Functional Characterization of Suppressor of Cytokine Signalling 6 and Its Interaction with Erythropoietin Receptor in Colorectal Cancer Cells
Simple Summary
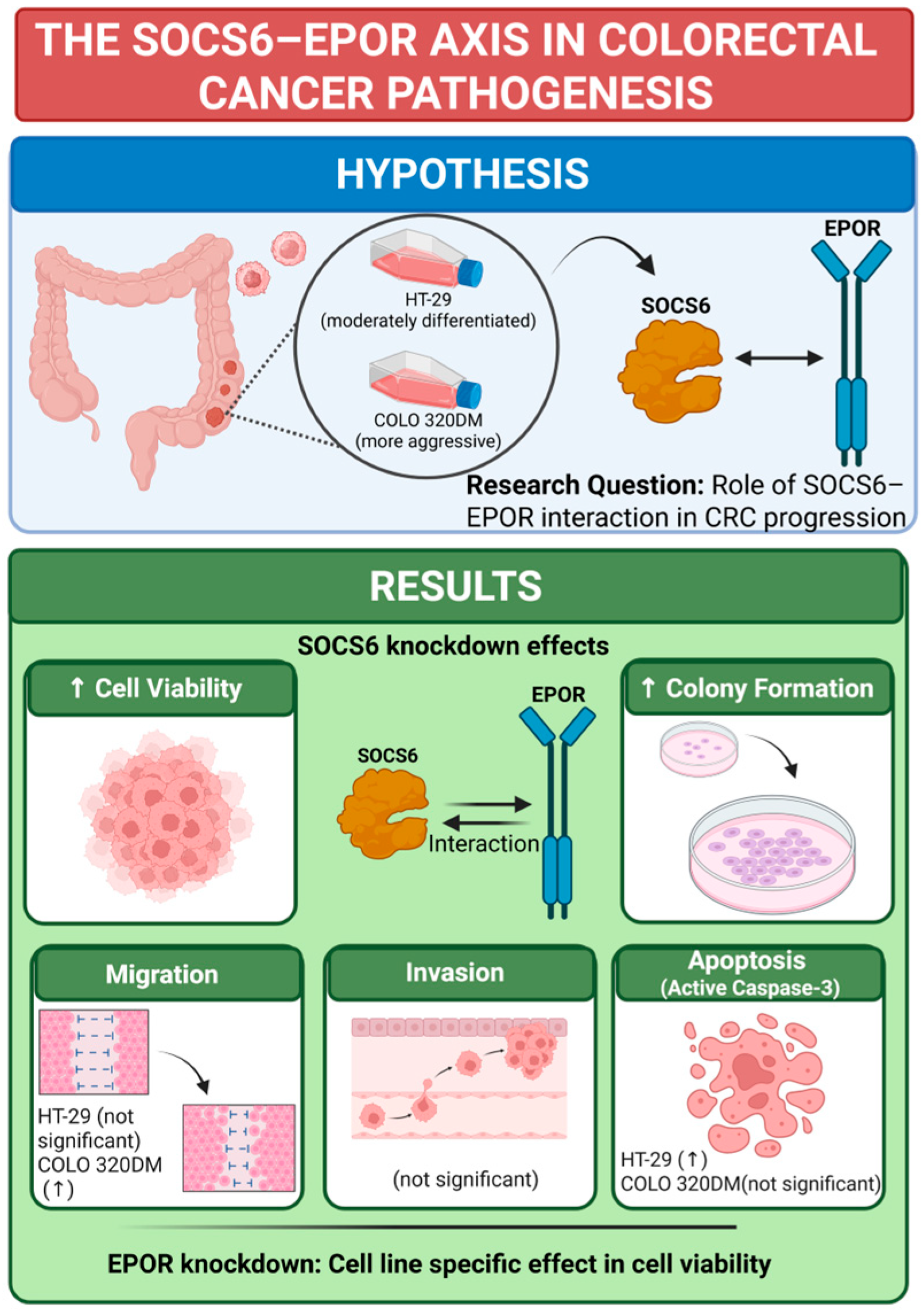
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics
2.2. Cell Lines and Cell Culture
2.3. SOCS6 and EPOR Knockdown Using siRNA
2.4. RNA Extraction and Quantitative RT-PCR
2.5. Viability Assay
2.6. Colony Formation Assay
2.7. Cell Migration Assay
2.8. Cell Invasion Assay
2.9. Assessment of Apoptosis Using Active Caspase-3 ELISA
2.10. Statistical Analysis
3. Results
3.1. Bioinformatics Analysis Revealed SOCS6 Interaction with EPOR
3.2. Effect of SOCS6 Knockdown on Cellular Proteins and Function
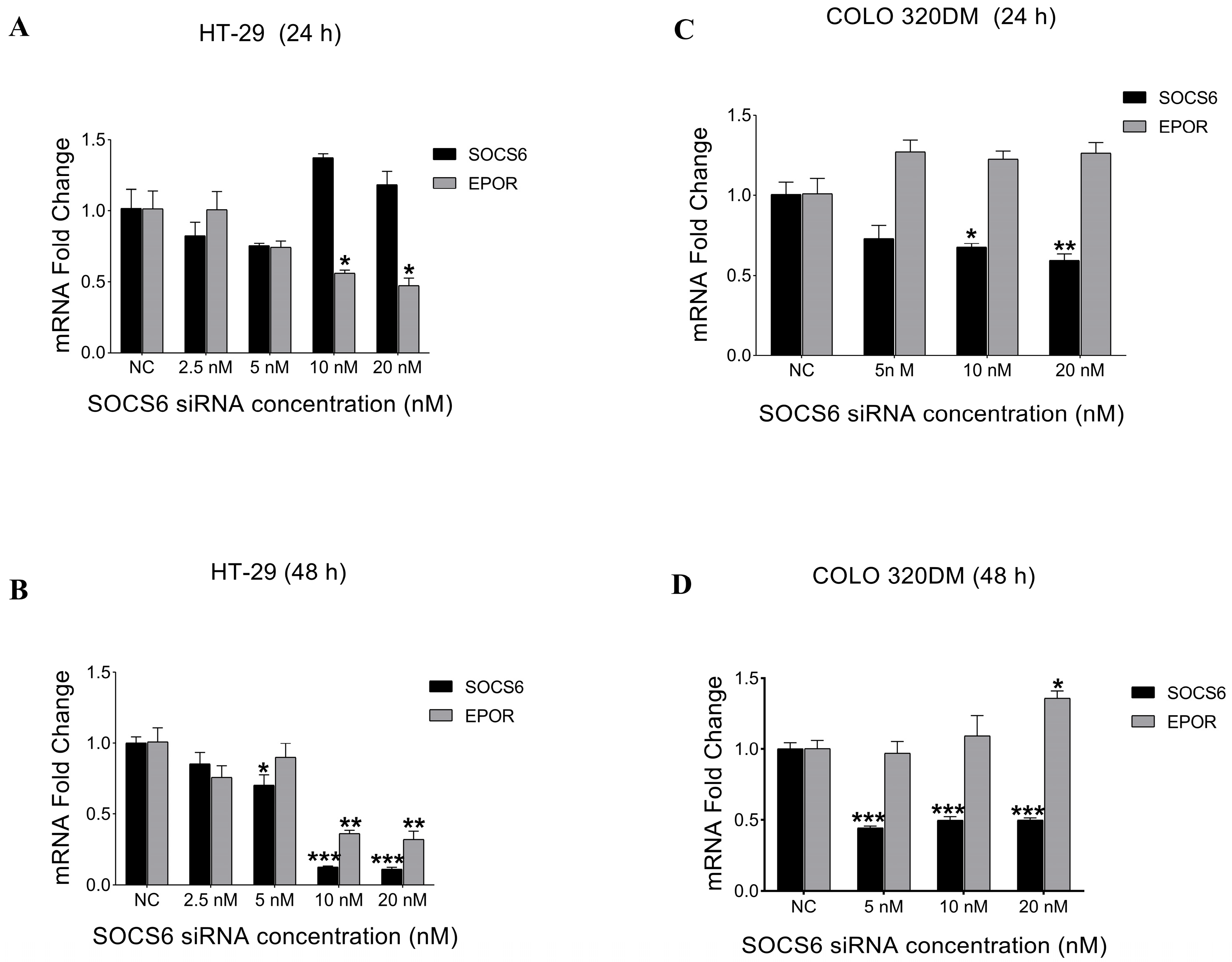
3.2.1. Expression Analysis of SOCS6 and EPOR After SOCS6 Silencing
3.2.2. SOCS6 Silencing Enhanced Cell Viability in HT-29 and COLO 320DM Cells
3.2.3. SOCS6 Silencing Enhanced Colony Formation Ability in HT-29 and COLO 320DM Cells
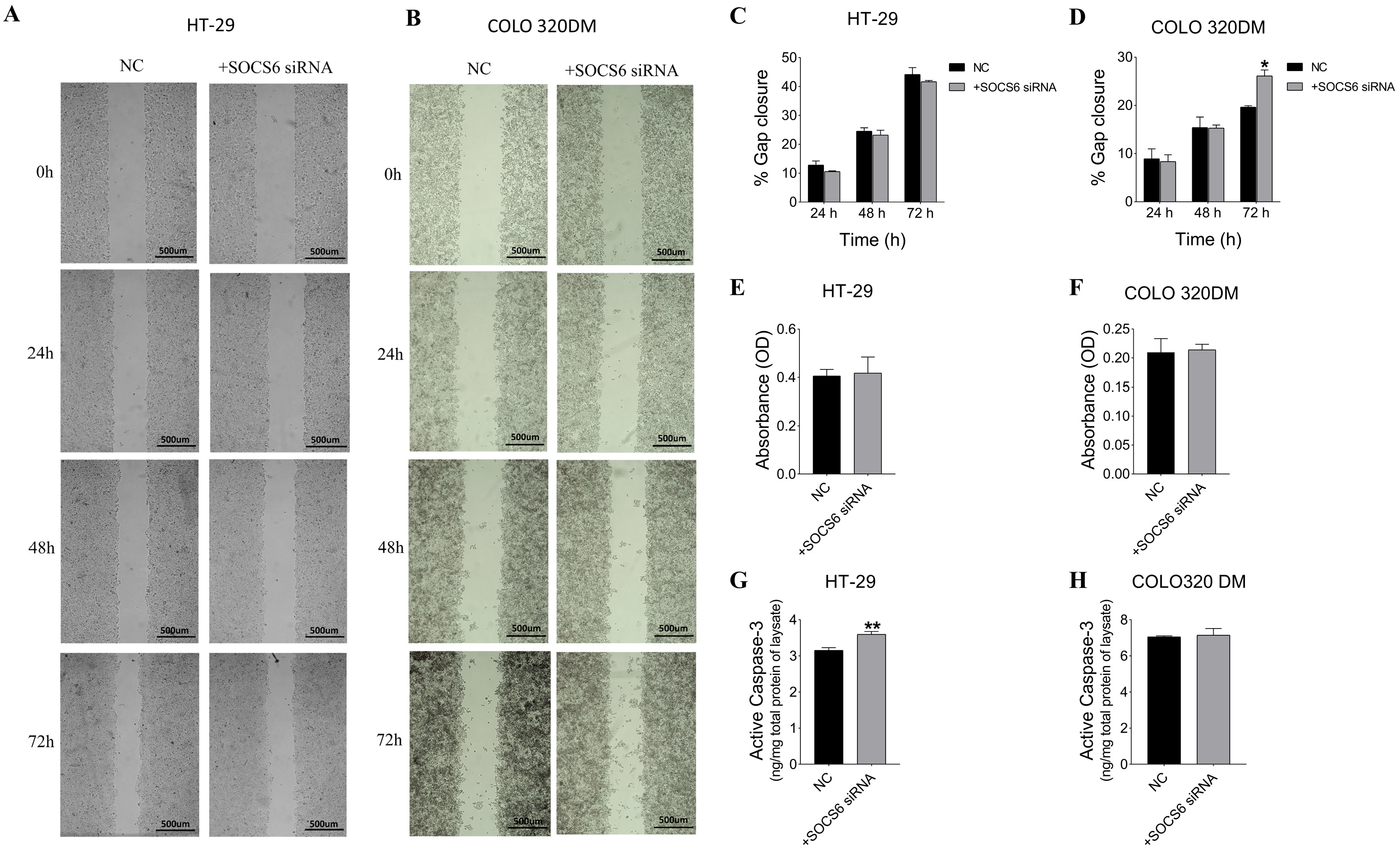
3.2.4. SOCS6 Silencing Enhanced Cell Migration of COLO 320DM Cells and Upregulated Caspase-3 Levels in HT-29 Cells, with Non-Significant Invasive Effects in Both Cells
3.3. Effect of EPOR Knockdown on Cells Viability
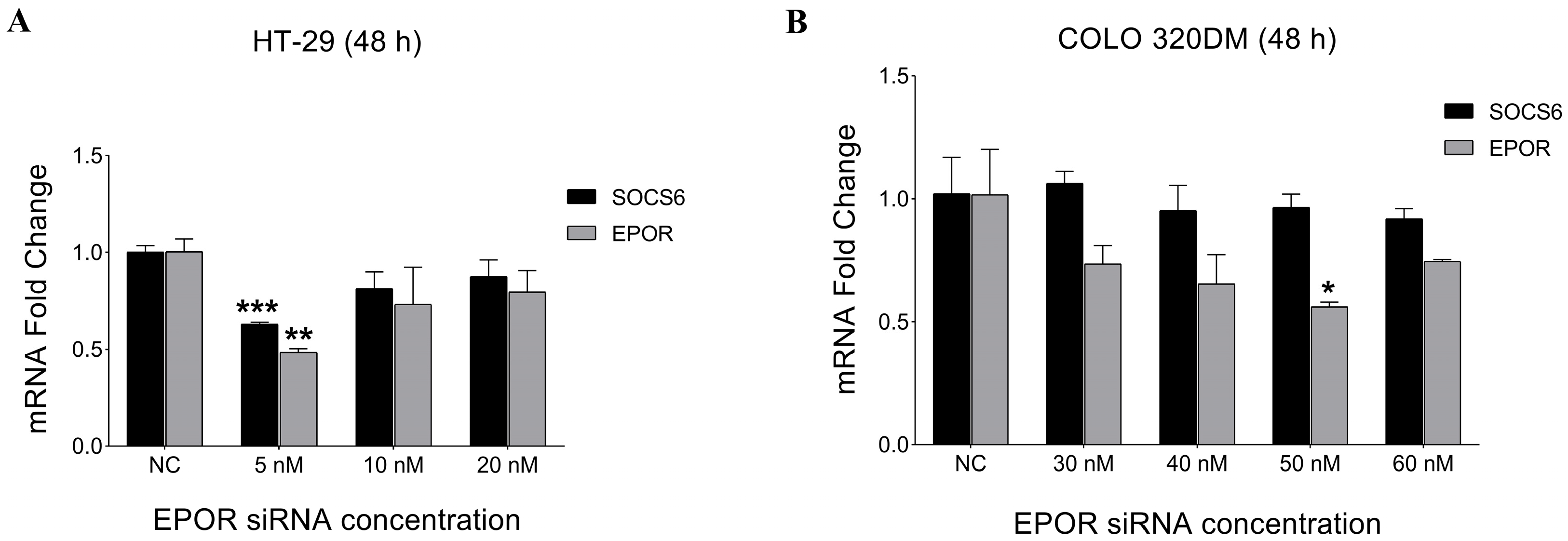
3.3.1. Expression Analysis of EPOR and SOCS6 After EPOR Silencing
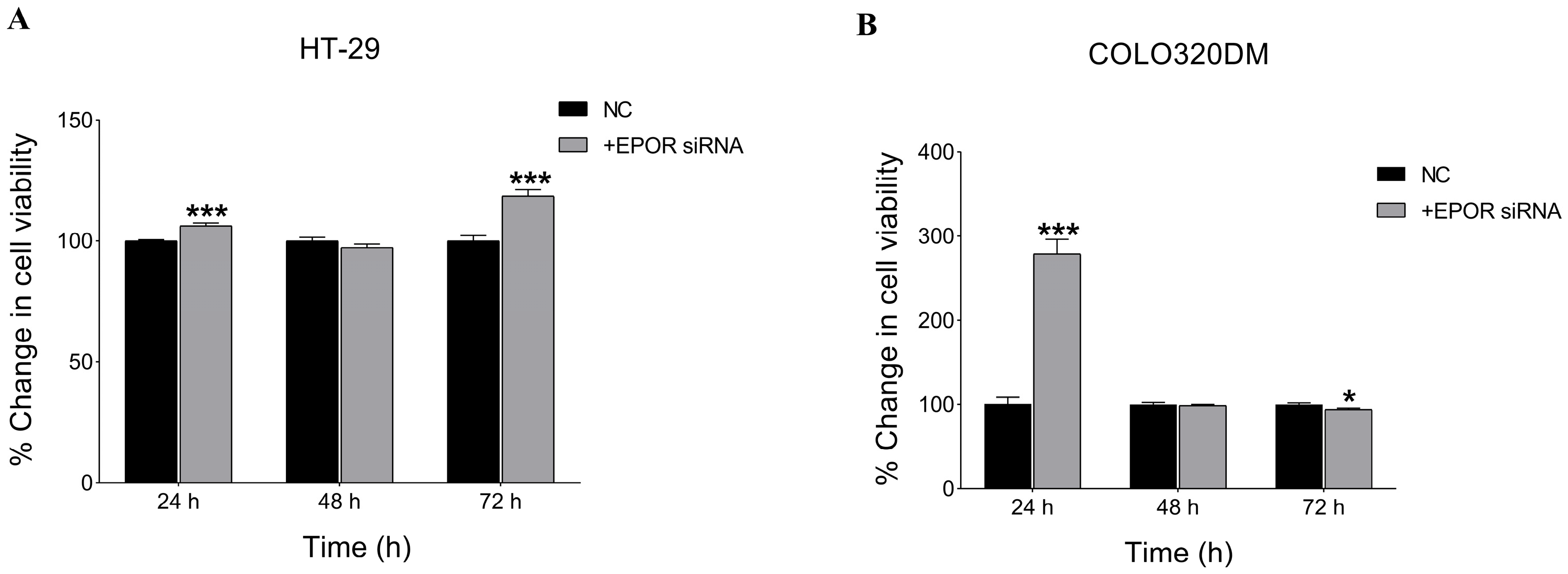
3.3.2. EPOR Silencing Enhanced Cell Viability in HT-29 and COLO 320DM Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Tissue Culture Collection |
| CEA | Carcinoembryonic antigen |
| CRC | Colorectal cancer |
| EndoG | Endonuclease G |
| EPOR | Erythropoietin receptor |
| ESAs | Erythropoiesis-stimulating agents |
| MSI | Microsatellite instability |
| NC | Negative Control |
| NRU | Neutral Red Uptake |
| SOCS | Suppressor of cytokine signalling |
| SOCS6 | Suppressor of cytokine signalling 6 |
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| WHO | World Health Organization |
References
- Kile, B.T.; Schulman, B.A.; Alexander, W.S.; Nicola, N.A.; Martin, H.M.E.; Hilton, D.J. The SOCS Box: A Tale of Destruction and Degradation. Trends Biochem. Sci. 2002, 27, 235–241. [Google Scholar] [CrossRef]
- Kazi, J.U.; Kabir, N.N.; Flores-Morales, A.; Rönnstrand, L. SOCS Proteins in Regulation of Receptor Tyrosine Kinase Signalling. Cell. Mol. Life Sci. 2014, 71, 3297–3310. [Google Scholar] [CrossRef]
- Kabir, N.N.; Sun, J.; Rönnstrand, L.; Kazi, J.U. SOCS6 Is a Selective Suppressor of Receptor Tyrosine Kinase Signalling. Tumour Biol. 2014, 35, 10581–10589. [Google Scholar] [CrossRef]
- Baker, B.J.; Akhtar, L.N.; Benveniste, E.N. SOCS1 and SOCS3 in the Control of CNS Immunity. Trends Immunol. 2009, 30, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Martens, N.; Wery, M.; Wang, P.; Braet, F.; Gertler, A.; Hooghe, R.; Vandenhaute, J.; Hooghe-Peters, E.L. The Suppressor of Cytokine Signalling (SOCS)-7 Interacts with the Actin Cytoskeleton through Vinexin. Exp. Cell Res. 2004, 298, 239–248. [Google Scholar] [CrossRef]
- Kazi, J.U.; Rönnstrand, L. SOCS2 Associates with FLT3 and Negatively Regulates Downstream Signalling. Mol. Oncol. 2013, 7, 693–703. [Google Scholar] [CrossRef]
- Lin, H.Y.; Lai, R.H.; Lin, S.T.; Lin, R.C.; Wang, M.J.; Lin, C.C.; Lee, H.C.; Wang, F.F.; Chen, J.Y. Suppressor of Cytokine Signalling 6 (SOCS6) Promotes Mitochondrial Fission via Regulating DRP1 Translocation. Cell Death Differ. 2013, 20, 139–153. [Google Scholar] [CrossRef]
- Cheng, L.; Kong, B.; Zhao, Y.; Jiang, J. MiR-494 Inhibits Cervical Cancer Cell Proliferation through Upregulation of SOCS6 Expression. Oncol. Lett. 2018, 15, 3075–3080. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Zhang, D.L.; Fu, C.; Wei, B.Z.; Li, G.J. MiR-183 Modulates Multi-Drug Resistance in Hepatocellular Cancer (HCC) Cells via MiR-183-IDH2/SOCS6-HIF-1α Feedback Loop. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2020–2027. [Google Scholar] [PubMed]
- Yuan, D.; Wang, W.; Su, J.; Zhang, Y.; Luan, B.; Rao, H.; Cheng, T.; Zhang, W.; Xiao, S.; Zhang, M.; et al. SOCS6 Functions as a Tumour Suppressor by Inducing Apoptosis and Inhibiting Angiogenesis in Human Prostate Cancer. Curr. Cancer Drug Targets 2018, 18, 894–904. [Google Scholar] [CrossRef]
- Qing, O.Y.; He, X.; Yang, A.; Li, B.; Xu, M. Interference with NTSR1 Expression Exerts an Anti-Invasion Effect via the Jun/MiR-494/SOCS6 Axis of Glioblastoma Cells. Cell. Physiol. Biochem. 2018, 49, 2382–2395. [Google Scholar] [CrossRef]
- Sun, X.; Sun, Y.; Li, J.; Zhao, X.; Shi, X.; Gong, T.; Pan, S.; Zheng, Z.; Zhang, X. SOCS6 Promotes Radiosensitivity and Decreases Cancer Cell Stemness in Esophageal Squamous Cell Carcinoma by Regulating C-Kit Ubiquitylation. Cancer Cell Int. 2021, 21, 165. [Google Scholar] [CrossRef]
- Xia, Y.; Wei, K.; Yang, F.M.; Hu, L.Q.; Pan, C.F.; Pan, X.L.; Wu, W.B.; Wang, J.; Wen, W.; He, Z.C.; et al. Correction: MiR-1260b, Mediated by YY1, Activates KIT Signalling by Targeting SOCS6 to Regulate Cell Proliferation and Apoptosis in NSCLC. Cell Death Dis. 2020, 11, 261, Erratum in Cell Death Dis. 2019, 10, 112. https://doi.org/10.1038/S41419-019-1390-Y. [Google Scholar] [CrossRef]
- Lai, R.H.; Hsiao, Y.W.; Wang, M.J.; Lin, H.Y.; Wu, C.W.; Chi, C.W.; Li, A.F.Y.; Jou, Y.S.; Chen, J.Y. SOCS6, down-Regulated in Gastric Cancer, Inhibits Cell Proliferation and Colony Formation. Cancer Lett. 2010, 288, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; An, T.; Wang, Y.; Lu, X.; Zhang, Y.; Wang, X.; Zhang, X.; Zhang, C. Down-Regulation of SOCS6: An Unfavorable Prognostic Factor for Gastrointestinal Stromal Tumour Proven by Survival Analysis. Diagn. Pathol. 2021, 16, 113. [Google Scholar] [CrossRef]
- Letellier, E.; Schmitz, M.; Baig, K.; Beaume, N.; Schwartz, C.; Frasquilho, S.; Antunes, L.; Marcon, N.; Nazarov, P.V.; Vallar, L.; et al. Identification of SOCS2 and SOCS6 as Biomarkers in Human Colorectal Cancer. Br. J. Cancer 2014, 111, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Luo, G.; Yang, Z.; Zhu, F.; An, Y.; Shi, Y.; Fan, D. MiR-17-5p Promotes Proliferation by Targeting SOCS6 in Gastric Cancer Cells. FEBS Lett. 2014, 588, 2055–2062. [Google Scholar] [CrossRef]
- Zhu, J.G.; Dai, Q.S.; Han, Z.D.; He, H.C.; Mo, R.J.; Chen, G.; Chen, Y.F.; Wu, Y.D.; Yang, S.B.; Jiang, F.N.; et al. Expression of SOCSs in Human Prostate Cancer and Their Association in Prognosis. Mol. Cell. Biochem. 2013, 381, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.-H.; Wang, M.-J.; Yang, S.-H.; Chen, J.-Y. Genomic Organization and Functional Characterization of the Promoter for the Human Suppressor of Cytokine Signalling 6 Gene. Gene 2009, 448, 64–73. [Google Scholar] [CrossRef]
- Yoon, S.; Yi, Y.S.; Kim, S.S.; Kim, J.H.; Park, W.S.; Nam, S.W. SOCS5 and SOCS6 Have Similar Expression Patterns in Normal and Cancer Tissues. Tumour Biol. 2012, 33, 215–221. [Google Scholar] [CrossRef]
- Sriram, K.B.; Larsen, J.E.; Francis, S.M.; Wright, C.M.; Clarke, B.E.; Duhig, E.E.; Brown, K.M.; Hayward, N.K.; Yang, I.A.; Bowman, R.V.; et al. Array-Comparative Genomic Hybridization Reveals Loss of SOCS6 Is Associated with Poor Prognosis in Primary Lung Squamous Cell Carcinoma. PLoS ONE 2012, 7, e30398. [Google Scholar] [CrossRef]
- Qiu, X.; Zheng, J.; Guo, X.; Gao, X.; Liu, H.; Tu, Y.; Zhang, Y. Reduced Expression of SOCS2 and SOCS6 in Hepatocellular Carcinoma Correlates with Aggressive Tumour Progression and Poor Prognosis. Mol. Cell. Biochem. 2013, 378, 99–106. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, Y.; Gao, J.; Zhu, G.; Gao, K.; Zhang, W.; Shi, F.; Zhang, Q. Expression of SHP-1 and SOCS6 in Patients with Acute Leukemia and Their Clinical Implication. Onco Targets Ther. 2017, 10, 1915–1920. [Google Scholar] [CrossRef]
- Zhang, P.; Guan, P.; Ye, X.; Lu, Y.; Hang, Y.; Su, Y.; Hu, W. SOCS6 Promotes Mitochondrial Fission and Cardiomyocyte Apoptosis and Is Negatively Regulated by Quaking-Mediated MiR-19b. Oxidative Med. Cell. Longev. 2022, 2022, 1121323. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Q.; Hong, H.; Zhang, H.; Zhang, T. MiR-19 Promotes Osteosarcoma Progression by Targeting SOCS6. Biochem. Biophys. Res. Commun. 2018, 495, 1363–1369. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Wu, X.; Long, F.; Yue, W.; Wu, D.; Xie, Y. Circular RNA _0015278 Inhibits the Progression of Non-Small Cell Lung Cancer through Regulating the MicroRNA 1278/SOCS6 Gene Axis. Ann. Transl. Med. 2021, 9, 1255. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Liu, M.; Wang, Y.; Yang, Y.; Li, Z.; Shi, R.; Miao, Y. MicroRNA-494-3p Exacerbates Renal Epithelial Cell Dysfunction by Targeting SOCS6 under High Glucose Treatment. Kidney Blood Press. Res. 2022, 47, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Liu, Y.; Wang, Y.; Meng, M.; Wang, K.; Zang, X.; Zhao, S.; Sun, X.; Cui, L.; Pan, L.; et al. MiR-21 and MiR-155 Promote Non-Small Cell Lung Cancer Progression by Downregulating SOCS1, SOCS6, and PTEN. Oncotarget 2016, 7, 84508–84519. [Google Scholar] [CrossRef]
- Shen, R.; Wang, Y.; Wang, C.X.; Yin, M.; Liu, H.L.; Chen, J.P.; Han, J.Q.; Wang, W.B. MiRNA-155 Mediates TAM Resistance by Modulating SOCS6-STAT3 Signalling Pathway in Breast Cancer. Am. J. Transl. Res. 2015, 7, 2115–2126. [Google Scholar]
- Ma, J.; Xu, L.Y.; Sun, Q.H.; Wan, X.Y.; BingLi. Inhibition of MiR-1298-5p Attenuates Sepsis Lung Injury by Targeting SOCS6. Mol. Cell. Biochem. 2021, 476, 3745–3756. [Google Scholar] [CrossRef]
- Li, Z.; Fan, H.; Chen, W.; Xiao, J.; Ma, X.; Ni, P.; Xu, Z.; Yang, L. MicroRNA-653-5p Promotes Gastric Cancer Proliferation and Metastasis by Targeting the SOCS6-STAT3 Pathway. Front. Mol. Biosci. 2021, 8, 655580. [Google Scholar] [CrossRef]
- Zhang, J.; Pu, X.-M.; Xiong, Y. Kshv-Mir-K12-1-5p Promotes Cell Growth and Metastasis by Targeting SOCS6 in Kaposi’s Sarcoma Cells. Cancer Manag. Res. 2019, 11, 4985–4995. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Shi, M.; Hu, J.; Zhao, L.N. Inhibition of SOCS6 Confers Radioresistance in Esophageal Squamous Cell Carcinoma. Biochem. Biophys. Res. Commun. 2021, 550, 92–98. [Google Scholar] [CrossRef]
- Zadjali, F.; Pike, A.C.W.; Vesterlund, M.; Sun, J.; Wu, C.; Li, S.S.C.; Rönnstrand, L.; Knapp, S.; Bullock, A.N.; Flores-Morales, A. Structural Basis for C-KIT Inhibition by the Suppressor of Cytokine Signalling 6 (SOCS6) Ubiquitin Ligase. J. Biol. Chem. 2011, 286, 480–490. [Google Scholar] [CrossRef] [PubMed]
- Kazi, J.U.; Sun, J.; Phung, B.; Zadjali, F.; Flores-Morales, A.; Rönnstrand, L. Suppressor of Cytokine Signalling 6 (SOCS6) Negatively Regulates Flt3 Signal Transduction through Direct Binding to Phosphorylated Tyrosines 591 and 919 of Flt3. J. Biol. Chem. 2012, 287, 36509–36517. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kang, C.B. Erythropoietin Receptor Structural Domains. Vitam. Horm. 2017, 105, 1–17. [Google Scholar] [CrossRef]
- Anagnostou, A.; Liu, Z.; Steiner, M.; Chin, K.; Lee, E.S.; Kessimian, N.; Noguchi, C.T. Erythropoietin Receptor MRNA Expression in Human Endothelial Cells. Proc. Natl. Acad. Sci. USA 1994, 91, 3974–3978. [Google Scholar] [CrossRef]
- Farrell, F.; Lee, A. The Erythropoietin Receptor and Its Expression in Tumour Cells and Other Tissues. Oncologist 2004, 9, 18–30. [Google Scholar] [CrossRef]
- Ostrowski, D.; Heinrich, R. Clinical Medicine Alternative Erythropoietin Receptors in the Nervous System. J. Clin. Med. 2018, 7, 24. [Google Scholar] [CrossRef]
- Bretz, C.A.; Ramshekar, A.; Kunz, E.; Wang, H.; Hartnett, M.E. Signalling Through the Erythropoietin Receptor Affects Angiogenesis in Retinovascular Disease. Investig. Ophthalmol. Vis. Sci. 2020, 61, 23. [Google Scholar] [CrossRef]
- Katavetin, P.; Tungsanga, K.; Eiam-Ong, S.; Nangaku, M. Antioxidative Effects of Erythropoietin. Kidney Int. 2007, 72, S10–S15. [Google Scholar] [CrossRef]
- Bond, W.S.; Rex, T.S. Evidence That Erythropoietin Modulates Neuroinflammation through Differential Action on Neurons, Astrocytes, and Microglia. Front. Immunol. 2014, 5, 112627. [Google Scholar] [CrossRef]
- Vittori, D.C.; Chamorro, M.E.; Hernández, Y.V.; Maltaneri, R.E.; Nesse, A.B. Erythropoietin and Derivatives: Potential Beneficial Effects on the Brain. J. Neurochem. 2021, 158, 1032–1057. [Google Scholar] [CrossRef]
- Iwata, Y.; Sakai, N.; Nakajima, Y.; Oshima, M.; Nakagawa-Yoneda, S.; Ogura, H.; Sato, K.; Minami, T.; Kitajima, S.; Toyama, T.; et al. Anti-Fibrotic Potential of Erythropoietin Signalling on Bone Marrow Derived Fibrotic Cell. BMC Nephrol. 2021, 22, 203. [Google Scholar] [CrossRef]
- Acs, G.; Zhang, P.J.; Rebbeck, T.R.; Acs, P.; Verma, A. Immunohistochemical Expression of Erythropoietin and Erythropoietin Receptor in Breast Carcinoma. Cancer 2002, 95, 969–981. [Google Scholar] [CrossRef]
- Hardee, M.E.; Arcasoy, M.O.; Blackwell, K.L.; Kirkpatrick, J.P.; Dewhirst, M.W. Erythropoietin Biology in Cancer. Clin. Cancer Res. 2006, 12, 332–339. [Google Scholar] [CrossRef]
- Elliott, S.; Busse, L.; Bass, M.B.; Lu, H.; Sarosi, I.; Sinclair, A.M.; Spahr, C.; Um, M.; Van, G.; Glenn Begley, C. Anti-Epo Receptor Antibodies Do Not Predict Epo Receptor Expression. Blood 2006, 107, 1892–1895. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Sawasaki, Y.; Takeuchi, K.; Kato, S.; Imai, N.; Kato, Y.; Shibata, N.; Kobayashi, M.; Moriguchi, Y.; Higuchi, M.; et al. Erythropoietin-Induced Proliferation of Gastric Mucosal Cells. World J. Gastroenterol. 2006, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Szenajch, J.; Wcislo, G.; Jeong, J.Y.; Szczylik, C.; Feldman, L. The Role of Erythropoietin and Its Receptor in Growth, Survival and Therapeutic Response of Human Tumour Cells from Clinic to Bench—A Critical Review. Biochim. Biophys. Acta-Rev. Cancer 2010, 1806, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Henke, M.; Laszig, R.; Rübe, C.; Schäfer, U.; Haase, K.D.; Schilcher, B.; Mose, S.; Beer, K.T.; Burger, U.; Dougherty, C.; et al. Erythropoietin to Treat Head and Neck Cancer Patients with Anaemia Undergoing Radiotherapy: Randomised, Double-Blind, Placebo-Controlled Trial. Lancet 2003, 362, 1255–1260. [Google Scholar] [CrossRef]
- Colorectal Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer?utm_source=chatgpt.com (accessed on 14 December 2025).
- Zhao, J.; Ji, H.; Li, K.; Yu, G.; Zhou, S.; Xiao, Q.; Dunlop, M.; Theodoratou, E.; Li, X.; Ding, K. Decoding the Genetic and Environmental Forces in Propelling the Surge of Early-Onset Colorectal Cancer. Chin. Med. J. (Engl.) 2025, 138, 1163. [Google Scholar] [CrossRef] [PubMed]
- Binefa, G.; Rodríguez-Moranta, F.; Teule, À.; Medina-Hayas, M. Colorectal Cancer: From Prevention to Personalized Medicine. World J. Gastroenterol. 2014, 20, 6786–6808. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumour DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Phipps, A.I.; Buchanan, D.D.; Makar, K.W.; Win, A.K.; Baron, J.A.; Lindor, N.M.; Potter, J.D.; Newcomb, P.A. KRAS-Mutation Status in Relation to Colorectal Cancer Survival: The Joint Impact of Correlated Tumour Markers. Br. J. Cancer 2013, 108, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Fearon, E.R.; Hamilton, S.R.; Kern, S.E.; Preisinger, A.C.; Leppert, M.; Smits, A.M.; Bos, J.L. Genetic Alterations during Colorectal-Tumour Development. N. Engl. J. Med. 1988, 319, 525–532. [Google Scholar] [CrossRef]
- Storojeva, I.; Boulay, J.L.; Ballabeni, P.; Buess, M.; Terracciano, L.; Laffer, U.; Mild, G.; Herrmann, R.; Rochlitz, C. Prognostic and Predictive Relevance of DNAM-1, SOCS6 and CADH-7 Genes on Chromosome 18q in Colorectal Cancer. Oncology 2005, 68, 246–255. [Google Scholar] [CrossRef]
- Patterson, S.D.; Rossi, J.M.; Paweletz, K.L.; Dan Fitzpatrick, V.; Begley, C.G.; Busse, L.; Elliott, S.; McCaffery, I. Functional EpoR Pathway Utilization Is Not Detected in Primary Tumour Cells Isolated from Human Breast, Non-Small Cell Lung, Colorectal, and Ovarian Tumour Tissues. PLoS ONE 2015, 10, e0122149. [Google Scholar] [CrossRef] [PubMed]
- Sobah, M.L.; Liongue, C.; Ward, A.C. SOCS Proteins in Immunity, Inflammatory Diseases, and Immune-Related Cancer. Front. Med. 2021, 8, 727987. [Google Scholar] [CrossRef]
- Trengove, M.C.; Ward, A.C. Proteins in Development and Diseases. Am. J. Clin. Exp. Immunol. 2013, 272, 264–297. [Google Scholar] [CrossRef]
- Narci, K.; Kahraman, D.C.; Koyas, A.; Ersahin, T.; Tuncbag, N.; Atalay, R.C. Context Dependent Isoform Specific PI3K Inhibition Confers Drug Resistance in Hepatocellular Carcinoma Cells. BMC Cancer 2022, 22, 320. [Google Scholar] [CrossRef]
- Sun, L.; Ke, M.; Yin, M.; Zeng, Y.; Ji, Y.; Hu, Y.; Fu, S.; Zhang, C. Extracellular Vesicle-Encapsulated MicroRNA-296-3p from Cancer-Associated Fibroblasts Promotes Ovarian Cancer Development through Regulation of the PTEN/AKT and SOCS6/STAT3 Pathways. Cancer Sci. 2024, 115, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huang, S.; Zhao, L. Suppressor of Cytokine Signalling 6 in Cancer Development and Therapy: Deciphering Its Emerging and Suppressive Roles. Cytokine Growth Factor Rev. 2022, 64, 21–32. [Google Scholar] [CrossRef]
- Dai, L.; Li, Z.; Liang, W.; Hu, W.; Zhou, S.; Yang, Z.; Tao, Y.; Hou, X.; Xing, Z.; Mao, J.; et al. SOCS Proteins and Their Roles in the Development of Glioblastoma. Oncol. Lett. 2022, 23, 5. [Google Scholar] [CrossRef]
- Croker, B.A.; Kiu, H.; Nicholson, S.E. SOCS Regulation of the JAK/STAT Signalling Pathway. Semin. Cell Dev. Biol. 2008, 19, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Li, J.; Zhou, C.; Lv, C.; Tian, M. MIR-142-3p Suppresses SOCS6 Expression and Promotes Cell Proliferation in Nasopharyngeal Carcinoma. Cell. Physiol. Biochem. 2015, 36, 1743–1752. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wu, S.; Song, Z.H.; Yang, Y.; Li, Y.L.; Li, J. Unveiling the Pathological Functions of SOCS in Colorectal Cancer: Current Concepts and Future Perspectives. Pathol. Res. Pract. 2024, 262, 155564. [Google Scholar] [CrossRef]
- van de Geijn, G.-J.M.; Gits, J.; Touw, I.P. Distinct Activities of Suppressor of Cytokine Signalling (SOCS) Proteins and Involvement of the SOCS Box in Controlling G-CSF Signalling. J. Leukoc. Biol. 2004, 76, 237–244. [Google Scholar] [CrossRef]
- Keewan, E.; Matlawska-Wasowska, K. The Emerging Role of Suppressors of Cytokine Signalling (Socs) in the Development and Progression of Leukemia. Cancers 2021, 13, 4000. [Google Scholar] [CrossRef]
- Maharati, A.; Moghbeli, M. PI3K/AKT Signalling Pathway as a Critical Regulator of Epithelial-Mesenchymal Transition in Colorectal Tumour Cells. Cell Commun. Signal. 2023, 21, 201. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Ding, S.; Zhang, X.; Di, W.; Wang, X.; Zhang, H.; Chen, Y.; Zhang, Y.; Hu, Y. To Investigate the Occurrence and Development of Colorectal Cancer Based on the PI3K/AKT/MTOR Signalling Pathway. Front. Biosci. (Landmark Ed.) 2023, 28, 37. [Google Scholar] [CrossRef]
- Stefani, C.; Miricescu, D.; Stanescu-Spinu, I.I.; Nica, R.I.; Greabu, M.; Totan, A.R.; Jinga, M. Growth Factors, PI3K/AKT/MTOR and MAPK Signalling Pathways in Colorectal Cancer Pathogenesis: Where Are We Now? Int. J. Mol. Sci. 2021, 22, 10260. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.Q.; Luo, Q.; Wang, L.; Song, G.B.; Sheng, J.P.; Xu, B. Signalling Pathways Involved in Colorectal Cancer: Pathogenesis and Targeted Therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Sun, B.; Xiang, J.; Chen, Z. MiR-301a Promotes Colorectal Cancer Cell Growth and Invasion by Directly Targeting SOCS6. Cell. Physiol. Biochem. 2015, 35, 227–236. [Google Scholar] [CrossRef]
- Enane, F.O.; Saunthararajah, Y.; Korc, M. Differentiation Therapy and the Mechanisms That Terminate Cancer Cell Proliferation without Harming Normal Cells. Cell Death Dis. 2018, 9, 912. [Google Scholar] [CrossRef]
- Zhu, C.; Fan, F.; Li, C.Y.; Xiong, Y.; Liu, X. Caspase-3 Promotes Oncogene-Induced Malignant Transformation via EndoG-Dependent Src-STAT3 Phosphorylation. Cell Death Dis. 2024, 15, 486. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; Li, Z.; Huang, Q.; Li, F.; Li, C.Y. Caspase-3 Regulates the Migration, Invasion and Metastasis of Colon Cancer Cells. Int. J. Cancer 2018, 143, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Cell Line-EPOR-The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000187266-EPOR/cell+line (accessed on 21 October 2025).
- Kershaw, N.J.; Murphy, J.M.; Lucet, I.S.; Nicola, N.A.; Babon, J.J. Regulation of Janus Kinases by SOCS Proteins. Biochem. Soc. Trans. 2013, 41, 1042–1047. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, T.; Buzdin, A.; Khabusheva, E.; Spirin, P.; Suntsova, M.; Sorokin, M.; Popenko, V.; Rubtsov, P.; Prassolov, V. Subtype of Neuroblastoma Cells with High KIT Expression Are Dependent on KIT and Its Knockdown Induces Compensatory Activation of Pro-Survival Signalling. Int. J. Mol. Sci. 2022, 23, 7724. [Google Scholar] [CrossRef]
- Alemán, L.M.; Doench, J.; Sharp, P.A. Comparison of SiRNA-Induced off-Target RNA and Protein Effects. RNA 2007, 13, 385. [Google Scholar] [CrossRef]



| Primer | Sequence |
|---|---|
| SOCS6 primers (forward) | 5′ ATCACGGAGCTATTGTCTGGA |
| SOCS6 primers (reverse) | 5′ CTGACTCTCATCCTCGGGGA |
| GAPDH primer (forward) | 5′ GGATTTGGTCGTATTGGG |
| GAPDH primer (reverse) | 5′ GGAAGATGGTGATGGGATT |
| EPOR primer (forward) | 5′ CAAGTTCGAGAGCAAAGCGG |
| EPOR primer (reverse) | 5′ TTCCTCCCAGAAACACAAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Al-Bahri, A.; Zadjali, F.; Hanif, S.; Alharthi, Z.; Sakr, H.; Al-Kharusi, A. Functional Characterization of Suppressor of Cytokine Signalling 6 and Its Interaction with Erythropoietin Receptor in Colorectal Cancer Cells. Cancers 2026, 18, 171. https://doi.org/10.3390/cancers18010171
Al-Bahri A, Zadjali F, Hanif S, Alharthi Z, Sakr H, Al-Kharusi A. Functional Characterization of Suppressor of Cytokine Signalling 6 and Its Interaction with Erythropoietin Receptor in Colorectal Cancer Cells. Cancers. 2026; 18(1):171. https://doi.org/10.3390/cancers18010171
Chicago/Turabian StyleAl-Bahri, Asma, Fahad Zadjali, Shika Hanif, Zaina Alharthi, Hussein Sakr, and Amira Al-Kharusi. 2026. "Functional Characterization of Suppressor of Cytokine Signalling 6 and Its Interaction with Erythropoietin Receptor in Colorectal Cancer Cells" Cancers 18, no. 1: 171. https://doi.org/10.3390/cancers18010171
APA StyleAl-Bahri, A., Zadjali, F., Hanif, S., Alharthi, Z., Sakr, H., & Al-Kharusi, A. (2026). Functional Characterization of Suppressor of Cytokine Signalling 6 and Its Interaction with Erythropoietin Receptor in Colorectal Cancer Cells. Cancers, 18(1), 171. https://doi.org/10.3390/cancers18010171








