Avelumab Maintenance Therapy for Advanced Urothelial Carcinoma with Low Tumor Burden
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Population
2.2. Statistical Methods and Analytical Approach
3. Results
3.1. Patient Characteristics
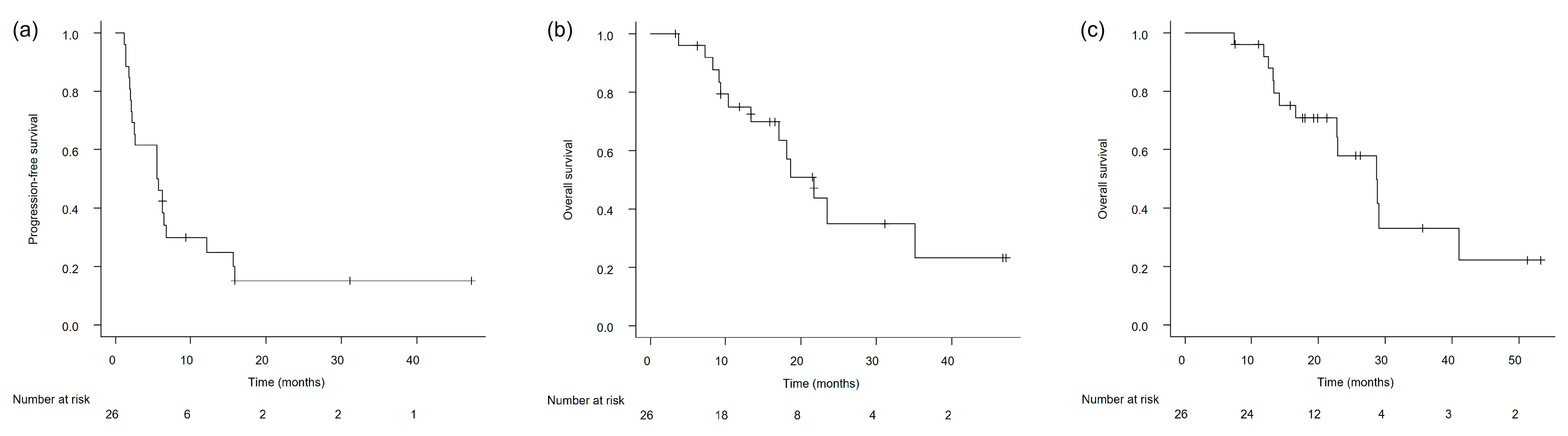
3.2. Clinical Outcomes in the Overall Study Population
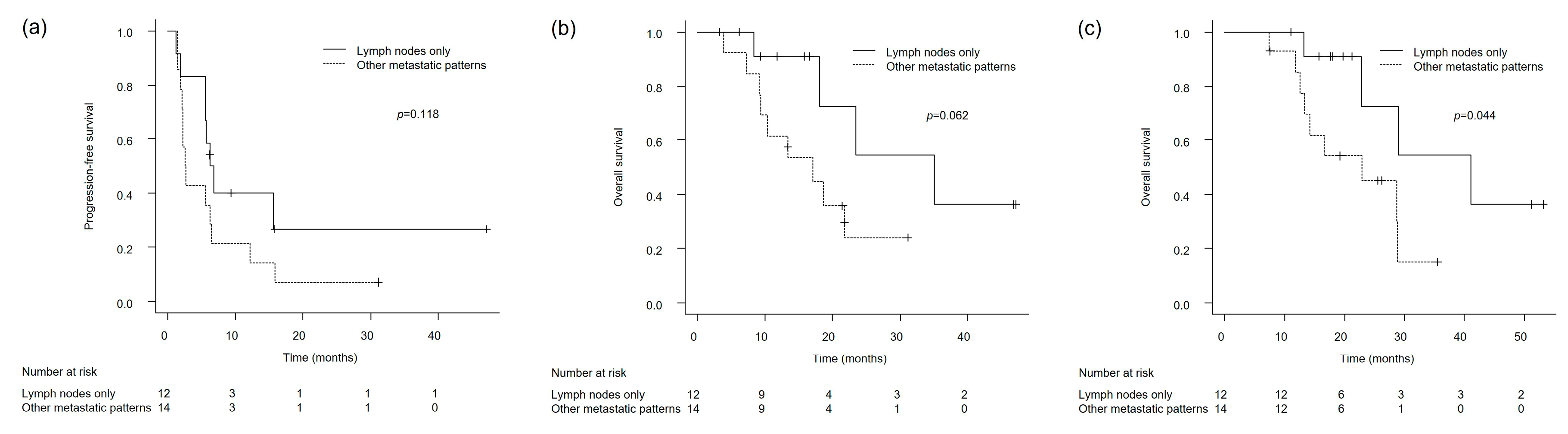
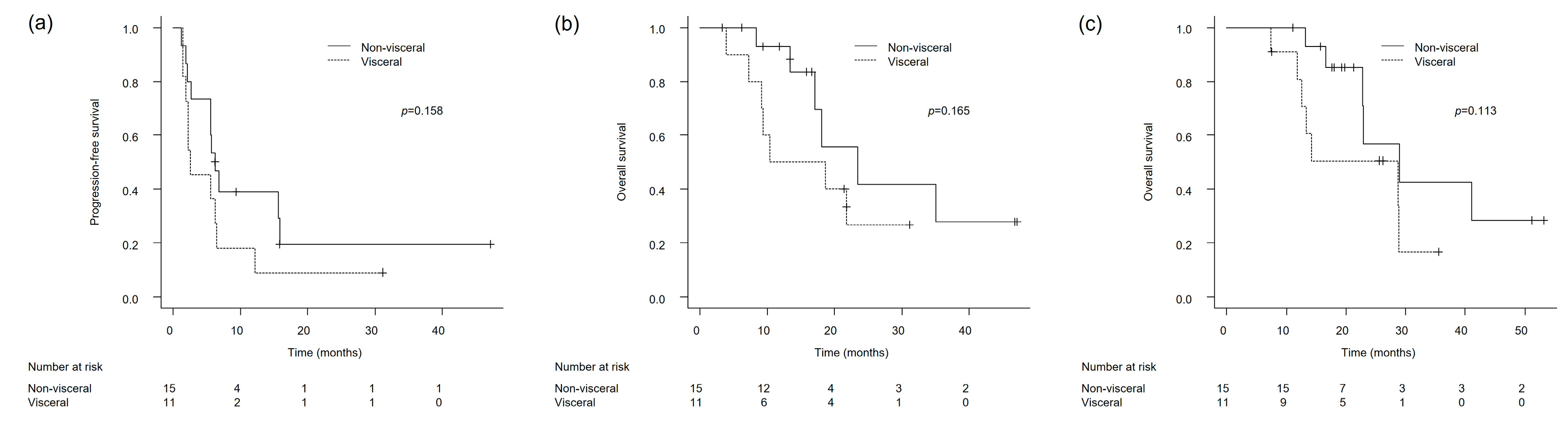
3.3. Clinical Outcomes in Patients with Lymph Node-Only and Non-Visceral Metastases
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Bladder Cancer; Version 1.2025; NCCN: Plymouth Meeting, PA, USA; Available online: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf (accessed on 1 June 2025).
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.S.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus gemcitabine–cisplatin in advanced urothelial carcinoma. N. Engl. J. Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- Grande, E.; Hussain, S.A.; Barthélémy, P.; Kanesvaran, R.; Giannatempo, P.; Benjamin, D.J.; Hoffman, J.; Birtle, A. Individualizing first-line treatment for advanced urothelial carcinoma: A favorable dilemma for patients and physicians. Cancer Treat. Rev. 2025, 134, 102900. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.M.; Gulley, J.L. Product review: Avelumab, an anti-PD-L1 antibody. Hum. Vaccin. Immunother. 2019, 15, 891–908. [Google Scholar] [CrossRef] [PubMed]
- Barthélémy, P.; Thibault, C.; Fléchon, A.; Gross-Goupil, M.; Voog, E.; Eymard, J.-C.; Abraham, C.; Chasseray, M.; Lorgis, V.; Hilgers, W.; et al. Real-world study of avelumab first-line maintenance treatment in patients with advanced urothelial carcinoma in France: Overall results from the noninterventional AVENANCE study and analysis of outcomes by second-line treatment. Eur. Urol. Oncol. 2025, 8, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.; Barata, P.; Moon, H.; Gupta, S.; Hutson, T.; Sternberg, C.N.; Brown, J.R.; Dave, V.; Downey, C.; Shillington, A.C.; et al. Avelumab first-line maintenance for locally advanced or metastatic urothelial carcinoma: Results from the real-world US PATRIOT-II study. Clin. Genitourin. Cancer 2024, 22, 102238. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Ullén, A.; Loriot, Y.; Sridhar, S.S.; Sternberg, C.N.; Bellmunt, J.; et al. Avelumab first-line maintenance for advanced urothelial carcinoma: Results from the JAVELIN Bladder 100 trial after ≥2 years of follow-up. J. Clin. Oncol. 2023, 41, 3486–3492. [Google Scholar] [CrossRef] [PubMed]
- Furubayashi, N.; Minato, A.; Tomoda, T.; Hori, Y.; Kiyoshima, K.; Negishi, T.; Haraguchi, Y.; Koga, T.; Kuroiwa, K.; Fujimoto, N.; et al. Organ-specific tumor response to avelumab maintenance therapy for advanced urothelial carcinoma: A multicenter retrospective study. Anticancer. Res. 2023, 43, 5689–5698. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.; Huber, C.; Pawar, V.; Roach, M.; May, S.G.; Desai, I.; Chang, J.; Bharmal, M. Management of Patients with Advanced Urothelial Carcinoma in an Evolving Treatment Landscape: A Qualitative Study of Provider Perspectives of First-Line Therapies. Clin. Genitourin. Cancer 2022, 20, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Elwyn, G.; Frosch, D.; Thomson, R.; Joseph-Williams, N.; Lloyd, A.; Kinnersley, P.; Cording, E.; Tomson, D.; Dodd, C.; Rollnick, S.; et al. Shared decision making: A model for clinical practice. J. Gen. Intern. Med. 2012, 27, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Terada, N.; Saito, T.; Yokomizo, A.; Kohei, N.; Goto, T.; Kawamura, S.; Hashimoto, Y.; Takahashi, A.; Kimura, T.; et al. Differential prognostic factors in low- and high-burden de novo metastatic hormone-sensitive prostate cancer patients. Cancer Sci. 2021, 112, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, C.J.; Chen, Y.-H.; Carducci, M.; Liu, G.; Jarrard, D.F.; Eisenberger, M.; Wong, Y.-N.; Hahn, N.; Kohli, M.; Cooney, M.M.; et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N. Engl. J. Med. 2015, 373, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Barthelemy, P.; Loriot, Y.; Thibault, C.; Flechon, A.; Voog, E.; Eymard, J.C.; Simon, C.; Pouessel, D.; Chasseray, M.; Lorgis, V.; et al. AVENANCE real-world study of avelumab first-line maintenance treatment for advanced urothelial carcinoma: Analyses in low tumor burden subgroups. J. Clin. Oncol. 2025, 43 (Suppl. S5), 718. [Google Scholar] [CrossRef]
- Bellmunt, J.; Théodore, C.; Demkov, T.; Komyakov, B.; Sengelov, L.; Daugaard, G.; Caty, A.; Carles, J.; Jagiello-Gruszfeld, A.; Karyakin, O.; et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J. Clin. Oncol. 2009, 27, 4454–4461. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsson, K.; Brandt, S.B.; Jensen, J.B. Patients with metastatic or locally advanced bladder cancer not undergoing systemic oncological treatment—Characteristics and long-term outcome in a single-center Danish cohort. Cancers 2025, 17, 1105. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.; Grande, E.; Davis, I.D.; Moon, H.H.; Grimm, M.-O.; Gupta, S.; Barthélémy, P.; Thibault, C.; Guenther, S.; Hanson, S.; et al. Avelumab first-line maintenance treatment for advanced urothelial carcinoma: Review of evidence to guide clinical practice. ESMO Open 2023, 8, 102050. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Powles, T.; Park, S.H.; Voog, E.; Valderrama, B.P.; Gurney, H.; Ullén, A.; Loriot, Y.; Sridhar, S.S.; Tsuchiya, N.; et al. Avelumab first-line maintenance for advanced urothelial carcinoma: Long-term outcomes from the JAVELIN Bladder 100 trial in patients with nonvisceral or lymph node-only disease. Eur. Urol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://ascopubs.org/doi/10.1200/JCO.2025.43.5_suppl.664 (accessed on 1 June 2025).
- Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/visceral (accessed on 1 June 2025).
- Zhang, J.; Cai, D.; Hong, S. Prevalence and prognosis of bone metastases in common solid cancers at initial diagnosis: A population-based study. BMJ Open 2023, 13, e069908. [Google Scholar] [CrossRef] [PubMed]
- Makrakis, D.; Talukder, R.; Lin, G.I.; Diamantopoulos, L.N.; Dawsey, S.; Gupta, S.; Carril-Ajuria, L.; Castellano, D.; de Kouchkovsky, I.; Koshkin, V.S.; et al. Association Between Sites of Metastasis and Outcomes With Immune Checkpoint Inhibitors in Advanced Urothelial Carcinoma. Clin. Genitourin. Cancer 2022, 20, e440–e452. [Google Scholar] [CrossRef] [PubMed]
- Ruatta, F.; Derosa, L.; Escudier, B.; Colomba, E.; Guida, A.; Baciarello, G.; Loriot, Y.; Fizazi, K.; Albiges, L. Prognosis of renal cell carcinoma with bone metastases: Experience from a large cancer centre. Eur. J. Cancer 2019, 107, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Stellato, M.; Santini, D.; Cursano, M.C.; Foderaro, S.; Tonini, G.; Procopio, G. Bone metastases from urothelial carcinoma: The dark side of the moon. J. Bone Oncol. 2021, 31, 100405. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xie, C.; Li, Q.; Huang, X.; Huang, W.; Yin, D. Prognostic factors and nomogram for the overall survival of bladder cancer bone metastasis: A SEER-based study. Medicine 2023, 102, e33275. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Gu, H.; Zhang, D.; Wen, M.; Yan, Z.; Song, B.; Xie, C. Establishment and validation of a nomogram for predicting overall survival of upper-tract urothelial carcinoma with bone metastasis: A population-based study. BMC Urol. 2024, 24, 100. [Google Scholar] [CrossRef] [PubMed]
| Characteristic (n = 26) | |
|---|---|
| Age (years), median (IQR) | 72 (65–77) |
| Sex, no. (%) | |
| Male | 17 (65.4) |
| Female | 9 (35.6) |
| ECOG PS score, no. (%) | |
| 0 | 22 (84.6) |
| ≥1 | 4 (15.4) |
| Primary tumor site, no. (%) | |
| Upper urinary tract | 10 (38.5) |
| Lower urinary tract | 14 (53.8) |
| Both | 2 (7.7) |
| Surgical treatment for the primary tumor, no. (%) | 13 (50.0) |
| Pure UC in histologic testing, no. (%) | 19 (73.1) |
| Disease site, no. (%) | |
| Lymph node | 21 (80.8) |
| Primary tumor organ (pelvis, ureter, and bladder) | 13 (50.0) |
| Lung | 4 (15.4) |
| Bone | 4 (15.4) |
| Peritoneal dissemination | 3 (11.5) |
| Liver | 2 (7.7) |
| Brain | 2 (7.7) |
| Others (uterus or heart) | 2 (7.7) |
| Response for platinum-based chemotherapy, no. (%) | |
| CR + PR | 15 (57.7) |
| SD | 11 (42.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furubayashi, N.; Tsujita, J.; Takayama, A.; Nakashima, S.; Nakamura, M.; Negishi, T. Avelumab Maintenance Therapy for Advanced Urothelial Carcinoma with Low Tumor Burden. Cancers 2025, 17, 2447. https://doi.org/10.3390/cancers17152447
Furubayashi N, Tsujita J, Takayama A, Nakashima S, Nakamura M, Negishi T. Avelumab Maintenance Therapy for Advanced Urothelial Carcinoma with Low Tumor Burden. Cancers. 2025; 17(15):2447. https://doi.org/10.3390/cancers17152447
Chicago/Turabian StyleFurubayashi, Nobuki, Jiro Tsujita, Azusa Takayama, Shin Nakashima, Motonobu Nakamura, and Takahito Negishi. 2025. "Avelumab Maintenance Therapy for Advanced Urothelial Carcinoma with Low Tumor Burden" Cancers 17, no. 15: 2447. https://doi.org/10.3390/cancers17152447
APA StyleFurubayashi, N., Tsujita, J., Takayama, A., Nakashima, S., Nakamura, M., & Negishi, T. (2025). Avelumab Maintenance Therapy for Advanced Urothelial Carcinoma with Low Tumor Burden. Cancers, 17(15), 2447. https://doi.org/10.3390/cancers17152447






