Oncologic Outcomes of Breast-Conserving Surgery in a Colombian Cancer Center: An Observational, Analytical, Retrospective Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
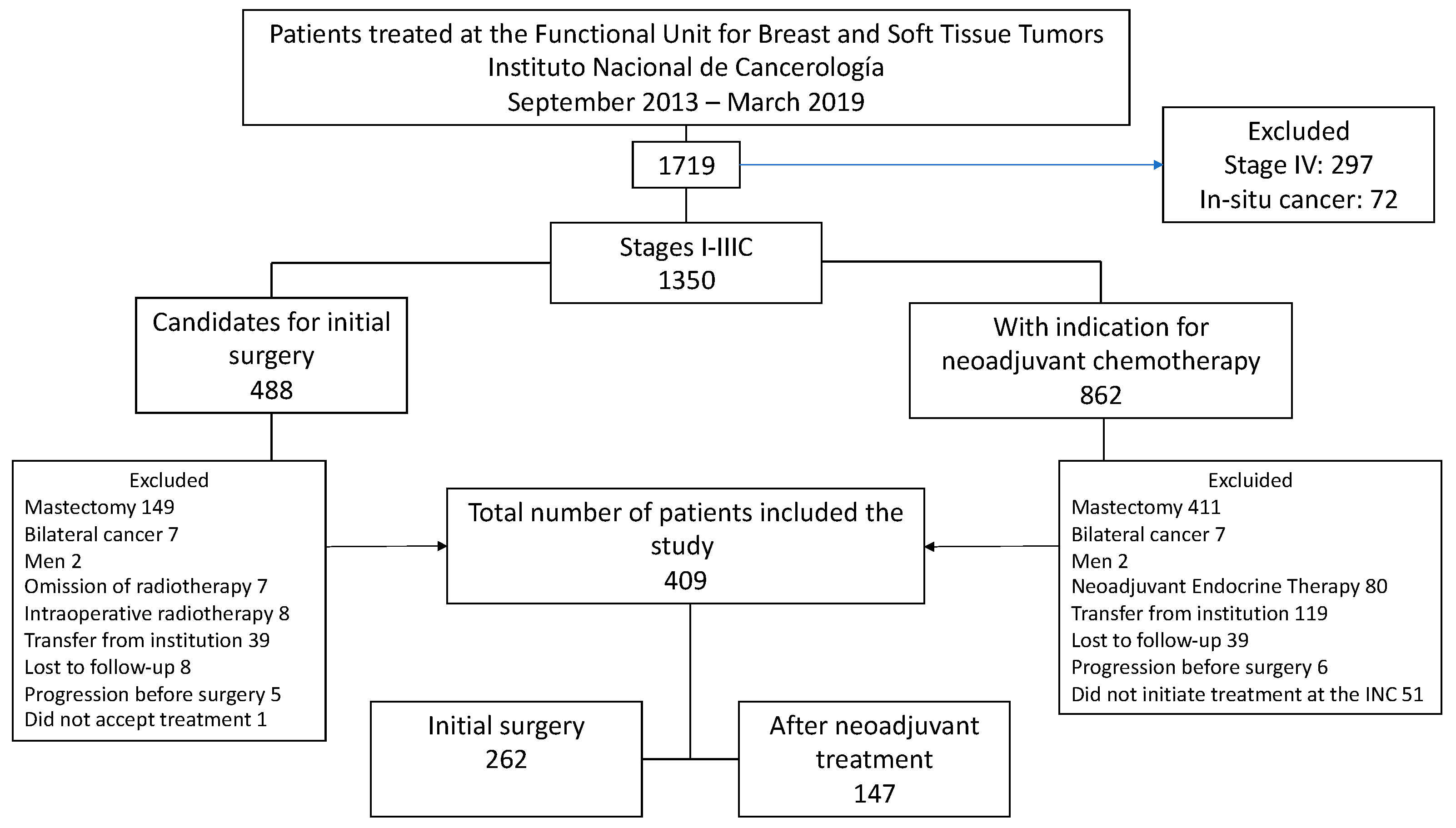
2.1. Study Design and Patient Eligibility
2.2. Statistical Analysis
3. Results
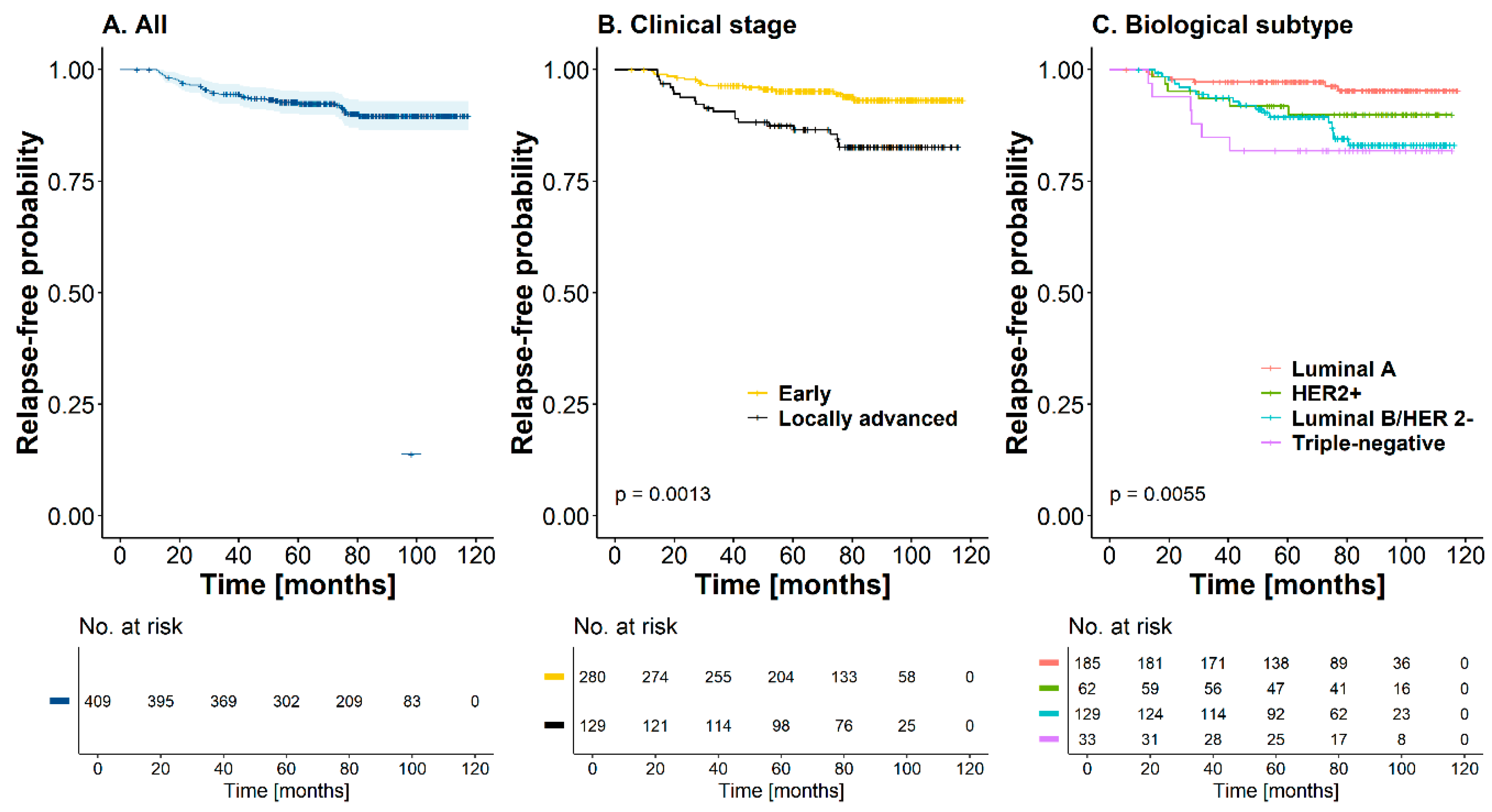
3.1. Local, Distant, Regional, or Mixed Recurrence
3.2. Univariate and Multivariate Analysis of Recurrences
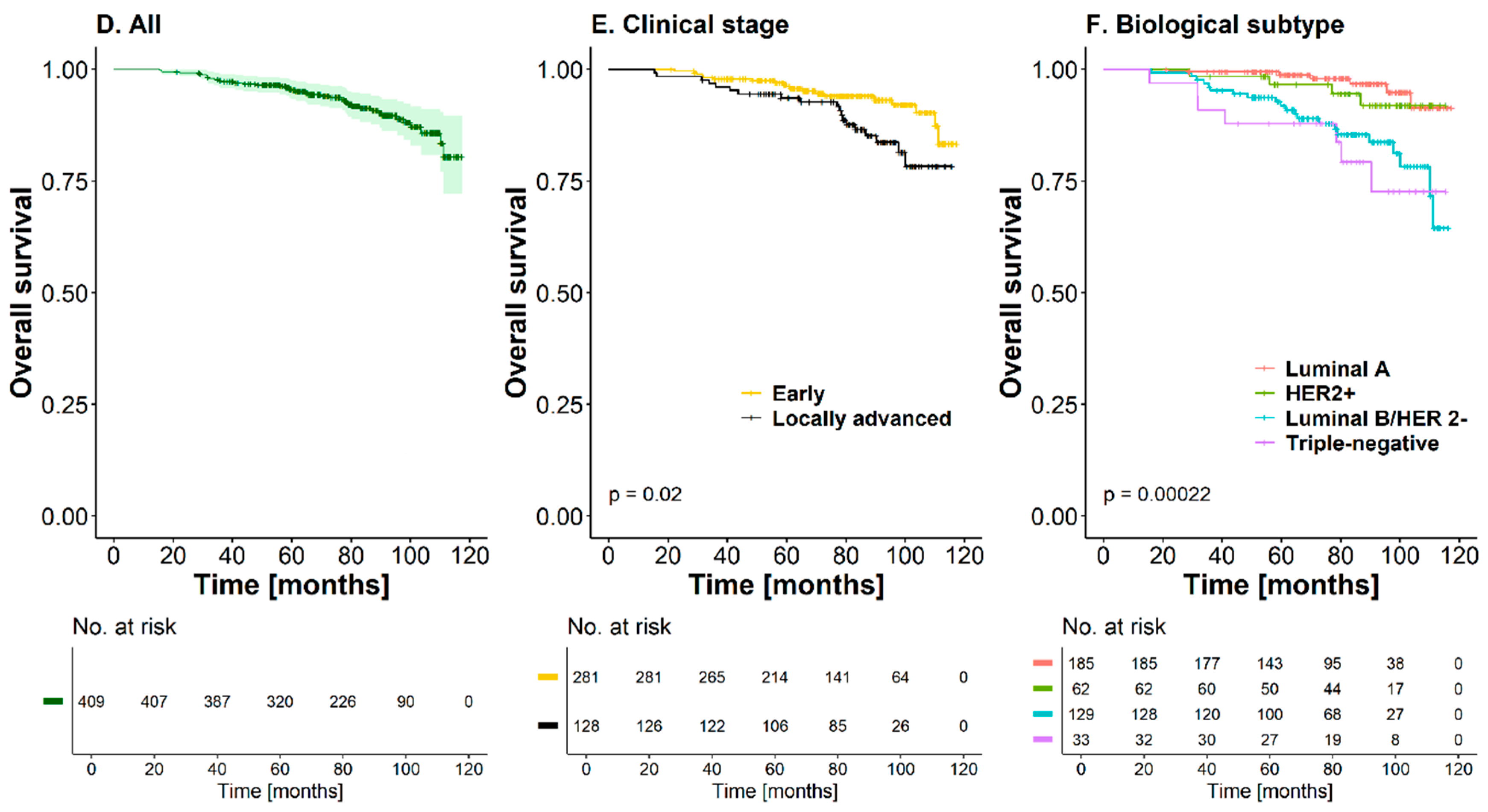
3.3. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D-CRT | Three-dimensional conformal radiotherapy |
| AC | Anthracycline, cyclophosphamide |
| AI | Aromatase inhibitor |
| BCS | Breast-conserving surgery |
| CI | Confidence intervals |
| IMRT | Intensity-modulated radiation therapy |
| INC | Instituto Nacional de Cancerología |
| IQR | Interquartile range |
| NACT | Neoadjuvant chemotherapy |
| OS | Overall survival |
| pCR | Pathologic complete response |
| RT | Adjuvant radiotherapy |
| SD | Standard deviation |
| T | Taxanes |
| TC | Taxane and cyclophosphamide |
| TH | Taxanes and trastuzumab |
| TR | Time to recurrence |
| VMAT | Volumetric intensity-modulated arc therapy |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; International Agency for Research on Cancer. Global Cancer Observatory; Cancer Today 2022; Colombia. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/170-colombia-fact-sheet.pdf (accessed on 25 March 2025).
- Kesson, E.M.; Allardice, G.M.; George, W.D.; Burns, H.J.G.; Morrison, D.S. Effects of multidisciplinary team working on breast cancer survival: Retrospective, comparative, interventional cohort study of 13 722 women. BMJ 2012, 344, e2718. [Google Scholar] [CrossRef]
- Halsted, W.S. The results of radical operations for the cure of carcinoma of the breast. Ann. Surg. 1907, 46, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef]
- Veronesi, U.; Volterrani, F.; Luini, A.; Saccozzi, R.; Del Vecchio, M.; Zucali, R.; Galimberti, V.; Rasponi, A.; Di Re, E.; Squicciarini, P.; et al. Quadrantectomy versus lumpectomy for small size breast cancer. Eur. J. Cancer Clin. Oncol. 1990, 26, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, U.; Luini, A.; Del Vecchio, M.; Greco, M.; Galimberti, V.; Merson, M.; Rilke, F.; Sacchini, V.; Saccozzi, R.; Savio, T.; et al. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N. Engl. J. Med. 1993, 328, 1587–1591. [Google Scholar] [CrossRef]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef]
- Hudis, C.A.; Barlow, W.E.; Costantino, J.P.; Gray, R.J.; Pritchard, K.I.; Chapman, J.A.W.; Sparano, J.A.; Hunsberger, S.; Enos, R.A.; Gelber, R.D.; et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: The STEEP system. J. Clin. Oncol. 2007, 25, 2127–2132. [Google Scholar] [CrossRef]
- Jacobson, J.A.; Danforth, D.N.; Cowan, K.H.; d’Angelo, T.; Steinberg, S.M.; Pierce, L.; Lippman, M.E.; Lichter, A.S.; Glatstein, E.; Okunieff, P. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N. Engl. J. Med. 1995, 332, 907–911. [Google Scholar] [CrossRef]
- Sarrazin, D.; Lê, M.; Arriagada, R.; Contesso, G.; Fontaine, F.; Spielmann, M.; Rochard, F.; Le Chevalier, T.; Lacour, J. Ten-year results of a randomized trial comparing a conservative treatment to mastectomy in early breast cancer. Radiother. Oncol. 1989, 14, 177–184. [Google Scholar] [CrossRef]
- Blichert-Toft, M.; Nielsen, M.; Düring, M.; Møller, S.; Rank, F.; Overgaard, M.; Mouridsen, H.T. Long-term results of breast conserving surgery vs. mastectomy for early stage invasive breast cancer: 20-year follow-up of the Danish randomized DBCG-82TM protocol. Acta Oncol. 2008, 47, 672–681. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: Meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet 2011, 378, 1707–1716. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014, 383, 2127–2135. [Google Scholar] [CrossRef]
- Van Dongen, J.A. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J. Natl. Cancer Inst. 2000, 92, 1143–1150. [Google Scholar] [CrossRef]
- Landercasper, J.; Whitacre, E.; Degnim, A.C.; Al-Hamadani, M. Reasons for re-excision after lumpectomy for breast cancer: Insight from the American Society of Breast Surgeons MasterySM Database. Ann. Surg. Oncol. 2014, 21, 3185–3191. [Google Scholar] [CrossRef]
- Bear, H.D.; Anderson, S.; Smith, R.E.; Geyer, C.E.; Mamounas, E.P.; Fisher, B.; Brown, A.M.; Robidoux, A.; Margolese, R.; Kahlenberg, M.S.; et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J. Clin. Oncol. 2006, 24, 2019–2027. [Google Scholar] [CrossRef]
- Mamounas, E.P. Impact of neoadjuvant chemotherapy on locoregional surgical treatment of breast cancer. Ann. Surg. Oncol. 2015, 22, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- King, T.A.; Morrow, M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat. Rev. Clin. Oncol. 2015, 12, 335–343. [Google Scholar] [CrossRef]
- Cooperating Investigators of the EORTC; Van Nes, J.G.H.; Putter, H.; Julien, J.P.; Tubiana-Hulin, M.; Van De Vijver, M.; Bogaerts, J.; de Vos, M.; van de Velde, C.J.H. Preoperative chemotherapy is safe in early breast cancer, even after 10 years of follow-up; clinical and translational results from the EORTC trial 10902. Breast Cancer Res. Treat. 2009, 115, 101–113. [Google Scholar] [CrossRef]
- Díaz-Casas, S.E.; Castilla-Tarra, J.A.; Pena-Torres, E.; Orozco-Ospino, M.; Mendoza-Diaz, S.; Nuñez-Lemus, M.; García-Angulo, O.; García-Mora, M.; Guzmán-AbiSaab, L.; Lehmann-Mosquera, C.; et al. Pathological response to neoadjuvant chemotherapy and the molecular classification of locally advanced breast cancer in a Latin American cohort. Oncologist 2019, 24, e1360–e1370. [Google Scholar] [CrossRef]
- Cho, J.H.; Park, J.M.; Park, H.S.; Park, S.; Kim, S.I.; Park, B. Oncologic safety of breast-conserving surgery compared to mastectomy in patients receiving neoadjuvant chemotherapy for locally advanced breast cancer. J. Surg. Oncol. 2013, 108, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.C.; Han, W.; Moon, H.G.; Im, S.A.; Moon, W.K.; Park, I.A.; Park, S.J.; Noh, D.Y. Breast-conserving surgery after tumor downstaging by neoadjuvant chemotherapy is oncologically safe for stage III breast cancer patients. Ann. Surg. Oncol. 2013, 20, 2582–2589. [Google Scholar] [CrossRef]
- Gwark, S.; Kim, H.; Kim, J.; Chung, I.; Kim, H.J.; Ko, B.; Lee, J.W.; Son, B.H.; Ahn, S.H.; Lee, S.B. Survival after breast-conserving surgery compared with that after mastectomy in breast cancer patients receiving neoadjuvant chemotherapy. Ann. Surg. Oncol. 2023, 30, 2845–2853. [Google Scholar] [CrossRef]
- Hage, A.N.; Capriccioso, C.; Brennan, J.; Heiden, B.; Zheutlin, A.; Sabel, M.S. Impact of neoadjuvant chemotherapy on surgical outcomes among patients with hormone receptor positive breast cancer. J. Surg. Oncol. 2017, 116, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Eiermann, W.; Semiglazov, V.; Manikhas, A.; Lluch, A.; Tjulandin, S.; Zambetti, M.; Vazquez, F.; Byakhow, M.; Lichinister, M.; et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): A randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 2010, 375, 377–384. [Google Scholar] [CrossRef]
- De Azambuja, E.; Holmes, A.P.; Piccart-Gebhart, M.; Holmes, E.; Di Cosimo, S.; Swaby, R.F.; Untch, M.; Jackisch, C.; Lang, I.; Smith, I.; et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): Survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response. Lancet Oncol. 2014, 15, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Schneeweiss, A.; Loibl, S.; Salat, C.; Denkert, C.; Rezai, M.; Blohmer, J.U.; Jackisch, C.; Paepke, S.; Gerber, B.; et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): A randomised phase 2 trial. Lancet Oncol. 2014, 15, 747–756. [Google Scholar] [CrossRef]
- Shepherd, J.H.; Ballman, K.; Polley, M.Y.C.; Campbell, J.D.; Fan, C.; Selitsky, S.; Fernandez-Martinez, A.; Parker, J.; Hoadley, K.A.; Hu, Z.; et al. CALGB 40603 (Alliance): Long-term outcomes and genomic correlates of response and survival after neoadjuvant chemotherapy with or without carboplatin and bevacizumab in triple-negative breast cancer. J. Clin. Oncol. 2022, 40, 1323–1334. [Google Scholar] [CrossRef]
- Loibl, S.; O’Shaughnessy, J.; Untch, M.; Sikov, W.M.; Rugo, H.S.; McKee, M.D.; Huober, J.; Golshan, M.; Von Minckwitz, G.; Maag, D.; et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 497–509. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Starosławska, E.; de la Haba-Rodriguez, J.; Im, S.A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Swain, S.M.; Ewer, M.S.; Viale, G.; Delaloge, S.; Ferrero, J.M.; Verrill, M.; Colomer, R.; Vieira, C.; Werner, T.L.; Douthwaite, H.; et al. Pertuzumab, trastuzumab, and standard anthracycline- and taxane-based chemotherapy for the neoadjuvant treatment of patients with HER2-positive localized breast cancer (BERENICE): A phase II, open-label, multicenter, multinational cardiac safety study. Ann. Oncol. 2018, 29, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Van Ramshorst, M.S.; Van Der Voort, A.; Van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Dezentjé, V.O.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1630–1640. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Corradini, S.; Reitz, D.; Pazos, M.; Schönecker, S.; Braun, M.; Harbeck, N.; Matuschek, C.; Bölke, E.; Ganswindt, U.; Alongi, F.; et al. Mastectomy or breast-conserving therapy for early breast cancer in real-life clinical practice: Outcome comparison of 7565 cases. Cancers 2019, 11, 160. [Google Scholar] [CrossRef]
- Almahariq, M.F.; Quinn, T.J.; Siddiqui, Z.; Jawad, M.S.; Chen, P.Y.; Gustafson, G.S.; Dilworth, J.T. Breast conserving therapy is associated with improved overall survival compared to mastectomy in early-stage, lymph node-negative breast cancer. Radiother. Oncol. 2020, 142, 186–194. [Google Scholar] [CrossRef]
- Sun, Z.H.; Chen, C.; Kuang, X.; Song, J.; Sun, S.; Wang, W.X. Breast surgery for young women with early-stage breast cancer. Medicine 2021, 100, e25880. [Google Scholar] [CrossRef]
- Allison, K.H. Prognostic and predictive parameters in breast pathology: A pathologist’s primer. Mod. Pathol. 2021, 34, 94–106. [Google Scholar] [CrossRef]
| Clinical and Histopathological Data | ||
|---|---|---|
| Age (Years) | Mean ± SD | 58.7 ± 10.7 |
| Tumor size (T), n (%) | T1 | 127 (31.3) |
| T2 | 230 (56.2) | |
| T3 | 20 (4.90) | |
| T4b | 32 (7.80) | |
| Node involvement (N), n (%) | N0 | 284 (69.4) |
| N1 | 86 (21.0) | |
| N2a | 37 (9.00) | |
| N2b | 1 (0.24) | |
| N3c | 1 (0.24) | |
| Clinical stage, n (%) | I | 115 (28.1) |
| IIA | 166 (40.6) | |
| IIB | 60 (14.7) | |
| IIIA | 36 (8.80) | |
| IIIB | 31 (7.60) | |
| IIIC | 1 (0.24) | |
| Histological type †, n (%) | Ductal (NOS) | 362 (88.5) |
| Lobular | 18 (4.43) | |
| Medullary | 1 (0.24) | |
| Mucinous | 8 (1.97) | |
| Papillary | 5 (1.20) | |
| Tubular | 12 (2.90) | |
| Other | 3 (0.73) | |
| Histological grade, n (%) | I | 70 (17.1) |
| II | 265 (64.8) | |
| III | 74 (18.1) | |
| Molecular subtype, n (%) | Luminal A | 185 (45.2) |
| Luminal B HER2-positive | 53 (13.0) | |
| Luminal B HER2-negative | 129 (31.5) | |
| Pure HER | 9 (2.20) | |
| Triple-negative | 33 (8.10) | |
| Prognostic data of the surgical specimen | ||
| Tumor extension §, n (%) | ||
| Unifocal | 383 (93.6) | |
| Multifocal | 26 (6.4) | |
| Sentinel lymph node report §, n (%) | 256 (62.6) | |
| Sentinel lymph node result | Negative | 178 (69.5) |
| Positive | 78 (30.5) | |
| Macrometastasis | 54 (69.2) | |
| Micrometastasis | 21 (26.9) | |
| Isolated tumor cells | 3 (3.80) | |
| Number of positive lymph nodes | 1 | 55 (70.5) |
| [2,3] | 20 (25.6) | |
| ≥4 | 3 (3.80) | |
| Number of lymph nodes at axillary dissection § | ||
| 0 | 264 (64.5) | |
| [1–3] | 102 (24.9) | |
| ≥4 | 43 (10.5) | |
| Positive resection margins ¶, n (%) | 52 (12.7) | |
| Resection margin status | 1 margin | 44 (84.6) |
| 2 margins | 6 (11.5) | |
| 3 margins or more | 2 (3.80) | |
| Pathological response *, n (%) | 147 (35.9) | |
| Class 1 | 37 (25.2) | |
| Class 2 | 16 (10.9) | |
| Class 3 | 88 (59.9) | |
| Class 4 | 6 (4.10) | |
| Treatment Type § | n (%) | |
|---|---|---|
| Initial breast-conserving surgery § | 262 (64.1) | |
| Type of axillary surgery in initial breast-conserving surgery | Sentinel lymph node | 238 (90.8) |
| Axillary dissection | 24 (9.20) | |
| Surgery after neoadjuvant chemotherapy § | 147 (35.9) | |
| Type of axillary surgery following neoadjuvant chemotherapy | Sentinel lymph node | 18 (12.2) |
| Axillary dissection | 129 (87.8) | |
| Neoadjuvant chemotherapy, n (%) | ||
| Chemotherapy regimen | 147 (35.9) | |
| AC-T | 88 (59.9) | |
| AC-TC | 7 (4.80) | |
| AC-TH | 38 (25.9) | |
| Others | 14 (5.40) | |
| Treatments for positive margins | 52 (12.7) | |
| Simple mastectomy | 4 (7.70) | |
| Re-quadrantectomy | 34 (65.4) | |
| Radiotherapy | 14 (26.9) | |
| Management of positive sentinel lymph node § | 78 (30.5) | |
| Management type | Omission of dissection | 35 (44.9) |
| Lymph node dissection | 43 (55.1) | |
| Adjuvant systemic therapy, n (%) | 156 (38.1) | |
| Chemotherapy alone | 92 (58.9) | |
| Chemotherapy + target therapy | 28 (17.9) | |
| Target therapy alone | 35 (22.4) | |
| Other | 1 (0.64) | |
| Adjuvant radiotherapy § | 407 (99.5) | |
| Radiotherapy technique used | 3D-CRT | 370 (90.9) |
| IMRT | 31 (7.60) | |
| VMAT | 6 (1.50) | |
| Adjuvant endocrine therapy § | 365 (89.2) | |
| Type of endocrine therapy used | Tamoxifen | 189 (51.8) |
| Aromatase inhibitor | 105 (28.8) | |
| Tamoxifen + aromatase inhibitor | 70 (19.2) | |
| GnRH analog + AI | 1 (0.30) |
| Characteristic | Primary Treatment | ||
|---|---|---|---|
| Total | Initial Surgery | Neoadjuvant Chemotherapy | |
| Local | 12 (2.93) | ||
| Regional | 9 (2.2) | ||
| Distant | 23 (5.62) | ||
| Type of recurrence § | |||
| Local §, n (%) | 12 (27.3) | 5 (41.6) | 7 (58.3) |
| Recurrence site | |||
| Same quadrant | 10 (83.3) | 4 (80.0) | 6 (85.7) |
| Different quadrant | 2 (16.7) | 1 (20.0) | 1 (14.2) |
| Regional §, n (%) | 9 (18.1) | 6 (62.5) | 3 (37.5) |
| Distant §, n (%) | 23 (52.3) | 8 (34.8) | 15 (65.2) |
| Recurrence site | |||
| Bone | 12 (52.2) | 5 (62.5) | 7 (46.6) |
| Lung | 3 (13.0) | 1 (12.5) | 2 (13.3) |
| Pleura | 3 (13.0) | 1 (12.5) | 2 (13.3) |
| Liver | 2 (8.70) | 0 (0.00) | 2 (13.3) |
| Central nervous system | 2 (8.70) | 0 (0.00) | 2 (13.3) |
| Non-regional lymph nodes + bone | 1 (4.30) | 1 (12.5) | 0 (0.00) |
| Treatments used †, n (%) | |||
| Re-quadrantectomy | 1 (2.70) | 1 (100) | 0 (0.00) |
| Simple mastectomy | 8 (21.6) | 3 (37.5) | 5 (62.5) |
| Axillary dissection | 4 (10.8) | 3 (75.0) | 1 (25.0) |
| Chemotherapy | 6 (16.2) | 2 (33.3) | 4 (66.7) |
| Chemotherapy + target therapy | 3 (8.10) | 0 (0.00) | 3 (100) |
| Endocrine therapy | 15 (40.5) | 8 (53.3) | 7 (46.7) |
| Endocrine therapy + target therapy | 12 (32.4) | 6 (50.0) | 6 (50.0) |
| Radiotherapy | 11 (29.7) | 3 (27.3) | 8 (72.7) |
| Metastasectomy | 3 (8.10) | 0 (0.00) | 3 (100) |
| Other | 2 (5.40) | 0 (0.00) | 2 (100) |
| Overall Survival (OS) | Time to Recurrence (TR) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | HR a [CI95%] | p-Value | HR b [CI95%] | p-Value | HR a [CI95%] | p-Value | HR b [CI95%] | p-Value |
| Age (years) | ||||||||
| <50 | Ref. | Ref. | ||||||
| ≥50 | 1.21 [0.55; 3.08] | 0.654 | 0.87 [0.43; 1.91] | 0.707 | ||||
| Clinical stage | ||||||||
| Early | Ref. | Ref. | Ref. | Ref. | ||||
| Locally advanced | 2.09 [1.11; 3.98] | 0.023 | 5.13 [1.49; 17.6] | <0.01 | 2.78 [1.47; 5.38] | <0.01 | 2.21 [0.83; 5.90] | 0.112 |
| Tumor size | ||||||||
| T1-T2 | Ref. | Ref. | Ref. | Ref. | ||||
| T3-T4 | 1.53 [0.67; 3.49] | 0.106 | 0.55 [0.13; 2.33] | 0.420 | 1.98 [0.87; 4.06] | 0.100 | 0.76 [0.31; 1.91] | 0.566 |
| Histological grade | ||||||||
| I-II | Ref. | Ref. | Ref. | |||||
| III | 2.01 [0.96; 4.20] | 0.064 | 0.74 [0.25; 2.19] | 0.590 | 1.54 [0.70; 3.10] | 0.264 | ||
| Biological subtype | ||||||||
| Luminal A | Ref. | Ref. | Ref. | Ref. | ||||
| Her2+ | 1.72 [0.48; 6.09] | 0.401 | 0.44 [0.05; 4.30] | 0.480 | 2.45 [0.82; 7.08] | 0.103 | 1.78 [0.56; 5.64] | 0.330 |
| Luminal B HER2- | 4.90 [1.97; 12.1] | <0.01 | 2.97 [0.89; 9.91] | 0.077 | 3.58 [1.59; 8.94] | <0.01 | 3.06 [1.24; 7.56] | 0.016 |
| Triple-negative | 6.13 [2.05; 18.3] | <0.01 | 8.02 [1.79; 35.9] | <0.01 | 4.99 [1.68; 14.4] | <0.01 | 4.46 [1.37; 14.6] | 0.013 |
| Negative | Ref. | Ref. | Ref. | Ref. | ||||
| [1-3] | 1.40 [0.64; 3.04] | 0.391 | 0.82 [0.26; 2.58] | 0.730 | 1.19 [0.53; 2.51] | 0.651 | 1.03 [0.44; 2.41] | 0.946 |
| ≥4 | 2.25 [0.89; 5.67] | 0.086 | 4.00 [1.44; 11.1] | <0.01 | 2.85 [1.21; 6.13] | 0.018 | 2.56 [1.08; 6.06] | 0.033 |
| Tumor extensión | ||||||||
| Unifocal | Ref. | Ref. | Ref. | |||||
| Multifocal | 2.06 [0.66; 4.96] | 0.189 | 3.44 [1.34; 7.50] | 0.012 | 3.31 [1.37; 8.04] | <0.01 | ||
| Initial treatment | ||||||||
| Surgery | Ref. | Ref. | Ref. | |||||
| Neoadjuvant CT | 2.35 [1.18; 4.66] | 0.015 | 1.22 [0.36; 4.18] | 0.750 | 2.45 [1.30; 4.81] | <0.01 | 1.13 [0.42; 3.01] | 0.810 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Casas, S.E.; Rosero-Díazdel Castillo, F.J.; Mendoza-Díaz, S.; Sáenz-Ladino, A.; Sánchez-Pedraza, R.; Silva-Cárdenas, S.P.; Zuluaga-Liberato, A.; Briceño-Morales, X.; Guzmán-AbiSaab, L.; Gamboa-Garay, Ó.; et al. Oncologic Outcomes of Breast-Conserving Surgery in a Colombian Cancer Center: An Observational, Analytical, Retrospective Cohort Study. Cancers 2025, 17, 1131. https://doi.org/10.3390/cancers17071131
Díaz-Casas SE, Rosero-Díazdel Castillo FJ, Mendoza-Díaz S, Sáenz-Ladino A, Sánchez-Pedraza R, Silva-Cárdenas SP, Zuluaga-Liberato A, Briceño-Morales X, Guzmán-AbiSaab L, Gamboa-Garay Ó, et al. Oncologic Outcomes of Breast-Conserving Surgery in a Colombian Cancer Center: An Observational, Analytical, Retrospective Cohort Study. Cancers. 2025; 17(7):1131. https://doi.org/10.3390/cancers17071131
Chicago/Turabian StyleDíaz-Casas, Sandra E., Flavio J. Rosero-Díazdel Castillo, Sara Mendoza-Díaz, Andersson Sáenz-Ladino, Ricardo Sánchez-Pedraza, Sonia P. Silva-Cárdenas, Andrea Zuluaga-Liberato, Ximena Briceño-Morales, Luis Guzmán-AbiSaab, Óscar Gamboa-Garay, and et al. 2025. "Oncologic Outcomes of Breast-Conserving Surgery in a Colombian Cancer Center: An Observational, Analytical, Retrospective Cohort Study" Cancers 17, no. 7: 1131. https://doi.org/10.3390/cancers17071131
APA StyleDíaz-Casas, S. E., Rosero-Díazdel Castillo, F. J., Mendoza-Díaz, S., Sáenz-Ladino, A., Sánchez-Pedraza, R., Silva-Cárdenas, S. P., Zuluaga-Liberato, A., Briceño-Morales, X., Guzmán-AbiSaab, L., Gamboa-Garay, Ó., Ángel-Aristizábal, J., Mariño-Lozano, I., Suárez-Rodríguez, R., García-Mora, M., Duarte-Torres, C., & Núñez-Lemus, M. (2025). Oncologic Outcomes of Breast-Conserving Surgery in a Colombian Cancer Center: An Observational, Analytical, Retrospective Cohort Study. Cancers, 17(7), 1131. https://doi.org/10.3390/cancers17071131






