Salivary Gland Volume Changes and Dry Mouth Symptom Following Definitive Radiation Therapy in Oropharyngeal Cancer Patients—A Comparison of Two Different Approaches: Intensity-Modulated Radiation Therapy Versus Intensity-Modulated Radiation Therapy/Intensity-Modulated Proton Therapy Combination
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients’ Characteristics
3.2. Oncologic Outcomes
3.3. Dosimetric Parameters
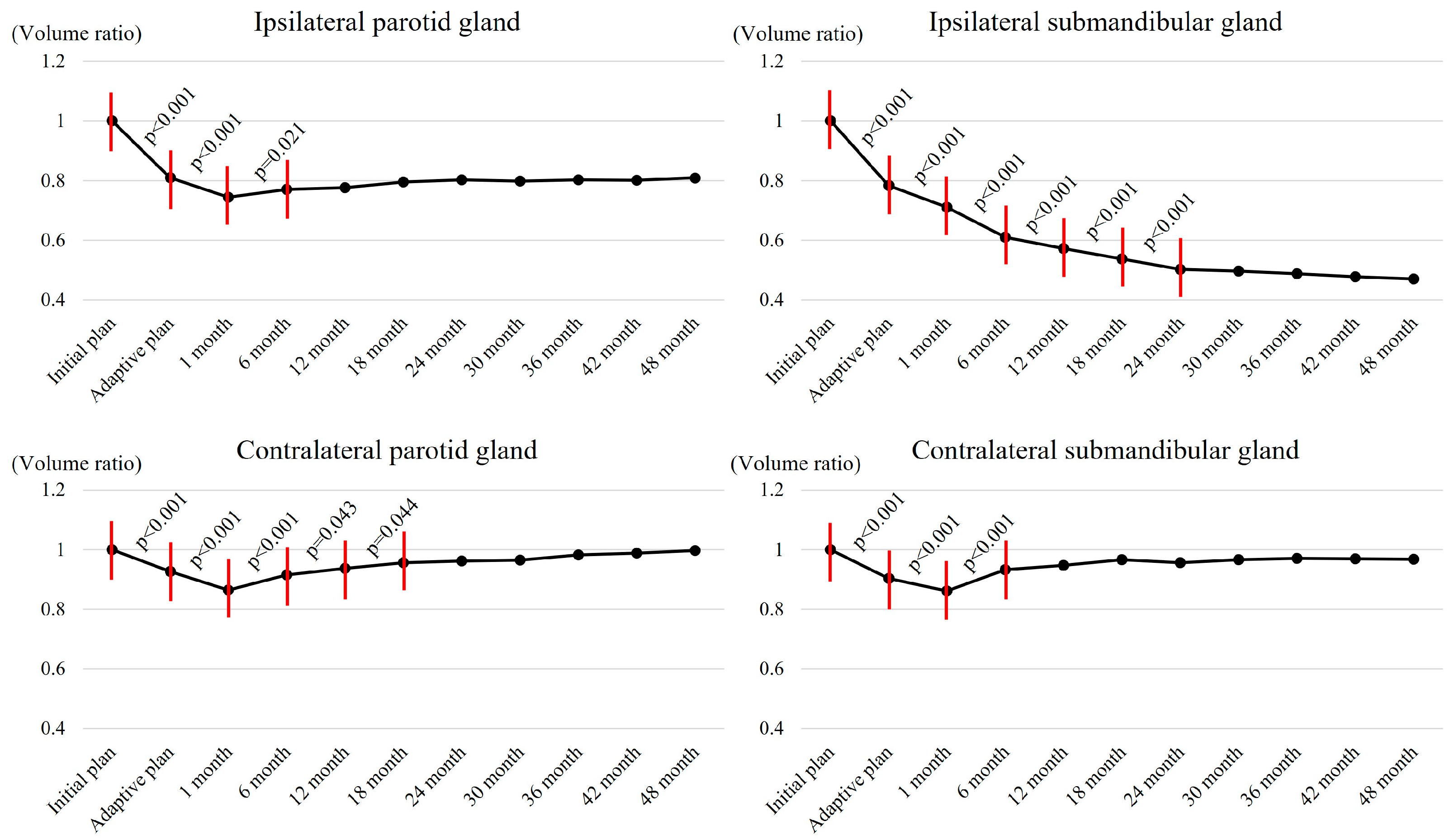
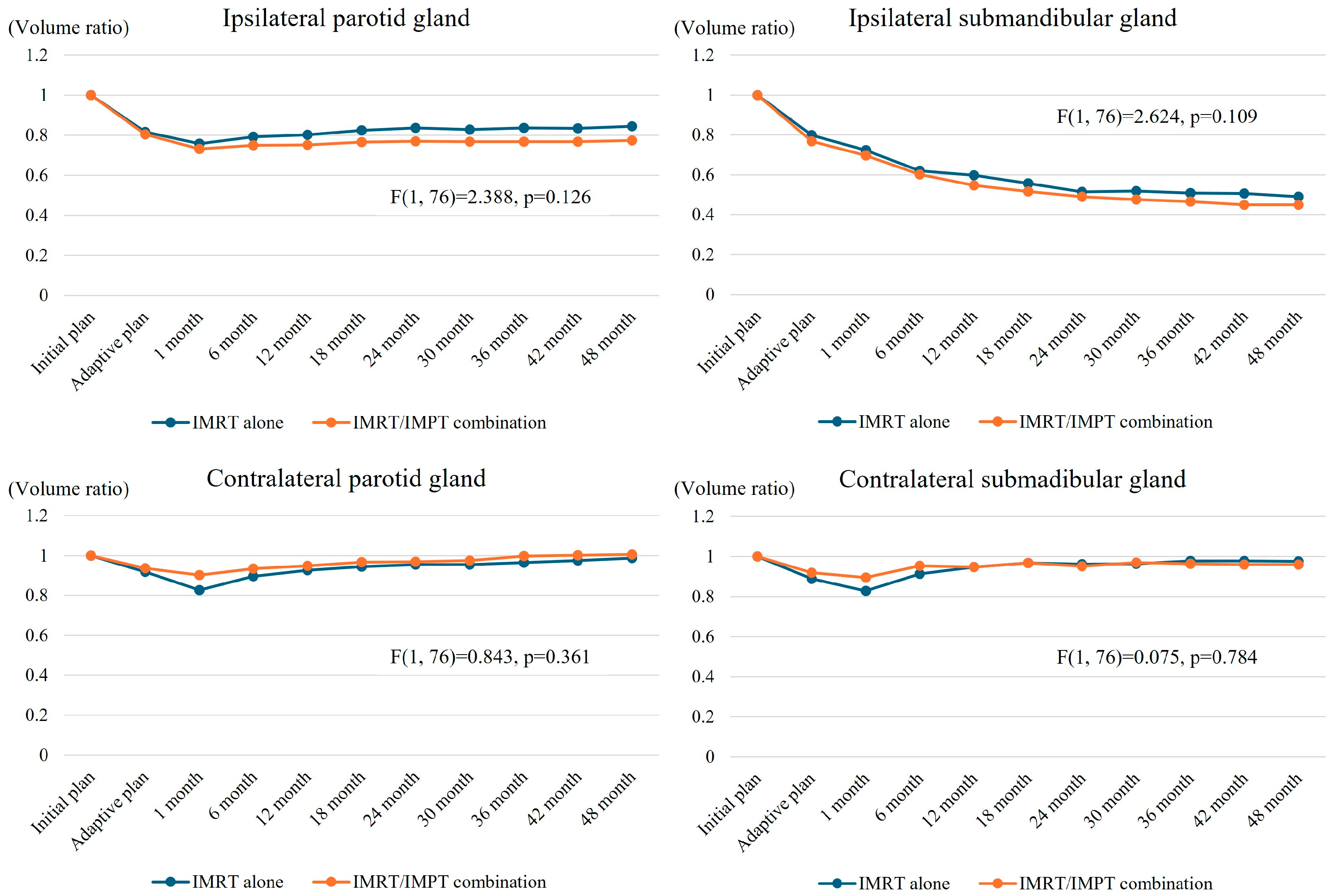
3.4. Salvary Gland Volume Changes
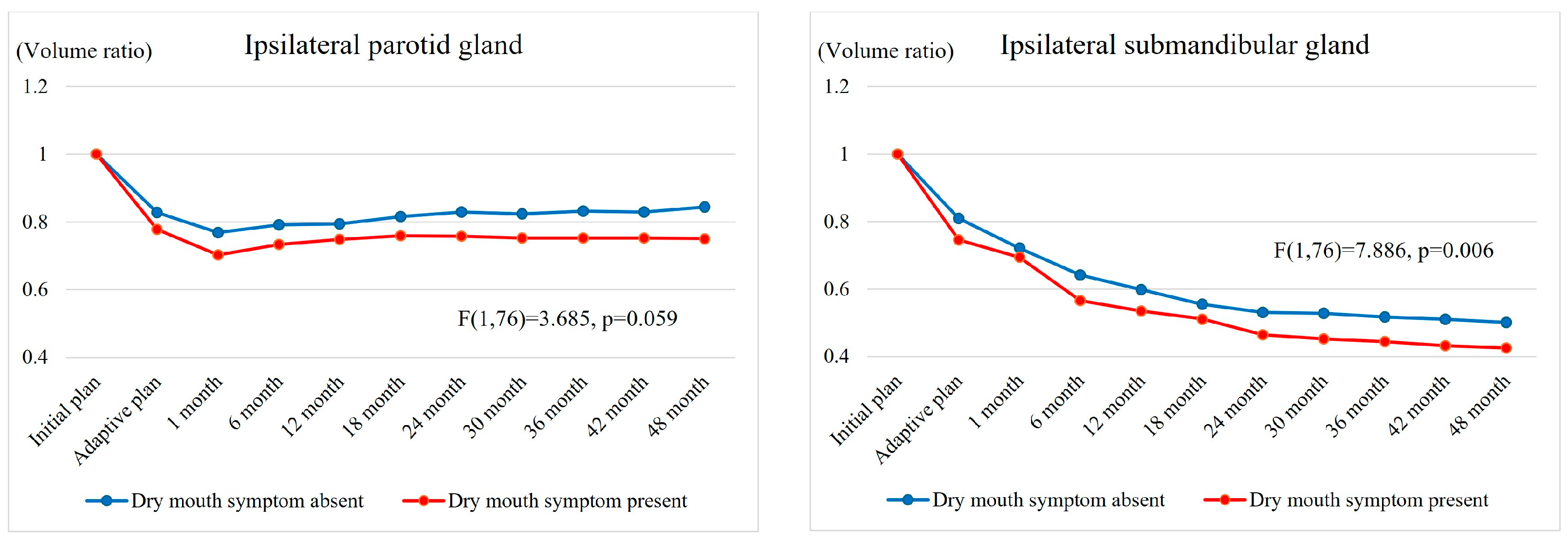
3.5. Changes in Dry Mouth Symptom
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Schlichting, J.A.; Pagedar, N.A.; Chioreso, C.; Lynch, C.F.; Charlton, M.E. Treatment trends in head and neck cancer: Surveillance, Epidemiology, and End Results (SEER) Patterns of Care analysis. Cancer Causes Control 2019, 30, 721–732. [Google Scholar] [CrossRef]
- Jensen, S.B.; Vissink, A.; Limesand, K.H.; Reyland, M.E. Salivary Gland Hypofunction and Xerostomia in Head and Neck Radiation Patients. J. Natl. Cancer Inst. Monogr. 2019, 53, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.B.; Pedersen, A.M.; Vissink, A.; Andersen, E.; Brown, C.G.; Davies, A.N.; Dutilh, J.; Fulton, J.S.; Jankovic, L.; Lopes, N.N.; et al. A systematic review of salivary gland hypofunction and xerostomia induced by cancer therapies: Preva-lence, severity and impact on quality of life. Support. Care Cancer 2010, 18, 1039–1060. [Google Scholar] [CrossRef] [PubMed]
- Gjini, M.; Ahmed, S.; Kalnicki, S.; Tomé, W.A.; Garg, M.K.; Kabarriti, R.; Brodin, N.P. Volumetric changes of the parotid gland during IMRT based on mid-treatment imaging: Implications for parotid stem cell sparing strategies in head and neck cancer. Acta Oncol. 2022, 61, 1069–1074. [Google Scholar] [CrossRef]
- Teshima, K.; Murakami, R.; Tomitaka, E.; Nomura, T.; Toya, R.; Hiraki, A.; Nakayama, H.; Hirai, T.; Shinohara, M.; Oya, N.; et al. Radiation-induced Parotid Gland Changes in Oral Cancer Patients: Correlation Between Parotid Volume and Saliva Production. Ultrasound Med. Biol. 2009, 40, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, Y.; Kreiborg, S.; Murakami, S.; Tsujimoto, T.; Sumida, I. Changes in the Submandibular Gland in Patients with Head and Neck Cancer After Radiation Therapy: A Preliminary Study. Anticancer Res. 2017, 37, 3239–3242. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Zhang, C.; Lv, X. Structural evaluation of Parotid gland in post radiotherapy oral cancer patients: A prospective study. Int. J. Radiat. Res. 2021, 19, 521–529. [Google Scholar] [CrossRef]
- Walters, R.; Kutuk, T.; Williams, A.; Rosen, E.; Contreras, J.; Coutinho, L.; Reyes, E.G.; Hobson, M.; Kaiser, A.; Kalman, N. Proton Therapy Specific Salivary Gland Volume Changes After Head and Neck Radiotherapy. Int. J. Radiat. Oncol. 2021, 111, e416. [Google Scholar] [CrossRef]
- Rwigema, J.M.; Langendijk, J.A.; Paul van der Laan, H.; Lukens, J.N.; Swisher-McClure, S.D.; Lin, A. A Model-Based Approach to Predict Short-Term Toxicity Benefits With Proton Therapy for Oro-pharyngeal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 553–562. [Google Scholar] [CrossRef] [PubMed]
- van de Water, T.A.; Lomax, A.J.; Bijl, H.P.; de Jong, M.E.; Schilstra, C.; Hug, E.B.; Langendijk, J.A. Potential Benefits of Scanned Intensity-Modulated Proton Therapy Versus Advanced Photon Therapy With Regard to Sparing of the Salivary Glands in Oropharyngeal Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1216–1224. [Google Scholar] [CrossRef]
- Park, S.G.; Ahn, Y.C.; Oh, D.; Noh, J.M.; Ju, S.G.; Kwon, D.; Jo, K.; Chung, K.; Chung, E.; Lee, W.; et al. Early clinical outcomes of helical tomotherapy/intensity-modulated proton therapy combination in naso-pharynx cancer. Cancer Sci. 2019, 110, 2867–2874. [Google Scholar] [CrossRef]
- Yoon, H.G.; Ahn, Y.C.; Oh, D.; Noh, J.M.; Park, S.G.; Nam, H.; Ju, S.G.; Kwon, D.; Park, S. Early Clinical Outcomes of Intensity Modulated Radiation Therapy/Intensity Modulated Proton Therapy Combination in Comparison with Intensity Modulated Radiation Therapy Alone in Oropharynx Cancer Patients. Cancers 2021, 13, 1549. [Google Scholar] [CrossRef] [PubMed]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.G.; Ahn, Y.C.; Kim, Y.B.; Park, S.G.; Choi, Y.M.; Na, C.H.; Hong, C.S.; Oh, D.; Kwon, D.Y.; Kim, C.C.; et al. Development of a Tongue Immobilization Device Using a 3D Printer for IMRT of Nasopharyngeal Cancer Patients. Cancer Res. Treat. 2020, 53, 45–54. [Google Scholar] [CrossRef]
- Cho, W.K.; Oh, D.; Lee, E.; Kim, T.G.; Lee, H.; Nam, H.; Noh, J.M.; Ahn, Y.C. Feasibility of Selective Neck Irradiation with Lower Elective Radiation Dose in Treating Nasopharynx Cancer Patients. Cancer Res. Treat. 2019, 51, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, Q.; Chen, M.; Lu, W. Dynamic tomotherapy delivery. Med. Phys. 2011, 38, 3013–3024. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services; N.I.o.H.; National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0; U.S. Department of Health and Human Services: Washington, DC, USA, 2017.
- Strojan, P.; Hutcheson, K.A.; Eisbruch, A.; Beitler, J.J.; Langendijk, J.A.; Lee, A.W.; Corry, J.; Mendenhall, W.M.; Smee, R.; Rinaldo, A.; et al. Treatment of late sequelae after radiotherapy for head and neck cancer. Cancer Treat. Rev. 2017, 59, 79–92. [Google Scholar] [CrossRef]
- Nagler, R. The enigmatic mechanism of irradiation-induced damage to the major salivary glands. Oral Dis. 2002, 8, 141–146. [Google Scholar] [CrossRef]
- Kam, M.K.; Leung, S.F.; Zee, B.; Chau, R.M.; Suen, J.J.; Mo, F.; Lai, M.; Ho, R.; Cheung, K.Y.; Yu, B.K.; et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early stage nasopharyngeal carcinoma patients. J. Clin. Oncol. 2007, 25, 4873–4879. [Google Scholar] [CrossRef] [PubMed]
- Vásquez Osorio, E.M.; Hoogeman, M.S.; Al-Mamgani, A.; Teguh, D.N.; Levendag, P.C.; Heijmen, B.J. Local anatomic changes in parotid and submandibular glands during radiotherapy for oro-pharynx cancer and correlation with dose, studied in detail with nonrigid registration. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Sanguineti, G.; Ricchetti, F.; Thomas, O.; Wu, B.; McNutt, T. Pattern and predictors of volumetric change of parotid glands during intensity modulated radiother-apy. Br. J. Radiol. 2013, 86, 20130363. [Google Scholar] [CrossRef]
- Lou, J.; Huang, P.; Ma, C.; Zheng, Y.; Chen, J.; Liang, Y.; Li, H.; Yin, Y.; Liu, D.; Yu, G.; et al. Parotid gland radiation dose-xerostomia relationships based on actual delivered dose for nasopharyngeal carcinoma. J. Appl. Clin. Med Phys. 2018, 19, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.T.C.; Cheng, S.C.H.; Wu, V.W.C.; Kwong, D.L.W. Post-radiotherapy morphological changes of parotid gland are dose- and radiotherapy tech-nique-dependent. Br. J. Radiol. 2011, 84, 1157. [Google Scholar] [CrossRef]
- Hiraoka, S.; Yoshimura, M.; Nakajima, A.; Nakashima, R.; Mizowaki, T. Long-term outcomes of stimulated salivary flow and xerostomia after definitive intensity-modulated radiation therapy for patients with head and neck cancerdagger. J. Radiat. Res. 2024, 65, 71–77. [Google Scholar] [CrossRef]
- Li, Y.; Taylor, J.M.; Ten Haken, R.K.; Eisbruch, A. The impact of dose on parotid salivary recovery in head and neck cancer patients treated with radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 660–669. [Google Scholar] [CrossRef]
- Gupta, T.; Hotwani, C.; Kannan, S.; Master, Z.; Rangarajan, V.; Murthy, V.; Budrukkar, A.; Ghosh-Laskar, S.; Agarwal, J.P. Prospective longitudinal assessment of parotid gland function using dynamic quantitative pertechnate scintigraphy and estimation of dose–response relationship of parotid-sparing radiotherapy in head-neck cancers. Radiat. Oncol. 2015, 10, 67. [Google Scholar] [CrossRef]
- Hey, J.; Setz, J.; Gerlach, R.; Janich, M.; Hildebrandt, G.; Vordermark, D.; Gernhardt, C.R.; Kuhnt, T. Parotid gland-recovery after radiotherapy in the head and neck region—36 months follow-up of a prospective clinical study. Radiat. Oncol. 2011, 6, 125. [Google Scholar] [CrossRef]
- Steenbakkers, R.J.; van Rijn–Dekker, M.I.; Stokman, M.A.; Kierkels, R.G.; van der Schaaf, A.; Hoek, J.G.v.D.; Bijl, H.P.; Kramer, M.C.; Coppes, R.P.; Langendijk, J.A.; et al. Parotid Gland Stem Cell Sparing Radiation Therapy for Patients With Head and Neck Cancer: A Double-Blind Randomized Controlled Trial. Int. J. Radiat. Oncol. 2021, 112, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Alterio, D.; Marvaso, G.; Ferrari, A.; Volpe, S.; Orecchia, R.; Jereczek-Fossa, B.A. Modern radiotherapy for head and neck cancer. Semin. Oncol. 2019, 46, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; A Bhide, S.; Clark, C.; A Miles, E.; Miah, A.B.; Newbold, K.; Tanay, M.; et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, L.; Qiu, T.; Gao, H.; Wang, L.; Huang, S.; He, X.; Wu, L. Long-term follow-up of protective effects on salivary and swallowing structures and improvement of late xerostomia and dysphagia by level IIb optimisation in clinical target volume of nasopharyngeal carcinoma. BMC Cancer 2024, 24, 648. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.B.; Ly, D.; Dan, T.D.; Ondos, J.; Ning, H.; Belard, A.; O’Connell, J.; Miller, R.W.; Simone, N.L. Comparison of intensity-modulated radiotherapy, adaptive radiotherapy, proton radiotherapy, and adaptive proton radiotherapy for treatment of locally advanced head and neck cancer. Radiother. Oncol. 2011, 101, 376–382. [Google Scholar] [CrossRef]
- Oh, D. Proton Therapy for Head and Neck Cancer: Current Clinical Applications and Future Direction. Korean Soc. Head Neck Oncol. 2021, 37, 1–10. [Google Scholar] [CrossRef]
- Mohan, R. A review of proton therapy—Current status and future directions. Precis. Radiat. Oncol. 2022, 6, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Newhauser, W. International Commission on Radiation Units and Measurements Report 78: Prescribing, Recording and Reporting Proton-beam Therapy. Radiat. Prot. Dosim. 2009, 133, 60–62. [Google Scholar] [CrossRef]
- Safai, S.; Bortfeld, T.; Engelsman, M. Comparison between the lateral penumbra of a collimated double-scattered beam and uncollimated scanning beam in proton radiotherapy. Phys. Med. Biol. 2008, 53, 1729–1750. [Google Scholar] [CrossRef] [PubMed]
- Romesser, P.B.; Cahlon, O.; Scher, E.; Zhou, Y.; Berry, S.L.; Rybkin, A.; Sine, K.M.; Tang, S.; Sherman, E.J.; Wong, R.; et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensi-ty-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother. Oncol. 2016, 118, 286–292. [Google Scholar] [CrossRef]
| Early Scheme Until 2018 28 Patients (35.9%) | Later Scheme Since 2018 50 Patients (64.1%) | |||||
|---|---|---|---|---|---|---|
| Initial Plan | Adaptive Re-Plan | Total Dose (EQD2) | Initial Plan | Adaptive Re-Plan | Total Dose (EQD2) | |
| GTV | 2.2 Gy × 18 Fxs | 2.4 Gy × 12 Fxs | 68.4 Gy (70.0 Gy) | 2.4 Gy × 16 Fxs | 2.4 Gy × 12 Fxs | 67.2 Gy (69.4 Gy) |
| HR-CTV | 2.0 Gy × 18 Fxs | 2.0 Gy × 12 Fxs | 60.0 Gy (60.0 Gy) | 2.0 Gy × 16 Fxs | 2.0 Gy × 12 Fxs | 56.0 Gy (56.0 Gy) |
| LR-CTV | 2.0 Gy × 18 Fxs | -- | 36.0 Gy (36.0 Gy) | 2.0 Gy × 16 Fxs | -- | 32.0 Gy (32.0 Gy) |
| All Patients | IMRT Alone Group | IMRT/IMPT Combination Group | |||
|---|---|---|---|---|---|
| (n = 78) | (n = 39) | (n = 39) | p-Value | ||
| Age (years) | |||||
| Mean ± SD | 59.4 ± 10.1 | 58.4 ± 10.4 | 60.4 ± 9.9 | 0.202 | |
| Median (range, years) | 60 (39~89) | 60 (40~85) | 59 (39~89) | ||
| Gender | |||||
| Male | 67 (85.9%) | 35 (89.7%) | 32 (82.1%) | 0.329 | |
| Female | 11 (14.1%) | 4 (10.3%) | 7 (17.9%) | ||
| Smoking history | |||||
| >10 pack-years | 51 (65.4%) | 29 (74.4%) | 22 (56.4%) | 0.096 | |
| ≤10 pack-years | 27 (34.6%) | 10 (25.6%) | 17 (43.6%) | ||
| Primary tumor size (cm) | |||||
| Mean ± SD | 2.2 ± 0.9 | 2.2 ± 0.9 | 2.1 ± 0.9 | 0.300 | |
| Largest LN size (cm) | |||||
| Mean ± SD | 2.8 ± 1.2 | 2.9 ± 1.2 | 2.6 ± 1.2 | 0.244 | |
| HPV status | |||||
| Positive | 60 (76.9%) | 25 (64.1%) | 35 (89.7%) | 0.007 | |
| Negative | 3 (3.8%) | 1 (2.6%) | 2 (5.1%) | ||
| Unknown | 15 (19.2%) | 13 (33.3%) | 2 (5.1%) | ||
| AJCC 7th T stage | |||||
| cT1 | 36 (46.2%) | 18 (46.2%) | 18 (46.2%) | 1.000 | |
| cT2 | 42 (53.8%) | 21 (53.8%) | 21 (53.8%) | ||
| AJCC 7th N stage | |||||
| cN0 | 7 (9.0%) | 4 (10.3%) | 3 (7.7%) | 0.184 | |
| cN1 | 16 (20.5%) | 6 (15.4%) | 10 (25.6%) | ||
| cN2 | 52 (66.7%) | 29 (74.4%) | 23 (59.0%) | ||
| cN3 | 3 (3.8%) | 0 (0.0%) | 3 (7.7%) | ||
| AJCC 7th stage | |||||
| Stage I | 2 (2.6%) | 2 (5.1%) | 0 (0.0%) | 0.339 | |
| Stage II | 5 (6.4%) | 2 (5.1%) | 3 (7.7%) | ||
| Stage III | 16 (20.5%) | 6 (15.4%) | 10 (25.6%) | ||
| Stage IV | 55 (70.5%) | 29 (74.4%) | 26 (66.7%) | ||
| GTV (cc) | |||||
| Mean ± SD | 22.8 ± 24.6 | 20.9 ± 10.5 | 24.6 ± 33.3 | 0.511 | |
| Treatment | |||||
| CCRT | 67 (85.9%) | 35 (89.7%) | 32 (82.1%) | 0.329 | |
| RT alone | 11 (14.1%) | 4 (10.3%) | 7 (17.9%) | ||
| IMRT Alone | IMRT/IMPT Combination | p-Value | |
|---|---|---|---|
| Ipsilateral parotid gland | |||
| Mean dose (Gy) | 24.8 ± 7.4 | 30.3 ± 10.4 | 0.011 |
| V10Gy (%) | 81.3 ± 47.9 | 77.0 ± 14.2 | 0.614 |
| V20Gy (%) | 48.5 ± 14.6 | 59.7 ± 18.1 | 0.008 |
| V30Gy (%) | 34.9 ± 13.5 | 45.3 ± 21.2 | 0.024 |
| V40Gy (%) | 23.9 ± 11.8 | 31.8 ± 21.6 | 0.079 |
| V50Gy (%) | 15.6 ± 8.8 | 16.9 ± 10.9 | 0.600 |
| V60Gy (%) | 8.6 ± 5.3 | 10.4 ± 9.5 | 0.345 |
| Ipsilateral submandibular gland | |||
| Mean dose (Gy) | 56.3 ± 4.8 | 55.8 ± 5.5 | 0.689 |
| V10Gy (%) | 100.0 ± 0.0 | 98.4 ± 8.3 | 0.294 |
| V20Gy (%) | 99.6 ± 1.2 | 97.5 ± 8.4 | 0.178 |
| V30Gy (%) | 96.5 ± 5.1 | 94.5 ± 9.4 | 0.300 |
| V40Gy (%) | 85.5 ± 12.0 | 83.4 ± 16.1 | 0.559 |
| V50Gy (%) | 61.3 ± 15.8 | 54.9 ± 17.8 | 0.135 |
| V60Gy (%) | 49.6 ± 66.7 | 34.4 ± 10.9 | 0.183 |
| Contralateral parotid gland | |||
| Mean dose (Gy) | 8.2 ± 5.4 | 3.5 ± 0.9 | <0.001 |
| V10Gy (%) | 20.8 ± 22.8 | 8.1 ± 7.9 | 0.003 |
| V20Gy (%) | 2.1 ± 5.0 | 0.0 ± 0.1 | 0.015 |
| V30Gy (%) | 0 | 0 | NA |
| V40Gy (%) | 0 | 0 | NA |
| V50Gy (%) | 0 | 0 | NA |
| V60Gy (%) | 0 | 0 | NA |
| Contralateral submandibular gland | |||
| Mean dose (Gy) | 11.3 ± 5.2 | 6.7 ± 3.0 | <0.001 |
| V10Gy (%) | 51.8 ± 32.6 | 33.8 ± 13.3 | 0.004 |
| V20Gy (%) | 11.2 ± 16.0 | 4.5 ± 11.8 | 0.058 |
| V30Gy (%) | 0.7 ± 2.4 | 1.5 ± 5.4 | 0.466 |
| V40Gy (%) | 0.2 ± 1.3 | 0.3 ± 1.2 | 0.856 |
| V50Gy (%) | 0.1 ± 0.6 | 0.0 ± 0.1 | 0.335 |
| V60Gy (%) | 0.0 ± 0.2 | 0.0 ± 0.0 | 0.277 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.G.; Ahn, Y.C.; Oh, D.; Yang, K.; Ju, S.G.; Kim, J.M.; Kwon, D.; Choi, E.; Yoon, H.G. Salivary Gland Volume Changes and Dry Mouth Symptom Following Definitive Radiation Therapy in Oropharyngeal Cancer Patients—A Comparison of Two Different Approaches: Intensity-Modulated Radiation Therapy Versus Intensity-Modulated Radiation Therapy/Intensity-Modulated Proton Therapy Combination. Cancers 2025, 17, 554. https://doi.org/10.3390/cancers17030554
Park SG, Ahn YC, Oh D, Yang K, Ju SG, Kim JM, Kwon D, Choi E, Yoon HG. Salivary Gland Volume Changes and Dry Mouth Symptom Following Definitive Radiation Therapy in Oropharyngeal Cancer Patients—A Comparison of Two Different Approaches: Intensity-Modulated Radiation Therapy Versus Intensity-Modulated Radiation Therapy/Intensity-Modulated Proton Therapy Combination. Cancers. 2025; 17(3):554. https://doi.org/10.3390/cancers17030554
Chicago/Turabian StylePark, Seung Gyu, Yong Chan Ahn, Dongryul Oh, Kyungmi Yang, Sang Gyu Ju, Jin Man Kim, Dongyeol Kwon, Euncheol Choi, and Han Gyul Yoon. 2025. "Salivary Gland Volume Changes and Dry Mouth Symptom Following Definitive Radiation Therapy in Oropharyngeal Cancer Patients—A Comparison of Two Different Approaches: Intensity-Modulated Radiation Therapy Versus Intensity-Modulated Radiation Therapy/Intensity-Modulated Proton Therapy Combination" Cancers 17, no. 3: 554. https://doi.org/10.3390/cancers17030554
APA StylePark, S. G., Ahn, Y. C., Oh, D., Yang, K., Ju, S. G., Kim, J. M., Kwon, D., Choi, E., & Yoon, H. G. (2025). Salivary Gland Volume Changes and Dry Mouth Symptom Following Definitive Radiation Therapy in Oropharyngeal Cancer Patients—A Comparison of Two Different Approaches: Intensity-Modulated Radiation Therapy Versus Intensity-Modulated Radiation Therapy/Intensity-Modulated Proton Therapy Combination. Cancers, 17(3), 554. https://doi.org/10.3390/cancers17030554







