Contemporary Trends and Predictors Associated with Adverse Pathological Upstaging Among Non-Metastatic Localized Clinical T2 Muscle-Invasive Bladder Cancers Undergoing Radical Cystectomy: Outcomes from a Single Tertiary Centre in the United Kingdom
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Cohort and Radical Cystectomy Registry
2.2. Surgical Procedure and Pathological Upstaging Definition
2.3. Statistical Analyses
3. Results
3.1. Study Cohort Characteristics According to Final Path Upstaging
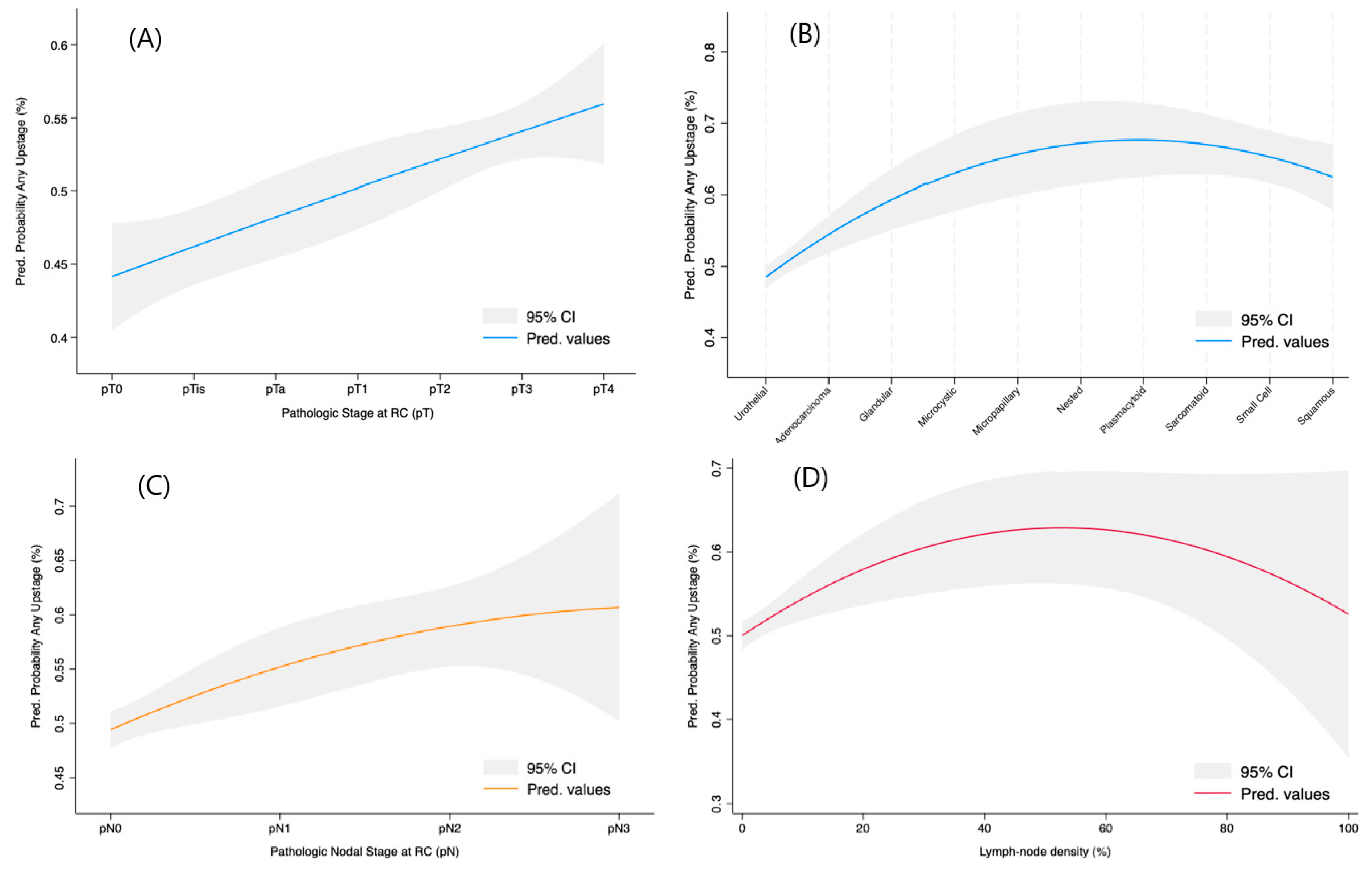
3.2. Any Pathological Upstaging at RC Specimen
3.3. Predictors for Only pT Upstaging
3.4. Predictors for Only pN Upstaging
3.5. Multivariable Regression Modelling for Independent Upstaging Predictors
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.; Luengo-Fernandez, R.; Sullivan, R.; Witjes, J.A. Economic Burden of Bladder Cancer Across the European Union. Eur. Urol. 2016, 69, 438–447. [Google Scholar] [CrossRef]
- Witjes, J.A.; Bruins, H.M.; Carrión, A.; Cathomas, R.; Compérat, E.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Lorch, A.; Martini, A.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2023 Guidelines. Eur. Urol. 2023, 85, 17–31. [Google Scholar] [CrossRef]
- Pfail, J.; Lichtbroun, B.; Golombos, D.M.; Jang, T.L.; Packiam, V.T.; Ghodoussipour, S. The role of radical cystectomy and lymphadenectomy in the management of bladder cancer with clinically positive lymph node involvement. Curr. Opin. Urol. 2024, 35, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A. The quality of life in men after radical cystectomy with a continent cutaneous diversion or orthotopic bladder sub-stitution: Is there a difference? J. Urol. 2003, 170, 331. [Google Scholar] [PubMed]
- de Angelis, M.; Basile, G.; Scornajenghi, C.M.; Asero, V.; Del Giudice, F.; Moschini, M. Bladder-sparing strategies in patients with clinically localized muscle-invasive bladder cancer. Curr. Opin. Urol. 2023, 33, 354–359. [Google Scholar] [CrossRef]
- Kamat, A.M.; Black, P.C. Bladder Cancer: A Practical Guide; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Bayraktar, Z.; Gurbuz, G.; Taşci, A.I.; Sevin, G. Staging error in the bladder tumor: The correlation between stage of TUR and cys-tectomy. Int. Urol. Nephrol. 2001, 33, 627–629. [Google Scholar] [CrossRef]
- Mclaughlin, S.; Shephard, J.; Wallen, E.; Maygarden, S.; Carson, C.C.; Pruthi, R.S. Comparison of the clinical and pathologic staging in patients undergoing radical cystectomy for bladder cancer. Int. Braz. J. Urol. 2007, 33, 25–32. [Google Scholar] [CrossRef]
- Svatek, R.S.; Shariat, S.F.; Novara, G.; Skinner, E.C.; Fradet, Y.; Bastian, P.J.; Kamat, A.M.; Kassouf, W.; Karakiewicz, P.I.; Fritsche, H.; et al. Discrepancy between clinical and pathological stage: External validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int. 2011, 107, 898–904. [Google Scholar] [CrossRef]
- Turker, P.; Bostrom, P.J.; Wroclawski, M.L.; van Rhijn, B.; Kortekangas, H.; Kuk, C.; Mirtti, T.; Fleshner, N.E.; Jewett, M.A.; Finelli, A.; et al. Upstaging of urothelial cancer at the time of radical cystectomy: Factors associated with upstaging and its effect on outcome. BJU Int. 2012, 110, 804–811. [Google Scholar] [CrossRef]
- McFadden, J.; Tachibana, I.; Adra, N.; Collins, K.; Cary, C.; Koch, M.; Kaimakliotis, H.; Masterson, T.; Rice, K. Impact of variant histology on upstaging and survival in patients with nonmuscle invasive bladder cancer undergoing radical cystectomy. Urol. Oncol. 2024, 42, 69.e11–69.e16. [Google Scholar] [CrossRef] [PubMed]
- van Hoogstraten, L.M.C.; Man, C.C.O.; Witjes, J.A.; Meijer, R.P.; Mulder, S.F.; Smilde, T.J.; Ripping, T.M.; Kiemeney, L.A.; Aben, K.K.H.; BlaZIB Study Group; et al. Low adherence to recommended use of neoadjuvant chemotherapy for muscle-invasive bladder cancer. World J. Urol. 2023, 41, 1837–1845. [Google Scholar] [CrossRef]
- Albisinni, S.; Orecchia, L.; Mjaess, G.; Aoun, F.; Del Giudice, F.; Antonelli, L.; Moschini, M.; Soria, F.; Mertens, L.S.; Gallioli, A.; et al. Enhanced Recovery After Surgery for patients undergoing radical cystectomy: Surgeons’ perspectives and recommendations ten years after its implementation. Eur. J. Surg. Oncol. (EJSO) 2024, 51, 109543. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, V.; Carino, D.; Corvino, R.; Salciccia, S.; De Berardinis, E.; Krajewski, W.; Nowak, Ł.; Łaszkiewicz, J.; Szydełko, T.; Nair, R.; et al. Surgical Technique and Perioperative Outcomes of the “Sapienza” Urology Residency Program’s Trocar Placement Configuration During Robotic-Assisted Radical Prostatectomy (RARP): A Retrospective, Single-Centre Observational Study Comparing Experienced Attendings vs. Post-Graduate Year I–III Residents as Bedside Assistants. Cancers 2024, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- Khetrapal, P.; Catto, J.; Ambler, G.; Ricciardi, F.; Khan, S.; Feber, A.; Dixon, S.; Williams, N.; Ahmed, I.; Charlesworth, P.; et al. PD42-02 Results of the Intracorporeal Robotic vs Open Cystectomy (IROC) Multi-Centre Randomised Trial. J. Urol. 2022, 207, e695. [Google Scholar] [CrossRef]
- Ge, P.; Wang, L.; Lu, M.; Mao, L.; Li, W.; Wen, R.; Lin, J.; Wang, J.; Chen, J. Oncological Outcome of Primary and Secondary Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-analysis. Sci. Rep. 2018, 8, 7543. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Compérat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef]
- El-Adawy, M.S.; Ibrahim, H.; Zanaty, F.; Kotb, S. Factors related to upstaging of clinical stage T2 organ-confined bladder cancer following radical cystectomy: A multicenter study. Urol. Ann. 2022, 14, 232–235. [Google Scholar] [CrossRef]
- Yafi, F.A.; Aprikian, A.G.; Chin, J.L.; Fradet, Y.; Izawa, J.; Estey, E.; Fairey, A.; Rendon, R.; Cagiannos, I.; Lacombe, L.; et al. Impact of concomitant carcinoma in situ on upstaging and outcome following radical cystectomy for bladder cancer. World J. Urol. 2013, 32, 1295–1301. [Google Scholar] [CrossRef]
- Bouchelouche, K. PET/CT in Bladder Cancer: An Update. Semin. Nucl. Med. 2022, 52, 475–485. [Google Scholar] [CrossRef]
- Mertens, L.S.; Fioole-Bruining, A.; Vegt, E.; Vogel, W.V.; van Rhijn, B.W.; Horenblas, S. Impact of (18) F-fluorodeoxyglucose (FDG)-positron-emission tomography/computed tomography (PET/CT) on management of patients with carcinoma invading bladder muscle. BJU Int. 2013, 112, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Tufano, A.; Rosati, D.; Moriconi, M.; Santarelli, V.; Canale, V.; Salciccia, S.; Sciarra, A.; Franco, G.; Cantisani, V.; Di Pierro, G.B. Diagnostic Accuracy of Contrast-Enhanced Ultra-sound (CEUS) in the Detection of Muscle-Invasive Bladder Cancer: A Systematic Review and Diagnostic Meta-Analysis. Curr. Oncol. 2024, 31, 818–827. [Google Scholar] [CrossRef]
- Amenyogbe, A.; Lemire, F.; Yachnin, D.; Carrier, M.; McAlpine, K.; Breau, R.H.; Bossé, D.; Wang, T.-F.; Morash, C.; Cagiannos, I.; et al. A survey of physician perception and practices regarding pharmacological thromboprophylaxis during chemotherapy for bladder cancer. Can. Urol. Assoc. J. 2022, 16, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Proietti, F.; Flammia, R.S.; Licari, L.C.; Bologna, E.; Bove, A.M.; Brassetti, A.; Tuderti, G.; Mastroianni, R.; Tufano, A.; Simone, G.; et al. Impacts of Neoadjuvant Chemotherapy on Perioperative Outcomes in Patients with Bladder Cancer Treated with Radical Cystectomy: A Single High-Volume Center Experience. J. Pers. Med. 2024, 14, 212. [Google Scholar] [CrossRef]
- de Angelis, M.; Jannello, L.M.I.; Siech, C.; Baudo, A.; Di Bello, F.; Goyal, J.A.; Tian, Z.; Longo, N.; de Cobelli, O.; Chun, F.K.; et al. Neoadjuvant chemotherapy before radical cystectomy in patients with organ-confined and non-organ-confined urothelial carcinoma. Urol. Oncol. 2025, 43, 62.e1–62.e6. [Google Scholar] [CrossRef]
- Audenet, F.; Sfakianos, J.P.; Waingankar, N.; Ruel, N.H.; Galsky, M.D.; Yuh, B.E.; Gin, G.E. A delay ≥8 weeks to neoadjuvant chemotherapy before radical cystectomy increases the risk of upstaging. Urol. Oncol. Semin. Orig. Investig. 2018, 37, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Campbell, R.A.; Michael, P.D.; Wood, A.; Haywood, S.C.; Eltemamy, M.; Kaouk, J.; Campbell, S.C.; Haber, G.-P.; Weight, C.J.; et al. Clinical Upstaging After Neoadjuvant Chemotherapy Impacting Eligibility for Vaginal-sparing Cystectomy: Identifying Bladder Cancer Patients Who May Benefit From Interim Imaging. Urology 2024, 191, 102–109. [Google Scholar] [CrossRef]
- Boeri, L.; Soligo, M.; Frank, I.; Boorjian, S.A.; Thompson, R.H.; Tollefson, M.; Tarrel, R.; Quevedo, F.J.; Cheville, J.C.; Karnes, R.J. Clinical predictors and survival outcome of patients receiving suboptimal neoadjuvant chemotherapy and radical cystectomy for muscle-invasive bladder cancer: A single-center experience. World J. Urol. 2019, 37, 2409–2418. [Google Scholar] [CrossRef]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef]
- Liu, K.X.; Haas-Kogan, D.; Laprie, A. Role of Radiotherapy in the Era of Targeted Therapy and Precision Oncology; Frontiers Media SA: Lausanne, Switzerland, 2022. [Google Scholar]
- Fan, X.; He, W.; Huang, J. Bladder-sparing approaches for muscle invasive bladder cancer: A narrative review of current evidence and future perspectives. Transl. Androl. Urol. 2023, 12, 802–808. [Google Scholar] [CrossRef]
- Renner, A.; Burotto, M.; Valdes, J.M.; Roman, J.C.; Walton-Diaz, A. Neoadjuvant immunotherapy for muscle invasive urothelial bladder carcinoma: Will it change current standards? Ther. Adv. Urol. 2021, 13, 17562872211029779. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Meghani, K.; Cooley, L.F.; McLaughlin, K.A.; Fall, L.A.; Yu, Y.; Castro, M.A.A.; Groeneveld, C.S.; de Reyniès, A.; Nazarov, V.I.; et al. Expression-based subtypes define pathologic response to neoadjuvant immune-checkpoint inhibitors in muscle-invasive bladder cancer. Nat. Commun. 2023, 14, 2126. [Google Scholar] [CrossRef]
- Moschini, M.; Gandaglia, G.; Dehò, F.; Salonia, A.; Briganti, A.; Montorsi, F. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 561–563. [Google Scholar] [CrossRef]
- Zhu, Z.; Xiao, Y.; Hu, S.; Wang, Z.; Zhu, Z. Neoadjuvant and Adjuvant Chemotherapy for Variant Histology Bladder Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 907454. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.A.; Khanna, A.; Boorjian, S.A.; Knorr, J.; Cox, R.; Nicholas, M.; Cheville, J.; Sharma, V.; Murthy, P.B.; Tarrell, R.; et al. Impact of Neoadjuvant Chemotherapy on Pathologic Downstaging in Patients with Variant Histology Undergoing Radical Cystectomy. Clin. Genitourin. Cancer 2023, 22, 157–163.e1. [Google Scholar] [CrossRef] [PubMed]
- Tabayoyong, W.; Li, R.; Gao, J.; Kamat, A. Optimal Timing of Chemotherapy and Surgery in Patients with Muscle-Invasive Bladder Cancer and Upper Urinary Tract Urothelial Carcinoma. Urol. Clin. N. Am. 2018, 45, 155–167. [Google Scholar] [CrossRef]
- Krasnow, R.E.; Drumm, M.; Roberts, H.J.; Niemierko, A.; Wu, C.-L.; Wu, S.; Zhang, J.; Heney, N.M.; Wszolek, M.F.; Blute, M.L.; et al. Clinical Outcomes of Patients with Histologic Variants of Urothelial Cancer Treated with Trimodality Bladder-sparing Therapy. Eur. Urol. 2017, 72, 54–60. [Google Scholar] [CrossRef]
- Fischer-Valuck, B.W.; Michalski, J.M.; Contreras, J.A.; Brenneman, R.; Christodouleas, J.P.; Abraham, C.D.; Kim, E.H.; Arora, V.K.; Bullock, A.D.; Carmona, R.; et al. A propensity analysis comparing definitive chemo-radiotherapy for muscle-invasive squamous cell carcinoma of the bladder vs. urothelial carcinoma of the bladder using the National Cancer Database. Clin. Transl. Radiat. Oncol. 2018, 15, 38–41. [Google Scholar] [CrossRef]
| Total | pT/N Non-Upstaging (i.e., ≤pT2, pN0) | pT/N Upstaging (i.e., >pT2, >pN0) | p Value | |
|---|---|---|---|---|
| Sample Size, n (%) | N = 275 | N = 134 (47.2%) | N = 141(51.3%) | |
| Gender, n (%) | 0.7 | |||
| Male | 202 (73.5%) | 100 (74.6%) | 102 (72.3%) | |
| Female | 73 (26.5%) | 34 (25.4%) | 39 (27.7%) | |
| Age, median (IQR) | 69 (55–78) | 68 (55–76) | 68 (55–78) | 0.5 |
| BMI, median (IQR) | 27.4 (24–31) | 27.5 (24–31) | 27.3 (24.1–30) | 0.8 |
| CCI, median (IQR) | 5 (2–8) | 5 (2–8) | 5 (2–7) | 0.8 |
| ASA, median (IQR) | 2 (1–3) | 2 (1–3) | 2 (1–3) | 0.9 |
| No. of previous TURBTs, n (%) | 0.9 | |||
| 1 | 235 (85.5%) | 115 (85.8%) | 120 (65.2%) | |
| ≥2 | 40 (14.5%) | 19 (14.2%) | 21 (14.8%) | |
| Concomitant CIS, n (%) | 0.8 | |||
| No | 197 (71.6%) | 97 (72.4%) | 100 (71%) | |
| Yes | 78 (28.4%) | 37 (27.6%) | 41 (29%) | |
| TURBT Grade, n (%) | 0.02 | |||
| Low grade | 12 (4.4%) | 10 (7.7%) | 2 (2.2%) | |
| High grade | 260 (95.6%) | 123 (92.5%) | 137 (97.8%) | |
| Previous BCG exposure, n (%) | 0.48 | |||
| No | 240 (87.3%) | 115 (85.8%) | 125 (88.6%) | |
| Yes | 35 (12.7%) | 19 (14.2%) | 16 (11.4%) | |
| mpMRI, n (%) | 0.98 | |||
| No | 240 (87.3%) | 117 (87.3%) | 123 (87.3%) | |
| Yes | 35 (12.7%) | 17 (12.7%) | 18 (12.7%) | |
| FDG-PET, n (%) | 0.58 | |||
| No | 199 (72.4%) | 99 (68.4%) | 100 (71%) | |
| Yes | 76 (27.6%) | 35 (31.6%) | 41 (29%) | |
| MDT discussion, n (%) | 0.01 | |||
| No | 52 (18.9%) | 17 (12.7%) | 35 (24.8%) | |
| Yes | 223 (81.1%) | 117 (87.3%) | 106 (75.2%) | |
| NAC, n (%) | 0.001 | |||
| No | 187 (68%) | 78 (58.2%) | 109 (78.3%) | |
| Yes | 88 (32%) | 56 (41.8%) | 32 (22.7%) | |
| RC surgical approach, n (%) | 0.57 | |||
| ORC | 79 (28.4%) | 40 (29.9%) | 39 (27.7%) | |
| RARC | 196 (71.6%) | 94 (70.1%) | 102 (72.3%) | |
| PLND, n (%) | 0.5 | |||
| Standard | 211 (73%) | 101 (75.4%) | 110 (78%) | |
| Extended | 64 (23%) | 33 (24.6%) | 31 (22%) | |
| Urinary diversion, n (%) | 0.5 | |||
| UCS | 6 (2.2%) | 3 (2.2%) | 3 (2.1%) | |
| IC intracorporeal | 200 (72.7%) | 95 (70.9%) | 105 (74.5%) | |
| IC extracorporeal | 53 (19.3%) | 26 (19.4%) | 27 (19.1%) | |
| Neobladder | 16 (5.8%) | 10 (7.5%) | 6 (4.3%) | |
| Any intraop. complications, n (%) | 0.65 | |||
| No | 252 (91.7%) | 123 (91.8%) | 129 (91.5%) | |
| Yes | 23 (8.3%) | 11 (8.2%) | 12 (8.5%) | |
| Clavien–Dindo grade, n (%) | 0.4 | |||
| Grade I | 5 (21.5%) | 2 (18.2%) | 3 (25%) | |
| Grade II | 13 (53.2%) | 7 (63.6%) | 6 (50%) | |
| Grade III | 4 (17.4%) | 2 (18.2%) | 2 (16.6%) | |
| Grade IV | 1 (4.3%) | 0 | 1 (8.3%) | |
| Grade V | 0 | 0 | 0 | |
| Length of stay, median (IQR) | 7 (4–11) | 7 (4–11) | 8 (5–11) | 0.5 |
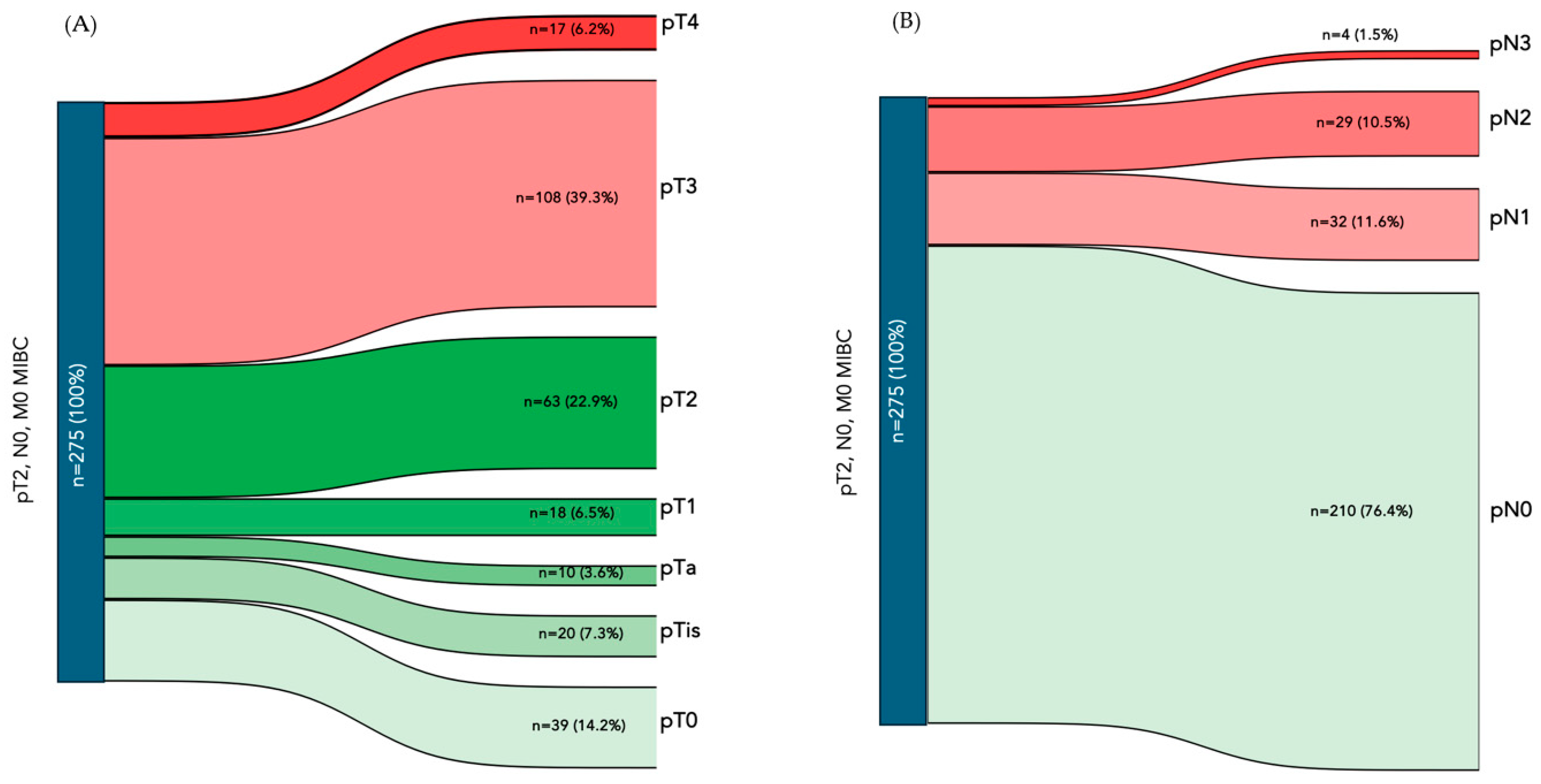
| pT stage, n (%) | <0.001 | |||
| pT0 | 39 (14.2%) | 38 (56.1%) | 1 (0.7%) | |
| pTa | 10 (3.6%) | 10 (14.9%) | 0 (0%) | |
| pTis | 20 (7.3%) | 17 (25.4%) | 3 (2.1%) | |
| pT1 | 18 (6.5%) | 15 (23.6%) | 3 (2.1%) | |
| pT2 | 63 (22.9%) | 54 (81.1%) | 9 (6.4%) | |
| pT3 | 108 (39.3%) | 0 (0%) | 108 (76.6%) | |
| pT4 | 17 (6.2%) | 0 (0%) | 17 (12%) | |
| pN stage, n (%) | <0.001 | |||
| pN0 | 210 (76.4%) | 134 (100%) | 76 (53.9%) | |
| pN1 | 32 (11.6%) | 0 (0%) | 32 (22.7%) | |
| pN2 | 29 (10.5%) | 0 (0%) | 29 (20.6%) | |
| pN3 | 4 (1.5%) | 0 (0%) | 4 (2.8%) | |
| Grade at RC, n (%) | NA | |||
| Low-grade | 1 (0.4%) | 1 (0.8%) | 0 | |
| High-grade | 272 (99.6%) | 132 (99.2%) | 140 (100%) | |
| Surgical Margins, n (%) | <0.001 | |||
| Negative | 256 (93.1%) | 133 (99.3%) | 123 (87.2%) | |
| Positive | 19 (6.9%) | 1 (0.7%) | 18 (12.8%) | |
| Variant Histology at RC, n (%) | 0.05 | |||
| Absent | 226 (82.2%) | 117 (87.3%) | 109 (77.2%) | |
| Present | 49 (17.8%) | 17 (12.7%) | 32 (22.7%) |
| Any Upstaging | pT Upstaging | pN Upstaging | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | OR | 95%CI | p Value | OR | 95%CI | p Value | OR | 95%CI | p Value |
| Gender | |||||||||
| Male | Ref. | Ref. | Ref. | ||||||
| Female | 0.89 | 0.5–1.5 | 0.39 | 0.9 | 0.5–1.5 | 0.36 | 0.9 | 0.5–1.7 | 0.5 |
| No. of previous TURBTs | |||||||||
| 1 | Ref. | Ref. | Ref. | ||||||
| 2+ | 1.06 | 0.54–2.07 | 0.5 | 0.9 | 0.4–1.7 | 0.41 | 1.1 | 0.5–2.4 | 0.5 |
| Concomitant CIS | |||||||||
| No | Ref. | Ref. | Ref. | ||||||
| Yes | 1.08 | 0.64–1.82 | 0.45 | 0.97 | 0.6–1.64 | 0.51 | 0.9 | 0.5–1.6 | 0.4 |
| Grade at TURBT | NA | NA | NA | ||||||
| Low-grade | Ref. | Ref. | |||||||
| High-grade | 2.1 | 1.5–7 | 0.04 | 5.6 | 1.2–37 | 0.002 | |||
| Previous BCG exposure | |||||||||
| No | Ref. | Ref. | Ref. | ||||||
| Yes | 0.78 | 0.38–1.58 | 0.3 | 0.78 | 0.38–1.6 | 0.31 | 1 | 0.4–2.2 | 0.6 |
| mpMRI | |||||||||
| No | Ref. | Ref. | Ref. | ||||||
| Yes | 1.01 | 0.5–2.05 | 0.56 | 1.01 | 0.5–2 | 0.6 | 0.8 | 0.3–1.9 | 0.4 |
| FDG-PET | |||||||||
| No | Ref. | Ref. | Ref. | ||||||
| Yes | 1.16 | 0.7–1.97 | 0.34 | 0.83 | 0.5–1.4 | 0.3 | 1.6 | 0.9–3 | 0.1 |
| MDT discussion | |||||||||
| No | Ref. | Ref. | Ref. | ||||||
| Yes | 0.51 | 0.2–0.9 | 0.01 | 0.6 | 0.35–0.96 | 0.02 | 0.4 | 0.2–0.8 | 0.005 |
| NAC | |||||||||
| No | Ref. | Ref. | Ref. | ||||||
| Yes | 0.41 | 0.24–0.7 | 0.001 | 0.41 | 0.24–0.7 | 0.001 | 0.3 | 0.1–0.6 | 0.001 |
| RC surgical approach | |||||||||
| ORC | Ref. | Ref. | Ref. | ||||||
| RARC | 1.17 | 0.68–1.99 | 0.33 | 1 | 0.6–1.8 | 0.5 | 0.9 | 0.5–1.6 | 0.4 |
| Any intraop. complications | |||||||||
| No | Ref. | Ref. | Ref. | ||||||
| Yes | 0.71 | 0.16–3.22 | 0.5 | 0.9 | 0.2–4.1 | 0.6 | 0.8 | 0.7–0.8 | 0.15 |
| Variant Histology at RC | |||||||||
| Absent | Ref. | Ref. | Ref. | ||||||
| Present | 1.82 | 1.1–3.43 | 0.04 | 4 | 2.5–6.5 | <0.001 | 1.5 | 0.8–3 | 0.2 |
| Any Upstaging | pT Upstaging | pN Upstaging | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameter | OR | 95%CI | p Value | OR | 95%CI | p Value | OR | 95%CI | p Value |
| Gender | |||||||||
| Male | Ref. | Ref. | Ref. | ||||||
| Female | 0.9 | 0.5–1.6 | 0.75 | 0.9 | 0.5–1.6 | 0.7 | 0.9 | 0.5–1.8 | 0.8 |
| Concomitant CIS | |||||||||
| No | Ref. | Ref. | Ref. | ||||||
| Yes | 1.2 | 0.7–2.2 | 0.5 | 1.1 | 0.6–1.9 | 0.9 | 1 | 0.5–2 | 0.9 |
| Grade at TURBT | Ref. | Ref. | NA | NA | NA | ||||
| Low-grade | |||||||||
| High-grade | 4.3 | 1.1–17 | 0.04 | 5.6 | 1.3–36 | 0.02 | |||
| Previous BCG exposure | |||||||||
| No | Ref. | Ref. | Ref. | ||||||
| Yes | 0.7 | 0.4–1.6 | 0.4 | 0.7 | 0.3–1 | 0.4 | 1 | 0.4–2.4 | 1 |
| mpMRI | |||||||||
| No | Ref. | Ref. | Ref. | ||||||
| Yes | 1 | 0.43–2.1 | 0.9 | 0.9 | 0.4–2 | 0.8 | 0.9 | 0.3–2.5 | 0.9 |
| FDG-PET | |||||||||
| No | Ref. | Ref. | Ref. | ||||||
| Yes | 1.3 | 0.7–2.3 | 0.4 | 0.9 | 0.5–1.5 | 0.6 | 1.8 | 1–3.3 | 0.05 |
| MDT discussion | |||||||||
| No | Ref. | Ref. | Ref. | ||||||
| Yes | 0.5 | 0.3–0.9 | 0.02 | 0.5 | 0.3–0.9 | 0.02 | 0.44 | 0.2–0.9 | 0.02 |
| NAC | |||||||||
| No | Ref. | Ref. | Ref. | ||||||
| Yes | 0.4 | 0.2–0.7 | 0.002 | 0.4 | 0.2–0.7 | 0.02 | 0.3 | 0.1–0.6 | 0.001 |
| Variant Histology at RC | |||||||||
| Absent | Ref. | Ref. | Ref. | ||||||
| Present | 2.3 | 1.1–4.6 | 0.02 | 2.5 | 1.3–5 | 0.01 | 1.7 | 0.8–3.6 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Giudice, F.; Abu-Ghanem, Y.; Nair, R.; Mensah, E.; Kam, J.; Ibrahim, Y.; Gad, M.; Chatterton, K.; Amery, S.; Alao, R.; et al. Contemporary Trends and Predictors Associated with Adverse Pathological Upstaging Among Non-Metastatic Localized Clinical T2 Muscle-Invasive Bladder Cancers Undergoing Radical Cystectomy: Outcomes from a Single Tertiary Centre in the United Kingdom. Cancers 2025, 17, 1477. https://doi.org/10.3390/cancers17091477
Del Giudice F, Abu-Ghanem Y, Nair R, Mensah E, Kam J, Ibrahim Y, Gad M, Chatterton K, Amery S, Alao R, et al. Contemporary Trends and Predictors Associated with Adverse Pathological Upstaging Among Non-Metastatic Localized Clinical T2 Muscle-Invasive Bladder Cancers Undergoing Radical Cystectomy: Outcomes from a Single Tertiary Centre in the United Kingdom. Cancers. 2025; 17(9):1477. https://doi.org/10.3390/cancers17091477
Chicago/Turabian StyleDel Giudice, Francesco, Yasmin Abu-Ghanem, Rajesh Nair, Elsie Mensah, Jonathan Kam, Youssef Ibrahim, Mohamed Gad, Kathryn Chatterton, Suzanne Amery, Romerr Alao, and et al. 2025. "Contemporary Trends and Predictors Associated with Adverse Pathological Upstaging Among Non-Metastatic Localized Clinical T2 Muscle-Invasive Bladder Cancers Undergoing Radical Cystectomy: Outcomes from a Single Tertiary Centre in the United Kingdom" Cancers 17, no. 9: 1477. https://doi.org/10.3390/cancers17091477
APA StyleDel Giudice, F., Abu-Ghanem, Y., Nair, R., Mensah, E., Kam, J., Ibrahim, Y., Gad, M., Chatterton, K., Amery, S., Alao, R., Challacombe, B., Hegazy, M., Crocetto, F., Santarelli, V., Łaszkiewicz, J., Rocco, B., Sciarra, A., Chung, B. I., Thurairaja, R., & Khan, M. S. (2025). Contemporary Trends and Predictors Associated with Adverse Pathological Upstaging Among Non-Metastatic Localized Clinical T2 Muscle-Invasive Bladder Cancers Undergoing Radical Cystectomy: Outcomes from a Single Tertiary Centre in the United Kingdom. Cancers, 17(9), 1477. https://doi.org/10.3390/cancers17091477






