Is the Addition of Chemotherapy to Adjuvant Radiation in Merkel Cell Cancer Beneficial? Real-World Data with Long-Term Follow-Up
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Statistical Analysis
3. Results
3.1. Study Population
3.2. Sample Size
3.3. Disease and Treatment Characteristics
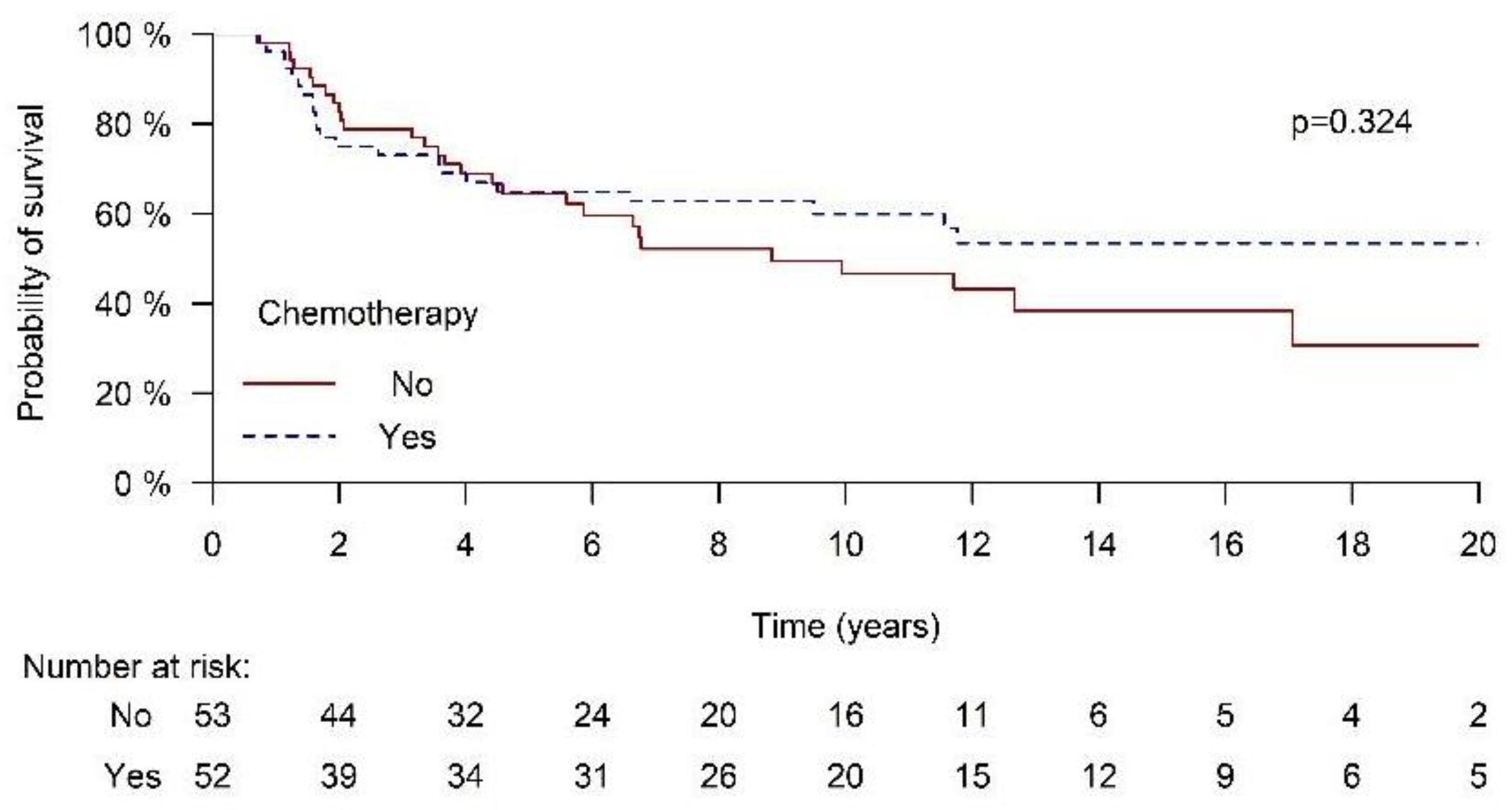
3.4. Survival Outcomes
3.5. Disease-Free Survival
3.6. Survival Outcomes in MCC Patients Excluding Unknown Primary
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schneider, S.; Thurnher, D.; Erovic, B.M. Merkel cell carcinoma: Interdisciplinary management of a rare disease. J. Ski. Cancer 2013, 2013, 189342. [Google Scholar] [CrossRef]
- Bleicher, J.; Asare, E.A.; Flores, S.; Bowles, T.L.; Bowen, G.M.; Hyngstrom, J.R. Oncologic outcomes of patients with Merkel Cell Carcinoma (MCC): A multi-institutional cohort study. Am. J. Surg. 2021, 221, 844–849. [Google Scholar] [CrossRef]
- Harms, P.W.; Harms, K.L.; Moore, P.S.; DeCaprio, J.A.; Nghiem, P.; Wong, M.K.K.; Brownell, I.; International Workshop on Merkel Cell Carcinoma Research (IWMCC) Working Group. The biology and treatment of Merkel cell carcinoma: Current understanding and research priorities. Nat. Rev. Clin. Oncol. 2018, 15, 763–776. [Google Scholar] [CrossRef]
- Becker, J.C.; Stang, A.; Hausen, A.Z.; Fischer, N.; DeCaprio, J.A.; Tothill, R.W.; Lyngaa, R.; Hansen, U.K.; Ritter, C.; Nghiem, P.; et al. Epidemiology, biology and therapy of Merkel cell carcinoma: Conclusions from the EU project IMMOMEC. Cancer Immunol. Immunother. CII 2018, 67, 341–351. [Google Scholar] [CrossRef]
- Fitzgerald, T.L.; Dennis, S.; Kachare, S.D.; Vohra, N.A.; Wong, J.H.; Zervos, E.E. Dramatic Increase in the Incidence and Mortality from Merkel Cell Carcinoma in the United States. Am. Surg. 2015, 81, 802–806. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Batich, K.; Chable-Montero, F.; Sagy, N.; Schwartz, A.M.; Henson, D.E. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: A population based study. J. Cutan. Pathol. 2010, 37, 20–27. [Google Scholar] [CrossRef]
- Hodgson, N.C. Merkel cell carcinoma: Changing incidence trends. J. Surg. Oncol. 2005, 89, 1–4. [Google Scholar] [CrossRef]
- Paulson, K.G.; Park, S.Y.; Vandeven, N.A.; Lachance, K.; Thomas, H.; Chapuis, A.G.; Harms, K.L.; Thompson, J.A.; Bhatia, S.; Stang, A.; et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2018, 78, 457–463.e2. [Google Scholar] [CrossRef]
- Tello, T.L.; Coggshall, K.; Yom, S.S.; Yu, S.S. Merkel cell carcinoma: An update and review: Current and future therapy. J. Am. Acad. Dermatol. 2018, 78, 445–454. [Google Scholar] [CrossRef]
- Heath, M.; Jaimes, N.; Lemos, B.; Mostaghimi, A.; Wang, L.C.; Peñas, P.F.; Nghiem, P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J. Am. Acad. Dermatol. 2008, 58, 375–381. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef]
- Schadendorf, D.; Lebbé, C.; Zur Hausen, A.; Avril, M.F.; Hariharan, S.; Bharmal, M.; Becker, J.C. Merkel cell carcinoma: Epidemiology, prognosis, therapy and unmet medical needs. Eur. J. Cancer 2017, 71, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.C.; Busam, K.J.; Chou, J.F.; Panageas, K.S.; Pulitzer, M.P.; Allen, P.J.; Kraus, D.H.; Brady, M.S.; Coit, D.G. Recurrence after complete resection and selective use of adjuvant therapy for stage I through III Merkel cell carcinoma. Cancer 2012, 118, 3311–3320. [Google Scholar] [CrossRef]
- Bhatia, S.; Storer, B.E.; Iyer, J.G.; Moshiri, A.; Parvathaneni, U.; Byrd, D.; Sober, A.J.; Sondak, V.K.; Gershenwald, J.E.; Nghiem, P. Adjuvant Radiation Therapy and Chemotherapy in Merkel Cell Carcinoma: Survival Analyses of 6908 Cases from the National Cancer Data Base. J. Natl. Cancer Inst. 2016, 108, djw042. [Google Scholar] [CrossRef]
- Lewis, K.G.; Weinstock, M.A.; Weaver, A.L.; Otley, C.C. Adjuvant local irradiation for Merkel cell carcinoma. Arch. Dermatol. 2006, 142, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Roman, S.A.; Sosa, J.A.; Judson, B.L. The role of adjuvant therapy in the management of head and neck merkel cell carcinoma: An analysis of 4815 patients. JAMA Otolaryngol.-Head Neck Surg. 2015, 141, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Garneski, K.M.; Nghiem, P. Merkel cell carcinoma adjuvant therapy: Current data support radiation but not chemotherapy. J. Am. Acad. Dermatol. 2007, 57, 166–169. [Google Scholar] [CrossRef]
- Tarabadkar, E.S.; Fu, T.; Lachance, K.; Hippe, D.S.; Pulliam, T.; Thomas, H.; Li, J.Y.; Lewis, C.W.; Doolittle-Amieva, C.; Byrd, D.R.; et al. Narrow excision margins are appropriate for Merkel cell carcinoma when combined with adjuvant radiation: Analysis of 188 cases of localized disease and proposed management algorithm. J. Am. Acad. Dermatol. 2021, 84, 340–347. [Google Scholar] [CrossRef]
- Strom, T.; Carr, M.; Zager, J.S.; Naghavi, A.; Smith, F.O.; Cruse, C.W.; Messina, J.L.; Russell, J.; Rao, N.G.; Fulp, W.; et al. Radiation Therapy is Associated with Improved Outcomes in Merkel Cell Carcinoma. Ann. Surg. Oncol. 2016, 23, 3572–3578. [Google Scholar] [CrossRef]
- Hasan, S.; Liu, L.; Triplet, J.; Li, Z.; Mansur, D. The role of postoperative radiation and chemoradiation in merkel cell carcinoma: A systematic review of the literature. Front. Oncol. 2013, 3, 276. [Google Scholar] [CrossRef]
- Wong, W.G.; Stahl, K.; Olecki, E.J.; Holguin, R.P.; Pameijer, C.; Shen, C. Survival Benefit of Guideline-Concordant Postoperative Radiation for Local Merkel Cell Carcinoma. J. Surg. Res. 2021, 266, 168–179. [Google Scholar] [CrossRef]
- Iyer, J.G.; Blom, A.; Doumani, R.; Lewis, C.; Tarabadkar, E.S.; Anderson, A.; Ma, C.; Bestick, A.; Parvathaneni, U.; Bhatia, S.; et al. Response rates and durability of chemotherapy among 62 patients with metastatic Merkel cell carcinoma. Cancer Med. 2016, 5, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.C.; Stillie, A.L.; Seel, M.; Ainslie, J. Merkel cell carcinoma: 27-year experience at the Peter MacCallum Cancer Centre. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1430–1435. [Google Scholar] [CrossRef] [PubMed]
- Fenig, E.; Brenner, B.; Katz, A.; Rakovsky, E.; Hana, M.B.; Sulkes, A. The role of radiation therapy and chemotherapy in the treatment of Merkel cell carcinoma. Cancer 1997, 80, 881–885. [Google Scholar] [CrossRef]
- Poulsen, M.G.; Rischin, D.; Porter, I.; Walpole, E.; Harvey, J.; Hamilton, C.; Keller, J.; Tripcony, L. Does chemotherapy improve survival in high-risk stage I and II Merkel cell carcinoma of the skin? Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 114–119. [Google Scholar] [CrossRef]
- Paulson, K.G.; Iyer, J.G.; Blom, A.; Warton, E.M.; Sokil, M.; Yelistratova, L.; Schuman, L.; Nagase, K.; Bhatia, S.; Asgari, M.M.; et al. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J. Investig. Dermatol. 2013, 133, 642–646. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Russell, J.; Lebbé, C.; Chmielowski, B.; Gambichler, T.; Grob, J.J.; Kiecker, F.; Rabinowits, G.; Terheyden, P.; Zwiener, I.; et al. Efficacy and Safety of First-line Avelumab Treatment in Patients with Stage IV Metastatic Merkel Cell Carcinoma: A Preplanned Interim Analysis of a Clinical Trial. JAMA Oncol. 2018, 4, e180077. [Google Scholar] [CrossRef]
- Nghiem, P.T.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.K.; Daud, A.; Fling, S.P.; et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology: Merkel Cell Carcinoma (Version 2023). Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1444 (accessed on 27 February 2025).
- Poulsen, M.; Round, C.; Keller, J.; Tripcony, L.; Veness, M. Factors influencing relapse-free survival in Merkel cell carcinoma of the lower limb—A review of 60 cases. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 393–397. [Google Scholar] [CrossRef]
- D’Angelo, S.P.; Lebbé, C.; Mortier, L.; Brohl, A.S.; Fazio, N.; Grob, J.J.; Prinzi, N.; Hanna, G.J.; Hassel, J.C.; Kiecker, F.; et al. First-line avelumab treatment in patients with metastatic Merkel cell carcinoma: 4-year follow-up from part B of the JAVELIN Merkel 200 study. ESMO Open 2024, 9, 103461. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Chemoradiation (n = 52) | Radiation Only (n = 53) | p Value |
|---|---|---|---|
| Age, median (IQR [years]) | 65.9 (59–73) | 77.3(61.4–83.3) | 0.002 |
| Male (%) | 36 (69.2) | 30 (56.6) | 0.181 |
| Radiation (GY) median (IQR) [GY] | 50 (45–50) | 45 (38–50) | 0.002 |
| Tumor site | |||
| Head and Neck | 13 (25) | 15 (28.3) | 0.7 |
| Torso | 5 (9.6) | 7 (13.2) | |
| Upper limbs | 3 (5.8) | 14 (26.4) | |
| Lower limbs | 6 (11.5) | 12 (22.6) | |
| Buttocks | 7 (13.5) | 14 (7.5) | |
| Unknown primary (lower body) | 15 (28.8) | 1 (1.9) | |
| Unknown primary (upper body) | 3 (5.8) | 0 | |
| T staging | 0.035 | ||
| T1 | 13 (39.4) | 31 (59.6) | |
| T2 | 15 (45.5) | 18 (34.6) | |
| T3 | 4 (12.1) | 3 (5.8) | |
| T4 | 1 (3) | 0 (0) | |
| N staging | <0.001 | ||
| N0 | 6 (11.5) | 37 (71.2) | |
| N1 | 43 (82.7) | 14 (26.9) | |
| N2 | 3 (5.8) | 1 (1.9) | |
| TNM Stage | <0.001 | ||
| 1 | 3 (5.8) | 24 (45.3) | |
| 2a | 2 (3.8) | 14 (26.4) | |
| 2b | 1 (1.9) | 0 | |
| 3a | 16 (30.8) | 11 (20.8) | |
| 3b | 30 (57.2) | 4 (7.5) |
| OS-5 Years | OS-20 Years | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | p Value | HR | 95% CI | p Value |
| Chemotherapy | 1.547 | 0.656–3.65 | 0.319 | 1.358 | 0.614–3.003 | 0.450 |
| Age at diagnosis | 1.064 | 1.029–1.100 | <0.001 | 1.069 | 1.039–1.101 | <0.001 |
| TNM stage | 0.696 | 0.722 | ||||
| I | Ref * | Ref * | ||||
| II | 1.114 | 0.385–3.229 | 0.696 | 0.283–1.713 | ||
| III | 1.492 | 0.570–3.904 | 0.827 | 0.373–1.833 | ||
| Location | 0.113 | 0.528 | ||||
| Head and neck | Ref * | Ref * | ||||
| Rest of body | 0.452 | 0.213–0.960 | 0.688 | 0.360–1.315 | ||
| Unknown primary | 0.517 | 0.173–1.546 | 0.781 | |||
| DFS-5 Years | |||
|---|---|---|---|
| Variable | HR | 95% CI | p Value |
| Chemotherapy | 1.324 | 0.592–2.960 | 0.494 |
| Age at diagnosis | 1.048 | 1.018–1.078 | <0.001 |
| TNM stage | 0.819 | ||
| I | Ref * | ||
| II | 1.229 | 0.459–3.293 | |
| III | 1.336 | 0.539–3.311 | |
| Location | 0.17 | ||
| Head and neck | Ref * | ||
| Rest of body | 0.517 | 0.260–1.217 | |
| Unknown primary | 0.685 | 0.263–1.784 | |
| Chemoradiation (n = 34) | Radiation (n = 34) | p Value | |
|---|---|---|---|
| 5-Year OS (%) | 60.8 | 65.8 | 0.54 |
| 20-Year OS (%) | 51.6 | 31.2 | 0.58 |
| 5-Year DFS (%) | 55.3 | 59.9 | 0.64 |
| 20-Year DFS (%) | 46.7 | 29.9 | 0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shalata, W.; Edri, H.T.F.; Sarel, I.; Ievko, A.; Turaieva, S.; Tairov, T.; Berezhnov, I.; Fenig, S.; Fenig, E.; Ziv-Baran, T.; et al. Is the Addition of Chemotherapy to Adjuvant Radiation in Merkel Cell Cancer Beneficial? Real-World Data with Long-Term Follow-Up. Cancers 2025, 17, 945. https://doi.org/10.3390/cancers17060945
Shalata W, Edri HTF, Sarel I, Ievko A, Turaieva S, Tairov T, Berezhnov I, Fenig S, Fenig E, Ziv-Baran T, et al. Is the Addition of Chemotherapy to Adjuvant Radiation in Merkel Cell Cancer Beneficial? Real-World Data with Long-Term Follow-Up. Cancers. 2025; 17(6):945. https://doi.org/10.3390/cancers17060945
Chicago/Turabian StyleShalata, Walid, Hanna T. Frumin Edri, Ina Sarel, Anna Ievko, Sofiia Turaieva, Tanzilya Tairov, Ilia Berezhnov, Shlomit Fenig, Eyal Fenig, Tomer Ziv-Baran, and et al. 2025. "Is the Addition of Chemotherapy to Adjuvant Radiation in Merkel Cell Cancer Beneficial? Real-World Data with Long-Term Follow-Up" Cancers 17, no. 6: 945. https://doi.org/10.3390/cancers17060945
APA StyleShalata, W., Edri, H. T. F., Sarel, I., Ievko, A., Turaieva, S., Tairov, T., Berezhnov, I., Fenig, S., Fenig, E., Ziv-Baran, T., Yakobson, A., & Brenner, R. (2025). Is the Addition of Chemotherapy to Adjuvant Radiation in Merkel Cell Cancer Beneficial? Real-World Data with Long-Term Follow-Up. Cancers, 17(6), 945. https://doi.org/10.3390/cancers17060945







