Endometrial Intraepithelial Neoplasia, Concurrent Endometrial Cancer and Risk for Pelvic Sentinel Node Metastases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
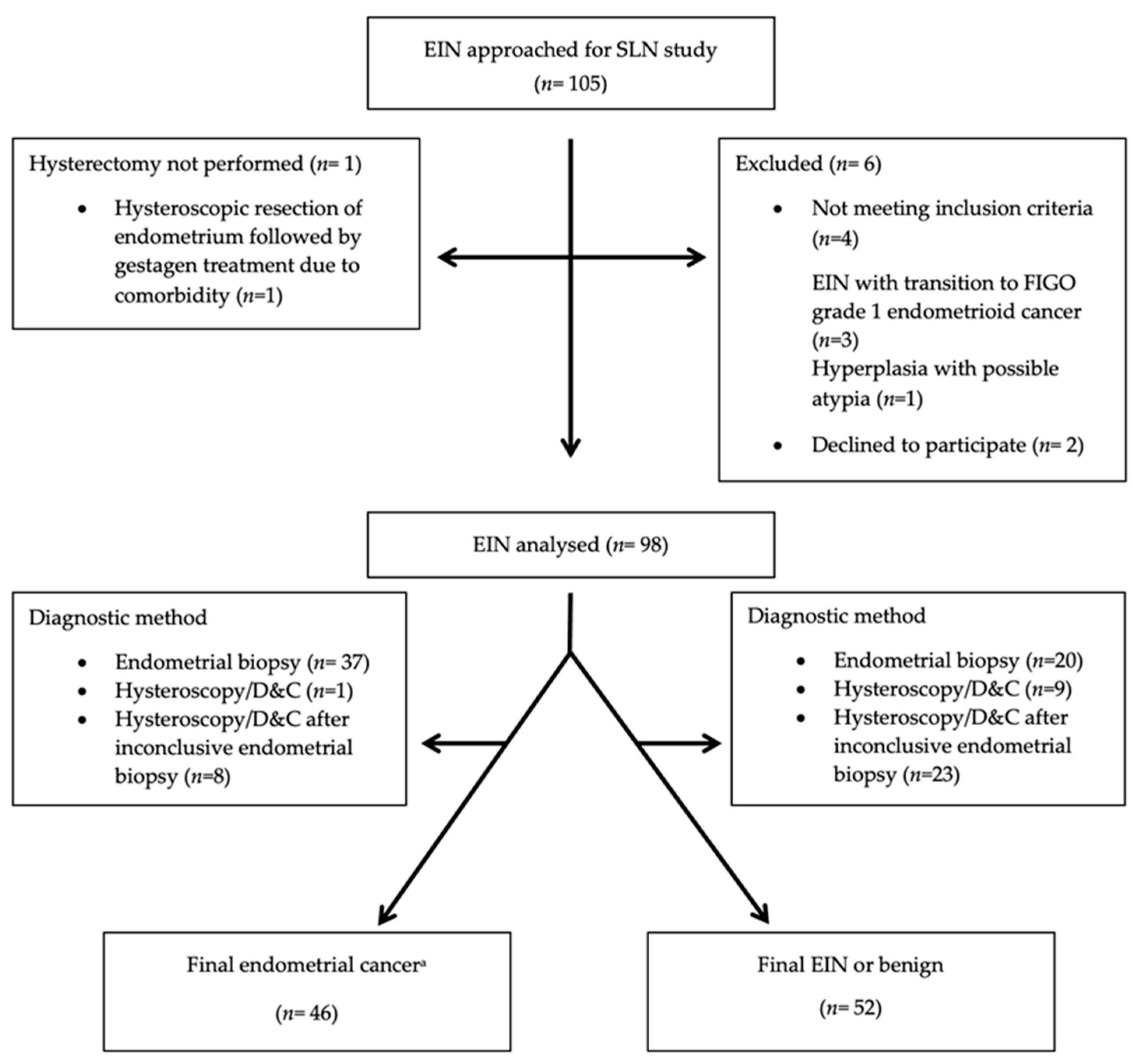
2.1. Study Design
2.2. Histological Analysis
2.3. Statistics
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mutter, G.L. Endometrial intraepithelial neoplasia (EIN): Will it bring order to chaos? The Endometrial Collaborative Group. Gynecol. Oncol. 2000, 76, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Jarboe, E.A.; Mutter, G.L. Endometrial intraepithelial neoplasia. Semin. Diagn. Pathol. 2010, 27, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Baak, J.P.; Mutter, G.L.; Robboy, S.; Van Diest, P.J.; Uyterlinde, A.M.; Ørbo, A.; Palazzo, J.; Fiane, B.; Løvslett, K.; Burger, C.; et al. The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer 2005, 103, 2304–2312. [Google Scholar] [CrossRef]
- Baak, J.P.; Mutter, G.L. EIN and WHO94. J. Clin. Pathol. 2005, 58, 1–6. [Google Scholar] [CrossRef]
- Chen, H.; Strickland, A.L.; Castrillon, D.H. Histopathologic diagnosis of endometrial precancers: Updates and future directions. Semin. Diagn. Pathol. 2022, 39, 137–147. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board (Ed.). World Health Organization Classification of Tumours, 5th ed.; Female Genital Tumours; IARC Press: Lyon, France, 2020. [Google Scholar]
- Ordi, J.; Bergeron, C.; Hardisson, D.; McCluggage, W.G.; Hollema, H.; Felix, A.; Soslow, R.A.; Oliva, E.; Tavassoli, F.A.; Alvarado-Cabrero, I.; et al. Reproducibility of current classifications of endometrial endometrioid glandular proliferations: Further evidence supporting a simplified classification. Histopathology 2014, 64, 284–292. [Google Scholar] [CrossRef]
- Trimble, C.L.; Kauderer, J.; Zaino, R.; Silverberg, S.; Lim, P.C.; Burke, J.J.; Alberts, D.; Curtin, J. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia. Cancer 2006, 106, 812–819. [Google Scholar] [CrossRef]
- Leitao, M.M., Jr.; Han, G.; Lee, L.X.; Abu-Rustum, N.R.; Brown, C.L.; Chi, D.S.; Sonoda, Y.; Levine, D.A.; Gardner, G.J.; Jewell, E.E.; et al. Complex atypical hyperplasia of the uterus: Characteristics and prediction of underlying carcinoma risk. Am. J. Obstet. Gynecol. 2010, 203, 349.e1–349.e6. [Google Scholar] [CrossRef]
- Vetter, M.H.; Smith, B.; Benedict, J.; Hade, E.M.; Bixel, K.; Copeland, L.J.; Cohn, D.E.; Fowler, J.M.; O’malley, D.; Salani, R.; et al. Preoperative predictors of endometrial cancer at time of hysterectomy for endometrial intraepithelial neoplasia or complex atypical hyperplasia. Am. J. Obstet. Gynecol. 2020, 222, 60.e1–60.e7. [Google Scholar] [CrossRef]
- Vetter, M.H.; Smith, B.; Benedict, J.; Hade, E.M.; Bixel, K.; Copeland, L.J.; Cohn, D.E.; Fowler, J.M.; O’malley, D.; Salani, R.; et al. Absolute risk of endometrial carcinoma during 20-year follow-up among women with endometrial hyperplasia. J. Clin. Oncol. 2010, 28, 788–792. [Google Scholar]
- Kurman, R.J.; Kaminski, P.F.; Norris, H.J. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer 1985, 56, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, M.; Caspani, G.; Bonazzi, C.; Chiappa, V.; Perego, P.; Mangioni, C. Fertility-sparing treatment in young women with endometrial cancer or atypical complex hyperplasia: A prospective single-institution experience of 21 cases. BJOG Int. J. Obstet. Gynaecol. 2009, 116, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Pal, N.M.; Broaddus, R.R.; Urbauer, D.L.; Balakrishnan, N.B.; Milbourne, A.; Schmeler, K.M.; Meyer, L.A.; Soliman, P.T.; Lu, K.H.; Ramirez, P.T.; et al. Treatment of low-risk endometrial cancer and complex atypical hyperplasia with the levonorgestrel-releasing intrauterine device. Obstet. Gynecol. 2018, 131, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef]
- Helgers, R.J.A.; Winkens, B.; Slangen, B.F.M.; Werner, H.M.J. Lymphedema and Post-Operative Complications after Sentinel Lymph Node Biopsy versus Lymphadenectomy in Endometrial Carcinomas-A Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 10, 120. [Google Scholar] [CrossRef]
- Leitao, M.M.; Zhou, Q.C.; Gomez-Hidalgo, N.R.; Iasonos, A.; Baser, R.; Mezzancello, M.; Chang, K.; Ward, J.; Chi, D.S.; Roche, K.L.; et al. Patient-reported outcomes after surgery for endometrial carcinoma: Prevalence of lower-extremity lymphedema after sentinel lymph node mapping versus lymphadenectomy. Gynecol. Oncol. 2020, 156, 147–153. [Google Scholar] [CrossRef]
- Persson, J.; Salehi, S.; Bollino, M.; Lönnerfors, C.; Falconer, H.; Geppert, B. Pelvic Sentinel lymph node detection in High-Risk Endometrial Cancer (SHREC-trial)-the final step towards a paradigm shift in surgical staging. Eur. J. Cancer 2019, 116, 77–85. [Google Scholar] [CrossRef]
- Sullivan, M.W.; Philp, L.; Kanbergs, A.N.; Safdar, N.; Oliva, E.; Bregar, A.; del Carmen, M.G.; Eisenhauer, E.L.; Goodman, A.; Muto, M.; et al. Lymph node assessment at the time of hysterectomy has limited clinical utility for patients with pre-cancerous endometrial lesions. Gynecol. Oncol. 2021, 162, 613–618. [Google Scholar] [CrossRef]
- Touhami, O.; Grégoire, J.; Renaud, M.-C.; Sebastianelli, A.; Grondin, K.; Plante, M. The utility of sentinel lymph node mapping in the management of endometrial atypical hyperplasia. Gynecol. Oncol. 2018, 148, 485–490. [Google Scholar] [CrossRef]
- Matanes, E.; Amajoud, Z.; Kogan, L.; Mitric, C.; Ismail, S.; Raban, O.; Knigin, D.; Levin, G.; Bahoric, B.; Ferenczy, A.; et al. Is sentinel lymph node assessment useful in patients with a preoperative diagnosis of endometrial intraepithelial neoplasia? Gynecol. Oncol. 2023, 168, 107–113. [Google Scholar] [CrossRef]
- Whyte, J.S.; Gurney, E.P.; Curtin, J.P.; Blank, S.V. Lymph node dissection in the surgical management of atypical endometrial hyperplasia. Am. J. Obstet. Gynecol. 2010, 202, 176.e1–176.e4. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Bollino, M.; Geppert, B.; Reynisson, P.; Lönnerfors, C.; Persson, J. Optimizing the Sensitivity of a Pelvic Sentinel Node Algorithm Requires a Hybrid Algorithm Combining Indocyanine Green Based Mapping and the Removal of Non-Mapped Nodes at Defined Anatomic Positions. Cancers 2024, 16, 3242. [Google Scholar] [CrossRef] [PubMed]
- Lührs, O.; Ekdahl, L.; Geppert, B.; Lönnerfors, C.; Persson, J. Resection of the upper paracervical lymphovascular tissue should be an integral part of a pelvic sentinel lymph node algorithm in early stage cervical cancer. Gynecol. Oncol. 2021, 163, 289–293. [Google Scholar] [CrossRef]
- Bizzarri, N.; Ianieri, M.M.; Rosati, A.; Anchora, L.P.; Ronsini, C.; Ladisa, I.; Cavinato, M.; Fanfani, F.; Fagotti, A.; Scambia, G.; et al. Consensus on the Gemelli terminology of surgical anatomy for radical hysterectomy. Int. J. Gynecol. Cancer 2023, 33, 876–881. [Google Scholar] [CrossRef]
- Querleu, D.; Cibula, D.; Abu-Rustum, N.R.; Fanfani, F.; Fagotti, A.; Anchora, L.P.; Ianieri, M.M.; Chiantera, V.; Bizzarri, N.; Scambia, G. International expert consensus on the surgical anatomic classification of radical hysterectomies. Am. J. Obstet. Gynecol. 2024, 230, 235.e1–235.e8. [Google Scholar] [CrossRef]
- Querleu, D.; Bizzarri, N.; Fanfani, F.; Fagotti, A.; Scambia, G. Simplified anatomical nomenclature of lateral female pelvic spaces. Int. J. Gynecol. Cancer 2022, 32, 1183–1188. [Google Scholar] [CrossRef]
- Bollino, M.; Geppert, B.; Lönnerfors, C.; Persson, J. A selective anatomically based lymph node sampling can replace a side specific pelvic lymphadenectomy in endometrial cancer with failed sentinel node mapping. Eur. J. Cancer 2024, 204, 114049. [Google Scholar] [CrossRef]
- Edge, S.B.; American Joint Committee on Cancer ACS. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual; Springer: New York, NY, USA, 2010. [Google Scholar]
- Bollino, M.; Geppert, B.; Lönnerfors, C.; Måsbäck, A.; Kasselaki, I.; Persson, J. Prevalence and size of pelvic sentinel lymph node metastases in endometrial cancer. Eur. J. Cancer 2024, 209, 114265. [Google Scholar] [CrossRef]
- Mueller, J.J.; Rios-Doria, E.; Park, K.J.; Broach, V.A.; Alektiar, K.M.; Jewell, E.L.; Zivanovic, O.; Sonoda, Y.; Abu-Rustum, N.R.; Leitao, M.M., Jr.; et al. Sentinel lymph node mapping in patients with endometrial hyperplasia: A practice to preserve or abandon? Gynecol. Oncol. 2023, 168, 1–7. [Google Scholar] [CrossRef]
- McCoy, C.A.; Coleman, H.G.; McShane, C.M.; McCluggage, W.G.; Wylie, J.; Quinn, D.; McMenamin, C. Factors associated with interobserver variation amongst pathologists in the diagnosis of endometrial hyperplasia: A systematic review. PLoS ONE 2024, 19, e0302252. [Google Scholar] [CrossRef]
- Palmér, M.; Åkesson, Å.; Marcickiewicz, J.; Blank, E.; Hogström, L.; Torle, M.; Mateoiu, C.; Dahm-Kähler, P.; Leonhardt, H. Accuracy of transvaginal ultrasound versus MRI in the PreOperative Diagnostics of low-grade Endometrial Cancer (PODEC) study: A prospective multicentre study. Clin. Radiol. 2023, 78, 70–79. [Google Scholar] [CrossRef]
| Characteristics Median (Min–Max) or n (%) as Appropriate | All Women | Final Pathologic Diagnosis | p-Value | |
|---|---|---|---|---|
| EC | EIN or Benign | |||
| Total | 98 | 46/98 (47%) | 52/98 (53%) | |
| Age (years) | 64 (31–86) | 70 (44–86) | 61 (31–80) | <0.001 |
| BMI (kg/m2) | 31.4 (19.5–51.9) | 29.4 (19.5–51.9) | 33.2 (20.9–51.0) | 0.123 |
| Parity | 2 (0–5) | 2 (0–4) | 2 (0–5) | 0.175 |
| Premenopausal | 15/98 (15%) | 5/46 (11%) | 10/52 (19%) | 0.251 |
| ASA | 0.408 | |||
| 1–2 | 82/98 (84%) | 40/46 (87%) | 42/52 (81%) | |
| 3–4 | 16/98 (16%) | 6/46 (13%) | 10/52 (19%) | |
| BMI ≥ 40 kg/m2 | 13/98 (13%) | 5/46 (11%) | 8/52 (15%) | |
| Diagnostic method | <0.001 a | |||
| Endometrial biopsy | 57/98 (58%) | 37/57 (65%) | 20/57 (35%) | |
| Hysteroscopy/D&C | 10/98 (10%) | 1/10 (10%) | 9/10 (90%) | 0.001 b |
| Hysteroscopy/D&C after inconclusive endometrial biopsy | 31/98 (32%) | 8/31 (26%) | 23/31 (74%) | <0.001 c |
| Endometrial thickness (mm) | 14 (3–50) | 13 (5–50) | 14 (3–33) | 0.625 |
| Sonographic evaluation | ||||
| MI > 50% | 4/98 (4%) | 4/4 (100%) | 0 | |
| Isolated polyp(s) | 34/98 (35%) | 8/34 (24%) | 26/34 (76%) | 0.001 d |
| General endometrial thickening | 61/98 (62%) | 35/61 (57%) | 26/61 (43%) | |
| N/A | 3/98 (3%) | 3/3 (100%) | 0 | |
| EC n = 46 Median (Min–Max) or n (%) as Appropriate | |
|---|---|
| Histology | |
| Endometrioid FIGO grade 1 | 42/46 (91%) (4 SLN+) |
| Endometrioid FIGO grade 2 | 3/46 (7%) (2 SLN+) |
| Carcinosarcoma | 1/46 (2%) (0 SLN+) |
| Diagnostic method | |
| Endometrial biopsy | 37/46 (80%) (5 SLN+) |
| Hysteroscopy/D&C | 1/46 (2%) (1 SLN+) |
| Hysteroscopy/D&C after inconclusive endometrial biopsy | 8/46 (17%) (0 SLN+) |
| Largest tumor diameter (mm) a | |
| All | 30 (3–100) |
| FIGO grade 1 | 30 (3–100) |
| FIGO grade 2 | 45 (30–45) |
| High grade carcinosarcoma | 30 (30–30) |
| Myometrial invasion ≥ 50% | |
| Total | 14/46 (30%) (5 SLN+) |
| Endometrioid FIGO grade 1 | 12 b/14 (86%) (3 SLN+) |
| Endometrioid FIGO grade 2 | 2/14 (14%) (2 SLN+) |
| Carcinosarcoma | 0 (0 SLN+) |
| LVSI | |
| Yes | 5/46 (11%) (5 SLN+) |
| No | 41/46 (89%) (1 SLN+) |
| 2009 FIGO stage | |
| IA | 30/46 (65%) |
| IB | 9/46 (20%) |
| II | 1/46 (2%) |
| IIIC1 | 4 c/46 (11%) |
| IIIC2 | 2 d/46 (2%) |
| Cancer recurrence e | |
| Yes | 2/46 (4%) (1 SLN+) |
| No | 44/46 (96%) (5 SLN+) |
| Preoperative | Postoperative | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case No. | Diagnostic Method | Type of Endometrial Lesion | Surgical Stage | Uterine Stage | MI (%) | Histology | LVSI (Y/N) | SLNs incl. PULT (n=) Metastatic/Total | Location and Size of Metastases (incl. SLNs) | Paraaortic Restaging (Y/N) | Cancer Recurrence a (Y/N) |
| 1 | EB | GET | IIIC1 | IB | >50 | Endometrioid FIGO grade 2 | Y | 1/4 | Obturator L, ITC. | N | Y. Lung metastasis. |
| 2 | EB | GET | IIIC2 | IA | <50 | Endometrioid FIGO grade 1 | Y | 2/3 | Obturator L and R, ITC. | Y. Two LN+. ITC. | N |
| 3 | EB | GET | IIIC1 | IB | >50 | Endometrioid FIGO grade 2 | Y | 2/3 | Extern iliac L and obturator L, ITC. | N | N |
| 4 | Hyst | GET | IIIC1 | IB | >50 | Endometrioid FIGO grade 1 | Y | 2/5 | Obturator L and R, ITC. | N | N |
| 5 | EB | N/A | IIIC1 | II | >50 | Endometrioid FIGO grade 1. 1.2 mm STIC in right fallopian tube | N | 1/7 | Extern iliac L, ITC. | N | N |
| 6 | EB | N/A | IIIC2 | IB | >50 | Endometrioid FIGO grade 1 w. mucinous differentiation | Y | 5/5 | Ovary R. PULT R, ITC. Extern iliac L, micro. Obturator L and R, unspecified whether micro or macro. Extern iliac R, ITC. | Y. One LN+. Macro. | N |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hawez, T.; Bollino, M.; Lönnerfors, C.; Persson, J. Endometrial Intraepithelial Neoplasia, Concurrent Endometrial Cancer and Risk for Pelvic Sentinel Node Metastases. Cancers 2024, 16, 4215. https://doi.org/10.3390/cancers16244215
Hawez T, Bollino M, Lönnerfors C, Persson J. Endometrial Intraepithelial Neoplasia, Concurrent Endometrial Cancer and Risk for Pelvic Sentinel Node Metastases. Cancers. 2024; 16(24):4215. https://doi.org/10.3390/cancers16244215
Chicago/Turabian StyleHawez, Tabayi, Michele Bollino, Celine Lönnerfors, and Jan Persson. 2024. "Endometrial Intraepithelial Neoplasia, Concurrent Endometrial Cancer and Risk for Pelvic Sentinel Node Metastases" Cancers 16, no. 24: 4215. https://doi.org/10.3390/cancers16244215
APA StyleHawez, T., Bollino, M., Lönnerfors, C., & Persson, J. (2024). Endometrial Intraepithelial Neoplasia, Concurrent Endometrial Cancer and Risk for Pelvic Sentinel Node Metastases. Cancers, 16(24), 4215. https://doi.org/10.3390/cancers16244215






