15-Day Duration of Venetoclax Combined with Azacitidine in Treatment-Naive Higher-Risk Myelodysplastic Syndromes: A Prospective Multicenter Study
Simple Summary
Abstract
1. Background
2. Methods
2.1. Patients
2.2. Procedures
2.3. Safety Assessment
2.4. Endpoints and Evaluation
2.5. Statistical Analysis
3. Results
3.1. Patient Demographics and Disease Characteristics
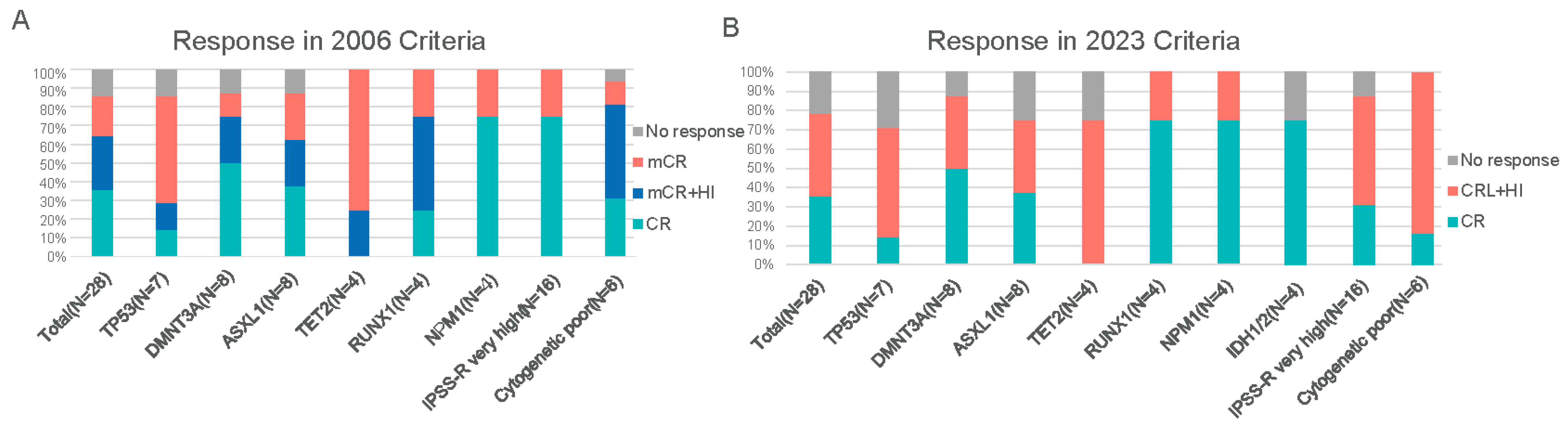
3.2. Treatment Response
3.3. Subgroup Analysis
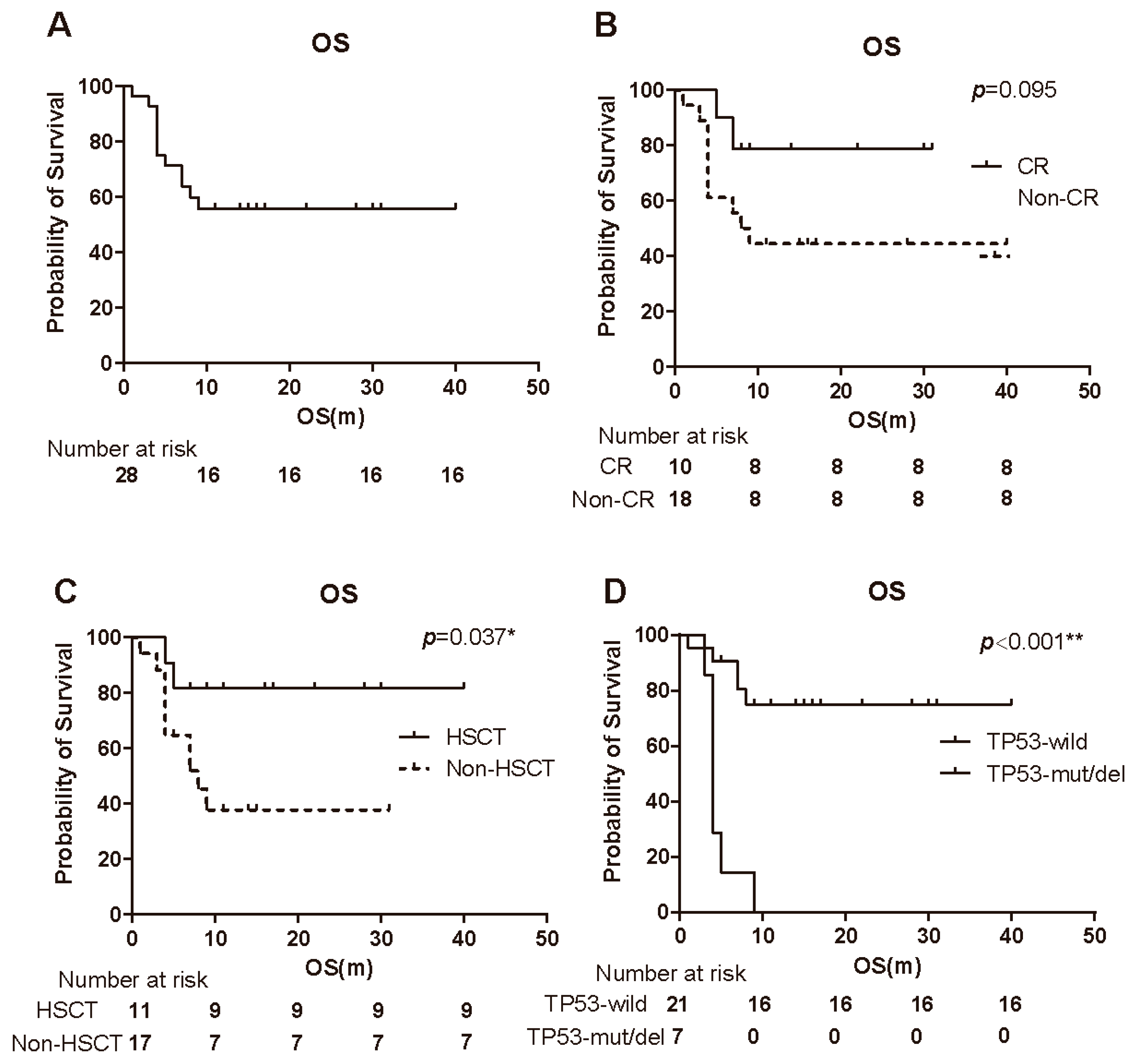
3.4. Overall Survival
3.5. Prognostic Factors
3.6. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AML | Acute myeloid leukemia |
| AZA | Azacitidine (5-azacitidine) |
| BCL-2 | B-cell lymphoma 2 (anti-apoptotic protein) |
| CR | Complete remission |
| HR-MDS | Higher-risk myelodysplastic syndromes |
| HSCT | Hematopoietic stem cell transplantation |
| ITD | Internal tandem duplication |
| IWG | International Working Group |
| MDS | Myelodysplastic syndromes |
| NCI CTCAE | National Cancer Institute Common Terminology Criteria for Adverse Events |
| NGS | Next-generation sequencing |
| OR | Overall response; also used as abbreviation for odds ratio (statistical term) |
| ORR | Objective response rate (overall response rate) |
| OS | Overall survival |
| PR | Partial remission |
| VA | Venetoclax plus azacitidine (combination regimen) |
| VAF | Variant allele frequency |
References
- Cazzola, M. Myelodysplastic Syndromes. N. Engl. J. Med. 2020, 383, 1358–1374. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef]
- Kröger, N. Treatment of high-risk myelodysplastic syndromes. Haematologica 2025, 110, 339–349. [Google Scholar] [CrossRef]
- Bernard, E.; Tuechler, H.; Greenberg Peter, L.; Hasserjian Robert, P.; Arango Ossa Juan, E.; Nannya, Y.; Devlin Sean, M.; Creignou, M.; Pinel, P.; Monnier, L.; et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evid. 2022, 1, EVIDoa2200008. [Google Scholar] [CrossRef]
- Sabile, J.; Pavletic, S.; Migdady, Y. Hematopoietic Stem Cell Transplantation for Myelodysplastic Syndromes: The Current Landscape and Future Directions. Cancer J. 2023, 29, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, P.; Cox, C.; LeBeau, M.M.; Fenaux, P.; Morel, P.; Sanz, G.; Sanz, M.; Vallespi, T.; Hamblin, T.; Oscier, D.; et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 1997, 89, 2079–2088. [Google Scholar] [CrossRef]
- Tomlinson, B.; de Lima, M.; Cogle, C.R.; Thompson, M.A.; Grinblatt, D.L.; Pollyea, D.A.; Komrokji, R.S.; Roboz, G.J.; Savona, M.R.; Sekeres, M.A.; et al. Transplantation Referral Patterns for Patients with Newly Diagnosed Higher-Risk Myelodysplastic Syndromes and Acute Myeloid Leukemia at Academic and Community Sites in the Connect® Myeloid Disease Registry: Potential Barriers to Care. Transpl. Cell. Ther. 2023, 29, 460.e1–460.e9. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Manero, G.; Fenaux, P. Hypomethylating agents and other novel strategies in myelodysplastic syndromes. J. Clin. Oncol. 2011, 29, 516–523. [Google Scholar] [CrossRef]
- Stomper, J.; Rotondo, J.C.; Greve, G.; Lübbert, M. Hypomethylating agents (HMA) for the treatment of acute myeloid leukemia and myelodysplastic syndromes: Mechanisms of resistance and novel HMA-based therapies. Leukemia 2021, 35, 1873–1889. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Kwon, J.; Fabiani, E.; Xiao, Z.; Liu, Y.V.; Follo, M.Y.; Liu, J.; Huang, H.; Gao, C.; Liu, J.; et al. Demethylation and Up-Regulation of an Oncogene after Hypomethylating Therapy. N. Engl. J. Med. 2022, 386, 1998–2010. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, Z.; Chen, L.; Wang, X. Comparison of Azacitidine and Decitabine in Myelodysplastic Syndromes and Acute Myeloid Leukemia: A Network Meta-analysis. Clin. Lymphoma Myeloma Leuk. 2021, 21, e530–e544. [Google Scholar] [CrossRef]
- Fenaux, P.; Mufti, G.J.; Hellstrom-Lindberg, E.; Santini, V.; Finelli, C.; Giagounidis, A.; Schoch, R.; Gattermann, N.; Sanz, G.; List, A.; et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomised, open-label, phase III study. Lancet Oncol. 2009, 10, 223–232. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Salimi, T.; Epstein, R.S. Real-world use and outcomes of hypomethylating agent therapy in higher-risk myelodysplastic syndromes: Why are we not achieving the promise of clinical trials? Future Oncol. 2021, 17, 5163–5175. [Google Scholar] [CrossRef]
- Konopleva, M.; Letai, A. BCL-2 inhibition in AML: An unexpected bonus? Blood 2018, 132, 1007–1012. [Google Scholar] [CrossRef]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef] [PubMed]
- DiNardo, C.D.; Jonas, B.A.; Pullarkat, V.; Thirman, M.J.; Garcia, J.S.; Wei, A.H.; Konopleva, M.; Döhner, H.; Letai, A.; Fenaux, P.; et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N. Engl. J. Med. 2020, 383, 617–629. [Google Scholar] [CrossRef]
- Jilg, S.; Reidel, V.; Müller-Thomas, C.; König, J.; Schauwecker, J.; Höckendorf, U.; Huberle, C.; Gorka, O.; Schmidt, B.; Burgkart, R.; et al. Blockade of BCL-2 proteins efficiently induces apoptosis in progenitor cells of high-risk myelodysplastic syndromes patients. Leukemia 2016, 30, 112–123. [Google Scholar] [CrossRef]
- Mei, C.; Ye, L.; Ren, Y.; Zhou, X.; Ma, L.; Xu, G.; Xu, W.; Lu, C.; Yang, H.; Luo, Y.; et al. 15-days duration of venetoclax combined with azacitidine in the treatment of relapsed/refractory high-risk myelodysplastic syndromes: A retrospective single-center study. Hematol. Oncol. 2023, 41, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.S.; Platzbecker, U.; Odenike, O.; Fleming, S.; Fong, C.Y.; Borate, U.; Jacoby, M.A.; Nowak, D.; Baer, M.R.; Peterlin, P.; et al. Efficacy and safety of venetoclax plus azacitidine for patients with treatment-naive high-risk myelodysplastic syndromes. Blood 2025, 145, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Greenberg, P.L.; Bennett, J.M.; Lowenberg, B.; Wijermans, P.W.; Nimer, S.D.; Pinto, A.; Beran, M.; de Witte, T.M.; Stone, R.M.; et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006, 108, 419–425. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Platzbecker, U.; Bewersdorf, J.P.; Stahl, M.; Adès, L.; Borate, U.; Bowen, D.; Buckstein, R.; Brunner, A.; Carraway, H.E.; et al. Consensus proposal for revised International Working Group 2023 response criteria for higher-risk myelodysplastic syndromes. Blood 2023, 141, 2047–2061. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, A.; Darbaniyan, F.; Jabbour, E.; Montalban-Bravo, G.; Ohanian, M.; Chien, K.; Kadia, T.; Takahashi, K.; Masarova, L.; Short, N.; et al. Azacitidine plus venetoclax in patients with high-risk myelodysplastic syndromes or chronic myelomonocytic leukaemia: Phase 1 results of a single-centre, dose-escalation, dose-expansion, phase 1–2 study. Lancet Haematol. 2022, 9, e756–e765. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Xue, S.; Wang, Y.; He, X.; Hu, X.; Miao, M.; Zhang, Y.; Tang, Z.; Xie, J.; Yang, X.; et al. Venetoclax and Decitabine vs Intensive Chemotherapy as Induction for Young Patients with Newly Diagnosed AML. Blood 2025, 145, 2645–2655. [Google Scholar] [CrossRef] [PubMed]
- Prébet, T.; Gore, S.D.; Esterni, B.; Gardin, C.; Itzykson, R.; Thepot, S.; Dreyfus, F.; Rauzy, O.B.; Recher, C.; Adès, L.; et al. Outcome of high-risk myelodysplastic syndrome after azacitidine treatment failure. J. Clin. Oncol. 2011, 29, 3322–3327. [Google Scholar] [CrossRef]
- Mina, A.; Komrokji, R.S. How I Treat Higher-Risk MDS. Blood 2025, 145, 2002–2011. [Google Scholar] [CrossRef]
- Komrokji, R.S.; Singh, A.M.; Ali, N.A.; Chan, O.; Padron, E.; Sweet, K.; Kuykendall, A.; Lancet, J.E.; Sallman, D.A. Assessing the role of venetoclax in combination with hypomethylating agents in higher risk myelodysplastic syndrome. Blood Cancer J. 2022, 12, 148. [Google Scholar] [CrossRef]
- Garcia-Manero, G.; Platzbecker, U.; Fenaux, P.; Roboz, G.; Fong, C.Y.; Hus, M.; Delage, R.; Brunner, A.; Lee, J.-H.; Turgut, M.; et al. Subgroup analyses from the randomized, Phase 3 VERONA study of venetoclax with azacitidine (Ven+Aza) versus placebo with azacitidine (Pbo+Aza) in patients with treatment-naïve, intermediate and higher-risk Myelodysplastic Syndromes (HR MDS). Blood 2025, 146, 235. [Google Scholar] [CrossRef]
| Characteristics | N (%) |
|---|---|
| Age, median (IQR), years | 63 (59–68) |
| <65, n (%) | 16 (57.1%) |
| ≥65, n (%) | 12 (42.9%) |
| Male, n (%) | 18 (64.3%) |
| ECOG PS, n (%) | |
| 0 | 1 (3.6%) |
| 1 | 18 (64.3%) |
| 2 | 9 (32.1%) |
| IPSS-R prognostic score, n (%) | |
| Intermediate | 5 (17.9%) |
| High | 7 (25.0%) |
| Very high | 16 (57.1%) |
| Bone marrow blast category, n (%) | |
| 5–9.5% | 6 (21.4%) |
| 10–19.5% | 22 (78.6%) |
| Bone marrow blast count, median IQR) | 12 (9.75–13.25) |
| Baseline transfusion dependence, n (%) | |
| RBC | 6 (21.4%) |
| Platelet | 2 (7.1%) |
| Cytogeneticrisk, n (%) | |
| Very good | 1 (3.6%) |
| Good | 14 (50.0%) |
| Intermediate | 7 (25.0%) |
| Poor | 1 (3.6%) |
| Very poor | 5 (17.9%) |
| IPSS-M risk, n (%) | |
| Very high | 18 (64.3%) |
| High | 8 (28.6%) |
| Moderate high | 2 (7.1%) |
| Baseline mutations, n (%) | |
| No mutations detected | 1 (3.6%) |
| DMNT3A | 8 (29.6%) |
| ASXL1 | 8 (29.6%) |
| TP53 | 7 (25.9%) |
| TET2 | 4 (14.8%) |
| RUNX1 | 4 (14.8%) |
| NPM1 | 4 (14.8%) |
| SRSF2 | 3 (11.1%) |
| SF3B1 | 3 (11.1%) |
| FLT3 | 3 (11.1%) |
| IDH1/2 | 4 (14.8%) |
| Overall Response | Complete Response | |||||
|---|---|---|---|---|---|---|
| Multivariate | Multivariate | |||||
| Characteristics | Univariate p-value | OR (95%CI) | p-value | Univariate p-value | OR (95%CI) | p-value |
| Gender | 0.525 | 0.639 | ||||
| Age | 0.445 | 0.953 | ||||
| LDH | 0.695 | 0.584 | ||||
| SF | 0.211 | 0.438 | ||||
| WBC | 0.191 | 1.568 (0.507–4.851) | 0.435 | 0.867 | ||
| N | 0.169 | 0.496 (0.094–2.621) | 0.409 | 0.946 | ||
| HB | 0.092 | 0.954 (0.877–1.037) | 0.269 | 0.389 | ||
| PLT | 0.900 | 0.126 | 1.006 (0.992–1.021) | 0.411 | ||
| BM BLAST | 0.851 | 0.891 | ||||
| IPSS-R | 0.190 | 0.352 (0.022–5.597) | 0.459 | 0.571 | ||
| cytogenetic | 1.000 | 0.292 | ||||
| DMNT3A | 0.865 | 0.324 | ||||
| ASXL1 | 0.742 | 0.856 | ||||
| TP53 | 1.000 | 0.197 | 1.386 (0.073–26.22) | 0.828 | ||
| TET2 | 0.999 | 0.999 | ||||
| NPM1 | 0.999 | 0.109 | 0.168 (0.012–2.326) | 0.183 | ||
| IDH1/2 | 0.999 | 0.109 | 0.260 (0.018–3.824) | 0.326 | ||
| RUNX1 | 0.999 | 0.633 | ||||
| FLT3 | 0.999 | 0.265 | ||||
| SF3B1 | 0.343 | 0.927 | ||||
| SRSF2 | 0.999 | 0.927 | ||||
| Overall Survival | |||
|---|---|---|---|
| Univariate | Multivariate | ||
| Characteristics | p-value | HR (95%CI) | p-value |
| Gender | 0.957 | ||
| Age | 0.053 | 1.022 (0.930–1.123) | 0.652 |
| LDH | 0.637 | ||
| SF | 0.625 | ||
| WBC | 0.465 | ||
| N | 0.110 | 1.413 (1.066–1.872) | 0.016 |
| HB | 0.388 | ||
| PLT | 0.159 | 0.997 (0.986–1.009) | 0.660 |
| BM BLAST | 0.018 | 1.236 (0.277–5.521) | 0.781 |
| IPSS-R | 0.205 | ||
| Cytogenetic | 0.047 | 0.069 (0.005–0.871) | 0.039 |
| DMNT3A | 0.422 | ||
| ASXL1 | 0.625 | ||
| TP53 | <0.001 | 128.908 (4.832–3438.954) | 0.004 |
| TET2 | 0.478 | ||
| NPM1 | 0.318 | ||
| IDH1/2 | 0.350 | ||
| HSCT | 0.059 | 0.158 (0.016–1.566) | 0.115 |
| OR | 0.455 | ||
| CR | 0.130 | 1.181 (0.170–8.201) | 0.866 |
| TEAE | Grade1/2 N (%) | Grade3/4 N (%) | Any Grade N (%) |
|---|---|---|---|
| Hematological | |||
| Leukopenia | 1 (3.6%) | 26 (92.9%) | 27 (96.4%) |
| Neutropenia | 1 (3.6%) | 27 (96.4%) | 28 (100.0%) |
| Anemia | 4 (14.3%) | 20 (71.4%) | 24 (85.7%) |
| Thrombocytopenia | 9 (32.1%) | 18 (64.3%) | 27 (96.4%) |
| Febrile neutropenia | 0 (0.0%) | 14 (50.0%) | 14 (50.0%) |
| Non-hematological | |||
| Pneumonia | 0 (0.0%) | 10 (35.7%) | 10 (35.7%) |
| Nausea | 13 (46.4%) | 0 (0.0%) | 13 (46.4%) |
| Vomiting | 3 (10.7%) | 0 (0.0%) | 3 (10.7%) |
| Constipation | 9 (32.1%) | 0 (0.0%) | 9 (32.1%) |
| Diarrhea | 2 (7.1%) | 0 (0.0%) | 2 (7.1%) |
| Liver injury | 3 (10.7%) | 0 (0.0%) | 3 (10.7%) |
| Renal injury | 3 (10.7%) | 0 (0.0%) | 3 (10.7%) |
| Cardiotoxicity | 3 (10.7%) | 0 (0.0%) | 3 (10.7%) |
| Skin/soft tissue infection | 1 (3.6%) | 0 (0.0%) | 1 (3.6%) |
| Sepsis | 0 (0.0%) | 1 (3.6%) | 1 (3.6%) |
| Tumor lysis syndrome | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Lai, B.; Mei, C.; Yan, X.; Chen, L.; Wang, Y.; Sheng, L.; Tang, S.; Mao, L.; Zhang, P.; Sun, Y.; et al. 15-Day Duration of Venetoclax Combined with Azacitidine in Treatment-Naive Higher-Risk Myelodysplastic Syndromes: A Prospective Multicenter Study. Cancers 2026, 18, 159. https://doi.org/10.3390/cancers18010159
Lai B, Mei C, Yan X, Chen L, Wang Y, Sheng L, Tang S, Mao L, Zhang P, Sun Y, et al. 15-Day Duration of Venetoclax Combined with Azacitidine in Treatment-Naive Higher-Risk Myelodysplastic Syndromes: A Prospective Multicenter Study. Cancers. 2026; 18(1):159. https://doi.org/10.3390/cancers18010159
Chicago/Turabian StyleLai, Binbin, Chen Mei, Xiao Yan, Lieguang Chen, Yi Wang, Lixia Sheng, Shanhao Tang, Liping Mao, Ping Zhang, Yongcheng Sun, and et al. 2026. "15-Day Duration of Venetoclax Combined with Azacitidine in Treatment-Naive Higher-Risk Myelodysplastic Syndromes: A Prospective Multicenter Study" Cancers 18, no. 1: 159. https://doi.org/10.3390/cancers18010159
APA StyleLai, B., Mei, C., Yan, X., Chen, L., Wang, Y., Sheng, L., Tang, S., Mao, L., Zhang, P., Sun, Y., Xie, W., Zhou, D., Mai, W., Wang, H., Ma, L., Lou, Y., Wu, W., Jiang, H., Zhang, J., ... Ouyang, G. (2026). 15-Day Duration of Venetoclax Combined with Azacitidine in Treatment-Naive Higher-Risk Myelodysplastic Syndromes: A Prospective Multicenter Study. Cancers, 18(1), 159. https://doi.org/10.3390/cancers18010159






