Immune Checkpoint Inhibitors and Antibody-Drug Conjugates in Urothelial Carcinoma: Current Landscape and Future Directions
Simple Summary
Abstract
1. Introduction
2. Immune Checkpoint Inhibitors
2.1. Mechanism of Action
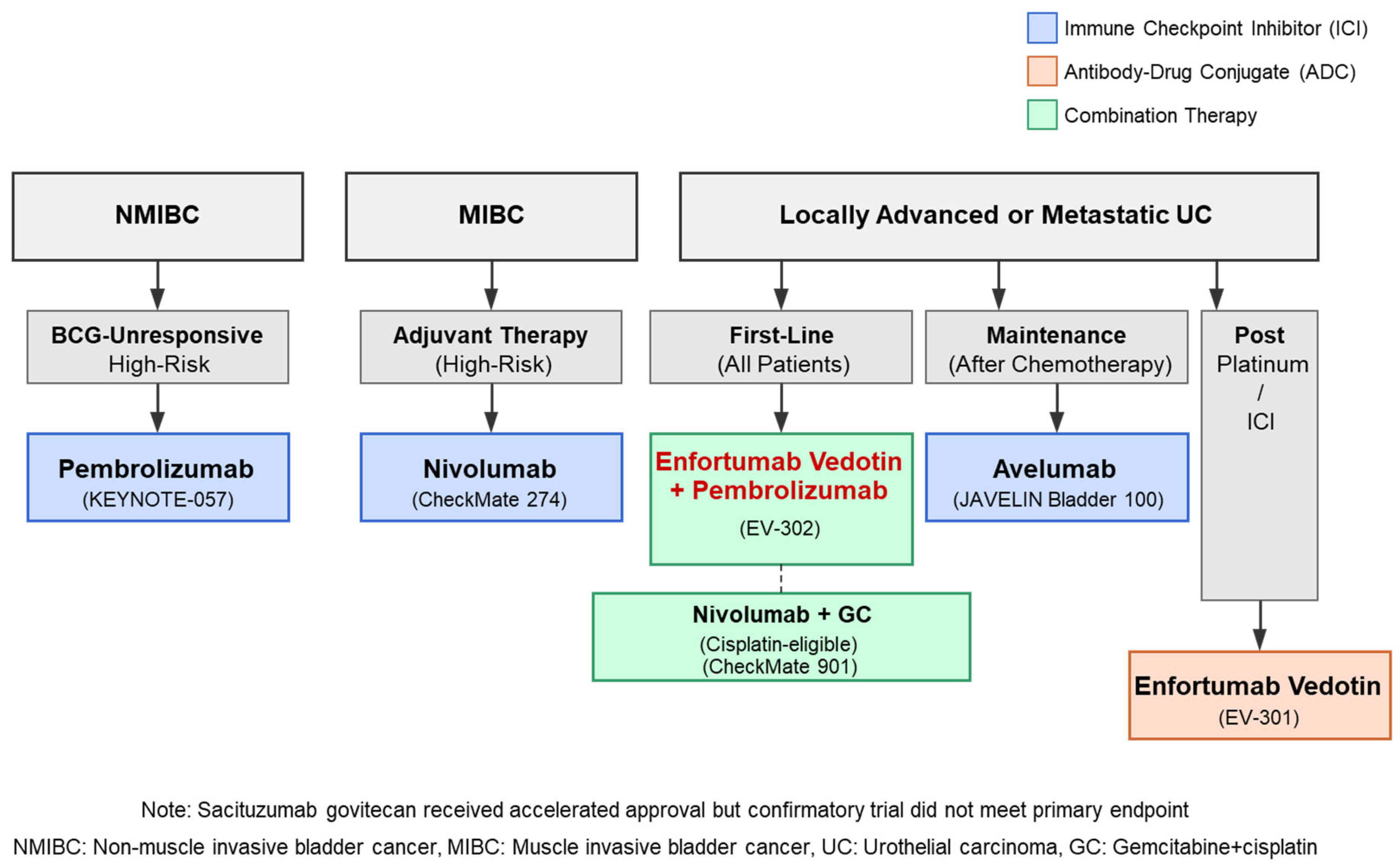
2.2. Clinical Evidence for ICIs in Different Treatment Settings
2.2.1. Second-Line Treatment After Platinum-Based Chemotherapy
2.2.2. Maintenance Therapy After First-Line Chemotherapy
2.2.3. First-Line Combination Strategies
2.2.4. Adjuvant Therapy for Muscle-Invasive Urothelial Carcinoma
2.2.5. BCG-Unresponsive Non-Muscle-Invasive Bladder Cancer
2.3. Management of Immune-Related Adverse Events
- Dermatologic: Rash, pruritus
- Gastrointestinal: Colitis, diarrhea
- Endocrine: Thyroid dysfunction, hypophysitis, adrenal insufficiency
- Hepatic: Hepatitis
- Pulmonary: Pneumonitis
- Less common but potentially serious: Neurological disorders, hematologic abnormalities, and myocarditis [26]
- Continue ICI therapy
- Implement symptomatic management
- Monitor symptoms closely
- Patient education on reporting worsening symptoms
- Temporarily suspend ICI treatment
- Initiate corticosteroids (prednisone 0.5–1 mg/kg/day)
- Consider specialist consultation based on the organ system involved
- Resume ICI once symptoms improve to Grade ≤ 1 and corticosteroid dose ≤ 10 mg/day
- Interrupt ICI treatment
- Administer high-dose corticosteroids (prednisone 1–2 mg/kg/day)
- Consider additional immunosuppressants for steroid-refractory cases
- Hospitalization may be required
- Evaluate the potential permanent discontinuation of ICI therapy
- Permanently discontinue ICI treatment (except for well-controlled endocrinopathies)
- High-dose corticosteroids with hospitalization
- Multidisciplinary management approach
- Consider early additional immunosuppression
- Taper corticosteroids gradually over 4–6 weeks once symptoms improve
- Consider prophylaxis against opportunistic infections during prolonged corticosteroid use
- Patient education on symptom recognition and prompt reporting is essential
2.4. Biomarkers and Predictors of Response
3. Antibody-Drug Conjugates
3.1. ADC Technology and Principles
- Monoclonal antibodies (mAbs): Provide target specificity by binding to antigens preferentially expressed on tumor cells. Ideal target antigens should be abundantly and homogeneously expressed on cancer cells, with minimal expression in normal tissues.
- Cytotoxic payload: Potent small-molecule drugs that induce cell death and are typically too toxic for conventional systemic delivery. Common payload classes include microtubule inhibitors (e.g., monomethyl auristatin E [MMAE]) and DNA-damaging agents (e.g., SN-38, a topoisomerase I inhibitor).
- Linker: Chemically connects the antibody to the payload. Linkers must remain stable in circulation while enabling efficient payload release within target cells. Linkers can be designed as cleavable (responding to environmental conditions like low pH or specific enzymes) or non-cleavable (requiring complete antibody degradation).
3.2. Enfortumab Vedotin
3.2.1. Structure and Target
3.2.2. Clinical Evidence
3.2.3. Safety Profile and Management
- Skin reactions: Occur in up to 55% of patients [42]
- Peripheral neuropathy: Usually sensory, cumulative with continued treatment
- Fatigue: Common but typically mild to moderate
- Gastrointestinal: Nausea, diarrhea (generally manageable)
- Alopecia: Generally reversible upon treatment discontinuation
- Metabolic: Hyperglycemia (monitor blood glucose, especially in diabetic patients) [11]
- Severe skin reactions: Grade ≥3 events in approximately 13% of patients, including maculopapular rash, bullous dermatitis, or exfoliative dermatitis [42]
- Severe peripheral neuropathy: Can be dose-limiting
- Ocular disorders: Including conjunctivitis and dry eye [11]
- Severe hyperglycemia: Particularly in patients with pre-existing diabetes
- Early dermatology consultation
- Topical emollients for mild cases
- Topical or systemic corticosteroids for moderate-severe cases
- Consider dose interruption for Grade ≥3 events until resolution to Grade ≤1
- Regular neurological assessment
- Dose reduction or treatment interruption for Grade ≥2 neuropathy
- Gabapentin or duloxetine may provide symptomatic relief
- Careful baseline and ongoing monitoring
- Prompt intervention at first signs of toxicity
- Appropriate dose modifications according to severity
- Patient education on symptom recognition and reporting
3.3. Sacituzumab Govitecan
3.3.1. Structure and Target
3.3.2. Clinical Evidence
3.3.3. Safety Profile and Management
3.4. Other ADCs in Development
4. Combination Strategies: ICIs and ADCs
4.1. Rationale for Combination Approaches
4.2. Enfortumab Vedotin Plus Pembrolizumab
4.3. Sacituzumab Govitecan Plus Pembrolizumab
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Kamat, A.M.; Hahn, N.M.; Efstathiou, J.A.; Lerner, S.P.; Malmström, P.U.; Choi, W.; Guo, C.C.; Lotan, Y.; Kassouf, W. Bladder cancer. Lancet 2016, 388, 2796–2810. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. SEER Cancer Statistics Review. Available online: https://seer.cancer.gov/statfacts/html/urinb.html (accessed on 5 April 2025).
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Felefly, T.; Kourie, H.R.; Saleh, K. Urothelial carcinoma in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 631–645. [Google Scholar]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Espinós, E.L.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- von der Maase, H.; Sengelov, L.; Roberts, J.T.; Ricci, S.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Zimmermann, A.; Arning, M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J. Clin. Oncol. 2005, 23, 4602–4608. [Google Scholar] [CrossRef]
- Galsky, M.D.; Hahn, N.M.; Rosenberg, J.; Sonpavde, G.; Hutson, T.; Oh, W.K.; Dreicer, R.; Vogelzang, N.J.; Sternberg, C.N.; Bajorin, D.F.; et al. Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy. J. Clin. Oncol. 2011, 29, 2432–2438. [Google Scholar] [CrossRef]
- Sonpavde, G.; Sternberg, C.N.; Rosenberg, J.E.; Hahn, N.M.; Galsky, M.D.; Vogelzang, N.J. Second-line systemic therapy and emerging drugs for metastatic transitional-cell carcinoma of the urothelium. Lancet Oncol. 2010, 11, 861–870. [Google Scholar] [CrossRef]
- Balar, A.V.; Galsky, M.D.; Rosenberg, J.E.; Powles, T.; Petrylak, D.P.; Bellmunt, J.; Loriot, Y.; Necchi, A.; Hoffman-Censits, J.; Perez-Gracia, J.L.; et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017, 389, 67–76. [Google Scholar] [CrossRef]
- Challita-Eid, P.M.; Satpayev, D.; Yang, P.; An, Z.; Morrison, K.; Shostak, Y.; Raitano, A.; Nadell, R.; Liu, W.; Lortie, D.R.; et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res. 2016, 76, 3003–3013. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef]
- Yajima, S.; Hirose, K.; Masuda, H. Enfortumab Vedotin With or Without Pembrolizumab in Metastatic Urothelial Carcinoma: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2025, 8, e250250. [Google Scholar] [CrossRef]
- Crispen, P.L.; Kusmartsev, S. Mechanisms of immune evasion in bladder cancer. Cancer Immunol. Immunother. 2020, 69, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.C.; Duffy, C.R.; Allison, J.P. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018, 8, 1069–1086. [Google Scholar] [CrossRef]
- Sharma, P.; Siefker-Radtke, A.; de Braud, F.; Basso, U.; Calvo, E.; Bono, P.; Morse, M.A.; Ascierto, P.A.; Lopez-Martin, J.; Brossart, P.; et al. Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 mg/kg Plus Ipilimumab 3 mg/kg Expansion Cohort Results. J. Clin. Oncol. 2019, 37, 1608–1616. [Google Scholar] [CrossRef]
- Bellmunt, J.; de Wit, R.; Vaughn, D.J.; Fradet, Y.; Lee, J.-L.; Fong, L.; Vogelzang, N.J.; Climent, M.A.; Petrylak, D.P.; Choueiri, T.K.; et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N. Engl. J. Med. 2017, 376, 1015–1026. [Google Scholar] [CrossRef]
- Sharma, P.; Retz, M.; Siefker-Radtke, A.; Baron, A.; Necchi, A.; Bedke, J.; Plimack, E.R.; Vaena, D.; Grimm, M.-O.; Bracarda, S.; et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): A multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017, 18, 312–322. [Google Scholar] [CrossRef]
- Powles, T.; Durán, I.; van der Heijden, M.S.; Loriot, Y.; Vogelzang, N.J.; De Giorgi, U.; Oudard, S.; Retz, M.M.; Castellano, D.; Bamias, A.; et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2018, 391, 748–757. [Google Scholar] [CrossRef]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- van der Heijden, M.S.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Witjes, J.A.; Gschwend, J.E.; Milowsky, M.I.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; et al. Adjuvant Nivolumab in High-Risk Muscle-Invasive Urothelial Carcinoma: Expanded Efficacy From CheckMate 274. J. Clin. Oncol. 2025, 43, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Balar, A.V.; Kamat, A.M.; Kulkarni, G.S.; Uchio, E.M.; Boormans, J.L.; Roumiguié, M.; Krieger, L.E.; Singer, E.A.; Bajorin, D.F.; Grivas, P.; et al. Pembrolizumab for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, J.; Lichtman, A.H.; Sharpe, A.H.; Galluzzi, L.; Kitsis, R.N. Immune checkpoint inhibitor-associated myocarditis: Manifestations and mechanisms. J. Clin. Investig. 2021, 131, e145186. [Google Scholar] [CrossRef]
- Schneider, B.J.; Naidoo, J.; Santomasso, B.D.; Lacchetti, C.; Adkins, S.; Anadkat, M.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 4073–4126. [Google Scholar] [CrossRef]
- Haanen, J.; Obeid, M.; Spain, L.; Carbonnel, F.; Wang, Y.; Robert, C.; Lyon, A.; Wick, W.; Kostine, M.; Peters, S.; et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1217–1238. [Google Scholar] [CrossRef]
- Rui, X.; Gu, T.T.; Pan, H.F.; Zhang, H.Z. Evaluation of PD-L1 biomarker for immune checkpoint inhibitor (PD-1/PD-L1 inhibitors) treatments for urothelial carcinoma patients: A meta-analysis. Int. Immunopharmacol. 2019, 67, 378–385. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef]
- Kato, M.; Uchida, J. Recent advances in immune checkpoint inhibitors in the treatment of urothelial carcinoma: A review. Int. J. Urol. 2023, 30, 1068–1077. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Li, S.; Han, S.; Shi, C.; Zhang, Y. Antibody drug conjugate: The “biological missile” for targeted cancer therapy. Signal Transduct. Target Ther. 2022, 7, 93. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, S.; Di Vittorio, G.; Pitari, G.; Cimini, A.M.; Ardini, M.; Gentile, R.; Iacobelli, S.; Sala, G.; Capone, E.; Flavell, D.J.; et al. Antibody-Drug Conjugates: The New Frontier of Chemotherapy. Int. J. Mol. Sci. 2020, 21, 5510. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, A.H.; Brown, M.P. Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br. J. Cancer 2017, 117, 1736–1742. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef]
- Hoffman-Censits, J.H.; Lombardo, K.A.; Parimi, V.; Kamanda, S.; Choi, W.; Hahn, N.M.; McConkey, D.J.; McGuire, B.M.; Bivalacqua, T.J.; Kates, M.; et al. Expression of Nectin-4 in Bladder Urothelial Carcinoma, in Morphologic Variants, and Nonurothelial Histotypes. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 619–625. [Google Scholar] [CrossRef]
- Klümper, N.; Ralser, D.J.; Zschäbitz, S.; Hahn, O.; Büttner, T.; Roghmann, F.; Bolenz, C.; Zengerling, F.; Schwab, C.; Nagy, D.; et al. NECTIN4 Amplification Is Frequent in Solid Tumors and Predicts Enfortumab Vedotin Response in Metastatic Urothelial Cancer. J. Clin. Oncol. 2024, 42, 802–812. [Google Scholar] [CrossRef]
- Rosenberg, J.; Sridhar, S.S.; Zhang, J.; Smith, D.; Ruether, D.; Flaig, T.W.; Baranda, J.; Lang, J.; Plimack, E.R.; Sangha, R.; et al. EV-101: A phase I study of single-agent enfortumab vedotin in patients with nectin-4-positive solid tumors, including metastatic urothelial carcinoma. J. Clin. Oncol. 2020, 38, 1041–1049. [Google Scholar] [CrossRef]
- Powles, T.; Rosenberg, J.E.; Sonpavde, G.P.; Loriot, Y.; Durán, I.; Lee, J.-L.; Matsubara, N.; Vulsteke, C.; Castellano, D.; Wu, C.; et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 1125–1135. [Google Scholar] [CrossRef]
- Brower, B.; McCoy, A.; Ahmad, H.; Eitman, C.; Bowman, I.A.; Rembisz, J.; Milowsky, M.I. Managing potential adverse events during treatment with enfortumab vedotin plus pembrolizumab in patients with advanced urothelial cancer. Cancer Treat. Rev. 2024, 14, 1326715. [Google Scholar]
- Lacouture, M.E.; Patel, A.B.; Rosenberg, J.E.; O’Donnell, P.H. Management of dermatologic events associated with the nectin-4-directed antibody-drug conjugate enfortumab vedotin. Oncologist 2022, 27, e223–e232. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Messersmith, W.A.; Kio, E.A.; Berlin, J.; Vahdat, L.; Masters, G.; Moroose, R.; Santin, A.; Kalinsky, K.; Picozzi, V.; et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann. Oncol. 2021, 32, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Shvartsur, A.; Bonavida, B. Trop2 and its overexpression in cancers: Regulation and clinical/therapeutic implications. Genes Cancer 2015, 6, 84–105. [Google Scholar] [CrossRef]
- Loriot, Y.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Grivas, P.; Fléchon, A.; Jain, R.K.; Swami, U.; Bupathi, M.; Barthélémy, P.; et al. Sacituzumab Govitecan Demonstrates Efficacy across Tumor Trop-2 Expression Levels in Patients with Advanced Urothelial Cancer. Clin. Cancer Res. 2024, 30, 3179–3188. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tagawa, S.T.; Jain, R.K.; Bupathi, M.; Balar, A.; Kalebasty, A.R.; George, S.; Palmbos, P.; Nordquist, L.; Davis, N.; et al. TROPHY-U-01 Cohort 2: A Phase II Study of Sacituzumab Govitecan in Cisplatin-Ineligible Patients With Metastatic Urothelial Cancer Progressing After Previous Checkpoint Inhibitor Therapy. J. Clin. Oncol. 2024, 42, 982–993. [Google Scholar] [CrossRef]
- Powles, T.; Tagawa, S.; Vulsteke, C.; Gross-Goupil, M.; Park, S.H.; Necchi, A.; De Santis, M.; Duran, I.; Morales-Barrera, R.; Guo, J.; et al. Sacituzumab govitecan in advanced urothelial carcinoma: TROPiCS-04, a phase III randomized trial. Ann. Oncol. 2025, 36, 561–571. [Google Scholar] [CrossRef]
- Bardia, A.; Mayer, I.A.; Diamond, J.R.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Schlam, I.; Tarantino, P.; Tolaney, S.M. Managing adverse events of sacituzumab govitecan. Expert Opin. Biol. Ther. 2023, 23, 1103–1111. [Google Scholar] [CrossRef]
- Scherrer, E.; Kang, A.; Bloudek, L.M.; Koshkin, V.S. HER2 expression in urothelial carcinoma, a systematic literature review. Front. Oncol. 2022, 12, 1011885. [Google Scholar] [CrossRef]
- Sheng, X.; Wang, L.; He, Z.; Shi, Y.; Luo, H.; Han, W.; Yao, X.; Shi, B.; Liu, J.; Hu, C.; et al. Efficacy and Safety of Disitamab Vedotin in Patients With Human Epidermal Growth Factor Receptor 2-Positive Locally Advanced or Metastatic Urothelial Carcinoma: A Combined Analysis of Two Phase II Clinical Trials. J. Clin. Oncol. 2024, 42, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.-W.; Zhang, J.; Yang, H.; Yang, J.; Zheng, T.; Sun, H.; Sun, Y.; Li, G.; Liu, F.; Wan, X.; et al. Clinical update related to the first-in-human trial of SYS6002 (CRB-701), a next-generation nectin-4 targeting antibody drug conjugate. J. Clin. Oncol. 2024, 42 (Suppl. 16), 3151. [Google Scholar] [CrossRef]
- Chang, H.L.; Schwettmann, B.; McArthur, H.L.; Chan, I.S. Antibody-drug conjugates in breast cancer: Overcoming resistance and boosting immune response. J. Clin. Investig. 2023, 133, e172156. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tang, K.; Zhang, Q.; Han, Q.; Quan, L.; Li, Y.; Cui, J.; Feng, N.; Gong, J.; Shang, B.; et al. Antibody-drug conjugate combinations in cancer treatment: Clinical efficacy and clinical study perspectives. Front. Pharmacol. 2025, 16, 1556245. [Google Scholar] [CrossRef]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef]
- O’Donnell, P.H.; Milowsky, M.I.; Petrylak, D.P.; Hoimes, C.J.; Flaig, T.W.; Mar, N.; Moon, H.H.; Friedlander, T.W.; McKay, R.R.; Bilen, M.A.; et al. Enfortumab vedotin with or without pembrolizumab in cisplatin-ineligible patients with previously untreated locally advanced or metastatic urothelial cancer. J. Clin. Oncol. 2023, 41, 4107–4117. [Google Scholar] [CrossRef]
- Grivas, P.; Pouessel, D.; Park, C.H.; Barthelemy, P.; Bupathi, M.; Petrylak, D.P.; Agarwal, N.; Gupta, S.; Fléchon, A.; Ramamurthy, C.; et al. Sacituzumab Govitecan in Combination With Pembrolizumab for Patients With Metastatic Urothelial Cancer That Progressed After Platinum-Based Chemotherapy: TROPHY-U-01 Cohort 3. J. Clin. Oncol. 2024, 42, 1415–1425. [Google Scholar] [CrossRef]
| Trial | Phase | Population | Treatment | Primary Endpoint(s) | Key Results | Grade ≥ 3 AEs | Reference |
|---|---|---|---|---|---|---|---|
| KEYNOTE-045 | III | Post-platinum | Pembrolizumab vs. Chemo | OS | 10.3 vs. 7.4 mo (HR 0.73) | 15% vs. 49% | [17] |
| IMvigor211 | III | Post-platinum | Atezolizumab vs. Chemo | OS | 11.1 vs. 10.6 mo (NS) | 20% vs. 43% | [19] |
| JAVELIN Bladder 100 | III | Maintenance after first-line | Avelumab + BSC vs. BSC | OS | 21.4 vs. 14.3 mo (HR 0.69) | 16.6% vs. 0% | [20] |
| CheckMate 274 | III | Adjuvant | Nivolumab vs. Placebo | DFS | HR 0.70, p < 0.001 | 17.9% vs. 7.2% | [22,23] |
| EV-301 | III | Post-platinum/post-ICI | EV vs. Chemo | OS | 12.9 vs. 9.0 mo (HR 0.70) | 51.4% vs. 49.8% | [40] |
| EV-302/KEYNOTE-A39 | III | First-line | EV + pembro vs. Chemo | OS, PFS | OS: 31.5 vs. 16.1 mo (HR 0.47) | 55.9% vs. 69.5% | [10] |
| CheckMate 901 | III | First-line (cisplatin-eligible) | Nivo + GC vs. GC | OS, PFS | OS: 21.7 vs. 18.9 mo (HR 0.78) | 61.8% vs. 51.7% | [21] |
| TROPiCS-04 | III | Post-platinum/post-ICI | SG vs. Chemo | OS | 10.3 vs. 9.0 mo (HR 0.86, NS) | 67% vs. 35% | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yajima, S.; Masuda, H. Immune Checkpoint Inhibitors and Antibody-Drug Conjugates in Urothelial Carcinoma: Current Landscape and Future Directions. Cancers 2025, 17, 1594. https://doi.org/10.3390/cancers17091594
Yajima S, Masuda H. Immune Checkpoint Inhibitors and Antibody-Drug Conjugates in Urothelial Carcinoma: Current Landscape and Future Directions. Cancers. 2025; 17(9):1594. https://doi.org/10.3390/cancers17091594
Chicago/Turabian StyleYajima, Shugo, and Hitoshi Masuda. 2025. "Immune Checkpoint Inhibitors and Antibody-Drug Conjugates in Urothelial Carcinoma: Current Landscape and Future Directions" Cancers 17, no. 9: 1594. https://doi.org/10.3390/cancers17091594
APA StyleYajima, S., & Masuda, H. (2025). Immune Checkpoint Inhibitors and Antibody-Drug Conjugates in Urothelial Carcinoma: Current Landscape and Future Directions. Cancers, 17(9), 1594. https://doi.org/10.3390/cancers17091594





