Simple Summary
The role of surgery in stage IV endometrial cancer with peritoneal carcinosis remains unclear. This systematic review and individual patient data meta-analysis compares two treatment approaches: primary debulking surgery followed by platinum-based chemotherapy, versus interval debulking surgery after neoadjuvant platinum-based chemotherapy. The analysis included 285 patients and found that primary debulking surgery resulted in longer progression-free survival (18 months vs. 12 months) but similar overall survival (around 30 months for both groups). The presence of no residual tumor after surgery was associated with better survival outcomes. This study suggests that primary debulking surgery should be considered the preferred option for advanced endometrial cancer with peritoneal metastasis when complete tumor removal is possible. Further studies are needed to refine treatment selection, potentially using molecular risk profiles.
Abstract
Objective. To compare the survival outcomes of primary debulking surgery and platinum-based adjuvant chemotherapy versus interval debulking surgery after platinum-based neoadjuvant chemotherapy in patients with stage IVb endometrial cancer and peritoneal carcinosis. Methods. The online search included the following data sources: PubMed, Scopus, WOS, and the Cochrane Library from 1990 to 2024 (PROSPERO registration code: CRD42023438602). A total of 3230 studies were identified, with the inclusion of 16. Individual patient data on survival outcomes, disease distribution, and residual tumors, as well as details of neoadjuvant chemotherapy and adjuvant treatment, were extracted. Results. A total of 285 patients were included: 197 (69%) underwent primary debulking surgery and 88 (31%) underwent interval debulking surgery. The pooled analysis revealed a median progression-free survival in the primary debulking surgery group of 18.0 months compared to 12.0 months in the interval debulking surgery group (p = 0.028; log-rank test), and a median overall survival of 30.92 months versus 28.73 months (p = 0.400; log-rank test). Among the 134 patients with available information on the residual tumor after primary debulking surgery or interval debulking surgery, 110 (82%) had no macroscopic residual tumor (residual tumor = 0). The median progression-free survival was 18.9 months in the residual tumor = 0 group compared to 6.19 months in the residual tumor > 0 group (p < 0.001; log-rank test); the median overall survival was 40.6 months versus 21 months (p = 0.028; log-rank test). Conclusions. These results indicate that primary debulking surgery should be considered the preferred treatment approach for advanced endometrial cancer with carcinosis, especially in carefully selected patients where complete cytoreduction is achievable. Further prospective studies are warranted to confirm these results and to establish standardized criteria for patient selection, incorporating molecular-integrated risk profiles for endometrial cancer.
1. Introduction
Endometrial cancer is the most common malignancy of the female genital tract in high-income countries, with an annual incidence of over 420,000 cases worldwide and an age-standardized rate of 15.5 per 100,000 women in Europe [1]. The disease is usually diagnosed when confined to the uterus; however, a proportion of patients have regional lymph node involvement (20%) or distant metastases (10%) at the time of diagnosis [2]. Stage IV endometrial cancer includes a heterogeneous patient population who may present with disease confined to the pelvis and infiltrating the bladder or the rectal mucosa (stage IVa), or with extrapelvic peritoneal metastases (stage IVb) and distant metastases (stage IVc according to the new International Federation of Gynaecology and Obstetrics (FIGO) 2023 endometrial cancer classification [3], previously classified as stage IVb).
Although the role of surgery in early-stage endometrial cancer is well established [4], its role in stage IV disease remains controversial. This is particularly relevant for patients with peritoneal metastasis who may present unresectable disease according to the surgical criteria used for advanced ovarian cancer with peritoneal spread [5], especially in the presence of extra-abdominal metastases. In this context, the hypothesis of applying the surgical algorithm [6,7] of the advanced ovarian cancer to stage IV endometrial cancer with peritoneal spread, opting for neoadjuvant chemotherapy and interval debulking surgery, appears to be a reasonable choice over primary debulking surgery. This option has been investigated in several retrospective studies [8,9,10,11]. According to the European Society of Gynecological Oncology (ESGO) guidelines [4], maximal cytoreduction should only be considered if a complete macroscopic resection can be achieved with acceptable morbidity. If upfront surgery is not feasible or acceptable and neoadjuvant chemotherapy is given, interval debulking surgery can only be considered if there is a response to chemotherapy. In both the adjuvant and neoadjuvant settings, the preferred regimen remains carboplatin/paclitaxel, as recommended by the National Comprehensive Cancer Network (NCCN) guidelines [12] and supported by the results of the GOG-0209 trial [13].
Nevertheless, due to the rarity of this condition, retrospective studies on advanced endometrial cancer treatment generally include stages III and IV patients in the same survival analysis. Stages IVa and IVb are usually merged, and there is a lack of precise descriptions of the initial disease extent when stage IVb is analyzed separately. Therefore, the guidelines do not provide clear guidance on the management of patients with endometrial cancer who present peritoneal carcinomatosis.
The aim of this systematic review and meta-analysis was to analyze the outcomes of primary debulking surgery and platinum-based adjuvant chemotherapy versus platinum-based neoadjuvant chemotherapy and interval debulking surgery in patients with advanced endometrial cancer and peritoneal carcinomatosis.
2. Methods
2.1. Database Queries and Study Selection
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14] and aimed to answer the following research question: “What is the impact of primary vs. interval debulking surgery on the prognosis of patients diagnosed with advanced endometrial cancer with carcinosis?”. The study was registered in PROSPERO on 3 July 2023 (code: CRD42023438602).
The literature review was conducted by systematically searching the library databases PubMed, Scopus, WOS, and the Cochrane Library. The last updated search was conducted on 30 June 2024, using a combination of relevant key terms such as “advanced endometrial cancer”, “uterine tumor”, “carcinosis”, “neoadjuvant chemotherapy”, “platinum”, “debulking surgery”, and “cytoreductive surgery”. Details of the construction and full search are reported in the Supplementary Materials.
Inclusion criteria were as follows: (i) possibility to extract survival data (progression-free survival, overall survival) of patients with stage IVb endometrial cancer (according to FIGO 1988 or 2009) [15,16] with peritoneal carcinomatosis, regardless of tumor histotype, with or without extra-abdominal metastases; (ii) studies including patients treated with primary debulking surgery followed by platinum-based adjuvant chemotherapy (at least 90% of cases) with or without associated adjuvant radiotherapy, or (iii) studies including patients treated with platinum-based neoadjuvant chemotherapy (at least 90% of cases) who later received interval debulking surgery followed by any adjuvant treatment; (iv) randomized controlled trials, observational prospective and retrospective studies; (v) English language.
Exclusion criteria were as follows: (i) studies that merged stages III and IV or stage IVa (patients with tumor confined to the pelvis, infiltrating the bladder and/or rectal mucosa) and stage IVb (patients with distant metastases including peritoneal carcinosis) in the same survival analyses; (ii) studies evaluating hyperthermic intraperitoneal chemotherapy (HIPEC) in endometrial cancer; (iii) no information available on the neoadjuvant or adjuvant chemotherapy regimen; (iv) neoadjuvant radiotherapy, no adjuvant treatment or only adjuvant radiotherapy or hormonal therapy after surgery in primary cytoreduction; (v) congress abstracts, case reports, editorials, letters to the editor, reviews, meta-analyses without any new patient data and book chapters.
2.2. Data Extraction, Quality Assessment, and Statistical Analysis
The quality of the selected studies was assessed using the Newcastle–Ottawa Scale instrument [17]. Data were extracted, summarized, and reported in the tables. Statistical analyses were conducted in R version 4.4.0 (2024-04-24 ucrt)—“Puppy Cup” [18]. Individual patient data on survival outcomes were extracted from each paper either from the tables provided in the results or Supplementary Data or extrapolated from the Kaplan–Meier curves using ScanIt Software by AmsterCHEM, (https://www.amsterchem.com/scanit.html, last accessed: 1 May 2024) and reconstructed using the KMtoIPD R package [19]. All results were stored in a text file, which was used to reconstruct the final curves and calculate the p-value using the log-rank test with the survminer package [20]. The significance threshold was set at a p-value < 0.05. Further details on study selection and data extraction are provided in the Supplementary Materials.
3. Results
3.1. Article Screening Selection, Quality Assessment, and Description of Included Studies
The results of the literature research are shown in the PRISMA flowchart (Figure 1). After removing duplicates, 3230 studies were selected for title and abstract screening, with 3044 being removed due to irrelevance toward the topic under investigation. All references were analyzed to evaluate additional eligible studies. A total of 16 studies met the selection criteria and were analyzed (Table 1, Table 2 and Table 3).
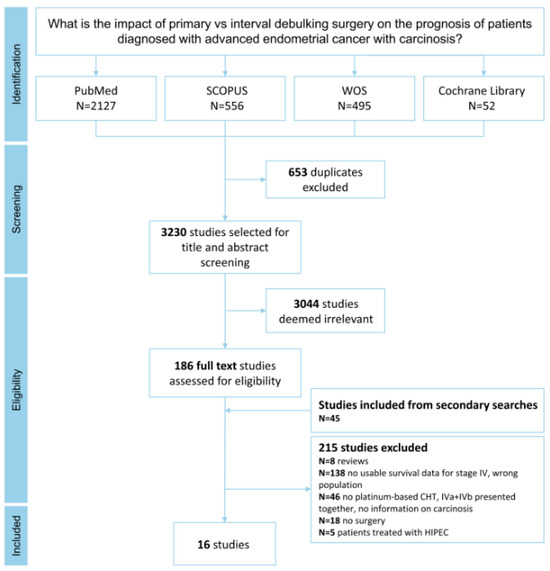
Figure 1.
PRISMA flowchart illustrating the selection and inclusion of articles in the meta-analysis.
The risk of bias analysis of the included studies is reported in Supplementary Table S1. All 16 studies were rated as “good”, with 11 (68.75%) scoring 11/14 points and 5 (31.25%) scoring 12/14 points.
Most of the included studies were conducted in the United States of America (n = 7; 44%), followed by Asia (n = 5; 31%), Europe (n = 3; 19%), and Australia (n = 1; 6%). All studies except one [21], were retrospective, and the majority were single-center studies (n = 11; 69%). The enrollment period was 40 years (from 1980 to 2022), with publication dates ranging between 1994 and 2022, with an increase from 2010 (44%).

Table 1.
Summary of selected studies, including only PDS patients. Legend: n: number; pts: patients; PDS: primary debulking surgery; RT: residual tumor; RTx: radiotherapy (may comprehend external pelvic radiotherapy, brachytherapy, whole abdominal irradiation); CT: chemotherapy; PFS: progression-free survival; OS: overall survival; MVA: multivariate analysis; HR: hazard ratio; HT: hormonal therapy; DFS: disease-free survival; N.A.: not available.
Table 1.
Summary of selected studies, including only PDS patients. Legend: n: number; pts: patients; PDS: primary debulking surgery; RT: residual tumor; RTx: radiotherapy (may comprehend external pelvic radiotherapy, brachytherapy, whole abdominal irradiation); CT: chemotherapy; PFS: progression-free survival; OS: overall survival; MVA: multivariate analysis; HR: hazard ratio; HT: hormonal therapy; DFS: disease-free survival; N.A.: not available.
| Author, Year, Country | Study Design, Enrollment Period | Age (Years) | Stages Included (Whole Cohort) | N. of Stage IVb pts with Peritoneal Dissemination | Extra-Abdominal Metastases | Abdominal Tumor Load | Histology |
|---|---|---|---|---|---|---|---|
| Bristow [22] 2000 USA | Retrospective Multicenter 1990–1998 | median 65 | IVb n = 65 | 65 | 9/65 Included in meta-analysis: 0/8 | pelvis 75.4% abdominal peritoneum 49.2% omentum 47.7% bowel serosa or mesentery 36.9% upper abdomen 24.6% (whole cohort) Included in meta-analysis: pelvis 7/8 abdominal peritoneum 5/8 omentum 3/8 bowel 2/8 | 21 serous 22 endometrioid 22 others (whole cohort) |
| Landrum [23] 2009 USA | Retrospective 1990–2006 | median 63 | IVb n = 55 | 55 | 0 | N.A. | 29 serous 24 endometrioid 2 clear cell (whole cohort) |
| Lee [24] 2014 USA | Retrospective Multicenter 1980–2011 | median 70 | IVb n = 48 | 48 | 0 | excluded unresectable disease 23 omentum only 25 extensive abdominal involvement | serous |
| Ueda [25] 2010 Japan | Retrospective 1991–2008 | median 63 | IVb n = 33 | 15 | 0 | abdominal peritoneum 42% omentum 39% retroperitoneal nodes 55% bowel/mesentery 21% (whole cohort) | 9 serous/clear cell 24 endometrioid (whole cohort) |
| Gehrig [26] 2004 USA | Retrospective 1990–2000 | median 68 | III–IV n = 24 | 11 | 0 | omental involvement | serous |
| Watari [27] 2005 Japan | Retrospective 1982–2002 | median 58 | IIIC–IV n = 55 | 11 | 0 | N.A. | 12 serous/clear cell 43 endometrioid (whole cohort) |
| Gitsch [28] 1994 Australia | Retrospective 1988–1993 | mean 73 | I–IV n = 18 | 4 | 0 | Included in meta-analysis: diaphragm 2/3 omentum 1/3 | serous |
| Nguyen [29] 2001 USA | Retrospective 1989–1998 | mean 65 | I–IV n = 22 | 1 | 0 | omental involvement | serous |
| Low [30] 2005 Singapore | Retrospective 1994–2003 | median 62 | I–IV n = 26 | 1 | 0 | N.A. | serous |
| Kelly [31] 2004 USA | Retrospective 1987–2002 | mean 68 | I–IV n = 51 | 9 | 0 | omental involvement | serous |
| Author, Year, Country | Abdominal RT After Surgery | Adjuvant Therapy | AC Regimens | Results | Included Patients For Meta-Analysis a | ||
| Bristow [22] 2000 USA | 26 RT = 0 cm 10 RT ≤ 1 cm 29 RT > 1 cm (4 no hysterectomy) Included in meta-analysis: 6 RT = 0 cm 1 RT ≤ 1 cm 1 RT > 1 cm | 27 CT 14 CT + RTx 11 RTx 3 HT 3 no therapy 7 unknown Included in meta-analysis: 3 CT 5 CT + RTx | platinum-based | Pts who received a treatment sequence of CT followed by RTx had a median survival rate of 40.0 months, compared to only 14.0 months for pts not receiving this combination (p = 0.004) The median survival of all pts undergoing optimal cytoreduction (≤1 cm RT) was 34.3 months, compared to 11.0 months for patients left with suboptimal RT (p = 0.0001) On MVA, only age and RT ≤ 1 cm retained significance as predictors of survival | 8 OS | ||
| Landrum [23] 2009 USA | 48 RT ≤ 1 cm (whole cohort) | 33 CT 14 CT + RTx 8 RTx | platinum-based | Median PFS for all patients (optimal and suboptimal cytoreduction) was 13 months Optimal cytoreduction was associated with a survival advantage with an HR of 2.4 At 2 years, OS for all patients treated with PDS and adjuvant CT was 53% | 47 OS | ||
| Lee [24] 2014 USA | 22 RT = 0 cm 14 RT < 1 cm 4 RT = 2–5 cm 5 RT > 5 cm 3 RT unknown (whole cohort) | 19 CT 16 CT + RTx 5 RTx 8 no therapy | platinum-based | At 5 years, DFS and OS rates were 12% and 19% On MVA, among pts treated with CT, optimal surgical cytoreduction (HR 0.09, 95% CI 0.02–0.35) and RTx (HR 0.36, 95% CI 0.15–0.80) were associated with a decreased rate of recurrence or progression Optimal cytoreductive surgery (HR 0.09, 95% CI 0.02–0.38) was the only significant prognostic factor for OS when the model was adjusted for age | 35 PFS | ||
| Ueda [25] 2010 Japan | 10 RT ≤ 2 cm 5 RT > 2 cm | 15 CT | platinum-based at least 90% c | Median OS RT ≤ 2 cm was 19 months vs. 6 months if RT > 2 cm (p = 0.0007) Median PFS RT ≤ 2 cm was 10 months vs. 1 months if RT > 2 cm (p = 0.0003) | 15 OS & PFS | ||
| Gehrig [26] 2004 USA | 7 RT = 0 cm 4 RT > 0 cm Included in meta-analysis: 6 RT = 0 cm, 4 RT > 0 cm | 10 CT 1 RTx | platinum-based | In the whole cohort (stages III and IV) time to progression for pts receiving RTx was 5.3 months as compared with 12.4 months for pts receiving CT (p = 0.01) Mean time to death for the RTx group was 8 months compared to 18 months in the CT group (p = 0.04) | 10 OS | ||
| Watari [27] 2005 Japan | N.A. | 11 CT | platinum-based | 5-year survival rate of stage IV pts was 20% | 11 OS | ||
| Gitsch [28] 1994 Australia | 2 RT < 2 cm 2 RT > 2 cm Included in meta-analysis: 2 RT < 2 cm, 1 RT > 2 cm | 1 CT 2 CT + RTx 1 no therapy | platinum-based | Of the pts with stages III and IV disease, 4 of 12 are alive with no evidence of disease after a mean follow-up of 22.5 months (range, 8–45 months) Eight of 12 women who received CT are alive with no evidence of disease, 4 of whom had stage III or IV disease | 3 OS | ||
| Nguyen [29] 2001 USA | RT = 0 | 1 CT | platinum-based | The projected 2-year survival was 40% for pts with stages III and IV as compared with 60% for pts with stages I and II | 1 OS | ||
| Low [30] 2005 Singapore | minimal RT | 1 CT + RTx | platinum-based | The OS at 5 years was 72.9% for stage I, 100% for stage II, 58.9% for stage III, and 0% for stage IV | 1 OS | ||
| Kelly [31] 2004 USA | 8 RT < 2 cm 1 RT > 2 cm | at least 8 CT b | platinum-based | Eight of the 10 stage IIIC/IV pts either progressed or recurred, and their median DFS was 6 months (range, 0–24 months) | 9 OS | ||
a pts meeting all inclusion criteria with available individual survival data; b one of the pts included may have not received adjuvant CT (not clear in the text); c a minority of patients may have received adjuvant irinotecan alone or oral medroxyprogesterone acetate (not clear in the text if these few cases belong to the group of pts included in our meta-analysis).

Table 2.
Summary of selected studies including only IDS patients. Legend: n: number; pts: patients; IDS: interval debulking surgery; RT: residual tumor; PR: partial response; PD: progressive disease; SD: stable disease; CT: chemotherapy; PFS: progression-free survival; OS: overall survival; MVA: multivariate analysis; UVA: univariate analysis; HR: hazard ratio; NACT: neoadjuvant chemotherapy; N.A.: not available.
Table 2.
Summary of selected studies including only IDS patients. Legend: n: number; pts: patients; IDS: interval debulking surgery; RT: residual tumor; PR: partial response; PD: progressive disease; SD: stable disease; CT: chemotherapy; PFS: progression-free survival; OS: overall survival; MVA: multivariate analysis; UVA: univariate analysis; HR: hazard ratio; NACT: neoadjuvant chemotherapy; N.A.: not available.
| Author, Year, Country | Study Design, Enrollment Period | Age (Years) | Stages Included (Whole Cohort) | N. of pts Stage IVb with Peritoneal Dissemination | Extra-Abdominal Metastases | Abdominal Tumor Load | Histology | N. of NACT Cycles | Response to NACT |
|---|---|---|---|---|---|---|---|---|---|
| Vandenput [21] 2009 Belgium | Prospective 1999–2007 | median 65 | IVb n = 30 | 30 | some may have had pleural effusion | N.A. | 27 serous 2 endometrioid 1 clear cell (whole cohort) | 3–4 | 2 CR 20 PR 6 SD 2 PD (no IDS) |
| Lim [32] 2022 Korea | Retrospective Multicenter 2008–2020 | median 56 | IIIC–IVb n = 32 | 10 | 8/10 | “unresectable” | 2 serous 5 endometrioid 3 carcinosarcoma | median 6 | 10 PR |
| Jani [33] 2021 USA | Retrospective 2003–2019 | median 63 | III–IV n = 40 | 22 b | pleural effusion 9/40 lung metastasis 3/40 liver 4/40 | carcinomatosis 32.5% omental caking 32.5% ascites 55% extensive nodal involvement 55% bowel/mesentery 7.5% (whole cohort) Included in meta-analysis: at least omentum 22/22 | 18 serous 2 endometrioid 6 clear cell 9 carcinosarcoma 2 mixed 3 undifferentiated (whole cohort) | 25 pts 3–4 15 pts ≥ 5 (whole cohort) | 3 CR 29 PR 6 SD 2 PD (whole cohort) |
| Author, Year, Country | NACT Regimens | Abdominal RT After Surgery | Adjuvant Therapy | AC Regimens | Results | Included Patients for Meta-Analysis a | |||
| Vandenput [21] 2009 Belgium | platinum-based | 22 RT = 0 cm 2 RT < 1 cm | 24 CT | 22 platinum-based 2 “switch” to other type | Histopathological features of chemoresponse in both uterus and omentum were related to a better PFS (p = 0.017, HR = 0.785) and OS (p = 0.014, HR = 0.707) The use of NACT resulted in a high rate (80%) of optimal IDS | 24 OS & PFS c | |||
| Lim [32] 2022 Korea | platinum-based | 9 RT = 0 cm 1 RT ≤ 1 cm | 10 CT | 9 platinum-based 1 ifosfamide–paclitaxel | On MVA, non-endometrioid histology and RT after IDS were independent poor prognostic factors for PFS (adjusted HR 7.322, p < 0.001; and 5.934, p = 0.001, respectively) On UVA non-endometrioid histology was the only factor associated with worse OS (adjusted HR 4.523, p = 0.0032) | 10 OS & PFS | |||
| Jani [33] 2021 USA | platinum-based | 23 RT = 0 6 RT < 1 cm 11 RT ≥ 1 cm (whole cohort) | N.A. | N.A. | Pts with higher chemotherapy response scores had longer PFS and OS and a higher rate of complete cytoreduction | 22 OS & PFS | |||
a pts meeting all inclusion criteria with available individual survival data; b omental chemotherapy response score feasible, thus surely with peritoneal dissemination; c of the 30 total stage IVb pts, 2 showed PD after NACT and, thus, did not undergo IDS, while 4 were deemed inoperable during surgery, so they were excluded from subsequent survival analysis.

Table 3.
Summary of selected studies, including both PDS and IDS patients. Legend: n: number; pts: patients; PDS: primary debulking surgery; IDS: interval debulking surgery; RT: residual tumor; RTx: radiotherapy (may comprehend external pelvic radiotherapy, brachytherapy, whole abdominal irradiation); CT: chemotherapy; PFS: progression-free survival; OS: overall survival; MVA: multivariate analysis; UVA: univariate analysis; HR: hazard ratio; NACT: neoadjuvant chemotherapy; DFS: disease-free survival; N.A.: not available.
Table 3.
Summary of selected studies, including both PDS and IDS patients. Legend: n: number; pts: patients; PDS: primary debulking surgery; IDS: interval debulking surgery; RT: residual tumor; RTx: radiotherapy (may comprehend external pelvic radiotherapy, brachytherapy, whole abdominal irradiation); CT: chemotherapy; PFS: progression-free survival; OS: overall survival; MVA: multivariate analysis; UVA: univariate analysis; HR: hazard ratio; NACT: neoadjuvant chemotherapy; DFS: disease-free survival; N.A.: not available.
| Author, Year, Country | Study Design, Enrollment Period | Age (Years) | Stages Included (Whole Cohort) | N. of pts Stage IVb with Peritoneal Dissemination | N. of pts PDS/IDS | Extra-Abdominal Metastases | Abdominal Tumor Load | Histology | N. of NACT Cycles | Response to NACT |
|---|---|---|---|---|---|---|---|---|---|---|
| Bogani [9] 2019 Italy | Retrospective propensity-matched 2005–2016 | PDS mean 65, IDS mean 63 | IVb n = 30 | 30 | 15/15 | 0 | unresectable disease in patients undergoing NACT | all serous | 3–6 | N.A. |
| Unsal [34] 2022 Turkey | Retrospective multicenter N.A. | median 64 | IVb n = 42 | 42 | 32/10 | 0 | omental involvement 88.1% no other information | all serous | 3–8 | N.A. |
| Rajkumar [35] 2019 UK | Retrospective multicenter 2010–2016 | 22 pts < 65, 23 pts ≥ 65 | IIIC–IVb n = 45 | 13 | 6/7 | 1/7 IDS | PDS: omentum 2/6, pelvis 5/6, bowel 1/6, retroperitoneal nodes 4/6 IDS: omentum 5/7, pelvis 5/7, bowel 1/7, retroperitoneal nodes 1/7, upper abdomen 1/7 | PDS: 3 serous, 3 endometrioid IDS: 2 serous, 3 endometrioid, 1 clear cell, 1 mixed | 3–6 | N.A. |
| Author, Year, Country | NACT regimens | Abdominal RT after Surgery | Adjuvant therapy | AC regimens | Results | Included Patients for Meta-Analysis a | ||||
| PDS | IDS | |||||||||
| Bogani [9] 2019 Italy | All platinum-based | PDS: 13 RT = 0 cm, 2 RT < 1 cm IDS: 14 RT = 0 cm, 1 RT < 1 cm | PDS: 15 CT IDS: 14 CT, 1 CT + RTx | All platinum-based except 1 treated with gemcitabine in the IDS group | Similar cytoreduction rate Median DFS was 12.0 vs. 15.3 months in the IDS vs. PDS group (p = 0.663) Median OS was 16.7 vs. 18.0 months in the IDS vs. PDS group (p = 0.349) | 15 OS & PFS b | 15 OS & PFS | |||
| Unsal [34] 2022 Turkey | All platinum-based | PDS: 26 RT = 0, 6 RT > 0 IDS: 8 RT = 0, 2 RT > 0 | PDS: 32 CT IDS: not clear | all platinum-based | Receiving NACT did not affect DFS and DSS in UVA | 32 OS & PFS | 10 OS & PFS | |||
| Rajkumar [35] 2019 UK | >90% Platinum-based 1 capecitabine in the whole NACT cohort | PDS: 5 RT ≤ 1 cm, 1 RT > 1 cm IDS: 6 RT ≤ 1 cm, 1 RT > 1 cm | PDS: 6 CT ± RTx c,d IDS: 7 CT ± RTx d | all platinum-based | Only poor performance status (p = 0.035), presence of bowel disease (p = 0.05) and suboptimal cytoreduction (p = 0.006) retained significance as predictors of poor survival on MVA Suboptimal cytoreduction surgery, compared to optimal cytoreduction, showed a 3.55-fold increased risk of death independent of performance status and anatomic region with disease (HR 3.55 (95% CI 1.44–8.73), p = 0.006) | 6 OS | 7 OS | |||
a pts meeting all inclusion criteria with available individual survival data; b individual survival and RT data on 4 more pts in the PDS group were obtained by direct request to the authors; c in the PDS group (whole cohort) 27 out of 28 women received postoperative CT, so it could be (not clear in the text) that 1 out of 6 stage IVb pts treated with PDS included in our meta-analysis did not receive postoperative CT; d 30 pts in the whole cohort also received extended beam pelvic RTx (not clear which pts).
3.2. Clinical and Pathological Features
A total of 285 patients with stage IVb endometrial cancer and peritoneal carcinomatosis were analyzed. Of these, 197 (69%) patients underwent primary debulking surgery, and 88 (31%) underwent neoadjuvant chemotherapy and interval debulking surgery. Specifically, patients were from 12 (75%) studies [9,21,22,23,24,25,26,27,32,33,34,35] that included stages III and/or IV, and 4 (25%) studies [28,29,30,31] that included all stages. In 12 (75%) studies [9,22,23,24,25,26,27,28,29,30,31,34], all patients had stage IVb endometrial cancer without extra-abdominal metastases. Considering all studies, in 230 (81%) patients, the disease was limited to the abdomen.
Considering the available information on histotype, 142 (50%) patients had serous stage IVb endometrial cancer, specifically 113 (57.4%) in the primary debulking surgery group and 29 (33%) in the interval debulking surgery group. Eight studies [9,24,26,28,29,30,31,34] included only serous endometrial cancer, and the remaining studies included all histotypes (Table 4).

Table 4.
Comparison between PDS and IDS groups of patients included in the meta-analysis, considering histology, residual tumor, and adjuvant treatment. Legend: PDS: primary debulking surgery; IDS: interval debulking surgery; RT: residual tumor; CT: chemotherapy; RTx: radiotherapy (may comprehend external pelvic radiotherapy, brachytherapy, whole abdominal irradiation); N.A.: not available.
Only Vandenput et al. [21] clearly defined the extent of disease using laparoscopy before offering neoadjuvant chemotherapy with interval debulking surgery, as opposed to primary debulking surgery. In contrast, seven other studies [9,22,23,25,26,31,35] relied on imaging (computed tomography scan) and/or exploratory laparotomy. Furthermore, in eight studies [23,27,28,29,30,32,33,34], there was a lack of clarity on how patients were assessed for cytoreducibility and, thus, addressed to a specific treatment.
3.3. Neoadjuvant/Adjuvant Treatment and Residual Tumor
Six studies included patients submitted to neoadjuvant chemotherapy [9,21,32,33,34,35], out of which, all patients but one received at least three cycles of platinum-based chemotherapy, and 56 (64%) received adjuvant chemotherapy. The adjuvant chemotherapy was platinum-based in all but four patients. Specifically, one patient received ifosfamide–paclitaxel, another received gemcitabine, and in two patients, the regimen was not specified. In the study by Bogani et al. [9], one patient also received adjuvant radiotherapy, while in the study by Rajkumar et al. [35], it is not clear whether multimodal adjuvant treatment was given. In two of the studies reporting on neoadjuvant chemotherapy [33,34], information on adjuvant treatment could not be obtained at all.
In 11 studies addressing primary debulking surgery [9,22,23,24,25,26,27,28,29,30,34], all patients except two definitively received adjuvant chemotherapy (n = 195). Postoperative chemotherapy was platinum-based in all patients in all studies except one [25], accounting for 180 (92%) out of 195 patients. Additionally, 38 (19%) out of 197 patients also received adjuvant radiotherapy. In 11 (5.6%) patients who underwent primary debulking surgery, it is not clear whether adjuvant radiotherapy was administered (Table 4).
Upon analyzing the data, we found that postoperative residual tumor was reported in 12 studies [9,21,22,25,26,28,29,30,31,32,34,35], comprising 134 patients, of whom 110 (82%) had no macroscopic residual tumor.
3.4. Individual Patient Data Meta-Analysis of Survival Outcomes
In the survival analysis on 285 patients, the median progression-free survival was 18.0 vs. 12.0 months in the primary debulking surgery vs. interval debulking surgery group (log-rank p = 0.028). Median overall survival was 30.92 vs. 28.73 months in the primary debulking surgery vs. interval debulking surgery group (log-rank p = 0.400) (Figure 2). Separate survival analysis by residual tumor type was only possible in 52 (no residual tumor) versus 22 patients (residual tumor > 0) when primary debulking surgery and interval debulking surgery were considered together. Median progression-free survival was 18.9 vs. 6.19 months in the residual tumor = 0 vs. residual tumor > 0 groups (log-rank p < 0.001) and median overall survival was 40.6 vs. 21 months in the residual tumor = 0 vs. residual tumor > 0 groups (log-rank p = 0.028) (Figure 3). Unfortunately, the number of patients with individual survival data and available information regarding the residual tumor at the end of surgery was too small to perform a separate evaluation between the primary debulking surgery and interval debulking surgery groups (Supplementary Figure S1).
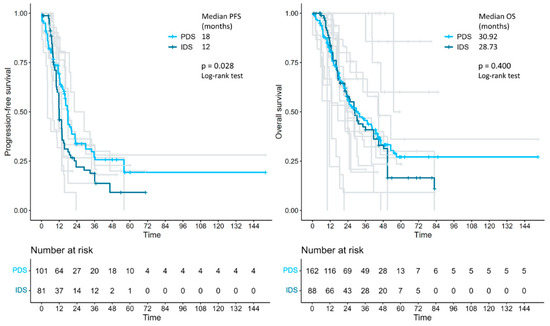
Figure 2.
Kaplan–Meier curves, reconstructed using individual patient data extracted from the articles, showing the PFS and OS of patients with advanced endometrial cancer who received either primary debulking surgery or interval debulking surgery. PFS: progression-free survival; OS: overall survival; PDS: primary debulking surgery; IDS: interval debulking surgery.
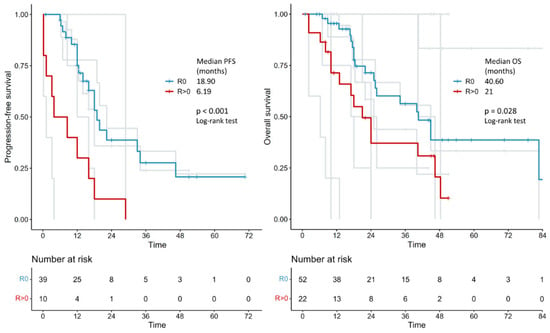
Figure 3.
Kaplan–Meier curves reconstructed using individual patient data extracted from the articles showing the PFS and OS of patients with advanced endometrial cancer who had no macroscopic tumoral residue (R0) after surgery vs. those with residue (R > 0). PFS: progression-free survival; OS: overall survival.
4. Discussion
4.1. Summary of Main Results
To the best of our knowledge, this is the first meta-analysis comparing survival outcomes of patients with stage IVb endometrial cancer with peritoneal spread treated with either primary debulking surgery and platinum-based adjuvant chemotherapy or with platinum-based neoadjuvant chemotherapy followed by interval debulking surgery. The results showed a 6-month survival benefit in terms of progression-free survival (but not in terms of overall survival) in the primary debulking surgery group compared to the interval debulking surgery group (18.0 vs. 12.0 months, p = 0.028). Importantly, regardless of the timing of surgery (primary or interval), complete cytoreduction (no residual tumor) results in a significant improvement in both progression-free survival (18.9 vs. 6.19 months, p < 0.001) and overall survival (40.6 vs. 21 months, p = 0.028).
4.2. Results in the Context of Published Literature
This systematic review highlights the lack of prospective randomized trials comparing primary debulking surgery versus neoadjuvant chemotherapy followed by interval debulking surgery in stage IVb endometrial cancer with peritoneal spread. Furthermore, the lack of clear guidance on when to choose one strategy over the other, as in the case of ovarian cancer with peritoneal metastases, represents a significant gap in the management of these patients [7]. One major issue is the absence of standardized surgical criteria for determining “optimal debulking” and the undefined thresholds for what constitutes “unresectable” or “inoperable” disease, which often leads to a switch to chemotherapy. Another critical question involves how the evaluation of “resectability” is conducted, as only Vandenput et al. [21] clearly evaluated the extent of disease with laparoscopy before offering neoadjuvant chemotherapy with interval debulking surgery. Another significant concern is the heterogeneity of adjuvant treatments that patients receive after surgery, which could potentially influence survival outcomes. Currently, there is insufficient high-quality evidence to determine whether multimodal treatment offers an oncologic benefit over chemotherapy alone in women with stage IV disease. Additionally, different characteristics of local tumor spread, such as vaginal, nodal, or parametrial involvement, may have influenced the choice of multimodal adjuvant treatments. Given these potential biases, these results should be interpreted with caution. Some authors [36] have reported a short-term overall survival advantage for patients treated with neoadjuvant chemotherapy and interval debulking surgery, attributing this benefit to fewer postoperative complications, which allow for more rapid reinitiation of medical therapies. In contrast, women treated with primary debulking surgery were at higher risk of early death but demonstrated a more favorable long-term prognosis [36]. This short-term survival advantage was confirmed in a recent systematic review by Huang et al. [10], which analyzed 5844 patients with stages III or IV endometrial cancer, including 1317 who underwent neoadjuvant chemotherapy. In our meta-analysis, only two studies [9,34] directly compared survival between the two treatment groups and found no significant advantage for either strategy. However, these studies were limited by small sample sizes and focused exclusively on serous histology. When reviewing the broader literature on this topic, we identified two additional studies [37,38] that were excluded from our analysis due to the lack of matching criteria, both of which reported contradictory results. Our results are more aligned with those of Eto et al. [37], reporting that the overall survival of patients treated with neoadjuvant chemotherapy followed by interval debulking surgery (n = 59) was comparable to that of patients undergoing primary debulking surgery (n = 279), although progression-free survival data were not reported. In contrast, the study by Wilkinson-Ryan et al. [38], which involved 44 patients, found no significant difference in median progression-free survival (primary debulking surgery: 10.4 vs. interval debulking surgery: 12 months, p = 0.29) or overall survival (primary debulking surgery: 17.3 vs. interval debulking surgery: 20.7 months, p = 0.23).
Moreover, we must emphasize the complexity of this issue and underscore the need for further research to determine the optimal treatment strategy. It is important to note that the patients included in our meta-analysis were highly selected, namely patients with advanced endometrial cancer with carcinomatosis, contrasting previous reports that included both stage IV and stage III patients.
Similarly to advanced ovarian cancer [6], and in line with our findings considering all patients, the presence of the residual tumor following surgery in endometrial cancer with peritoneal implants is one of the main prognostic factors [39,40,41]. In 2021, a systematic review and meta-analysis by Albright et al. on primary debulking surgery in the treatment of advanced-stage endometrial cancer (stages III and IV) [42] showed that submaximal (any gross residual disease vs. no residual tumor) and suboptimal (residual tumor ≥ 1 cm vs. residual tumor < 1 cm) cytoreduction were associated with worse progression-free survival and overall survival. When optimal cytoreduction is not feasible, neoadjuvant chemotherapy should be offered with the aim of achieving no residual tumor at interval debulking surgery, potentially improving prognosis while maintaining a low complication rate. In support of this concept, recently, Kanno et al. [43] found that, in stage IVb endometrial cancer, achieving resection without intra-abdominal macroscopic residue, whether before or after chemotherapy, could extend survival. The authors concluded that surgery should be pursued (primary or interval debulking surgery) when it can achieve no intra-abdominal residual tumor. Unfortunately, the lack of data on the residual tumor after primary debulking surgery or interval debulking surgery in a significant percentage of patients included in our meta-analysis (62% and 32%, respectively) prevented us from conducting a separate survival analysis based on the completeness of cytoreduction in the two treatment strategies.
4.3. Strengths and Weaknesses
One strength of our systematic review and meta-analysis is the strict inclusion criteria, which allowed us to focus specifically on advanced endometrial cancer with well-defined disease distribution and treatment protocols, rather than broadly including heterogenous patients as previously done by others. However, the lack of prospective studies to better define the selection criteria for primary debulking surgery or interval debulking surgery as well as the non-standardized surgical criteria for the achievement of optimal debulking in advanced endometrial cancer remain important limitations. It is worth discussing that patients with more advanced/unresectable carcinomatosis (and, therefore, with a worse prognosis) may have been referred to for neoadjuvant chemotherapy and interval debulking surgery rather than primary debulking surgery. Another limitation of our study was the inability to extract data on tumor histotype and residual tumor after surgery for a significant percentage of patients in both the primary and interval debulking surgery groups. Additionally, it is important to highlight the heterogeneity of adjuvant treatments, particularly the higher rate of postoperative radiotherapy in the primary debulking surgery group, with an unclear impact on survival outcomes. Furthermore, despite the difficulties in reconstructing survival curves, meta-analyses based on individual patient data are generally considered superior for validating and synthesizing study results [44].
4.4. Implications for Practice and Future Research
While a randomized controlled trial would ideally determine whether primary debulking surgery improves outcomes in “resectable” abdominal carcinomatosis of endometrial origin, the rarity of this condition makes such a trial impractical. In its place, well-designed multicentric retrospective or prospective studies are necessary. Additionally, in the era of molecular-integrated risk profiles for endometrial cancer [45,46], the role of targeted therapies, based on molecular characteristics like HER2 overexpression or mismatch repair deficiency, should be considered alongside standard chemotherapy. These innovative treatments may reduce the reliance on surgery in advanced cases.
5. Conclusions
The results of this meta-analysis indicate that primary debulking surgery should be considered the preferred treatment approach for advanced endometrial cancer with intra-abdominal peritoneal spread, especially in carefully selected patients where complete cytoreduction is achievable. Further prospective studies are necessary to confirm these results and to establish standardized criteria for patient selection for debulking surgery to ensure the best possible oncological outcomes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers17061026/s1, Table S1. Quality assessment of the selected studies. The assigned Quality Rating was Good, Fair, or Poor for each study. Figure S1. Kaplan-Meier curves reconstructed using individual patient data extracted from the articles showing the PFS and OS of patients with advanced EC divided by the timing of surgery and the macroscopic tumoral residue after surgery. Reference [47] is cited in the supplementary materials.
Author Contributions
Conceptualization: A.M.P.; methodology: A.M.P. and C.A.C.; software: C.A.C.; validation: F.M.; formal analysis: C.A.C.; investigation: G.M., C.A.C. and S.D.C.; resources: C.A.C. and S.D.C.; data curation: G.M., S.D.C., L.G., G.B., F.R., A.G.M. and C.A.C.; writing—original draft: G.M.; writing—review and editing: A.M.P. and C.A.C.; visualization: C.A.C.; supervision: A.M.P. and P.D.I.; project administration: P.D.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
The present study is a systematic review and meta-analysis of existing studies and did not involve the collection of new primary data or direct interaction with human participants or animals. As such, according to established guidelines and institutional review board policies, our study is exempt from ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data analysis is based on published data. No new data were generated in this article.
Acknowledgments
The authors would like to thank Claudia Cavicchi, our librarian, for her invaluable help with constructing the research queries and retrieving articles for screening. The icon #7049178 by kholifah, from the Noun Project, under the license type (CCBY3.0), was used in the graphical abstract.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cancer Today. Available online: https://gco.iarc.who.int/today/ (accessed on 16 May 2024).
- Cancer of the Endometrium—Cancer Stat Facts. SEER. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 16 May 2024).
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee; et al. FIGO staging of endometrial cancer: 2023. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Petrillo, M.; Vizzielli, G.; Fanfani, F.; Gallotta, V.; Cosentino, F.; Chiantera, V.; Legge, F.; Carbone, V.; Scambia, G.; Fagotti, A. Definition of a dynamic laparoscopic model for the prediction of incomplete cytoreduction in advanced epithelial ovarian cancer: Proof of a concept. Gynecol. Oncol. 2015, 139, 5–9. [Google Scholar] [CrossRef]
- Perrone, A.M.; Coada, C.A.; Ravegnini, G.; De Leo, A.; Damiano, G.; De Crescenzo, E.; Tesei, M.; Di Costanzo, S.; Genovesi, L.; Rubino, D.; et al. Post-operative residual disease and number of cycles of neoadjuvant chemotherapy in advanced epithelial ovarian carcinoma. Int. J. Gynecol. Cancer 2023, 33, 1270–1278. [Google Scholar] [CrossRef]
- Coada, C.A.; Dondi, G.; Ravegnini, G.; Di Costanzo, S.; Tesei, M.; Fiuzzi, E.; Di Stanislao, M.; Giunchi, S.; Zamagni, C.; Bovicelli, A.; et al. Optimal number of neoadjuvant chemotherapy cycles prior to interval debulking surgery in advanced epithelial ovarian cancer: A systematic review and meta-analysis of progression-free survival and overall survival. J. Gynecol. Oncol. 2023, 34, e82. [Google Scholar] [CrossRef]
- de Lange, N.M.; Ezendam, N.P.M.; Kwon, J.S.; Vandenput, I.; Mirchandani, D.; Amant, F.; van der Putten, L.J.M.; Pijnenborg, J.M.A. Neoadjuvant chemotherapy followed by surgery for advanced-stage endometrial cancer. Curr. Oncol. Tor. Ont. 2019, 26, e226–e232. [Google Scholar] [CrossRef]
- Bogani, G.; Ditto, A.; Leone Roberti Maggiore, U.; Scaffa, C.; Mosca, L.; Chiappa, V.; Martinelli, F.; Lorusso, D.; Raspagliesi, F. Neoadjuvant chemotherapy followed by interval debulking surgery for unresectable stage IVB Serous endometrial cancer. Tumori J. 2019, 105, 92–97. [Google Scholar] [CrossRef]
- Huang, A.B.; Wu, J.; Chen, L.; Albright, B.B.; Previs, R.A.; Moss, H.A.; Davidson, B.A.; Havrilesky, L.J.; Melamed, A.; Wright, J.D. Neoadjuvant chemotherapy for advanced stage endometrial cancer: A systematic review. Gynecol. Oncol. Rep. 2021, 38, 100887. [Google Scholar] [CrossRef]
- Capozzi, V.A.; Scarpelli, E.; De Finis, A.; Rotondella, I.; Scebba, D.; Gallinelli, A.; Montrucchio, C.; Martignon, G.; Leotta, M.; Ghi, T.; et al. Optimal Management for Stage IVB Endometrial Cancer: A Systematic Review. Cancers 2023, 15, 5123. [Google Scholar] [CrossRef]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic Version 1.2025—September 11, 2024 NCCN.org. NCCN. Available online: https://www.nccn.org/guidelines/guidelines-detail (accessed on 3 March 2024).
- Miller, D.S.; Filiaci, V.L.; Mannel, R.S.; Cohn, D.E.; Matsumoto, T.; Tewari, K.S.; DiSilvestro, P.; Pearl, M.L.; Argenta, P.A.; Powell, M.A.; et al. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 3841–3850. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Shepherd, J.H. Revised FIGO staging for gynaecological cancer. Br. J. Obstet. Gynaecol. 1989, 96, 889–892. [Google Scholar] [CrossRef]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef]
- Lo, C.K.; Mertz, D.; Loeb, M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014, 14, 45. [Google Scholar] [CrossRef]
- R Core Team. European Environment Agency, 2020. Available online: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 (accessed on 1 May 2024).
- Rogula, B.; Lozano-Ortega, G.; Johnston, K.M. A Method for Reconstructing Individual Patient Data From Kaplan-Meier Survival Curves That Incorporate Marked Censoring Times. MDM Policy Pract. 2022, 7, 23814683221077643. [Google Scholar] [CrossRef]
- Kassambara, A.; Kosinski, M.; Biecek, P.; Fabian, S. Survminer: Drawing Survival Curves using “ggplot2”. 9 March 2021. Available online: https://cran.r-project.org/web/packages/survminer/index.html (accessed on 7 July 2024).
- Vandenput, I.; Van Calster, B.; Capoen, A.; Leunen, K.; Berteloot, P.; Neven, P.; Moerman, P.; Vergote, I.; Amant, F. Neoadjuvant chemotherapy followed by interval debulking surgery in patients with serous endometrial cancer with transperitoneal spread (stage IV): A new preferred treatment? Br. J. Cancer 2009, 101, 244–249. [Google Scholar] [CrossRef]
- Bristow, R.E.; Zerbe, M.J.; Rosenshein, N.B.; Grumbine, F.C.; Montz, F.J. Stage IVB endometrial carcinoma: The role of cytoreductive surgery and determinants of survival. Gynecol. Oncol. 2000, 78, 85–91. [Google Scholar] [CrossRef]
- Landrum, L.M.; Moore, K.N.; Myers, T.K.; Lanneau, G.S., Jr.; McMeekin, D.S.; Walker, J.L.; Gold, M.A. Stage IVB endometrial cancer: Does applying an ovarian cancer treatment paradigm result in similar outcomes? A case-control analysis. Gynecol. Oncol. 2009, 112, 337–341. [Google Scholar] [CrossRef]
- Lee, L.J.; Demaria, R.; Berkowitz, R.; Matulonis, U.; Viswanathan, A.N. Clinical predictors of long-term survival for stage IVB uterine papillary serous carcinoma confined to the abdomen. Gynecol. Oncol. 2014, 132, 65–69. [Google Scholar] [CrossRef]
- Ueda, Y.; Enomoto, T.; Miyatake, T.; Egawa-Takata, T.; Ugaki, H.; Yoshino, K.; Fujita, M.; Kimura, T. Endometrial carcinoma with extra-abdominal metastasis: Improved prognosis following cytoreductive surgery. Ann. Surg. Oncol. 2010, 17, 1111–1117. [Google Scholar] [CrossRef]
- Gehrig, P.A.; Morris, D.E.; Van Le, L. Uterine serous carcinoma: A comparison of therapy for advanced-stage disease. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2004, 14, 515–520. [Google Scholar] [CrossRef]
- Watari, H.; Todo, Y.; Takeda, M.; Ebina, Y.; Yamamoto, R.; Sakuragi, N. Lymph-vascular space invasion and number of positive para-aortic node groups predict survival in node-positive patients with endometrial cancer. Gynecol. Oncol. 2005, 96, 651–657. [Google Scholar] [CrossRef]
- Gitsch, G.; Friedlander, M.L.; Wain, G.V.; Hacker, N.F. Uterine papillary serous carcinoma. A clinical study. Cancer 1995, 75, 2239–2243. [Google Scholar] [CrossRef]
- Nguyen, N.P.; Sallah, S.; Karlsson, U.; Vos, P.; Ludin, A.; Semer, D.; Tait, D.; Salehpour, M.; Jendrasiak, G.; Robiou, C. Prognosis for papillary serous carcinoma of the endometrium after surgical staging. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2001, 11, 305–311. [Google Scholar] [CrossRef]
- Low, J.S.; Wong, E.H.; Tan, H.S.; Yap, S.P.; Chua, E.J.; Sethi, V.K.; Soh, L.T.; Low, J.; Tay, E.H.; Chew, S.H. Adjuvant sequential chemotherapy and radiotherapy in uterine papillary serous carcinoma. Gynecol. Oncol. 2005, 97, 171–177. [Google Scholar] [CrossRef]
- Kelly, M.G.; O’Malley, D.; Hui, P.; McAlpine, J.; Dziura, J.; Rutherford, T.J.; Azodi, M.; Chambers, S.K.; Schwartz, P.E. Patients with uterine papillary serous cancers may benefit from adjuvant platinum-based chemoradiation. Gynecol. Oncol. 2004, 95, 469–473. [Google Scholar] [CrossRef]
- Lim, H.; Bang, S.H.; Kim, Y.; Cho, S.H.; Shin, W.; Kim, S.I.; Kim, T.H.; Suh, D.H.; Lim, M.C.; Kim, J.W.; et al. Clinical implications of neoadjuvant chemotherapy in advanced endometrial cancer: A multi-center retrospective cohort study. BMC Cancer 2022, 22, 703. [Google Scholar] [CrossRef]
- Jani, I.; Lastra, R.R.; Brito, K.S.; Liao, C.; Lazo, I.; Lee, N.K.; Yamada, S.D.; Kurnit, K.C. Chemotherapy response score as a prognostic tool in patients with advanced stage endometrial carcinoma treated with neoadjuvant chemotherapy. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2021, 31, 852–858. [Google Scholar] [CrossRef]
- Unsal, M.; Kilic, C.; Cakir, C.; Kilic, F.; Ersak, B.; Karakas, S.; Tokgozoglu, N.; Varli, B.; Oktar, O.; Kimyon Comert, G.; et al. Neoadjuvant chemotherapy in patients with stage IVB uterine serous carcinoma: A Turkish multicentric study. J. Obstet. Gynaecol. 2023, 43, 2151355. [Google Scholar] [CrossRef]
- Rajkumar, S.; Nath, R.; Lane, G.; Mehra, G.; Begum, S.; Sayasneh, A. Advanced stage (IIIC/IV) endometrial cancer: Role of cytoreduction and determinants of survival. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 26–31. [Google Scholar] [CrossRef]
- Tobias, C.J.; Chen, L.; Melamed, A.; St Clair, C.; Khoury-Collado, F.; Tergas, A.I.; Hou, J.Y.; Hur, C.; Ananth, C.V.; Neugut, A.I.; et al. Association of Neoadjuvant Chemotherapy With Overall Survival in Women With Metastatic Endometrial Cancer. JAMA Netw. Open 2020, 3, e2028612. [Google Scholar] [CrossRef] [PubMed]
- Eto, T.; Saito, T.; Shimokawa, M.; Hatae, M.; Takeshima, N.; Kobayashi, H.; Kasamatsu, T.; Yoshikawa, H.; Kamura, T.; Konishi, I.; et al. Status of treatment for the overall population of patients with stage IVb endometrial cancer, and evaluation of the role of preoperative chemotherapy: A retrospective multi-institutional study of 426 patients in Japan. Gynecol. Oncol. 2013, 131, 574–580. [Google Scholar] [CrossRef]
- Wilkinson-Ryan, I.; Frolova, A.I.; Liu, J.; Stewart Massad, L.; Thaker, P.H.; Powell, M.A.; Mutch, D.G.; Hagemann, A.R. Neoadjuvant chemotherapy versus primary cytoreductive surgery for stage IV uterine serous carcinoma. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2015, 25, 63–68. [Google Scholar] [CrossRef]
- Thomas, M.B.; Mariani, A.; Cliby, W.A.; Keeney, G.L.; Podratz, K.C.; Dowdy, S.C. Role of cytoreduction in stage III and IV uterine papillary serous carcinoma. Gynecol. Oncol. 2007, 107, 190–193. [Google Scholar] [CrossRef]
- Chi, D.S.; Welshinger, M.; Venkatraman, E.S.; Barakat, R.R. The role of surgical cytoreduction in Stage IV endometrial carcinoma. Gynecol. Oncol. 1997, 67, 56–60. [Google Scholar] [CrossRef]
- Goff, B.A.; Goodman, A.; Muntz, H.G.; Fuller, A.F., Jr.; Nikrui, N.; Rice, L.W. Surgical stage IV endometrial carcinoma: A study of 47 cases. Gynecol. Oncol. 1994, 52, 237–240. [Google Scholar] [CrossRef]
- Albright, B.B.; Monuszko, K.A.; Kaplan, S.J.; Davidson, B.A.; Moss, H.A.; Huang, A.B.; Melamed, A.; Wright, J.D.; Havrilesky, L.J.; Previs, R.A. Primary cytoreductive surgery for advanced stage endometrial cancer: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2021, 225, e1–e237. [Google Scholar] [CrossRef]
- Kanno, M.; Yunokawa, M.; Kurihara, N.; Aoki, Y.; Omi, M.; Tanigawa, T.; Kanao, H. Efficacy of intra-abdominal cytoreductive surgery in advanced endometrial cancer with distant metastasis. J. Gynecol. Oncol. 2023, 34, e77. [Google Scholar] [CrossRef]
- Lyman, G.H.; Kuderer, N.M. The strengths and limitations of meta-analyses based on aggregate data. BMC Med. Res. Methodol. 2005, 5, 14. [Google Scholar] [CrossRef]
- de Biase, D.; Maloberti, T.; Corradini, A.G.; Rosini, F.; Grillini, M.; Ruscelli, M.; Coluccelli, S.; Altimari, A.; Gruppioni, E.; Sanza, V.; et al. Integrated clinicopathologic and molecular analysis of endometrial carcinoma: Prognostic impact of the new ESGO-ESTRO-ESP endometrial cancer risk classification and proposal of histopathologic algorithm for its implementation in clinical practice. Front. Med. 2023, 10, 1146499. [Google Scholar] [CrossRef]
- Chacon, E.; Boria, F.; Lyer, R.R.; Fanfani, F.; Malzoni, M.; Bretová, P.; Luzarraga Aznar, A.; Fruscio, R.; Jedryka, M.A.; Tóth, R.; et al. SENECA study: Staging endometrial cancer based on molecular classification. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2024, 34, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A Web and Mobile App for Systematic Reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).