ruile, P.; et al. Photon-counting computed tomography—Clinical application in oncological, cardiovascular, and pediatric radiology. Rofo 2024, 196, 25–35. [Google Scholar] [CrossRef]
- Uhrig, M.; Simons, D.; Bonekamp, D.; Schlemmer, H.P. Improved detection of melanoma metastases by iodine maps from dual energy CT. Eur. J. Radiol. 2017, 90, 27–33. [Google Scholar] [CrossRef]
- Altenbernd, J.; Wetter, A.; Forsting, M.; Umutlu, L. Dual-energy CT of liver metastases in patients with uveal melanoma. Eur. J. Radiol. Open 2016, 3, 254–258. [Google Scholar] [CrossRef]
- Patnana, M.; Bronstein, Y.; Szklaruk, J.; Bedi, D.G.; Hwu, W.J.; Gershenwald, J.E.; Prieto, V.G.; Ng, C.S. Multimethod imaging, staging, and spectrum of manifestations of metastatic melanoma. Clin. Radiol. 2011, 66, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Wichmann, J.L.; Weyer, H.; Albrecht, M.H.; D’Angelo, T.; Leithner, D.; Lenga, L.; Booz, C.; Scholtz, J.E.; Bodelle, B.; et al. Dual-energy computed tomography in patients with cutaneous malignant melanoma: Comparison of noise-optimized and traditional virtual monoenergetic imaging. Eur. J. Radiol. 2017, 95, 1–8. [Google Scholar] [CrossRef]
- Chae, E.J.; Song, J.W.; Seo, J.B.; Krauss, B.; Jang, Y.M.; Song, K.S. Clinical utility of dual-energy CT in the evaluation of solitary pulmonary nodules: Initial experience. Radiology 2008, 249, 671–681. [Google Scholar] [CrossRef]
- Graser, A.; Becker, C.R.; Staehler, M.; Clevert, D.A.; Macari, M.; Arndt, N.; Nikolaou, K.; Sommer, W.; Stief, C.; Reiser, M.F.; et al. Single-phase dual-energy CT allows for characterization of renal masses as benign or malignant. Investig. Radiol. 2010, 45, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.; Babb, J.; Chandarana, H.; Macari, M. Dual source dual energy MDCT: Comparison of 80 kVp and weighted average 120 kVp data for conspicuity of hypo-vascular liver metastases. Invest. Radiol. 2010, 45, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, S.; Zanoni, M.; Sica, C.; Bagnacci, G.; Mancianti, N.; Galzerano, G.; Garosi, G.; Cacioppa, L.M.; Cellina, M.; Zamboni, G.A.; et al. Dual-Energy CT as a Well-Established CT Modality to Reduce Contrast Media Amount: A Systematic Review from the Computed Tomography Subspecialty Section of the Italian Society of Radiology. J. Clin. Med. 2024, 13, 6345. [Google Scholar] [CrossRef]
- Srinivas-Rao, S.; Cao, J.; Marin, D.; Kambadakone, A. Dual-Energy Computed Tomography to Photon Counting Computed Tomography: Emerging Technological Innovations. Radiol. Clin. N. Am. 2023, 61, 933–944. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Brochez, L.; Del Marmol, V.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics—Update 2024. Eur. J. Cancer 2025, 215, 115152. [Google Scholar] [CrossRef]
- Petralia, G.; Padhani, A.; Summers, P.; Alessi, S.; Raimondi, S.; Testori, A.; Bellomi, M. Whole-body diffusion-weighted imaging: Is. it all we need for detecting metastases in melanoma patients? Eur. Radiol. 2013, 23, 3466–3476. [Google Scholar] [CrossRef]
- Pfannenberg, C.; Schwenzer, N. Whole-body staging of malignant melanoma: Advantages, limitations and current importance of PET-CT, whole-body MRI and PET-MRI. Radiologe 2015, 55, 120–126. [Google Scholar] [CrossRef]
- Mosavi, F.; Ullenhag, G.; Ahlstrom, H. Whole-body MRI including diffusion-weighted imaging compared to CT for staging of malignant melanoma. Ups. J. Med. Sci. 2013, 118, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Jansen, Y.J.L.; Willekens, I.; Seremet, T.; Awada, G.; Schwarze, J.K.; De Mey, J.; Brussaard, C.; Neyns, B. Whole-Body MRI for the Detection of Recurrence in Melanoma Patients at High Risk of Relapse. Cancers 2021, 13, 442. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.; Taylor, S.; Janes, S.; Halligan, S.; Morton, A.; Navani, N.; Oliver, A.; Rockall, A.; Teague, J.; Miles, A.; et al. Patient experience and perceived acceptability of whole-body magnetic resonance imaging for staging colorectal and lung cancer compared with current staging scans: A qualitative study. BMJ Open 2017, 7, e016391. [Google Scholar] [CrossRef] [PubMed]
- Rata, M.; Blackledge, M.; Scurr, E.; Winfield, J.; Koh, D.M.; Dragan, A.; Candito, A.; King, A.; Rennie, W.; Gaba, S.; et al. Implementation of Whole-Body MRI (MY-RADS) within the OPTIMUM/MUKnine multi-centre clinical trial for patients with myeloma. Insights Imaging 2022, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Ullah, A.; Shah, D.; Ali, S.; Tahir, M. Artificial Intelligence in Melanoma Detection: A Review of Current Technologies and Future Directions. Int. J. Intell. Syst. 2025, 2025, 3164952. [Google Scholar] [CrossRef]
- Levy, J.; Barrett, D.L.; Harris, N.; Jeong, J.J.; Yang, X.; Chen, S.C. High-frequency ultrasound in clinical dermatology: A review. Ultrasound J. 2021, 13, 24. [Google Scholar] [CrossRef]
- Schwartz, F.R.; Sodickson, A.D.; Pickhardt, P.J.; Sahani, D.V.; Lev, M.H.; Gupta, R. Photon-Counting CT: Technology, Current and Potential Future Clinical Applications, and Overview of Approved Systems and Those in Various Stages of Research and Development. Radiology 2025, 314, e240662. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.; Nasir, M.U.; So, A.; Andrews, G.; Nicolaou, S.; Qamar, S.R. Clinical Applications of Dual-Energy CT. Korean J. Radiol. 2021, 22, 970–982. [Google Scholar] [CrossRef]
- Ahlawat, S.; Debs, P.; Amini, B.; Lecouvet, F.E.; Omoumi, P.; Wessell, D.E. Clinical Applications and Controversies of Whole-Body MRI: AJR Expert Panel Narrative Review. AJR Am. J. Roentgenol. 2023, 220, 463–475. [Google Scholar] [CrossRef]
- Grossarth, S.; Mosley, D.; Madden, C.; Ike, J.; Smith, I.; Huo, Y.; Wheless, L. Recent Advances in Melanoma Diagnosis and Prognosis Using Machine Learning Methods. Curr. Oncol. Rep. 2023, 25, 635–645. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Stafford, H.; Buell, J.; Chiang, E.; Ramesh, U.; Migden, M.; Nagarajan, P.; Amit, M.; Yaniv, D. Non-Melanoma Skin Cancer Detection in the Age of Advanced Technology: A Review. Cancers 2023, 15, 3094. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, B.W.; Lee, C.H.; Tseng, V.S. Development of a light-weight deep learning model for cloud applications and remote diagnosis of skin cancers. J. Dermatol. 2021, 48, 310–316. [Google Scholar] [CrossRef]
- Maron, R.C.; Utikal, J.S.; Hekler, A.; Hauschild, A.; Sattler, E.; Sondermann, W.; Haferkamp, S.; Schilling, B.; Heppt, M.V.; Jansen, P.; et al. Artificial Intelligence and Its Effect on Dermatologists’ Accuracy in Dermoscopic Melanoma Image Classification: Web-Based Survey Study. J. Med. Internet Res. 2020, 22, e18091. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, S.; Dent, A.; Faust, K.; Richer, M.; Djuric, U.; Van Ommeren, R.; Diamandis, P. Physician perspectives on integration of artificial intelligence into diagnostic pathology. NPJ Digit. Med. 2019, 2, 28. [Google Scholar] [CrossRef] [PubMed]
- Scheetz, J.; Rothschild, P.; McGuinness, M.; Hadoux, X.; Soyer, H.P.; Janda, M.; Condon, J.J.J.; Oakden-Rayner, L.; Palmer, L.J.; Keel, S.; et al. A survey of clinicians on the use of artificial intelligence in ophthalmology, dermatology, radiology and radiation oncology. Sci. Rep. 2021, 11, 5193. [Google Scholar] [CrossRef]
- Phillips, M.; Greenhalgh, J.; Marsden, H.; Palamaras, I. Detection of Malignant Melanoma Using Artificial Intelligence: An Observational Study of Diagnostic Accuracy. Dermatol. Pract. Concept. 2020, 10, e2020011. [Google Scholar] [CrossRef] [PubMed]
- Esteva, A.; Kuprel, B.; Novoa, R.A.; Ko, J.; Swetter, S.M.; Blau, H.M.; Thrun, S. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017, 542, 115–118. [Google Scholar] [CrossRef]
- Dercle, L.; Zhao, B.; Gonen, M.; Moskowitz, C.S.; Firas, A.; Beylergil, V.; Connors, D.E.; Yang, H.; Lu, L.; Fojo, T.; et al. Early Readout on Overall Survival of Patients with Melanoma Treated with Immunotherapy Using a Novel Imaging Analysis. JAMA Oncol. 2022, 8, 385–392. [Google Scholar] [CrossRef]
- Wang, Z.L.; Mao, L.L.; Zhou, Z.G.; Si, L.; Zhu, H.T.; Chen, X.; Zhou, M.J.; Sun, Y.S.; Guo, J. Pilot Study of CT-Based Radiomics Model for Early Evaluation of Response to Immunotherapy in Patients with Metastatic Melanoma. Front. Oncol. 2020, 10, 1524. [Google Scholar] [CrossRef]
- Brendlin, A.S.; Peisen, F.; Almansour, H.; Afat, S.; Eigentler, T.; Amaral, T.; Faby, S.; Calvarons, A.F.; Nikolaou, K.; Othman, A.E. A Machine learning model trained on dual-energy CT radiomics significantly improves immunotherapy response prediction for patients with stage IV melanoma. J. Immunother. Cancer 2021, 9, e003261. [Google Scholar] [CrossRef]
- Gabrys, H.S.; Basler, L.; Burgermeister, S.; Hogan, S.; Ahmadsei, M.; Pavic, M.; Bogowicz, M.; Vuong, D.; Tanadini-Lang, S.; Forster, R.; et al. PET/CT radiomics for prediction of hyperprogression in metastatic melanoma patients treated with immune checkpoint inhibitors. Front. Oncol. 2022, 12, 977822. [Google Scholar] [CrossRef]
- Bhatia, A.; Birger, M.; Veeraraghavan, H.; Um, H.; Tixier, F.; McKenney, A.S.; Cugliari, M.; Caviasco, A.; Bialczak, A.; Malani, R.; et al. MRI radiomic features are associated with survival in melanoma brain metastases treated with immune checkpoint inhibitors. Neuro Oncol. 2019, 21, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Shofty, B.; Artzi, M.; Shtrozberg, S.; Fanizzi, C.; DiMeco, F.; Haim, O.; Peleg Hason, S.; Ram, Z.; Bashat, D.B.; Grossman, R. Virtual biopsy using MRI radiomics for prediction of BRAF status in melanoma brain metastasis. Sci. Rep. 2020, 10, 6623. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.; Marinelli, L.; Patane, V.; Alessandrella, M.; Pezzella, M.C.; Troiani, T.; Brancaccio, G.; Scharf, C.; Argenziano, G.; Cappabianca, S.; et al. Whole-body magnetic resonance imaging for cutaneous melanoma staging: A scientific review. World J. Clin. Oncol. 2025, 16, 109206. [Google Scholar] [CrossRef]
- Guerrisi, A.; Miseo, L.; Falcone, I.; Messina, C.; Ungania, S.; Elia, F.; Desiderio, F.; Valenti, F.; Cantisani, V.; Soriani, A.; et al. Quantitative ultrasound radiomics analysis to evaluate lymph nodes in patients with cancer: A systematic review. Ultraschall Med. 2024, 45, 586–596. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, T.; Wang, L.; Xing, Y.; Hu, Y.; Xu, Z.; Lu, J.; Yang, J.; Chu, J.; Zhang, B.; et al. Robustness of radiomics within photon-counting detector CT: Impact of acquisition and reconstruction factors. Eur. Radiol. 2025, 35, 4661–4673. [Google Scholar] [CrossRef] [PubMed]
- Higgins, H.; Nakhla, A.; Lotfalla, A.; Khalil, D.; Doshi, P.; Thakkar, V.; Shirini, D.; Bebawy, M.; Ammari, S.; Lopci, E.; et al. Recent Advances in the Field of Artificial Intelligence for Precision Medicine in Patients with a Diagnosis of Metastatic Cutaneous Melanoma. Diagnostics 2023, 13, 3483. [Google Scholar] [CrossRef]
- Pinto-Coelho, L. How Artificial Intelligence Is Shaping Medical Imaging Technology: A Survey of Innovations and Applications. Bioengineering 2023, 10, 1435. [Google Scholar] [CrossRef]
- Avanzo, M.; Stancanello, J.; Pirrone, G.; Drigo, A.; Retico, A. The Evolution of Artificial Intelligence in Medical Imaging: From Computer Science to Machine and Deep Learning. Cancers 2024, 16, 3702. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, J.; Fantus, S. Medical artificial intelligence ethics: A systematic review of empirical studies. Digit. Health 2023, 9, 20552076231186064. [Google Scholar] [CrossRef] [PubMed]
- LEX, E. Homepage. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=OJ:L_202401689 (accessed on 14 November 2025).
| Guidelines | Follow-Up (Frequency) | Recommended Imaging | Specific Approach | References |
|---|---|---|---|---|
| AJCC 2018 | - Does not specify follow-up protocols; focused on staging. | - It does not provide specific recommendations on imaging. | -Detailed staging: provides criteria for TNM classification of melanoma. | [8] |
| AIOM 2023 | -Stage I–IIA: every 6 months for 5 years. - Stage IIB–IV: every 3–4 months for 2 years, then every 6 months until the 5th year. | - Ultrasound: every 6 months for 3 years (especially if no complete lymph node dissection) (Stage III). - Stage IIB–IV: CT chest-abdomen-pelvis every 6 months for 3 years, then annually until year 5. - Stage III–IV: consider annual brain MRI. | -Laboratory tests: LDH and CBC for stages IIB–IV. -Patient education: detailed instructions on self-examination and signs of recurrence. | [9] |
| SIGN 2023 | -Stage IA: every 12 months for 5 years. - Stage IB–IIA: every 6 months for 5 years. - Stage IIB–IV: every 3 months for 3 years, then every 6 months until the 5th year. | - Imaging is not routinely recommended. - Performed only if clinical symptoms or signs of recurrence are present. - Lymph node ultrasound may be considered if clinical suspicion arises. | -Psychological support: emphasis on the importance of emotional and psychological support. - Patient education: promotion of self-monitoring and symptom awareness. | [10] |
| ESMO 2024 | -Stage I–II: every 6 months for 5 years. - Stage III: every 3 months for 3 years, then every 6 months until the 5th year. -Stage IV: individualized follow-up. | - Ultrasound: may be performed every 3–6 months for regional disease (Stage III), as part of routine follow-up. - Stage III: CT or PET/CT scan every 6 months for 3 years, then annually. -Stage IV: imaging based on clinical presentation. | -Laboratory tests: LDH for stages III-IV. - Psychological support: attention to mental well-being during follow-up. | [11] |
| NCCN 2024 | -Stage 0–IIA: every 6–12 months for 5 years, then annually. - Stage IIB–IV: every 3–6 months for 2 years, every 3–12 months for the next 3 years, then annually. | -Ultrasound: may be used if physical exam is inconclusive or in high-risk patients. - Stage IIB–IV: consider CT with contrast or PET/CT every 3–12 months for 2–5 years. - Stage III: consider brain MRI every 3–12 months for 2–5 years. | -Laboratory tests: LDH for stages IIB–IV. - Patient education: monthly skin and lymph node self-examination. - Genetic counseling: for patients with significant family history. | [12] |
| Ultrasonography (US) | Computed Tomography (CT) | Magnetic Resonance Imaging (MRI) | Positron Emission Tomography (Pet) | |
|---|---|---|---|---|
| Sensitivity | ≈80–90% (regional lymph-node metastases) | ≈65–75% (pulmonary metastases; size-dependent) | ≈85–95% (brain metastases; high for marrow/soft tissue) | ≈80–90% (distant metastases in FDG-avid tumors) |
| Specificity | ≈85–98% | ≈80–90% | ≥90% | ≈85–95% |
| Key clinical Indication | Lesion thickness; nodal assessment; superficial metastases | Staging of chest/abdomen/pelvis; lung metastases; osseous lesions | Brain/spinal cord staging; soft-tissue & bone-marrow metastases; liver characterization | Advanced staging; occult metastases; treatment response; whole-body survey |
| Advantages | Non-invasive; high resolution for superficial tissues/nodes; bedside | Rapid whole-body overview; excellent for deep lesions; widely available | No ionizing radiation; excellent soft-tissue contrast; multiparametric | Metabolic + anatomic information; high whole-body sensitivity |
| Limitations | Operator-dependent; limited depth penetration; small nodules may be missed; inflammatory nodes false-positive | Lower accuracy for brain/small nodes; ionizing radiation; iodinated contrast risks | Longer exam; motion sensitivity; higher cost; less sensitive for lungs | Cost; limited spatial resolution; small/regional nodes may be missed; hyperglycemia degrades quality |
| Ionizing Radiation | No | Yes | No | Yes (radiotracer + CT) |
| Contrast Agent | None routinely (CEUS optional) | Iodinated IV often required | GBCA often useful | 18F-FDG; iodinated CT contrast sometimes used |
| Typical Duration | ≈10–20 min | ≈5–15 min | ≈20–45 min | ≈90–120 min (uptake + scan) |
| Contraindications/Precautions | No absolute contraindications; limited by obesity/subcutaneous air; CEUS: caution in severe cardiopulmonary instability | Pregnancy (relative); severe CKD or prior severe iodinated-contrast reaction | Non MR conditional implants; severe claustrophobia; severe renal impairment (rare NSF risk with some GBCAs) | Pregnancy/lactation (precautions); uncontrolled hyperglycemia |
| Technology | Clinical Use Status | Adoption in Clinical Centers | Level of Clinical Validation | Key Clinical Notes |
|---|---|---|---|---|
| HFUS | Used in specialized clinical settings | High in advanced dermatology centers | Moderate (thickness assessment, lymph nodes) | Excellent resolution for superficial lesions; useful in follow-up. |
| Elastography | Gradually entering clinical practice | Medium-high | Moderate (especially lymph node evaluation) | Useful for distinguishing reactive vs. metastatic tissues. |
| DECT | Limited use in research or specialized centers | Medium | Limited | Enhances contrast and metastasis detection with lower radiation. |
| PCCT | In preclinical and experimental use | Low | Low | High resolution; promising but costly and not yet standard. |
| WB-MRI | Selective use in advanced clinical settings | Medium | Good (prospective studies in follow-up) | Sensitive for distant and bone metastases; limited for lung assessment. |
| Parameter | Standard Imaging (US, CT, MRI, PET/CT) | Emerging Imaging (HFUS, Elastography, DECT, PCCT, WB-MRI) |
|---|---|---|
| Clinical Availability | High | Variable to low (depends on the technique and setting) |
| Radiation Exposure | Modality dependent: US/MRI none; CT/PET-CT ionizing (Overall Medium high) | Generally lower (WB-MRI, HFUS: none; DECT/PCCT: optimized) |
| Cost and Infrastructure | Moderate to high | High (advanced equipment and specialized training required) |
| Spatial Resolution | Good | Very high (e.g., HFUS and PCCT allow submillimeter resolution) |
| Sensitivity for Metastatic Spread | High for visceral and nodal metastases | High in selected settings-WB-MRI: bone/distant; HFUS/Elastography: superficial and nodal characterization; DECT/PCCT: small/low contrast lesions |
| Suitability for Long-Term Follow-Up | Limited by radiation and cumulative exposure | Favorable (especially WB-MRI and HFUS) |
| Integration with AI and Radiomics | Widely used in structured clinical settings (segmentation and reporting) | High potential-HFUS/Elastography: automated measurements; DECT/PCCT: spectral/radiomics; WB-MRI: quantitative DWI |
| Operator Dependency | Moderate (higher for US) | High (especially for HFUS and elastography); Moderate (DECT/PCCT and WB-MRI) |
| Level of Standardization | Well established protocols | Still under development in many cases |
| Exam Time/Workflow | Short/moderate; fully integrated in routine workflow | Moderate/long (WB-MRI longer); setup/training phases for new techniques |
| Clinical Evidence/Adoption | Guideline supported; widely adopted | Mixed: HFUS/Elastography with focused clinical use; DECT/PCCT/WB-MRI mainly research early clinical adoption |
| Melanoma specific strengths (examples) | PET/CT: systemic staging/therapy response; CT: lung/viscera; MRI: brain/soft tissue; US: nodal assessment | HFUS: sub-mm superficial and in transit disease; Elastography: nodal stiffness; DECT: iodine maps conspicuity; PCCT: high spatial/spectral, fewer artifacts; WB-MRI: whole-body marrow and bone metastasis |
| Main limitations/challenges | Radiation (CT/PET-CT); limited sensitivity for tiny/in transit lesions; cost of repeated imaging | HFUS: operator dependence, shallow penetration; Elastography: no shared cut-offs, vendor variability; DECT/PCCT: access, spectral harmonization, residual dose; WB-MRI: long exams, lower pulmonary performance, inter center heterogeneity |
| Near term development priorities | Dose/contrast optimization; structured reporting; value based follow-up; pragmatic AI QA/monitoring | Standardized training and protocols (HFUS); multicenter quantitative thresholds (Elastography); harmonized spectral protocols and iodine density biomarkers + radiomics (DECT/PCCT); abbreviated, quantitative DWI pathways and reporting templates (WB-MRI) |
| Modality | AI/Radiomics Task | Cohort/Setting | Key Metrics (If Reported) | Study |
|---|---|---|---|---|
| CT (standard) | Early survival estimation under ICI (anti PD-1) | n = 575; pooled KEYNOTE-002/-006; internal train/validation (validation pembrolizumab n = 287) | Time-dependent AUC at 6 months 0.92 (95% CI 0.89–0.95) vs. RECIST 1.1 0.80 (0.74–0.84) | [85] |
| CT (standard) | Early response/pseudoprogression identification | n = 50; single center; baseline + post-cycle 1–2 (train PD-1 n = 34/validation CTLA-4 n = 16) | Validation AUC 0.857 | [86] |
| Dual-energy CT (DECT) | Response prediction to immunotherapy (stage IV) | n = 140; baseline DECT; chronological split 70/70; 10-fold CV for tuning | Validation AUROC patient-level SECT 0.50 vs. DECT 0.75; lesion-level SECT 0.61 vs. DECT 0.85 | [87] |
| PET/CT (18F-FDG) | Hyper progression prediction (lesion-level) before ICI | 330 lesions in 56 patients; retrospective; single center; nested cross-validation; no external validation | Testing AUC ≈ 0.703 (CT) e ≈ 0.704 (PET/CT) a 3 mesi | [88] |
| Brain MRI | Prognosis in brain metastases (with ICI) | 88 pts; 196 metastases | Radiomic features associated with OS | [89] |
| Brain MRI | “Virtual biopsy”: BRAF mutation prediction in brain metastases | 53 pts (54 lesions); two centers | Accuracy 0.79 ± 0.13; AUC~0.78 | [90] |
| Whole-body MRI (WB-MRI) | Emerging role of radiomics/AI for risk stratification and personalization | Narrative/systematic review on WB-MRI in melanoma | synthesis of applications and potential (no AI model performance) | [91] |
| Ultrasound/Elastography | Melanoma specific AI: scarcity of published studies; evidence from oncologic lymph-node AI and melanoma elastography | US-radiomics review on oncologic lymphadenopathy; feasibility in cutaneous melanoma | Indirect/feasibility evidence; need melanoma specific validation | [42,92] |
| Photon-counting CT (PCCT) | No melanoma specific AI/radiomics studies identified to date | Robustness studies in PCD-CT (phantom/other diseases) | Focus on feature robustness (e.g., sensitivity to pitch/slice thickness), not melanoma outcomes | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenti, A.; Valenti, F.; Giuliani, S.; di Martino, S.; Neroni, L.; Sorino, C.; Sollena, P.; Desiderio, F.; Elia, F.; Maccallini, M.T.; et al. The Latest Diagnostic Imaging Technologies and AI: Applications for Melanoma Surveillance Toward Precision Oncology. Computers 2025, 14, 512. https://doi.org/10.3390/computers14120512
Valenti A, Valenti F, Giuliani S, di Martino S, Neroni L, Sorino C, Sollena P, Desiderio F, Elia F, Maccallini MT, et al. The Latest Diagnostic Imaging Technologies and AI: Applications for Melanoma Surveillance Toward Precision Oncology. Computers. 2025; 14(12):512. https://doi.org/10.3390/computers14120512
Chicago/Turabian StyleValenti, Alessandro, Fabio Valenti, Stefano Giuliani, Simona di Martino, Luca Neroni, Cristina Sorino, Pietro Sollena, Flora Desiderio, Fulvia Elia, Maria Teresa Maccallini, and et al. 2025. "The Latest Diagnostic Imaging Technologies and AI: Applications for Melanoma Surveillance Toward Precision Oncology" Computers 14, no. 12: 512. https://doi.org/10.3390/computers14120512
APA StyleValenti, A., Valenti, F., Giuliani, S., di Martino, S., Neroni, L., Sorino, C., Sollena, P., Desiderio, F., Elia, F., Maccallini, M. T., Russillo, M., Falcone, I., & Guerrisi, A. (2025). The Latest Diagnostic Imaging Technologies and AI: Applications for Melanoma Surveillance Toward Precision Oncology. Computers, 14(12), 512. https://doi.org/10.3390/computers14120512