Natural Language Processing of Radiology Reports to Assess Survival in Patients with Advanced Melanoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Classification of the Extent of Metastatic Disease
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
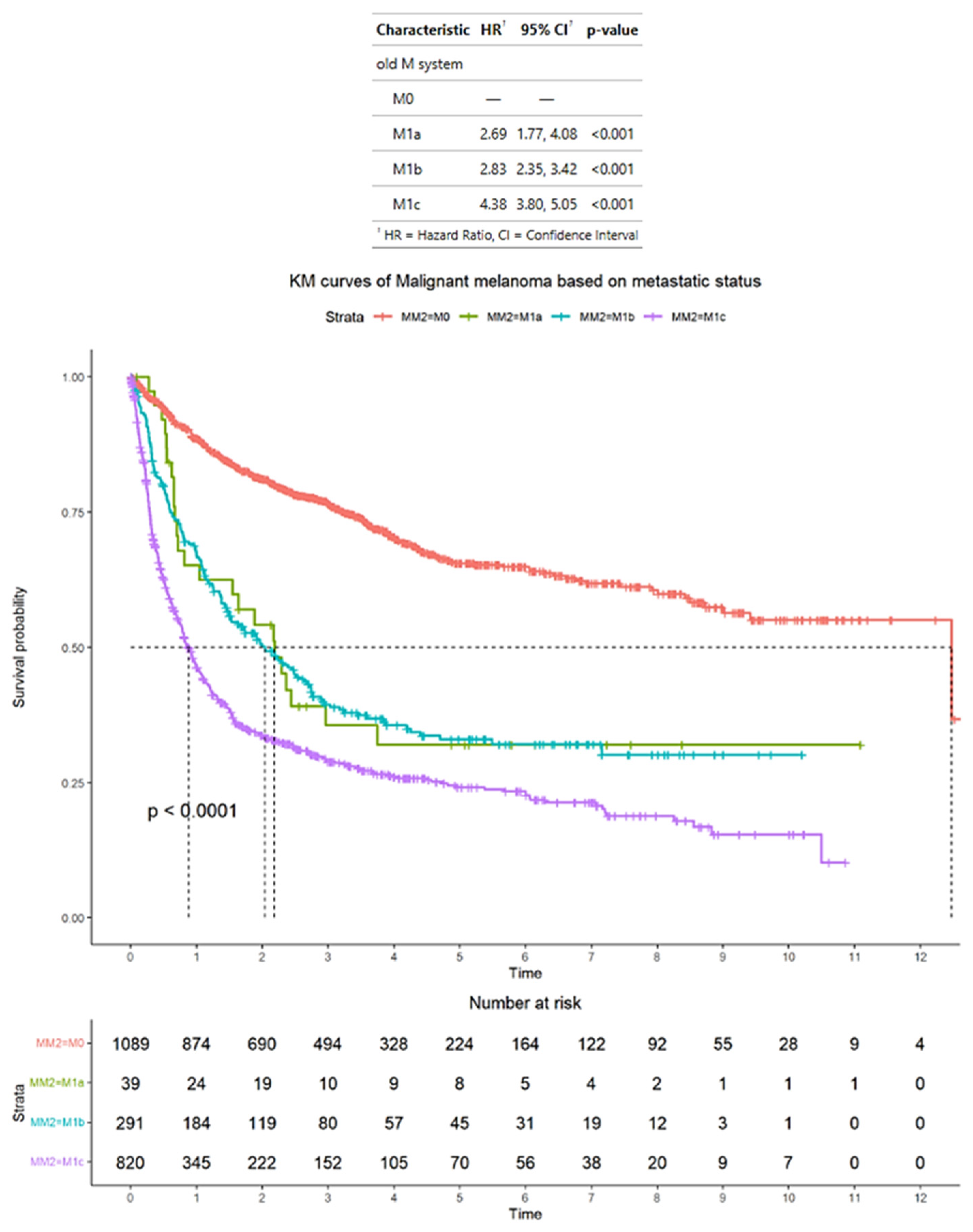
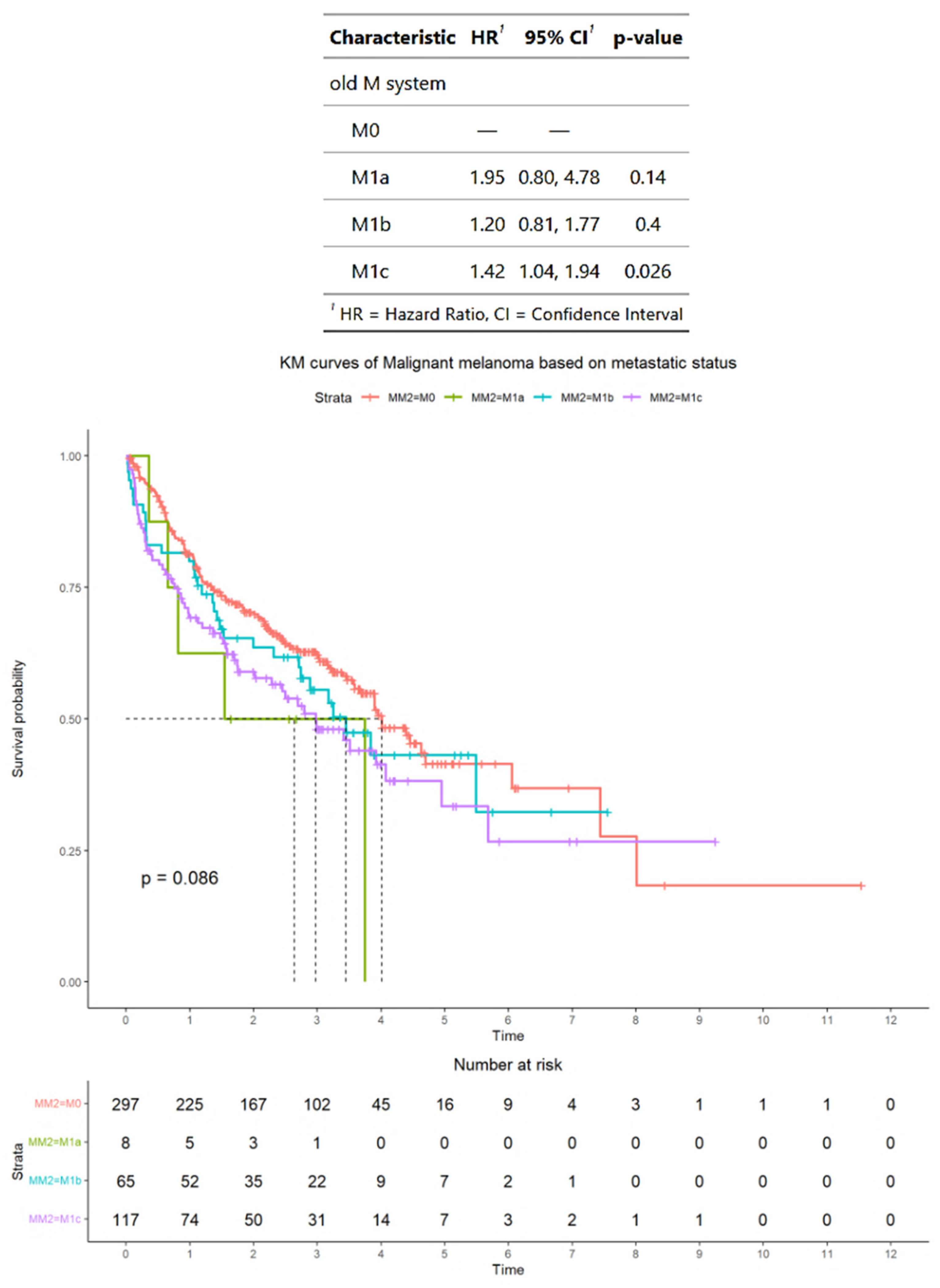
3.2. Survival Outcomes of Advanced Melanoma Classified According to AJCC Staging Criteria
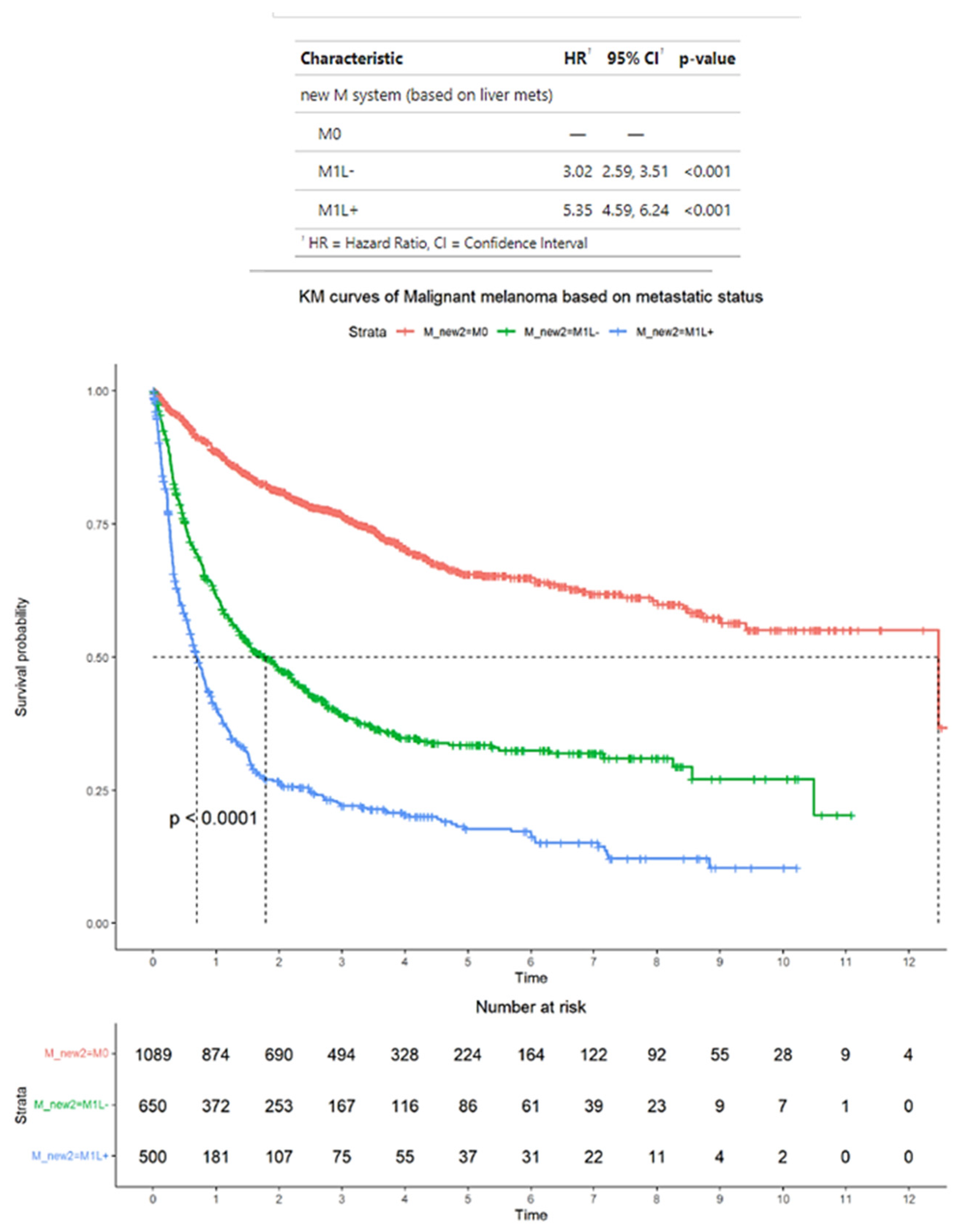
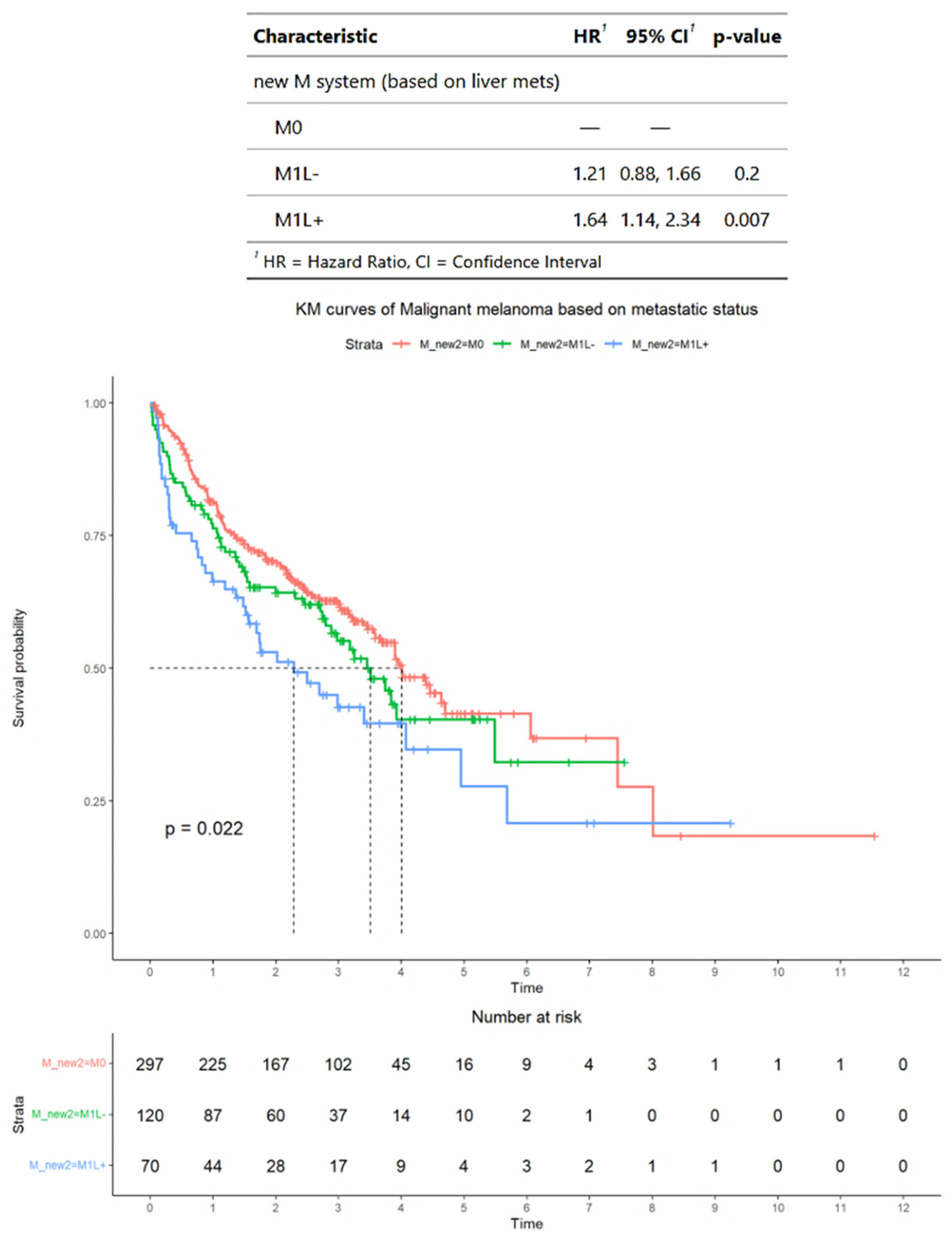
3.3. Survival Outcomes of Advanced Melanoma Classified According to Alternative Criteria
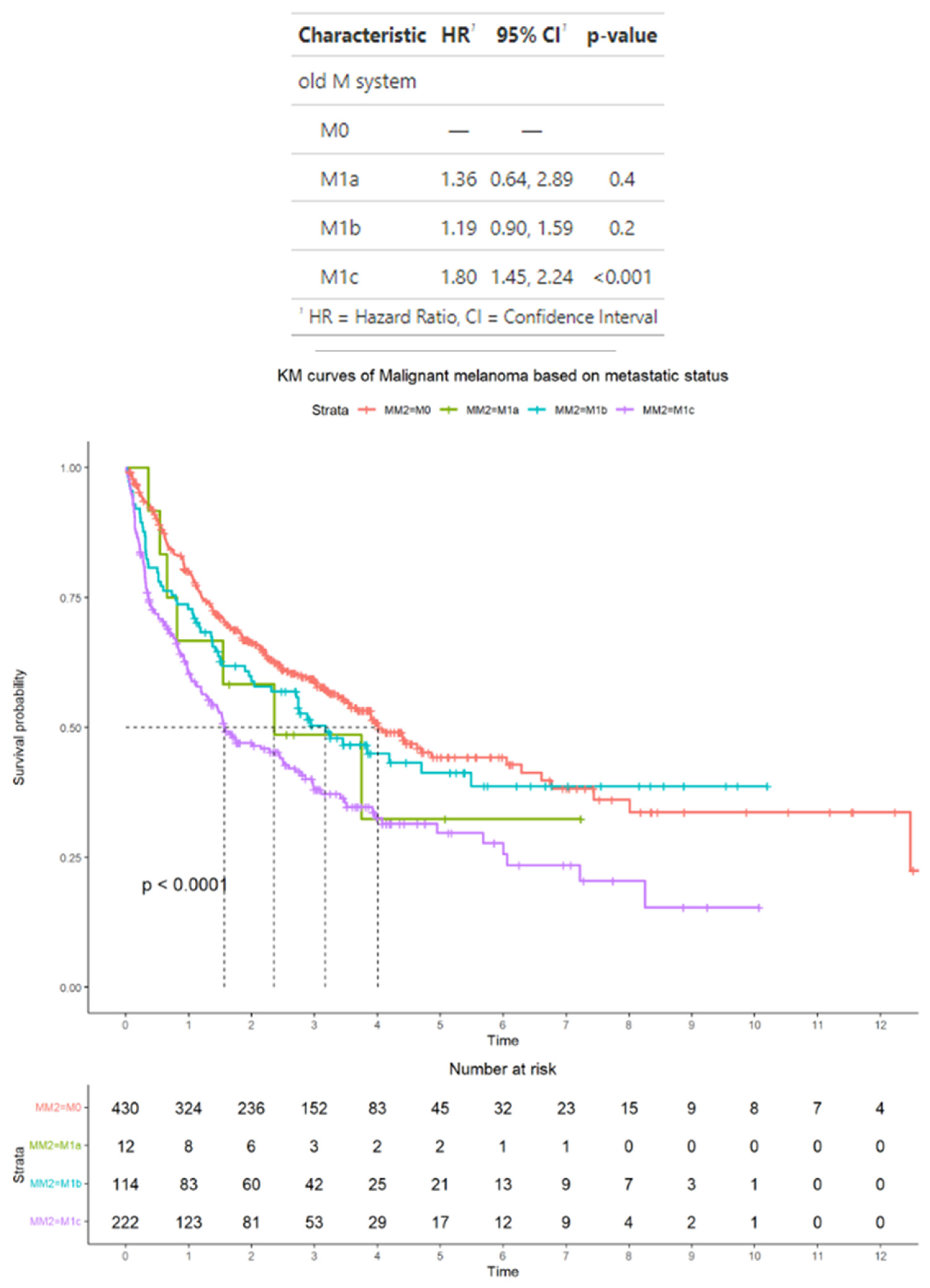
3.4. Survival Outcomes in the Subset of Advanced Melanoma Treated with Immunotherapy Classified According to AJCC Staging Criteria
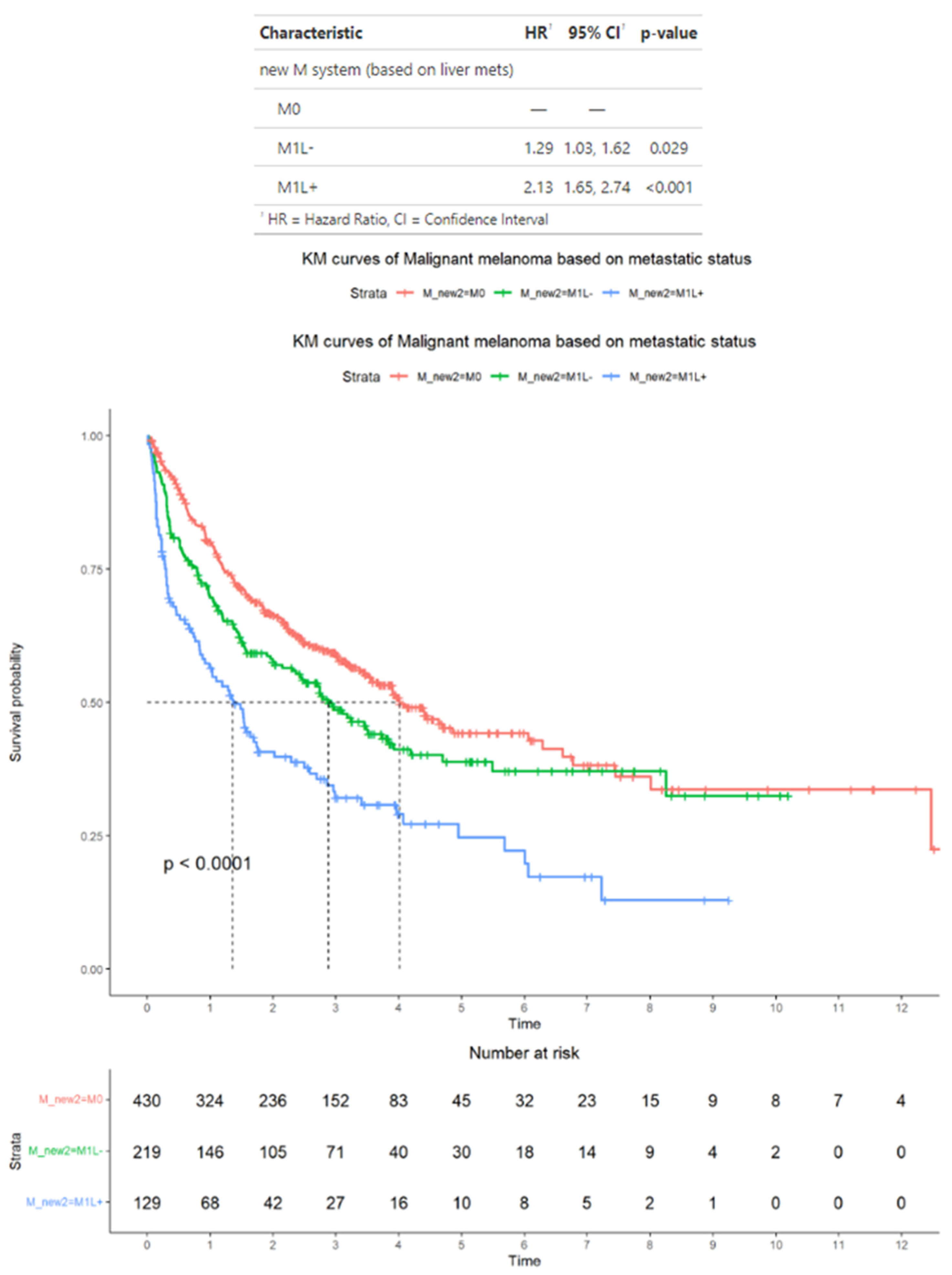
3.5. Survival Outcomes in the Subset of Advanced Melanoma Treated with Immunotherapy Classified According to Alternative Criteria
3.6. Survival Outcomes in the Subset of Advanced Melanoma Treated with Immunotherapy and with BRAF Mutation Status Available
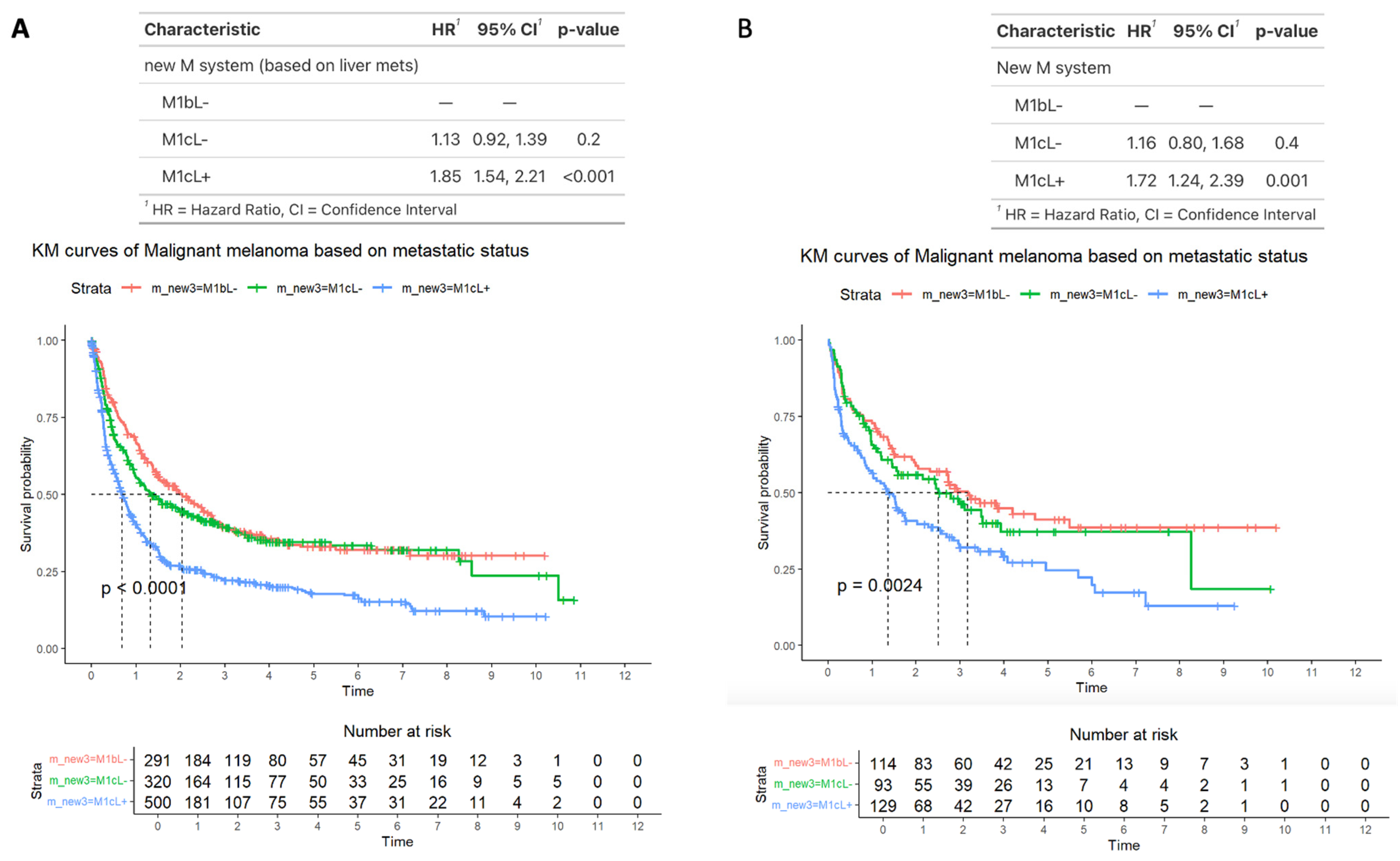
3.7. Survival Outcomes Comparing M1b, M1bL−, and M1cL+ Disease
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CNS | central nervous system |
| CT CAP | CT of the chest, abdomen, and pelvis |
| HR | hazard ratio |
| ICB | immune checkpoint blockade |
| OS | overall survival |
| NLP | natural language processing |
References
- Patel, J.K.; Didolkar, M.S.; Pickren, J.W.; Moore, R.H. Metastatic pattern of malignant melanoma. A study of 216 autopsy cases. Am. J. Surg. 1978, 135, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, R.R.; Postow, M.A. Combinatorial Approaches to the Treatment of Advanced Melanoma. Hematol. Oncol. Clin. North Am. 2021, 35, 145–158. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dréno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandalà, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Kirkwood, J.M.; Grob, J.J.; Simeone, E.; Grimaldi, A.M.; Maio, M.; Palmieri, G.; Testori, A.; Marincola, F.M.; Mozzillo, N. The role of BRAF V600 mutation in melanoma. J. Transl. Med. 2012, 10, 85. [Google Scholar] [CrossRef]
- Long, G.V.; Schachter, J.; Ribas, A.; Arance, A.M.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. 4-year survival and outcomes after cessation of pembrolizumab (pembro) after 2-years in patients (pts) with ipilimumab (ipi)-naive advanced melanoma in KEYNOTE-006. J. Clin. Oncol. 2018, 36, 9503. [Google Scholar] [CrossRef]
- Robert, C.; Flaherty, K.; Nathan, P.; Hersey, P.; Garbe, C.; Milhem, M.; Demidov, L.; Mohr, P.; Hassel, J.C.; Rutkowski, P.; et al. Five-year outcomes from a phase 3 METRIC study in patients with BRAF V600 E/K-mutant advanced or metastatic melanoma. Eur. J. Cancer 2019, 109, 61–69. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Bastholt, L.; Bataille, V.; Del Marmol, V.; Dréno, B.; Fargnoli, M.C.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: Diagnostics-Update 2019. Eur. J. Cancer 2020, 126, 141–158. [Google Scholar] [CrossRef]
- National Cancer Center Network. Melanoma (Version 2.2020). Available online: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma_blocks.pdf (accessed on 5 October 2024).
- Yu, J.; Green, M.D.; Li, S.; Sun, Y.; Journey, S.N.; Choi, J.E.; Rizvi, S.M.; Qin, A.; Waninger, J.J.; Lang, X.; et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 2021, 27, 152–164. [Google Scholar] [CrossRef]
- Bilen, M.A.; Shabto, J.M.; Martini, D.J.; Liu, Y.; Lewis, C.; Collins, H.; Akce, M.; Kissick, H.; Carthon, B.C.; Shaib, W.L.; et al. Sites of metastasis and association with clinical outcome in advanced stage cancer patients treated with immunotherapy. BMC Cancer 2019, 19, 857. [Google Scholar] [CrossRef]
- Betof, A.S.; Nipp, R.D.; Giobbie-Hurder, A.; Johnpulle, R.A.N.; Rubin, K.; Rubinstein, S.M.; Flaherty, K.T.; Lawrence, D.P.; Johnson, D.B.; Sullivan, R.J. Impact of Age on Outcomes with Immunotherapy for Patients with Melanoma. Oncologist 2017, 22, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Do, R.K.G.; Lupton, K.; Causa Andrieu, P.I.; Luthra, A.; Taya, M.; Batch, K.; Nguyen, H.; Rahurkar, P.; Gazit, L.; Nicholas, K.; et al. Patterns of Metastatic Disease in Patients with Cancer Derived from Natural Language Processing of Structured CT Radiology Reports over a 10-year Period. Radiology 2021, 301, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Causa Andrieu, P.; Golia Pernicka, J.S.; Yaeger, R.; Lupton, K.; Batch, K.; Zulkernine, F.; Simpson, A.L.; Taya, M.; Gazit, L.; Nguyen, H.; et al. Natural Language Processing of Computed Tomography Reports to Label Metastatic Phenotypes with Prognostic Significance in Patients with Colorectal Cancer. JCO Clin. Cancer Inform. 2022, 6, e2200014. [Google Scholar] [CrossRef]
- Pons, E.; Braun, L.M.; Hunink, M.G.; Kors, J.A. Natural Language Processing in Radiology: A Systematic Review. Radiology 2016, 279, 329–343. [Google Scholar] [CrossRef]
- Yim, W.W.; Yetisgen, M.; Harris, W.P.; Kwan, S.W. Natural Language Processing in Oncology: A Review. JAMA Oncol. 2016, 2, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Savova, G.K.; Danciu, I.; Alamudun, F.; Miller, T.; Lin, C.; Bitterman, D.S.; Tourassi, G.; Warner, J.L. Use of Natural Language Processing to Extract Clinical Cancer Phenotypes from Electronic Medical Records. Cancer Res. 2019, 79, 5463–5470. [Google Scholar] [CrossRef]
- Groot, O.Q.; Bongers, M.E.R.; Karhade, A.V.; Kapoor, N.D.; Fenn, B.P.; Kim, J.; Verlaan, J.J.; Schwab, J.H. Natural language processing for automated quantification of bone metastases reported in free-text bone scintigraphy reports. Acta Oncol. 2020, 59, 1455–1460. [Google Scholar] [CrossRef]
- Kehl, K.L.; Elmarakeby, H.; Nishino, M.; Van Allen, E.M.; Lepisto, E.M.; Hassett, M.J.; Johnson, B.E.; Schrag, D. Assessment of Deep Natural Language Processing in Ascertaining Oncologic Outcomes from Radiology Reports. JAMA Oncol. 2019, 5, 1421–1429. [Google Scholar] [CrossRef]
- Senders, J.T.; Karhade, A.V.; Cote, D.J.; Mehrtash, A.; Lamba, N.; DiRisio, A.; Muskens, I.S.; Gormley, W.B.; Smith, T.R.; Broekman, M.L.D.; et al. Natural Language Processing for Automated Quantification of Brain Metastases Reported in Free-Text Radiology Reports. JCO Clin. Cancer Inform. 2019, 3, 1–9. [Google Scholar] [CrossRef]
- Cheng, D.T.; Mitchell, T.N.; Zehir, A.; Shah, R.H.; Benayed, R.; Syed, A.; Chandramohan, R.; Liu, Z.Y.; Won, H.H.; Scott, S.N.; et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J. Mol. Diagn. 2015, 17, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.T.; Prasad, M.; Chekaluk, Y.; Benayed, R.; Sadowska, J.; Zehir, A.; Syed, A.; Wang, Y.E.; Somar, J.; Li, Y.; et al. Comprehensive detection of germline variants by MSK-IMPACT, a clinical diagnostic platform for solid tumor molecular oncology and concurrent cancer predisposition testing. BMC Med. Genom. 2017, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, M.; Kim, M.J.; Osman, I.; Jour, G. Impact of molecular testing in advanced melanoma on outcomes in a tertiary cancer center and as reported in a publicly available database. Cancer Rep. 2021, 4, e1380. [Google Scholar] [CrossRef]
- Mazzolini, G.; Ochoa, M.C.; Morales-Kastresana, A.; Sanmamed, M.F.; Melero, I. The liver, liver metastasis and liver cancer: A special case for immunotherapy with cytokines and immunostimulatory monoclonal antibodies. Immunotherapy 2012, 4, 1081–1085. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Hellmann, M.D.; Hamid, O.; Tsai, K.K.; Loo, K.L.; Gubens, M.A.; Rosenblum, M.; Harview, C.L.; Taube, J.M.; Handley, N.; et al. Liver Metastasis and Treatment Outcome with Anti-PD-1 Monoclonal Antibody in Patients with Melanoma and NSCLC. Cancer Immunol. Res. 2017, 5, 417–424. [Google Scholar] [CrossRef]
- Pires Da Silva, I.E.D.; Lo, S.; Gonzalez, M.; Guminski, A.; Long, G.V.; Menzies, A.M. Distinct patterns of response and toxicity (tox) by sites of metastases (mets) in patients (pts) treated with ipilimumab combined with PD-1 antibodies (ipi+PD1). J. Clin. Oncol. 2018, 36, 9553. [Google Scholar] [CrossRef]
- Rubatto, M.; Macagno, N.; Pastorin, G.; Fava, P.; Ceroni, L.; Ribero, S.; Quaglino, P. Liver metastases and immunotherapy: A barrier to response for metastatic melanoma. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e186–e188. [Google Scholar] [CrossRef] [PubMed]
- Najjar, Y.G.; Navrazhina, K.; Ding, F.; Bhatia, R.; Tsai, K.; Abbate, K.; Durden, B.; Eroglu, Z.; Bhatia, S.; Park, S.; et al. Ipilimumab plus nivolumab for patients with metastatic uveal melanoma: A multicenter, retrospective study. J. Immunother. Cancer 2020, 8, e000331. [Google Scholar] [CrossRef]
- Piulats, J.M.; Espinosa, E.; de la Cruz Merino, L.; Varela, M.; Alonso Carrión, L.; Martín-Algarra, S.; López Castro, R.; Curiel, T.; Rodríguez-Abreu, D.; Redrado, M.; et al. Nivolumab Plus Ipilimumab for Treatment-Naïve Metastatic Uveal Melanoma: An Open-Label, Multicenter, Phase II Trial by the Spanish Multidisciplinary Melanoma Group (GEM-1402). J. Clin. Oncol. 2021, 39, 586–598. [Google Scholar] [CrossRef]
- Dercle, L.; Ammari, S.; Roblin, E.; Bigorgne, A.; Champiat, S.; Taihi, L.; Plaian, A.; Hans, S.; Lakiss, S.; Tselikas, L.; et al. High serum LDH and liver metastases are the dominant predictors of primary cancer resistance to anti-PD(L)1 immunotherapy. Eur. J. Cancer 2022, 177, 80–93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, J.P.; Eichholz, J.; Sevilimedu, V.; Gangai, N.; Khalil, D.N.; Postow, M.A.; Do, R.K.G. Natural Language Processing of Radiology Reports to Assess Survival in Patients with Advanced Melanoma. Cancers 2025, 17, 1595. https://doi.org/10.3390/cancers17091595
Das JP, Eichholz J, Sevilimedu V, Gangai N, Khalil DN, Postow MA, Do RKG. Natural Language Processing of Radiology Reports to Assess Survival in Patients with Advanced Melanoma. Cancers. 2025; 17(9):1595. https://doi.org/10.3390/cancers17091595
Chicago/Turabian StyleDas, Jeeban P., Jordan Eichholz, Varadan Sevilimedu, Natalie Gangai, Danny N. Khalil, Michael A. Postow, and Richard K. G. Do. 2025. "Natural Language Processing of Radiology Reports to Assess Survival in Patients with Advanced Melanoma" Cancers 17, no. 9: 1595. https://doi.org/10.3390/cancers17091595
APA StyleDas, J. P., Eichholz, J., Sevilimedu, V., Gangai, N., Khalil, D. N., Postow, M. A., & Do, R. K. G. (2025). Natural Language Processing of Radiology Reports to Assess Survival in Patients with Advanced Melanoma. Cancers, 17(9), 1595. https://doi.org/10.3390/cancers17091595





