Correction: Dhillon et al. Molecular Insights and Therapeutic Advances in Low-Risk Myelodysplastic Neoplasms: A Clinical Review. Cancers 2025, 17, 3610
Error in Figure 1 Legend
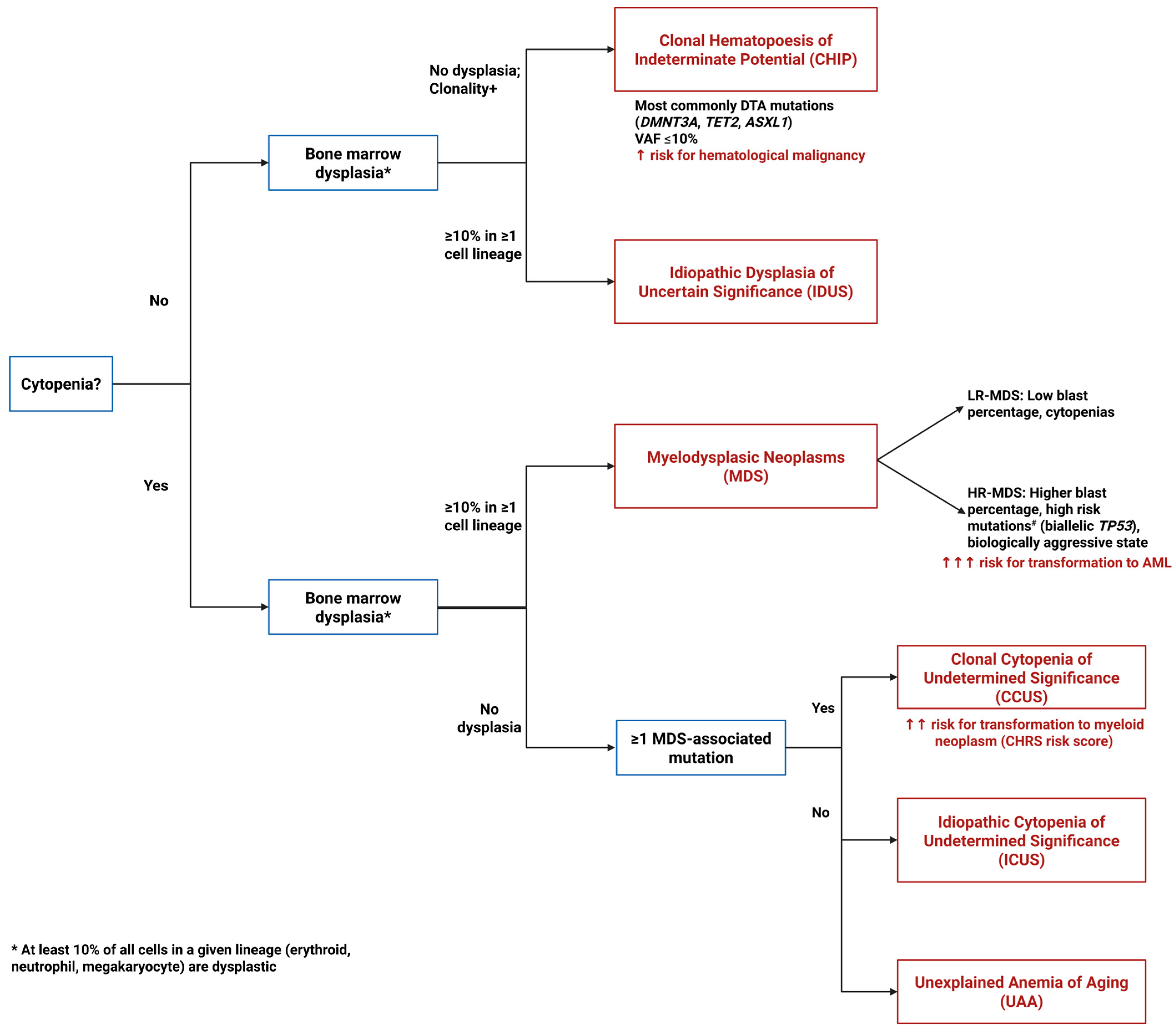
- Figure 1. Diagnostic algorithm for pre-MDS conditions. This flowchart illustrates the diagnostic pathway for distinguishing between clonal and non-clonal cytopenias and dysplasia that can precede or may progress to MDS. The algorithm differentiates four key entities based on the presence or absence of cytopenias, dysplastic features, and clonal mutations: ICUS (Idiopathic Cytopenias of Uncertain Significance)—cytopenia without clonal mutations or dysplasia; CCUS (Clonal Cytopenias of Uncertain Significance)—cytopenia with clonal mutations but without dysplasia; CHIP (Clonal Hematopoiesis of Indeterminate Potential)—clonal mutations without cytopenia or dysplasia; and IDUS (Idiopathic Dysplasia of Unknown Significance)—morphologic dysplasia without cytopenia or clonal mutations. # High risk mutations include ASXL1, CBL, DNMT3A, ETV6, EZH2, IDH2, KRAS, NPM1, NRAS, RUNX1, SF3B1, SRSF2, and U2AF1.
Error in Figure 1
Error in Figure 3 Legend
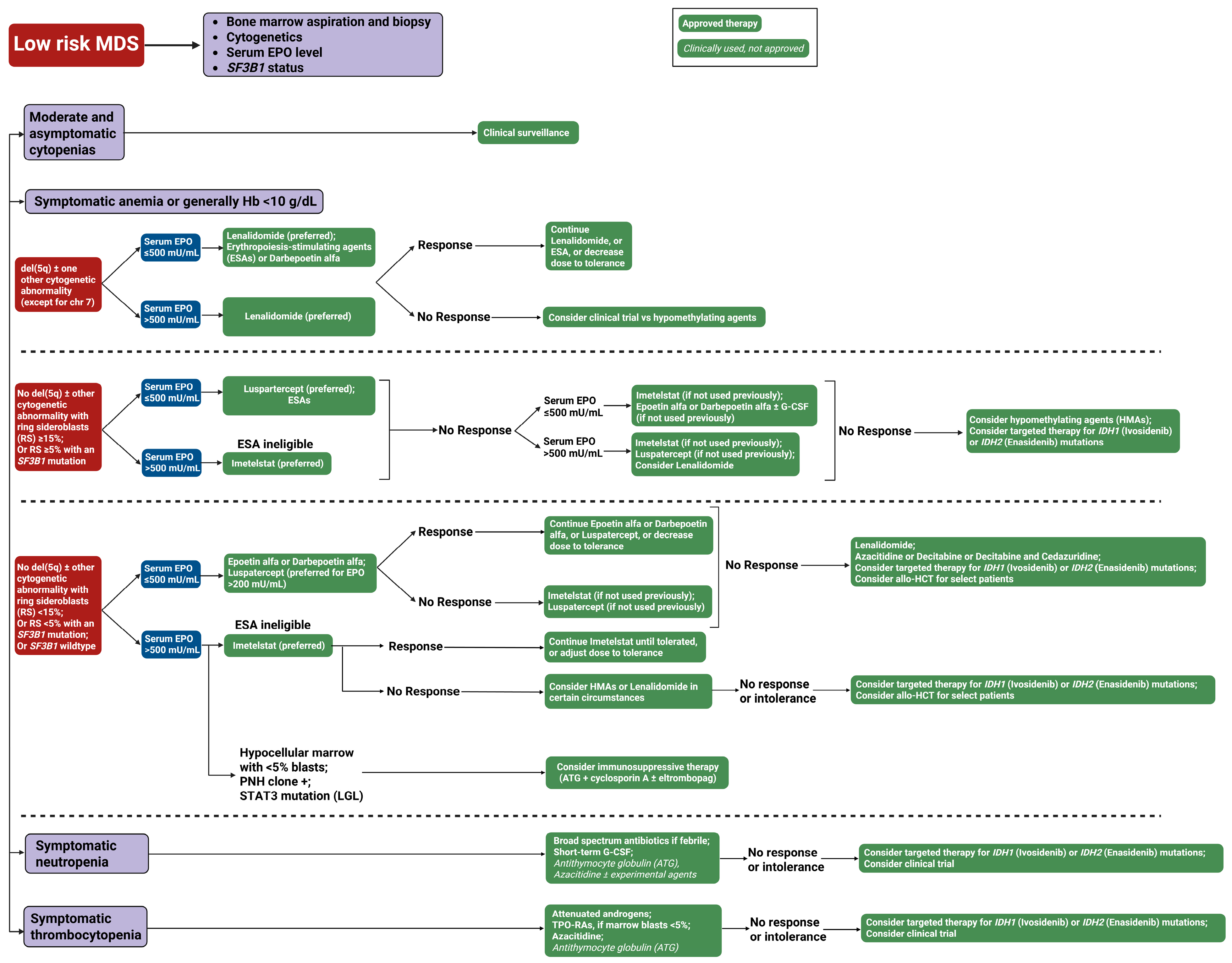
- Figure 3. Our treatment approach to low-risk myelodysplastic syndrome (LR-MDS). This flowchart outlines the therapeutic approach to low-risk MDS based on clinical presentation and laboratory parameters. The algorithm stratifies patients into four categories: Moderate and asymptomatic cytopenias (observation only), symptomatic anemia, symptomatic neutropenia, and symptomatic thrombocytopenia. Green boxes with solid backgrounds indicate FDA-approved therapies; green boxes with italicized text denote clinically used but not formally approved treatments. Blue boxes represent clinical decision points. Abbreviations: ATG, antithymocyte globulin; del(5q), deletion of chromosome 5q; chr, chromosome; EPO, erythropoietin; G-CSF, granulocyte colony-stimulating factor; Hb, hemoglobin; MDS, myelodysplastic syndrome; RBC, red blood cell; RS, ring sideroblasts; SF3B1, splicing factor 3B subunit 1 gene; TPO-RAs, thrombopoietin receptor agonists; and U/L, units per liter.
Error in Figure 3
Error in Table 1
Text Correction
Reference
- Dhillon, V.; Maciejewski, J.; Balasubramanian, S.K. Molecular Insights and Therapeutic Advances in Low-Risk Myelodysplastic Neoplasms: A Clinical Review. Cancers 2025, 17, 3610. [Google Scholar] [CrossRef]
| Agent | Class/Target | Included Patients | N | Efficacy Outcomes | Trial (Phase) |
|---|---|---|---|---|---|
| Erythropoietin alpha, Darbepoetin alpha | ESA | LR-MDS patients with anemia, low transfusion burden 1 | 130 | ER: 45.9% vs. 4.4% (placebo) | EPOANE3021 (Phase III) 2 |
| Lenalidomide | IMiDs | TD LR-MDS with del (5Q) | 205 | RBC-TI: 42.6 to 56.1% vs. 5.9% (placebo) | MDS-004 (Phase III) 3 |
| Deferasirox | ICT | TD LR-MDS with iron overload | 225 | EFS on ICT: 3.9 years vs. 3 (placebo) | TELESTO (Phase II) 4 |
| Etrombopag, Romiplostim | TPO | LR-MDS with severe thrombocytopenia | 169 | PLT-R: 47% vs. 11% (placebo) | EQOL-MDS (Phase II) 5 |
| Luspatercept | Erythroid maturation agents | TD LR-MDS, ESA-refractory 6 | 354; 229 | RBC-TI: 58.5% vs. 31.2% (placebo); 38% vs. 13% (placebo) | MEDALIST (Phase III) 6, COMMANDS (Phase III) 7 |
| Azacitidine, Decitabine, Guadecitabine | HMAs | TD LR-MDS, ESA-unresponsive | 113 | RBC-TI: 41% decitabine vs. 15% azacitadine | NCT01720225 (Phase II) 8 |
| Imetelstat | Telomerase inhibitor | TD LR-MDS, ESA-refractory | 178 | RBC-TI: 39.8% vs. 15% (placebo) | IMerge (Phase III) 9 |
| 1 Inclusion: Hb ≤ 10.0 g/dL, ≤4 RBC units/8 weeks, serum EPO < 500 mU/mL. | |||||
| 2 ESAs have been studied in multiple trials over decades (ECOG E1996 Trial, ARCADE Trial); no single definitive phase III trial established efficacy, though EPOANE3021 (NCT01381809) is one recent example showing ER of 45.9% vs. 4.4% at 24 weeks (N = 130). | |||||
| 3 Inclusion: TD patients with del(5Q). Primary endpoint: RBC-TI ≥ 26 weeks. MDS-003 (phase II, N = 148) was the initial registration trial. NCT00179621. | |||||
| 4 Inclusion: TD patients with serum ferritin > 2247 pmol/L (>1000 ng/mL) and prior receipt of 15–75 pRBCs. Primary endpoint: EFS from randomization to first nonfatal event (cardiac, hepatic, death, or AML transformation). Median EFS 1440 vs. 1091 days (p = 0.015). NCT00940602. | |||||
| 5 Inclusion: Platelet count < 30 × 103/mm3 with high bleeding risk. Primary endpoint: PLT response for ≥25 weeks. NCT02912208. | |||||
| 6 COMMANDS (NCT03682536, N = 354): ESA-naive TD patients with or without ring sideroblasts; <5% blasts, sEPO < 500 U/L. Primary endpoint: RBC-TI ≥ 12 weeks with Hb increase ≥ 1.5 g/dL within the first 24 weeks. Compared luspatercept vs. epoetin alfa (first-line, head-to-head comparison). First drug to demonstrate superiority over ESAs in first-line treatment of LR-MDS. | |||||
| 7 MEDALIST (NCT02631070, N = 229): Registration trial in ESA-refractory or failed patients with ring sideroblasts (≥15% RS or ≥5% with SF3B1 mutation); <5% blasts, sEPO ≤ 500 U/L. Primary endpoint: RBC-TI ≥ 8 weeks during weeks 1–24. Compared luspatercept vs. placebo. Led to initial FDA approval (2020) for ESA-refractory, RS+ disease. | |||||
| 8 Inclusion: TD patients unresponsive to ESAs with refractory anemia and ringed sideroblasts. Primary endpoint: ORR at 8 weeks. | |||||
| 9 Inclusion: TD patients relapsed, refractory, or ineligible for ESAs; non-del(5q); no prior lenalidomide or HMA. Primary endpoint: RBC-TI ≥ 8 weeks. Secondary endpoints included RBC-TI ≥ 24 weeks (28% vs. 3%). NCT02598661. | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Dhillon, V.; Maciejewski, J.; Balasubramanian, S.K. Correction: Dhillon et al. Molecular Insights and Therapeutic Advances in Low-Risk Myelodysplastic Neoplasms: A Clinical Review. Cancers 2025, 17, 3610. Cancers 2026, 18, 172. https://doi.org/10.3390/cancers18010172
Dhillon V, Maciejewski J, Balasubramanian SK. Correction: Dhillon et al. Molecular Insights and Therapeutic Advances in Low-Risk Myelodysplastic Neoplasms: A Clinical Review. Cancers 2025, 17, 3610. Cancers. 2026; 18(1):172. https://doi.org/10.3390/cancers18010172
Chicago/Turabian StyleDhillon, Vikram, Jaroslaw Maciejewski, and Suresh Kumar Balasubramanian. 2026. "Correction: Dhillon et al. Molecular Insights and Therapeutic Advances in Low-Risk Myelodysplastic Neoplasms: A Clinical Review. Cancers 2025, 17, 3610" Cancers 18, no. 1: 172. https://doi.org/10.3390/cancers18010172
APA StyleDhillon, V., Maciejewski, J., & Balasubramanian, S. K. (2026). Correction: Dhillon et al. Molecular Insights and Therapeutic Advances in Low-Risk Myelodysplastic Neoplasms: A Clinical Review. Cancers 2025, 17, 3610. Cancers, 18(1), 172. https://doi.org/10.3390/cancers18010172





