Novel Strategies and Therapeutic Advances for Bladder Cancer
Simple Summary
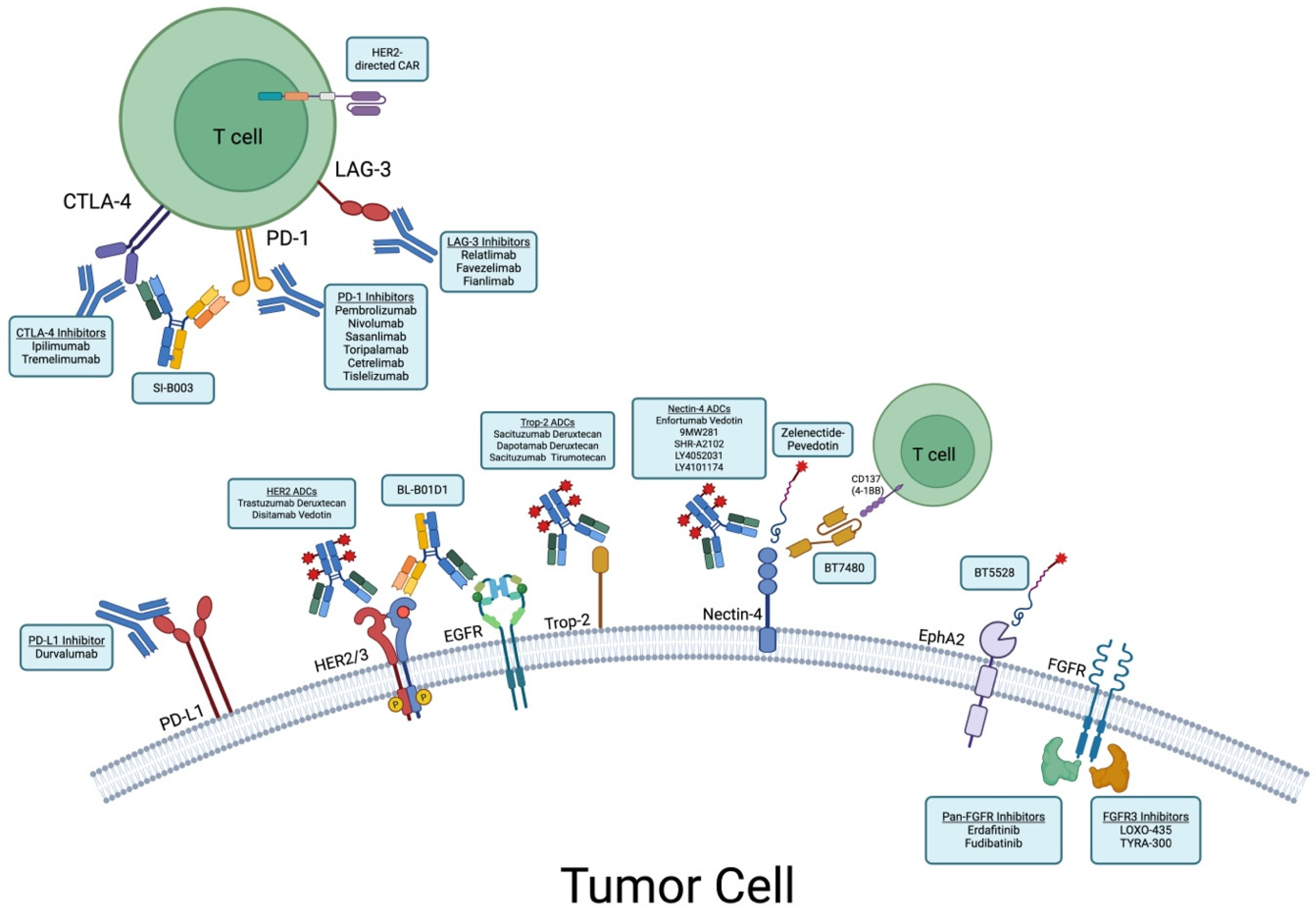
Abstract
1. Introduction
2. New Strategies for Muscle-Invasive Bladder Cancer
2.1. Perioperative Chemoimmunotherapy with Radical Cystectomy
2.2. Neoadjuvant Antibody–Drug Conjugates (ADC) with Radical Cystectomy
2.3. Targeted Therapies for Localized MIBC
2.4. Intravesical Therapy in Combination with Immunotherapy
2.5. Vaccine Therapy in Combination with Immunotherapy
2.6. Cytokine Therapies
3. Cystectomy-Sparing Approaches
3.1. Systemic Therapy
3.2. Trimodality Therapy (TMT)
4. Novel Therapeutics for Advanced Urothelial Cancer
4.1. Chemoimmunotherapy
4.2. The Next Wave of Antibody-Drug Conjugates
4.2.1. Nectin-4
4.2.2. HER2
4.2.3. Trop-2
4.2.4. Other Novel Targets
4.3. Small Molecule–Drug Conjugates
4.4. Targeted Therapies in Advanced/Metastatsic Urothelial Cancer
4.5. Cellular Therapies
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rumgay, H.; Li, M.; Yu, H.; Pan, H.; Ni, J. The Global Landscape of Bladder Cancer Incidence and Mortality in 2020 and Projections to 2040. J. Glob. Health 2023, 13, 04109. [Google Scholar] [CrossRef]
- Wéber, A.; Vignat, J.; Shah, R.; Morgan, E.; Laversanne, M.; Nagy, P.; Kenessey, I.; Znaor, A. Global Burden of Bladder Cancer Mortality in 2020 and 2040 According to GLOBOCAN Estimates. World J. Urol. 2024, 42, 237. [Google Scholar] [CrossRef] [PubMed]
- Pemov, A.; Wegman-Ostrosky, T.; Kim, J.; Koutros, S.; Douthitt, B.; Jones, K.; Zhu, B.; Baris, D.; Schwenn, M.; Johnson, A.; et al. Identification of Genetic Risk Factors for Familial Urinary Bladder Cancer: An Exome Sequencing Study. JCO Precis. Oncol. 2021, 5, 1830–1839. [Google Scholar] [CrossRef]
- Lenis, A.T.; Lec, P.M.; Chamie, K.; Mshs, M. Bladder Cancer: A Review. JAMA 2020, 324, 1980–1991. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Kirkali, Z.; Chan, T.; Manoharan, M.; Algaba, F.; Busch, C.; Cheng, L.; Kiemeney, L.; Kriegmair, M.; Montironi, R.; Murphy, W.M.; et al. Bladder Cancer: Epidemiology, Staging and Grading, and Diagnosis. Urology 2005, 66, 4–34. [Google Scholar] [CrossRef]
- Mitra, A.P.; Quinn, D.I.; Dorff, T.B.; Skinner, E.C.; Schuckman, A.K.; Miranda, G.; Gill, I.S.; Daneshmand, S. Factors Influencing Post-Recurrence Survival in Bladder Cancer Following Radical Cystectomy. BJU Int. 2012, 109, 846–854. [Google Scholar] [CrossRef]
- Surveillance, Epidemiology, and End Results Program. Available online: https://seer.cancer.gov/index.html (accessed on 7 May 2025).
- International Collaboration of Trialists on behalf of the Medical Research Council Advanced Bladder Cancer Working Party (Now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); The European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group; The Australian Bladder Cancer Study Group; The National Cancer Institute of Canada Clinical Trials Group; Finnbladder, Norwegian Bladder Cancer Study Group; Club Urologico Espanol de Tratamiento Oncologico Group. International Phase III Trial Assessing Neoadjuvant Cisplatin, Methotrexate, and Vinblastine Chemotherapy for Muscle-Invasive Bladder Cancer: Long-Term Results of the BA06 30894 Trial. J. Clin. Oncol. 2011, 29, 2171–2177. [Google Scholar] [CrossRef]
- Grossman, H.B.; Natale, R.B.; Tangen, C.M.; Speights, V.O.; Vogelzang, N.J.; Trump, D.L.; deVere White, R.W.; Sarosdy, M.F.; Wood, D.P.; Raghavan, D.; et al. Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer. N. Engl. J. Med. 2003, 349, 859–866. [Google Scholar] [CrossRef]
- Galsky, M.D.; Pal, S.K.; Chowdhury, S.; Harshman, L.C.; Crabb, S.J.; Wong, Y.-N.; Yu, E.Y.; Powles, T.; Moshier, E.L.; Ladoire, S.; et al. Comparative Effectiveness of Gemcitabine plus Cisplatin versus Methotrexate, Vinblastine, Doxorubicin, plus Cisplatin as Neoadjuvant Therapy for Muscle-Invasive Bladder Cancer. Cancer 2015, 121, 2586–2593. [Google Scholar] [CrossRef]
- Pfister, C.; Gravis, G.; Fléchon, A.; Chevreau, C.; Mahammedi, H.; Laguerre, B.; Guillot, A.; Joly, F.; Soulié, M.; Allory, Y.; et al. Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin or Gemcitabine and Cisplatin as Perioperative Chemotherapy for Patients With Nonmetastatic Muscle-Invasive Bladder Cancer: Results of the GETUG-AFU V05 VESPER Trial. J. Clin. Oncol. 2022, 40, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Catto, J.W.F.; Galsky, M.D.; Al-Ahmadie, H.; Meeks, J.J.; Nishiyama, H.; Vu, T.Q.; Antonuzzo, L.; Wiechno, P.; Atduev, V.; et al. Perioperative Durvalumab with Neoadjuvant Chemotherapy in Operable Bladder Cancer. N. Engl. J. Med. 2024, 391, 1773–1786. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Van Der Heijden, M.S.; Wang, Y.; Catto, J.W.F.; Meeks, J.J.; Al-Ahmadie, H.; Nishiyama, H.; Moeini Mortazavi, A.; Vu, T.Q.; Antonuzzo, L.; et al. Circulating Tumor DNA (ctDNA) in Patients with Muscle-Invasive Bladder Cancer (MIBC) Who Received Perioperative Durvalumab (D) in NIAGARA. J. Clin. Oncol. 2025, 43, 4503. [Google Scholar] [CrossRef]
- Siefker-Radtke, A.O.; Steinberg, G.D.; Bedke, J.; Nishiyama, H.; Fang, X.; Kataria, R.; Homet Moreno, B.; Hoimes, C.J. Phase III Study of Perioperative Pembrolizumab (Pembro) plus Neoadjuvant Chemotherapy (Chemo) versus Placebo plus Neoadjuvant Chemo in Cisplatin-Eligible Patients (Pts) with Muscle-Invasive Bladder Cancer (MIBC): KEYNOTE-866. J. Clin. Oncol. 2020, 38, TPS599. [Google Scholar] [CrossRef]
- Sonpavde, G.; Necchi, A.; Gupta, S.; Steinberg, G.D.; Gschwend, J.E.; Van Der Heijden, M.S.; Garzon, N.; Ibrahim, M.; Raybold, B.; Liaw, D.; et al. ENERGIZE: A Phase III Study of Neoadjuvant Chemotherapy Alone or with Nivolumab with/without Linrodostat Mesylate for Muscle-Invasive Bladder Cancer. Future Oncol. 2020, 16, 4359–4368. [Google Scholar] [CrossRef]
- Mercinelli, C.; Moschini, M.; Cigliola, A.; Mattorre, B.; Tateo, V.; Basile, G.; Cogrossi, L.L.; Maiorano, B.A.; Patanè, D.A.; Raggi, D.; et al. First Results of NURE-Combo: A Phase II Study of Neoadjuvant Nivolumab and Nab-Paclitaxel, Followed by Postsurgical Adjuvant Nivolumab, for Muscle-Invasive Bladder Cancer. J. Clin. Oncol. 2024, 42, 4196–4205. [Google Scholar] [CrossRef]
- Satkunasivam, R.; Lim, K.; Teh, B.S.; Esnaola, N.F.; Slawin, J.; Zhang, J.; Miles, B.; Brooks, M.A.; Anis, M.; Muhammad, T.; et al. A Phase II Clinical Trial of Neoadjuvant Sasanlimab and Stereotactic Body Radiation Therapy as an in Situ Vaccine for Cisplatin-Ineligible Muscle Invasive Bladder Cancer (RAD VACCINE MIBC). J. Clin. Oncol. 2022, 40, TPS4611. [Google Scholar] [CrossRef]
- Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Adjuvant Chemotherapy in Invasive Bladder Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data Advanced Bladder Cancer (ABC) Meta-Analysis Collaboration. Eur. Urol. 2005, 48, 189–199; discussion 199–201. [Google Scholar] [CrossRef]
- Sternberg, C.N.; Skoneczna, I.; Kerst, J.M.; Albers, P.; Fossa, S.D.; Agerbaek, M.; Dumez, H.; de Santis, M.; Théodore, C.; Leahy, M.G.; et al. Immediate versus Deferred Chemotherapy after Radical Cystectomy in Patients with pT3–pT4 or N+ M0 Urothelial Carcinoma of the Bladder (EORTC 30994): An Intergroup, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 2015, 16, 76–86. [Google Scholar] [CrossRef]
- Bajorin, D.F.; Witjes, J.A.; Gschwend, J.E.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; Park, S.H.; et al. Adjuvant Nivolumab versus Placebo in Muscle-Invasive Urothelial Carcinoma. N. Engl. J. Med. 2021, 384, 2102–2114. [Google Scholar] [CrossRef]
- Galsky, M.D.; Witjes, J.A.; Gschwend, J.E.; Milowsky, M.I.; Schenker, M.; Valderrama, B.P.; Tomita, Y.; Bamias, A.; Lebret, T.; Shariat, S.F.; et al. Adjuvant Nivolumab in High-Risk Muscle-Invasive Urothelial Carcinoma: Expanded Efficacy from CheckMate 274. J. Clin. Oncol. 2025, 43, 15–21. [Google Scholar] [CrossRef]
- Bellmunt, J.; Hussain, M.; Gschwend, J.E.; Albers, P.; Oudard, S.; Castellano, D.; Daneshmand, S.; Nishiyama, H.; Majchrowicz, M.; Degaonkar, V.; et al. Adjuvant Atezolizumab versus Observation in Muscle-Invasive Urothelial Carcinoma (IMvigor010): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2021, 22, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Assaf, Z.J.; Degaonkar, V.; Grivas, P.; Hussain, M.; Oudard, S.; Gschwend, J.E.; Albers, P.; Castellano, D.; Nishiyama, H.; et al. Updated Overall Survival by Circulating Tumor DNA Status from the Phase 3 IMvigor010 Trial: Adjuvant Atezolizumab Versus Observation in Muscle-Invasive Urothelial Carcinoma. Eur. Urol. 2024, 85, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Jackson-Spence, F.; Toms, C.; O’Mahony, L.F.; Choy, J.; Flanders, L.; Szabados, B.; Powles, T. IMvigor011: A Study of Adjuvant Atezolizumab in Patients with High-Risk MIBC Who Are ctDNA+ Post-Surgery. Future Oncol. 2023, 19, 509–515. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.P.; Flaig, T.W.; Mar, N.; Gourdin, T.S.; Srinivas, S.; Rosenberg, J.E.; Guseva, M.; Yu, Y.; Narayanan, S.; Hoimes, C.J. Study EV-103 Cohort H: Antitumor Activity of Neoadjuvant Treatment with Enfortumab Vedotin Monotherapy in Patients (Pts) with Muscle Invasive Bladder Cancer (MIBC) Who Are Cisplatin-Ineligible. J. Clin. Oncol. 2022, 40, 435. [Google Scholar] [CrossRef]
- O’Donnell, P.H.; Hoimes, C.J.; Rosenberg, J.E.; Petrylak, D.P.; Mar, N.; Barata, P.C.; Srinivas, S.; Gourdin, T.S.; Henry, E.; Bilen, M.A.; et al. Study EV-103: Neoadjuvant Treatment with Enfortumab Vedotin Monotherapy in Cisplatin-Ineligible Patients with Muscle Invasive Bladder Cancer (MIBC)—2-Year Event-Free Survival and Safety Data for Cohort H. J. Clin. Oncol. 2024, 42, 4564. [Google Scholar] [CrossRef]
- Sridhar, S.; O’Donnell, P.H.; Flaig, T.W.; Rosenberg, J.E.; Hoimes, C.J.; Milowsky, M.I.; Srinivas, S.; George, S.; McKay, R.R.; Petrylak, D.P.; et al. 2365MO Study EV-103 Cohort L: Perioperative Treatment w/ Enfortumab Vedotin (EV) Monotherapy in Cisplatin (Cis)-Ineligible Patients (Pts) w/ Muscle Invasive Bladder Cancer (MIBC). Ann. Oncol. 2023, 34, S1203. [Google Scholar] [CrossRef]
- Necchi, A.; Bedke, J.; Galsky, M.D.; Shore, N.D.; Xylinas, E.; Jia, C.; Dubrovsky, L.; Homet Moreno, B.; Witjes, J.A. KEYNOTE-905/EV-303: A Phase 3 Study to Evaluate the Efficacy and Safety of Perioperative Pembrolizumab or Pembrolizumab plus Enfortumab Vedotin (EV) for Muscle-Invasive Bladder Cancer (MIBC). J. Clin. Oncol. 2023, 41, TPS4601. [Google Scholar] [CrossRef]
- Powles, T.; Drakaki, A.; Teoh, J.Y.-C.; Grande, E.; Fontes-Sousa, M.; Porta, C.; Wu, E.; Goluboff, E.T.; Ho, S.; Hois, S.; et al. A Phase 3, Randomized, Open-Label, Multicenter, Global Study of the Efficacy and Safety of Durvalumab (D)+ Tremelimumab (T)+ Enfortumab Vedotin (EV) or D+ EV for Neoadjuvant Treatment in Cisplatin-Ineligible Muscle-Invasive Bladder Cancer (MIBC) (VOLGA). J. Clin. Oncol. 2022, 40, TPS579. [Google Scholar] [CrossRef]
- Zhang, T.; Woldu, S.; Qin, Q.; Cole, S.; Arafat, W.; Jiang, C.; Wang, J.; Courtney, K.; DeVilbiss, A.; Ploski, R.; et al. 2022TiP Stereotactic Treatment with Neoadjuvant Radiotherapy and Enfortumab Vedotin: A Phase I/II Study for Localized, Cisplatin Ineligible, Muscle Invasive Bladder Cancer (STAR-EV). Ann. Oncol. 2024, 35, S1165. [Google Scholar] [CrossRef]
- Hoimes, C.J.; Loriot, Y.; Bedke, J.; Nishiyama, H.; Kataria, R.S.; Homet Moreno, B.; Galsky, M.D. Perioperative Enfortumab Vedotin (EV) plus Pembrolizumab (Pembro) versus Chemotherapy in Cisplatin-Eligible Patients (Pts) with Muscle-Invasive Bladder Cancer (MIBC): Phase 3 KEYNOTE-B15/EV-304. J. Clin. Oncol. 2023, 41, TPS588. [Google Scholar] [CrossRef]
- Powles, T.; Tagawa, S.; Vulsteke, C.; Gross-Goupil, M.; Park, S.H.; Necchi, A.; De Santis, M.; Duran, I.; Morales-Barrera, R.; Guo, J.; et al. Sacituzumab Govitecan in Advanced Urothelial Carcinoma: TROPiCS-04, a Phase III Randomized Trial. Ann. Oncol. 2025, 36, 561–571. [Google Scholar] [CrossRef]
- Necchi, A.; Raggi, D.; Bandini, M.; Gallina, A.; Capitanio, U.; Gandaglia, G.; Cucchiara, V.; Fossati, N.; De Cobelli, F.; Salonia, A.; et al. SURE: An Open Label, Sequential-Arm, Phase II Study of Neoadjuvant Sacituzumab Govitecan (SG), and SG plus Pembrolizumab (Pembro) before Radical Cystectomy, for Patients with Muscle-Invasive Bladder Cancer (MIBC) Who Cannot Receive or Refuse Cisplatin-Based Chemotherapy. J. Clin. Oncol. 2021, 39, TPS506. [Google Scholar] [CrossRef]
- Maiorano, B.A.; Cigliola, A.; Moschini, M.; Tateo, V.; Patanè, D.A.; Mercinelli, C.; Nuccio, A.; Latini, G.; Borella, M.; Brembilla, G.; et al. 275MO Neoadjuvant Sacituzumab Govitecan, Followed by Radical Cystectomy, for Patients with Muscle-Invasive Bladder Cancer (MIBC): Updated Interim Results of SURE-01 Trial. Ann. Oncol. 2024, 35, S1510. [Google Scholar] [CrossRef]
- Sheng, X.; Zhang, C.; Ji, Y.; Zhou, L.; Zou, B.; Huang, H.; Wang, Y.; Yang, K.; Bai, X.; Feng, D.; et al. Neoadjuvant Treatment with Disitamab Vedotin plus Perioperative Toripalimab in Patients with Muscle-Invasive Bladder Cancer (MIBC) with HER2 Expression: Updated Efficacy and Safety Results from the Phase II RC48-C017 Trial. J. Clin. Oncol. 2025, 43, 665. [Google Scholar] [CrossRef]
- Hussain, S.A.; Loriot, Y.; de Velasco, G.; Necchi, A. SOGUG-NEOWIN: A Phase 2, Open-Label, Multi-Centre, Multi-National Interventional Trial Evaluating the Efficacy and Safety of Erdafitinib (ERDA) Monotherapy and ERDA and Cetrelimab (CET) as Neoadjuvant Treatment in Patients with Cisplatin-Ineligible Muscle-Invasive Bladder Cancer (MIBC) Whose Tumours Express FGFR Gene Alterations. J. Clin. Oncol. 2024, 42, TPS4623. [Google Scholar] [CrossRef]
- Galsky, M.D.; Sfakianos, J.P.; Ye, D.; He, D.; Hu, H.; Song, X.; Jiang, H.; Li, H.; Jiang, S.; Wang, B.; et al. Oral APL-1202 in Combination with Tislelizumab as Neoadjuvant Therapy in Patients with Muscle-Invasive Bladder Cancer (MIBC): Interim Analysis of ANTICIPATE Phase II Trial. J. Clin. Oncol. 2024, 42, 632. [Google Scholar] [CrossRef]
- Necchi, A.; Guerrero-Ramos, F.; Crispen, P.L.; Imbroda, B.H.; Garje, R.; Powles, T.B.; Peyton, C.C.; Pradere, B.; Ku, J.H.; Shore, N.D.; et al. LBA84 TAR-200 plus Cetrelimab (CET) or CET Alone as Neoadjuvant Therapy in Patients (Pts) with Muscle-Invasive Bladder Cancer (MIBC) Who Are Ineligible for or Refuse Neoadjuvant Cisplatin-Based Chemotherapy (NAC): Interim Analysis of SunRISe-4 (SR-4). Ann. Oncol. 2024, 35, S1271–S1272. [Google Scholar] [CrossRef]
- Li, R.; Villa, N.Y.; Yu, X.; Johnson, J.O.; Borjas, G.; Dhillon, J.; Moran-Segura, C.M.; Kim, Y.; Francis, N.; Dorman, D.; et al. Oncolytic Immunotherapy with Nivolumab in Muscle-Invasive Bladder Cancer: A Phase 1b Trial. Nat. Med. 2025, 31, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Saxena, M.; Anker, J.F.; Kodysh, J.; O’Donnell, T.; Kaminska, A.M.; Meseck, M.; Hapanowicz, O.; Niglio, S.A.; Salazar, A.M.; Shah, H.R.; et al. Atezolizumab plus Personalized Neoantigen Vaccination in Urothelial Cancer: A Phase 1 Trial. Nat. Cancer 2025, 1–12. [Google Scholar] [CrossRef]
- Sonpavde, G.P.; Valderrama, B.P.; Chamie, K.; Gupta, S.; De Santis, M.; Banerjee, J.K.; Ojalvo, L.; Ren, Y.; Bavle, A.; Powles, T. Phase 1/2 INTerpath-005 Study: V940 (mRNA-4157) plus Pembrolizumab with or without Enfortumab Vedotin (EV) for Resected High-Risk Muscle-Invasive Urothelial Carcinoma (MIUC). J. Clin. Oncol. 2025, 43, TPS893. [Google Scholar] [CrossRef]
- Grivas, P.; Van Der Heijden, M.S.; Necchi, A.; Siefker-Radtke, A.O.; Cutuli, H.; Qureshi, A.H.; Kreiser, S.; Hodari, M.; Ravimohan, S.; Zakharia, Y. PIVOT IO 009: A Phase 3, Randomized Study of Neoadjuvant and Adjuvant Nivolumab (NIVO) plus Bempegaldesleukin (BEMPEG; NKTR-214) versus NIVO Alone versus Standard of Care (SOC) in Patients (Pts) with Muscle-Invasive Bladder Cancer (MIBC) Who Are Cisplatin (Cis)-Ineligible. J. Clin. Oncol. 2022, 40, TPS596. [Google Scholar] [CrossRef]
- Marqueen, K.E.; Waingankar, N.; Sfakianos, J.P.; Mehrazin, R.; Niglio, S.A.; Audenet, F.; Jia, R.; Mazumdar, M.; Ferket, B.S.; Galsky, M.D. Early Mortality in Patients with Muscle-Invasive Bladder Cancer Undergoing Cystectomy in the United States. JNCI Cancer Spectr. 2019, 2, pky075. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Leow, J.J.; Sun, M.; Friedlander, D.F.; Seisen, T.; Abdollah, F.; Lipsitz, S.R.; Menon, M.; Kibel, A.S.; Bellmunt, J.; et al. Comparative Effectiveness of Robot-Assisted vs. Open Radical Cystectomy. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 88.e1–88.e9. [Google Scholar] [CrossRef]
- McKiernan, J.M.; Anderson, C.B. Complications of Radical Cystectomy and Urinary Diversion. In Complications of Urologic Surgery; Elsevier: Amsterdam, The Netherlands, 2018; Volume 41, pp. 433–444. [Google Scholar]
- Herr, H.W.; Bajorin, D.F.; Scher, H.I. Neoadjuvant Chemotherapy and Bladder-Sparing Surgery for Invasive Bladder Cancer: Ten-Year Outcome. J. Clin. Oncol. 1998, 16, 1298–1301. [Google Scholar] [CrossRef]
- Moran, G.W.; Li, G.; Robins, D.J.; Matulay, J.T.; McKiernan, J.M.; Anderson, C.B. Systematic Review and Meta-Analysis on the Efficacy of Chemotherapy with Transurethral Resection of Bladder Tumors as Definitive Therapy for Muscle Invasive Bladder Cancer. Bladder Cancer Amst. Neth. 2017, 3, 245–258. [Google Scholar] [CrossRef]
- Meyer, A.; Ghandour, R.; Bergman, A.; Castaneda, C.; Wosnitzer, M.; Hruby, G.; Benson, M.; McKiernan, J. The Natural History of Clinically Complete Responders to Neoadjuvant Chemotherapy for Urothelial Carcinoma of the Bladder. J. Urol. 2014, 192, 696–701. [Google Scholar] [CrossRef]
- Galsky, M.D.; Daneshmand, S.; Izadmehr, S.; Gonzalez-Kozlova, E.; Chan, K.G.; Lewis, S.; Achkar, B.E.; Dorff, T.B.; Cetnar, J.P.; Neil, B.O.; et al. Gemcitabine and Cisplatin plus Nivolumab as Organ-Sparing Treatment for Muscle-Invasive Bladder Cancer: A Phase 2 Trial. Nat. Med. 2023, 29, 2825–2834. [Google Scholar] [CrossRef]
- Geynisman, D.M.; Abbosh, P.H.; Ross, E.; Zibelman, M.R.; Ghatalia, P.; Anari, F.; Mark, J.R.; Stamatakis, L.; Hoffman-Censits, J.H.; Viterbo, R.; et al. Phase II Trial of Risk-Enabled Therapy After Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer (RETAIN 1). J. Clin. Oncol. 2025, 43, 1113–1122. [Google Scholar] [CrossRef]
- Ghatalia, P.; Ross, E.A.; Zibelman, M.R.; Anari, F.; Abbosh, P.; Tester, W.J.; Rose, T.L.; Cole, S.; Mark, J.R.; Viterbo, R.; et al. A Phase 2 Trial of Risk Enabled Therapy after Neoadjuvant Chemo-Immunotherapy for Muscle-Invasive Bladder Cancer (RETAIN-2). J. Clin. Oncol. 2025, 43, 815. [Google Scholar] [CrossRef]
- Iyer, G.; Ballman, K.V.; Atherton, P.J.; Murray, K.; Kwok, Y.; Steen, P.D.; Baghaie, S.; Rosenberg, J.E.; Morris, M.J. A Phase II Study of Gemcitabine plus Cisplatin Chemotherapy in Patients with Muscle-Invasive Bladder Cancer with Bladder Preservation for Those Patients Whose Tumors Harbor Deleterious DNA Damage Response (DDR) Gene Alterations (Alliance A031701). J. Clin. Oncol. 2022, 40, TPS4615. [Google Scholar] [CrossRef]
- Wei, A.Z.; Bakir, B.; Lenis, A.T.; Anderson, C.B.; Runcie, K.; DeCastro, G.J.; Pan, S.S.; Shen, M.M.; McKiernan, J.M.; Stein, M.N. A Phase 2, Randomized, Open-Label Study of Gemcitabine/Cisplatin plus Cemiplimab (REGN2810, Anti-PD-1) with or without Fianlimab (REGN3767, Anti-LAG-3) for Organ Preservation in Patients with Localized Muscle-Invasive Bladder Cancer (NeoSTOP-IT). J. Clin. Oncol. 2025, 43, TPS882. [Google Scholar] [CrossRef]
- Zlotta, A.R.; Ballas, L.K.; Niemierko, A.; Lajkosz, K.; Kuk, C.; Miranda, G.; Drumm, M.; Mari, A.; Thio, E.; Fleshner, N.E.; et al. Radical Cystectomy versus Trimodality Therapy for Muscle-Invasive Bladder Cancer: A Multi-Institutional Propensity Score Matched and Weighted Analysis. Lancet Oncol. 2023, 24, 669–681. [Google Scholar] [CrossRef]
- Economides, M.P.; Milowsky, M.I.; O’Donnell, P.H.; Alva, A.S.; Kollmeier, M.; Rose, T.L.; Pitroda, S.P.; Rosenberg, J.E.; Hochman, T.; Goldberg, J.D.; et al. Long-Term Outcomes of Pembrolizumab (Pembro) in Combination with Gemcitabine (Gem) and Concurrent Hypofractionated Radiation Therapy (RT) as Bladder Sparing Treatment for Muscle-Invasive Urothelial Cancer of the Bladder (MIUC): A Multicenter Phase 2 Trial. J. Clin. Oncol. 2023, 41, 4509. [Google Scholar] [CrossRef]
- Kougioumtzopoulou, A.; Koutsoukos, K.; Zakopoulou, R.; Tzannis, K.; Kyriazoglou, A.; Damatopoulou, A.; Liontos, M.; Pispirigkou, M.K.; Ntoumas, K.; Stravodimos, K.; et al. 1961O Nivolumab plus Chemoradiotherapy in Patients with Non-Metastatic Muscle-Invasive Bladder Cancer (nmMIBC), Not Undergoing Cystectomy: A Phase II, Randomized Study by the Hellenic GU Cancer Group. Ann. Oncol. 2024, 35, S1133–S1134. [Google Scholar] [CrossRef]
- Garcia-del-Muro, X.P.; Valderrama, B.; Medina-Colmenero, A.; Etxaniz, O.; Gironés Sarrió, R.; Juan-Fita, M.J.; Costa-García, M.; Moreno, R.; Miras Rodríguez, I.; Ortiz, I.; et al. Bladder Preservation with Durvalumab plus Tremelimumab and Concurrent Radiotherapy in Patients with Localized Muscle-Invasive Bladder Cancer (IMMUNOPRESERVE): A Phase II Spanish Oncology GenitoUrinary Group Trial. Clin. Cancer Res. 2025, 31, 659–666. [Google Scholar] [CrossRef]
- Singh, P.; Tangen, C.; Efstathiou, J.A.; Lerner, S.P.; Jhavar, S.G.; Hahn, N.M.; Costello, B.A.; Sridhar, S.S.; Du, W.; Meeks, J.J.; et al. INTACT: Phase III Randomized Trial of Concurrent Chemoradiotherapy with or without Atezolizumab in Localized Muscle Invasive Bladder Cancer—SWOG/NRG1806. J. Clin. Oncol. 2020, 38, TPS586. [Google Scholar] [CrossRef]
- Gupta, S.; Fujii, Y.; Van Der Heijden, M.S.; Weickhardt, A.J.; James, N.D.; Shariat, S.F.; Michalski, J.M.; Imai, K.; Fang, X.; Kapadia, E.; et al. Phase 3 KEYNOTE-992 Study of Pembrolizumab plus Chemoradiotherapy versus Placebo plus Chemoradiotherapy in Patients with Muscle-Invasive Bladder Cancer (MIBC). J. Clin. Oncol. 2024, 42, TPS720. [Google Scholar] [CrossRef]
- Gupta, S.; Almassi, N.; Bukavina, L.; Wee, C.E.; Stephans, K.L.; Diaz-Montero, C.M.; Tendulkar, R.D.; Mian, O.Y.; Chan, T.A. RAD-SG: Adaptive Radiation Therapy with Concurrent Sacituzumab Govitecan (SG) for Bladder Preservation in Patients (Pts) with Muscle Invasive Bladder Cancer (MIBC). J. Clin. Oncol. 2025, 43, TPS896. [Google Scholar] [CrossRef]
- Abidoye, O.; Jain, P.; Singh, P. Lines of Therapy for Locally Advanced/Metastatic Urothelial Carcinoma: The New Paradigm. JCO Oncol. Pract. 2025, in press. [Google Scholar] [CrossRef]
- von der Maase, H.; Hansen, S.W.; Roberts, J.T.; Dogliotti, L.; Oliver, T.; Moore, M.J.; Bodrogi, I.; Albers, P.; Knuth, A.; Lippert, C.M.; et al. Gemcitabine and Cisplatin Versus Methotrexate, Vinblastine, Doxorubicin, and Cisplatin in Advanced or Metastatic Bladder Cancer: Results of a Large, Randomized, Multinational, Multicenter, Phase III Study. J. Clin. Oncol. 2000, 18, 3068–3077. [Google Scholar] [CrossRef]
- De Santis, M.; Bellmunt, J.; Mead, G.; Kerst, J.M.; Leahy, M.; Maroto, P.; Gil, T.; Marreaud, S.; Daugaard, G.; Skoneczna, I.; et al. Randomized Phase II/III Trial Assessing Gemcitabine/Carboplatin and Methotrexate/Carboplatin/Vinblastine in Patients With Advanced Urothelial Cancer Who Are Unfit for Cisplatin-Based Chemotherapy: EORTC Study 30986. J. Clin. Oncol. 2012, 30, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, M.S.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus Gemcitabine–Cisplatin in Advanced Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef]
- Heijden, M.S.V.D.; Loriot, Y.; Bedke, J.; Valderrama, B.P.; Iyer, G.; Kikuchi, E.; Hoffman-Censits, J.; Vulsteke, C.; Drakaki, A.; Rausch, S.; et al. EV-302: Updated Analysis from the Phase 3 Global Study of Enfortumab Vedotin in Combination with Pembrolizumab (EV+P) vs Chemotherapy (Chemo) in Previously Untreated Locally Advanced or Metastatic Urothelial Carcinoma (La/mUC). J. Clin. Oncol. 2025, 43, 664. [Google Scholar] [CrossRef]
- Zhou, W.; Fang, P.; Yu, D.; Ren, H.; You, M.; Yin, L.; Mei, F.; Zhu, H.; Wang, Z.; Xu, H.; et al. Preclinical Evaluation of 9MW2821, a Site-Specific Monomethyl Auristatin E–Based Antibody–Drug Conjugate for Treatment of Nectin-4–Expressing Cancers. Mol. Cancer Ther. 2023, 22, 913–925. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, R.; Gao, S.; Yang, H.; Chen, J.; Yuan, F.; Liu, J.; Guo, H.; Zhang, S.; Li, X.; et al. 9MW2821, a Nectin-4 Antibody-Drug Conjugate (ADC), in Patients with Advanced Solid Tumor: Results from a Phase 1/2a Study. J. Clin. Oncol. 2024, 42, 3013. [Google Scholar] [CrossRef]
- Tang, B.; Sheng, X.; Guo, J.; Niu, H.; Shen, Y.; Jiang, S.; Fu, B.; Guo, J.; Wahafu, W.; Yao, K.; et al. Nectin-4 Targeted ADC, SHR-A2102, in Patients with Advanced or Metastatic Urothelial Carcinoma: A Phase 1 Study. J. Clin. Oncol. 2025, 43, 657. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Makker, V.; Oaknin, A.; Oh, D.-Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients with HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J. Clin. Oncol. 2024, 42, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Wang, L.; He, Z.; Shi, Y.; Luo, H.; Han, W.; Yao, X.; Shi, B.; Liu, J.; Hu, C.; et al. Efficacy and Safety of Disitamab Vedotin in Patients With Human Epidermal Growth Factor Receptor 2–Positive Locally Advanced or Metastatic Urothelial Carcinoma: A Combined Analysis of Two Phase II Clinical Trials. J. Clin. Oncol. 2024, 42, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, K.; Zhang, S.; Yan, X.; Li, S.; Xu, H.; Li, J.; Chi, Z.; Mao, L.; Lian, B.; et al. 1979P Disitamab Vedotin (DV) plus Toripalimab (T) in Unresectable Locally Advanced or Metastatic Urothelial Carcinoma (La/mUC): Long-Term Outcomes from a Phase Ib/II Study. Ann. Oncol. 2024, 35, S1145. [Google Scholar] [CrossRef]
- Galsky, M.D.; Koshkin, V.S.; Campbell, M.T.; Drakaki, A.; Bowman, I.; Rose, A.A.N.; Brown, J.R.; Aragon-Ching, J.B.; Gadde, S.; Harandi, A.; et al. 1967MO Preliminary Efficacy and Safety of Disitamab Vedotin (DV) with Pembrolizumab (P) in Treatment (Tx)-Naive HER2-Expressing, Locally Advanced or Metastatic Urothelial Carcinoma (La/mUC): RC48G001 Cohort C. Ann. Oncol. 2024, 35, S1138–S1139. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Alhalabi, O.; Lisberg, A.; Drakaki, A.; Garmezy, B.; Kogawa, T.; Spira, A.I.; Salkeni, M.A.; Gao, X.; Tolcher, A.W.; et al. Datopotamab Deruxtecan (Dato-DXd) in Locally Advanced/Metastatic Urothelial Cancer: Updated Results from the Phase 1 TROPIONPanTumor01 Study. J. Clin. Oncol. 2025, 43, 663. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Oaknin, A.; Lang, J.M.; Ciombor, K.K.; Ray-Coquard, I.L.; Oza, A.M.; Yonemori, K.; Xu, R.-H.; Zhao, J.; Gajavelli, S.; et al. TROPION-PanTumor03: Phase 2, Multicenter Study of Datopotamab Deruxtecan (Dato-DXd) as Monotherapy and in Combination with Anticancer Agents in Patients (Pts) with Advanced/Metastatic Solid Tumors. J. Clin. Oncol. 2023, 41, TPS3153. [Google Scholar] [CrossRef]
- Ye, D.; Jiang, S.; Yuan, F.; Zhou, F.; Jiang, K.; Zhang, X.; Li, X.; Seneviratne, L.C.; Yu, G.; Zhang, M.; et al. Efficacy and Safety of Sacituzumab Tirumotecan Monotherapy in Patients with Advanced Urothelial Carcinoma Who Progressed on or after Prior Anti-Cancer Therapies: Report from the Phase 1/2 MK-2870-001 Study. J. Clin. Oncol. 2025, 43, 796. [Google Scholar] [CrossRef]
- McGregor, B.A.; Sonpavde, G.P.; Kwak, L.; Regan, M.M.; Gao, X.; Hvidsten, H.; Mantia, C.M.; Wei, X.X.; Berchuck, J.E.; Berg, S.A.; et al. The Double Antibody Drug Conjugate (DAD) Phase I Trial: Sacituzumab Govitecan plus Enfortumab Vedotin for Metastatic Urothelial Carcinoma✩. Ann. Oncol. 2024, 35, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Bian, X.; Yang, T.; Jiang, S.; Cao, M.; Hua, X.; Xiao, S.; Wang, H.; Zhu, H.; Zhu, Y. 1959O BL-B01D1, an EGFR x HER3 Bispecific Antibody-Drug Conjugate (ADC), in Patients with Locally Advanced or Metastatic Urothelial Carcinoma (UC). Ann. Oncol. 2024, 35, S1133. [Google Scholar] [CrossRef]
- Torras, O.R.; Crouzet, L.; Necchi, A.; Baldini, C.; Bardaji, M.J.L.; de Spéville, B.D.; Italiano, A.; Verlingue, L.; Boni, V.; Carter, L.; et al. 652P BT8009 Monotherapy in Enfortumab Vedotin (EV)-Naïve Patients (Pts) with Metastatic Urothelial Carcinoma (mUC): Updated Results of Duravelo-1. Ann. Oncol. 2024, 35, S515–S516. [Google Scholar] [CrossRef]
- Loriot, Y.; Siefker-Radtke, A.O.; Friedlander, T.W.; Necchi, A.; Wei, A.Z.; Sridhar, S.S.; Garmezy, B.; Arroyo, S.; Gartside, E.; Liu, J.; et al. A Phase 2/3 Study of Bicycle Toxin Conjugate BT8009 Targeting Nectin-4 in Patients with Locally Advanced or Metastatic Urothelial Cancer (La/mUC): Duravelo-2. J. Clin. Oncol. 2024, 42, TPS4619. [Google Scholar] [CrossRef]
- Fontana, E.; Wang, J.S.; Mckean, M.; Aljumaily, R.; Machiels, J.-P.; de Spéville, B.D.; Vieito, M.; Carter, L.; Prenen, H.; Falchook, G.S.; et al. 647P EphA2-Targeting Bicycle Toxin Conjugate (BTC) BT5528 in Patients (Pts) with Advanced Solid Tumors: A Phase I/II Study. Ann. Oncol. 2024, 35, S511–S512. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Dowlati, A.; Lopez, J.S.; Rodon, J.; Spira, A.I.; Stein, M.; Zibelman, M.; Feliu, W.I.O.; Dickson, A.; De, A.; et al. 650P Initial Results from a Phase I/II Study of BT7480, a Novel Nectin-4/CD137 Bicycle Tumor-Targeted Immune Cell Agonist, in Patients (Pts) with Advanced Solid Tumors. Ann. Oncol. 2024, 35, S513–S514. [Google Scholar] [CrossRef]
- Ascione, C.M.; Napolitano, F.; Esposito, D.; Servetto, A.; Belli, S.; Santaniello, A.; Scagliarini, S.; Crocetto, F.; Bianco, R.; Formisano, L. Role of FGFR3 in Bladder Cancer: Treatment Landscape and Future Challenges. Cancer Treat. Rev. 2023, 115, 102530. [Google Scholar] [CrossRef] [PubMed]
- Knowles, M.A.; Hurst, C.D. Molecular Biology of Bladder Cancer: New Insights into Pathogenesis and Clinical Diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Matsubara, N.; Park, S.H.; Huddart, R.A.; Burgess, E.F.; Houede, N.; Banek, S.; Guadalupi, V.; Ku, J.H.; Valderrama, B.P.; et al. Erdafitinib or Chemotherapy in Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Siefker-Radtke, A.O.; Powles, T.; Moreno, V.; Kang, T.W.; Cicin, I.; Girvin, A.; Akapame, S.; Triantos, S.; O’Hagan, A.; Zhu, W.; et al. Erdafitinib (ERDA) vs ERDA plus Cetrelimab (ERDA+CET) for Patients (Pts) with Metastatic Urothelial Carcinoma (mUC) and Fibroblast Growth Factor Receptor Alterations (FGFRa): Final Results from the Phase 2 Norse Study. J. Clin. Oncol. 2023, 41, 4504. [Google Scholar] [CrossRef]
- Koshkin, V.S.; Barthelemy, P.; Gravis, G.; Goodman, O.; Duran, I.; Sarrio, R.G.; Hwang, C.; Garcia-Donas, J.; Barrera, R.M.; Zanetta, S.; et al. 1965MO Phase II Study of Futibatinib plus Pembrolizumab in Patients (Pts) with Advanced/Metastatic Urothelial Carcinoma (mUC): Final Analysis of Efficacy and Safety. Ann. Oncol. 2024, 35, S1136–S1137. [Google Scholar] [CrossRef]
- Iyer, G.; Ebi, H.; Cook, N.; Gao, X.; Kitano, S.; Matsubara, N.; Reimers, M.A.; Siefker-Radtke, A.O.; Kim, M.; Galsky, M.D.; et al. A First-in-Human Phase 1 Study of LY3866288 (LOXO-435), a Potent, Highly Isoform-Selective FGFR3 Inhibitor (FGFR3i) in Advanced Solid Tumors with FGFR3 Alterations: Initial Results from FORAGER-1. J. Clin. Oncol. 2025, 43, 662. [Google Scholar] [CrossRef]
- Hansen, A.R.; Zhang, A.Y.; Boni, V.; Mantia, C.; Yu, E.Y.; Weickhardt, A.J.; Robert, M.; Gupta, S.; Morales-Barrera, R.; Hoimes, C.J.; et al. A Multicenter, Open-Label Phase 1/2 Study of TYRA-300 in Advanced Urothelial Carcinoma and Other Solid Tumors with Activating FGFR3 Alterations (SURF301). J. Clin. Oncol. 2025, 43, TPS904. [Google Scholar] [CrossRef]
- Jain, R.K.; Ong, F.; Faltas, B.M.; Tagawa, S.T.; Jiang, D.M.; Heiligh, J.; Naqvi, S.M.H.; Kim, Y.; Pelosof, L.C.; Yang, Y.; et al. ETCTN 10483: Phase Ib Trial of Erdafitinib (E) Combined with Enfortumab Vedotin (EV) Following Platinum and PD-1/L1 Inhibitors for Metastatic Urothelial Carcinoma (mUC) with FGFR3/2 Genetic Alterations (GAs). J. Clin. Oncol. 2025, 43, 808. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Liu, J.; Luo, S.; Li, Q.; Zou, W.; Wang, Z.; Peng, Y.; Xiao, S.; Wang, H.; et al. SI-B003 (PD-1/CTLA-4) in Patients with Advanced Solid Tumors: A Phase I Study. J. Clin. Oncol. 2023, 41, e14668. [Google Scholar] [CrossRef]
- Safran, H.; Cassier, P.A.; Vicier, C.; Forget, F.; Gomez-Roca, C.A.; Penel, N.; Campone, M.; Romano, E.; Valerin, J.B.; Jerusalem, G.H.M.; et al. Phase 1/2 Study of DF1001, a Novel Tri-Specific, NK Cell Engager Therapy Targeting HER2, in Patients with Advanced Solid Tumors: Phase 1 DF1001 Monotherapy Dose-Escalation Results. J. Clin. Oncol. 2023, 41, 2508. [Google Scholar] [CrossRef]
- Makawita, S.; Gibbs, J.M.; McFadden, D.R.; Porter, C.; Shaw, A.R.; Robertson, C.; Woods, M.L.; Wang, T.; Grilley, B.J.; Heslop, H.E.; et al. Binary Oncolytic Adenovirus in Combination with HER2-Specific Autologous CAR VST for Treatment of Advanced HER2-Positive Solid Tumors (VISTA). J. Clin. Oncol. 2024, 42, TPS2679. [Google Scholar] [CrossRef]
| Trial Number | Investigational Drug | Mechanism | Cisplatin Eligibility | Trial Status | Location | Phase |
|---|---|---|---|---|---|---|
| NCT04876313 | Neoadjuvant Nab-paclitaxel + perioperative Nivolumab | Anti-PD-1 | Ineligible | Recruiting | Europe | 2 |
| NCT05241340 | Neoadjuvant Sasanlimab + concurrent SBRT | Anti-PD-1 | Ineligible | Recruiting | U.S. | 2 |
| NCT04813107 | Neoadjuvant Tislelizumab +/− APL-1202 | Anti-PD-1, MetAP2 inhibitor | Ineligible | Unknown | Global | 2 |
| NCT06571708 | Gemcitabine/Cisplatin with Cemiplimab +/− Fianlimab | Anti-PD-1, Anti-LAG-3 | Eligible | Not yet recruiting | U.S. | 2 |
| NCT05987241 | Adjuvant Nivolumab +/− Relatlimab | Anti-PD-1, Anti-LAG-3 | N/A | Recruiting | U.S. | 2/3 |
| NCT03924856 | Perioperative Pembrolizumab (with neoadjuvant chemotherapy) | Anti-PD-1 | Eligible | Active, not recruiting | Global | 3 |
| NCT02632409 | Adjuvant Nivolumab | Anti-PD-1 | N/A | Active, not recruiting | Global | 3 |
| NCT03661320 | Perioperative Nivolumab (with neoadjuvant chemotherapy) +/− Linrodostat | Anti-PD-1, IDO1 inhibitor | Eligible | Active, not recruiting | Global | 3 |
| NCT04209114 | Perioperative Nivolumab +/− Bempeg (NKTR-214) | Anti-PD-1, IL-2 prodrug | Ineligible | Completed | Global | 3 |
| NCT03732677 | Perioperative Durvalumab (with neoadjuvant chemotherapy) | Anti-PD-L1 | Eligible | Active, not recruiting | Global | 3 |
| NCT03288545 | Enfortumab Vedotin (various settings) | Nectin-4 ADC | Ineligible | Active, not recruiting | Global | 1b/2 |
| NCT06394570 | Neoadjuvant Enfortumab Vedotin + SBRT | Nectin-4 ADC | Ineligible | Recruiting | U.S. | 1/2 |
| NCT03924895 | Perioperative Enfortumab Vedotin +/− Pembrolizumab | Nectin-4 ADC, Anti-PD-1 | Ineligible | Active, not recruiting | Global | 3 |
| NCT04700124 | Perioperative Enfortumab Vedotin + Pembrolizumab | Nectin-4 ADC, Anti-PD-1 | Eligible | Active, not recruiting | Global | 3 |
| NCT04960709 | Neoadjuvant Enfortumab Vedotin with Durvalumab +/− Tremelimumab | Nectin-4 ADC, Anti-PD-L1, Anti-CTLA-4 | Ineligible | Active, not recruiting | Global | 3 |
| NCT05226117 | Neoadjuvant Sacituzumab Govitecan | Trop-2 ADC | Ineligible | Unknown | Europe | 2 |
| NCT05535218 | Neoadjuvant Sacituzumab Govitecan + perioperative Pembrolizumab | Trop-2 ADC, Anti-PD-1 | Ineligible | Active, not recruiting | Europe | 2 |
| NCT05297552 | Neoadjuvant Disitamab Vedotin + Toripalimab | HER2 ADC, Anti-PD-1 | Ineligible | Recruiting | Asia | 2 |
| NCT06511648 | Neoadjuvant Erdafitinib +/− Cetrelimab | FGFR inhibitor, Anti-PD-1 | Ineligible | Recruiting | Europe | 2 |
| NCT04919512 | Neoadjuvant Cetrelimab +/− TAR-200 gemcitabine | Anti-PD-1, intravesical gemictabine delivery system | Ineligible | Active, not recruiting | Global | 2 |
| NCT04610671 | Neoadjuvant GC0070 (Cremostigene) + Nivolumab | Oncolytic adenovirus, Anti-PD-1 | Ineligible | Active, not recruiting | U.S. | 1b |
| NCT03359239 | Adjuvant PGV001 + Atezolizumab | Multi-peptide neoantigen vaccine, Anti-PD-1 | N/A | Completed | U.S. | 1 |
| NCT06305767 | Perioperative V940 + Pembrolizumab and Enfortumab Vedotin (phase 1), Adjuvant Pembrolizumab +/− V940 (phase 2) | mRNA vaccine, Anti-PD-1, Nectin-4 ADC | Ineligible | Recruiting | Global | 1/2 |
| NCT06534983 | Adjuvant Autogene Cevumeran +/− Nivolumab | mRNA vaccine, Anti-PD-1 | Ineligible | Recruiting | Global | 2 |
| NCT | Investigational Drug | Mechanism | Line of Therapy | Trial Status | Location | Phase |
|---|---|---|---|---|---|---|
| NCT05845814 | Pembrolizumab + Favezelimab | Anti-PD-1, Anti-LAG-3 | First-line | Active, not recruiting | Global | 1/2 |
| NCT03036098 | Ipilimumab/Nivolumab (Arm A), Gemcitabine/Cisplatin +/− nivolumab (Arm C) | Anti-PD-1, Anti-CTLA-4 | First-line | Active, not recruiting | Global | 3 |
| NCT05216965 | 9MW2821 | Nectin-4 ADC | Unspecified | Recruiting | Asia | 1a/2 |
| NCT05735275 | SHR-A2102 | Nectin-4 ADC | Unspecified | Recruiting | Asia | 1 |
| NCT06465069 | LY4052031 | Nectin-4 ADC | Refractory | Suspended | Global | 1 |
| NCT06238479 | LY4101174 | Nectin-4 ADC | Refractory | Recruiting | Global | 1 |
| NCT06196736 | 9MW2821 + Toripalimab | Nectin-4 ADC, Anti-PD-1 | Refractory | Recruiting | Asia | 3 |
| NCT06823427 | 9MW2821 +/− Toripalimab | Nectin-4 ADC, Anti-PD-1 | First-line | Recruiting | Asia | 2 |
| NCT06592326 | 9MW2821 + Toripalimab | Nectin-4 ADC, Anti-PD-1 | First-line | Recruiting | Asia | 3 |
| NCT04223856 | Enfortumab Vedotin + Pembrolizumab | Nectin-4 ADC, Anti-PD-1 | First-line | Active, not recruiting | Global | 3 |
| NCT04264936 | Disitamab Vedotin + Toripalimab | HER2 ADC, Anti-PD-1 | Refractory | Unknown | Asia | 1b/2 |
| NCT04482309 | Trastuzumab Deruxtecan | HER2 ADC | Refractory | Recruiting | Global | 2 |
| NCT04879329 | Distiamab Vedotin + Pembrolizumab | HER2 ADC, Anti-PD-1 | Any | Recruiting | Global | 2 |
| NCT05911295 | Disitamab Vedotin + Pembrolizumab | HER2 ADC, Anti-PD-1 | First-line | Recruiting | Global | 3 |
| NCT05302284 | Disitamab Vedotin + Toripalimab | HER2 ADC, Anti-PD-1 | First-line | Recruiting | Asia | 3 |
| NCT05460273 | Datopotamab Deruxtecan | Trop-2 ADC | Unspecified | Active, not recruiting | Asia | 1/2 |
| NCT05489211 | Datopotamab Deruxtecan +/− other agents | Trop-2 ADC | Unspecified | Recruiting | Global | 2 |
| NCT03401385 | Datopotamab Deruxtecan | Trop-2 ADC | Refractory | Active, not recruiting | Global | 1 |
| NCT04152499 | Sacituzumab Tirumotecan | Trop-2 ADC | Refractory | Recruiting | Global | 1/2 |
| NCT06483334 | Sacituzumab Tirumotecan + Enfortumab Vedotin +/− Pembrolizumab | Trop-2 ADC, Nectin-4 ADC, Anti-PD-1 | Any | Recruiting | Global | 1/2 |
| NCT05785039 | BL-B01D1 | Bispecific EGFR-HER3 ADC | Refractory | Recruiting | Asia | 2 |
| NCT06857175 | BL-B01D1 | Bispecific EGFR-HER3 ADC | Refractory | Recruiting | Asia | 3 |
| NCT06405425 | BL-B01D1 + Anti-PD-1 (unspecified) | Bispecific EGFR-HER3 ADC, Anti-PD-1 | First-line | Recruiting | Asia | 2 |
| NCT04561362 | Zelenectide Pevedotin +/− Pembrolizumab | Nectin-4 toxin conjugate, Anti-PD-1 | Refractory | Recruiting | Global | 1/2 |
| NCT06225596 | Zelenectide Pevedotin +/− Pembrolizumab | Nectin-4 toxin conjugate, Anti-PD-2 | Any | Recruiting | Global | 2/3 |
| NCT04180371 | BT5528 +/− Nivolumab | EphA2 toxin conjugate, Anti-PD-1 | Refractory | Recruiting | Global | 1/2 |
| NCT05163041 | BT7480 +/− Nivolumab | Nectin-4/CD137 toxin conjugate, Anti-PD-1 | Refractory | Active, not recruiting | Global | 1/2 |
| NCT05544552 | TYRA-300 | FGFR3 inhibitor | Refractory | Recruiting | Global | 1/2 |
| NCT04963153 | Erdafitinib + Enfortumab Vedotin | Pan-FGFR inhibitor, Nectin-4 ADC | Refractory | Recruiting | Global | 1/2 |
| NCT03390504 | Erdafitinib | Pan-FGFR inhibitor | Refractory | Active, not recruiting | Global | 3 |
| NCT05614739 | LOXO-435 +/− Pembrolizumab +/− Enfortumab Vedotin | FGFR3 inhibitor, Anti-PD-1, Nectin-4 ADC | Any | Recruiting | Global | 1 |
| NCT03473743 | Erdafintib + either platinum-based chemotherapy or Cetrelimab | Pan-FGFR inhibitor, Anti-PD-1 | Any | Active, not recruiting | Global | 2 |
| NCT04601857 | Fudibatinib + Pembrolizumab | Pan-FGFR inhibitor | First-line | Active, not recruiting | Global | 2 |
| NCT04606472 | SI-B003 | PD-1/CTLA-4 bispecific antibody | Refractory | Recruiting | Asia | 1 |
| NCT05965856 | SI-B003 +/− BL-B01D1 | PD-1/CTLA-4 bispecific antibody, Bispecific EGFR-HER3 ADC | Refractory | Recruiting | Asia | 2 |
| NCT04143711 | DF1001 +/− Nivolumab, chemotherapy or Sacitizumab Govitecan | Trispecific HER2 NK cell engager, Anti-PD-1 | Refractory | Recruiting | Global | 1/2 |
| NCT03740256 | CAdVEC + HER2-directed CAR-T | Oncolytic adenovirus, CAR-T | Refractory | Recruiting | U.S. | 1 |
| NCT03359239 | PGV001 + Atezolizumab | Multi-peptide neoantigen vaccine, Anti-PD-1 | Refractory or switch-maintenance | Completed | U.S. | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehrlich, M.I.; Fox, R.D.; Runcie, K.D.; Stein, M.N.; Wei, A.Z. Novel Strategies and Therapeutic Advances for Bladder Cancer. Cancers 2025, 17, 2070. https://doi.org/10.3390/cancers17132070
Ehrlich MI, Fox RD, Runcie KD, Stein MN, Wei AZ. Novel Strategies and Therapeutic Advances for Bladder Cancer. Cancers. 2025; 17(13):2070. https://doi.org/10.3390/cancers17132070
Chicago/Turabian StyleEhrlich, Matthew I., Robert D. Fox, Karie D. Runcie, Mark N. Stein, and Alexander Z. Wei. 2025. "Novel Strategies and Therapeutic Advances for Bladder Cancer" Cancers 17, no. 13: 2070. https://doi.org/10.3390/cancers17132070
APA StyleEhrlich, M. I., Fox, R. D., Runcie, K. D., Stein, M. N., & Wei, A. Z. (2025). Novel Strategies and Therapeutic Advances for Bladder Cancer. Cancers, 17(13), 2070. https://doi.org/10.3390/cancers17132070






