Optimizing Preclinical Models for Oral Cancer: The Influence of 4NQO Administration Routes on Tumor Development
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Overall Health of the Wistar Rats
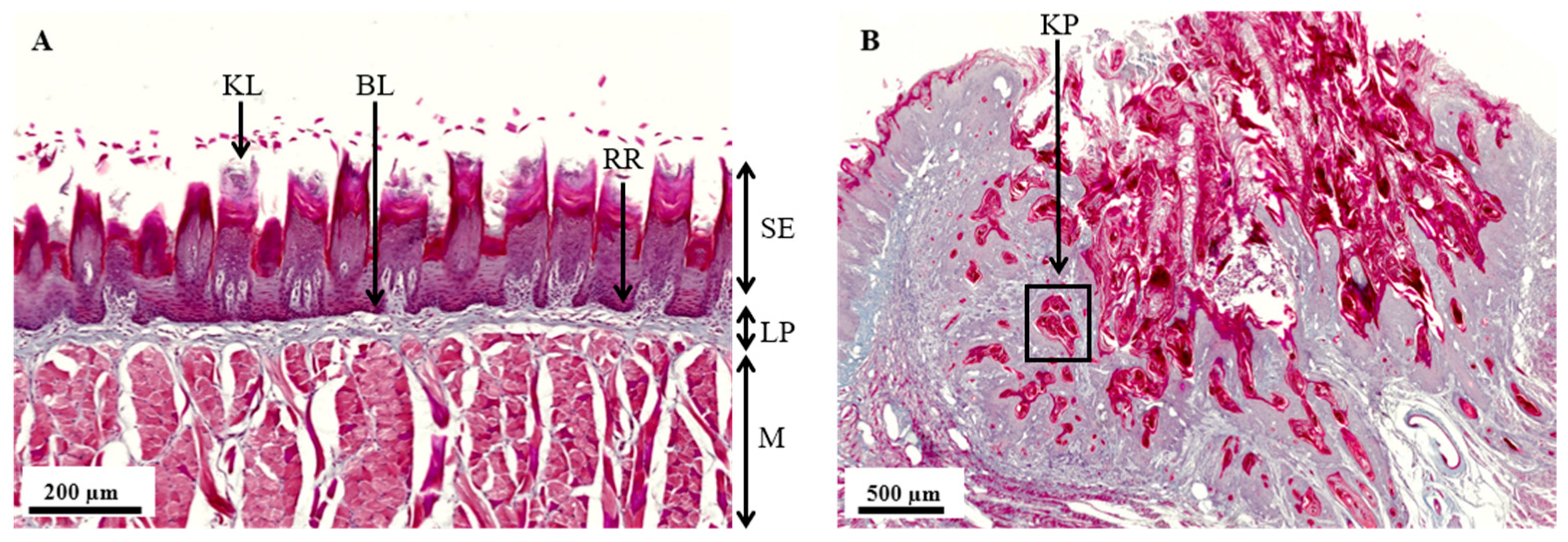
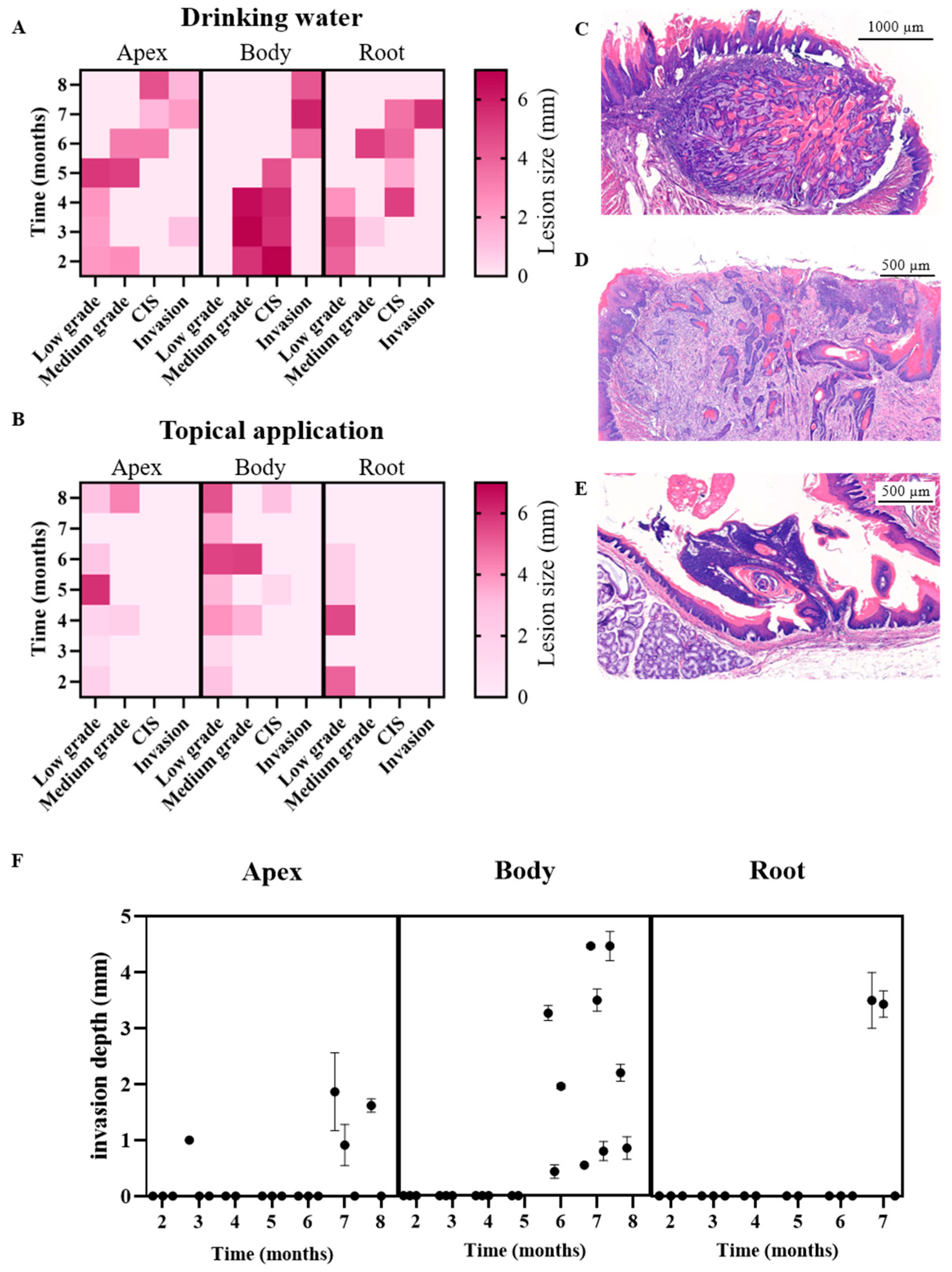
3.2. Histopathological Analysis of the 4NQO Rat Model
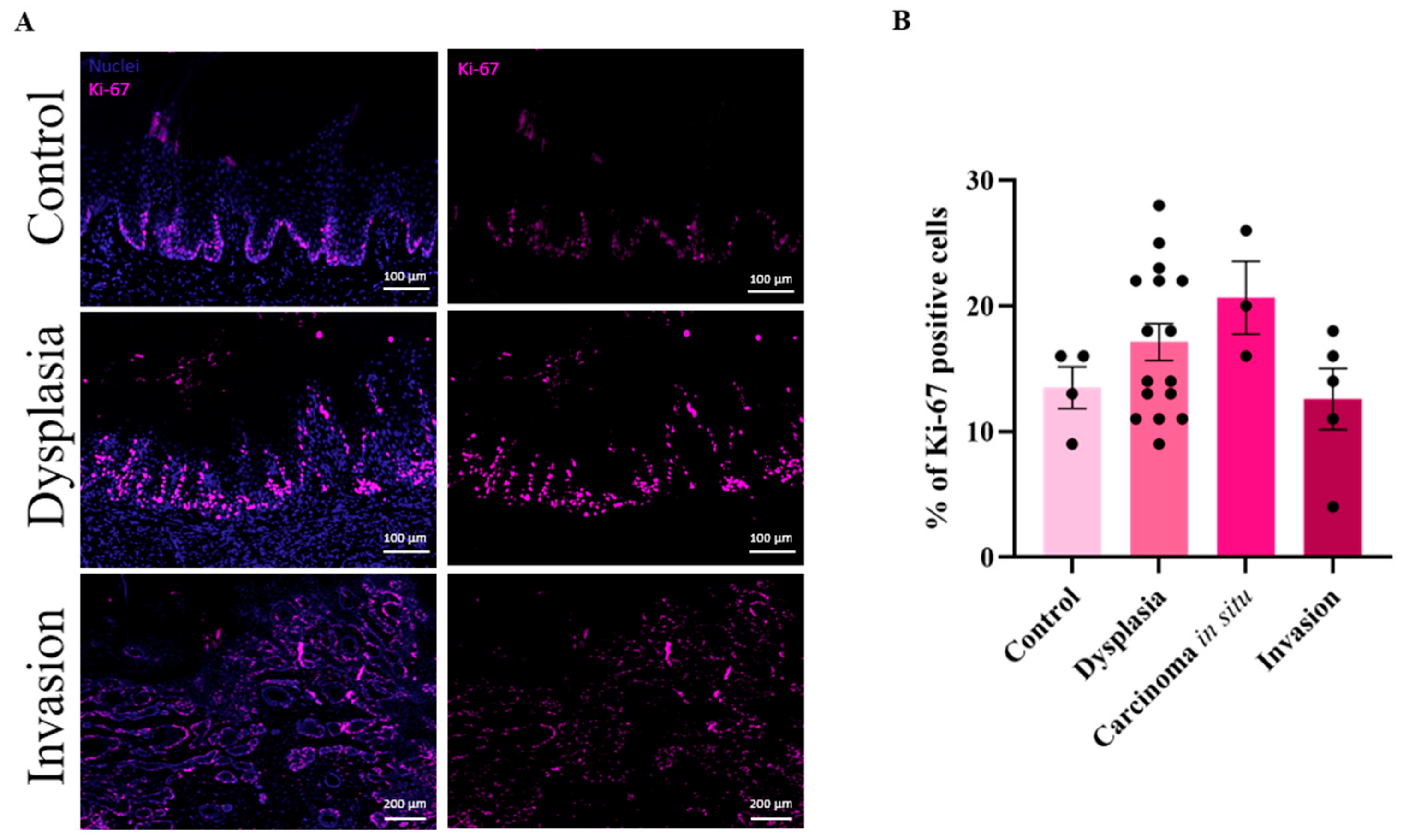
3.3. High Cell Proliferation in Dysplastic Lesions
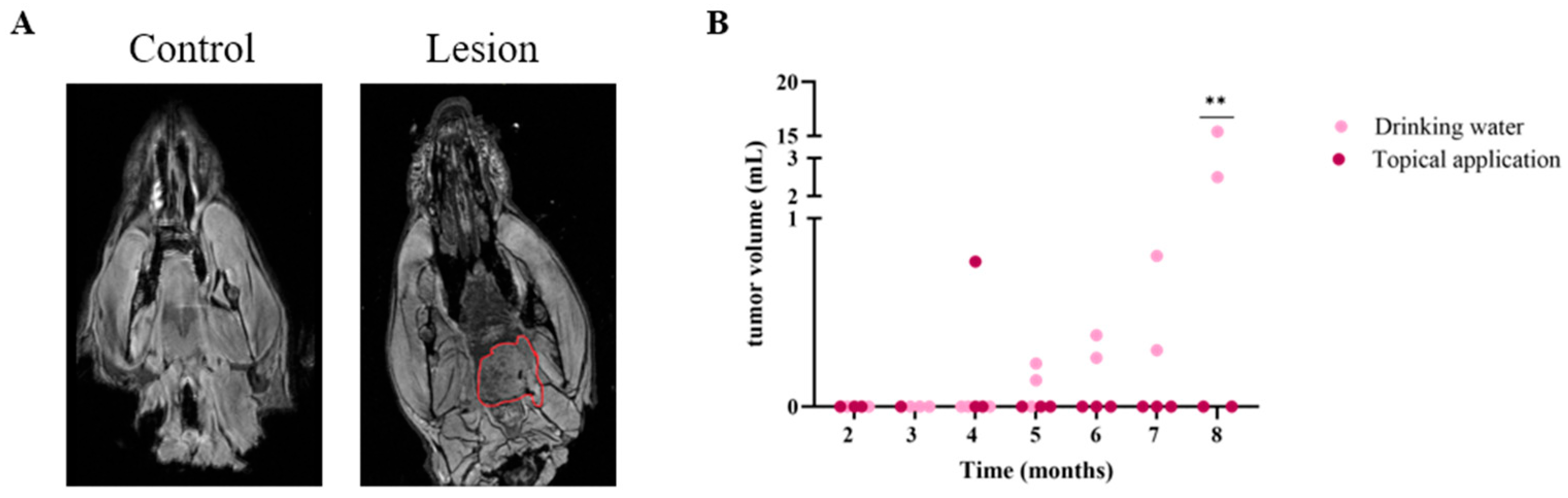
3.4. MRI Successfully Detects Oral Malignancies After 4NQO Treatment in Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OSCC | Oral squamous cell carcinoma |
| CIS | Carcinoma in situ |
| ISCC | Invasive squamous cell carcinoma |
| DMBA | 9,10 dimethyl-1,2,benzanthracene |
| 4NQO | 4-Nitroquinoline 1-oxide |
| MRI | Magnetic resonance imaging |
| H&E | Hematoxillin & eosin |
| SE | Squamous epithelium |
| LP | Lamina propria |
| M | Muscular layer |
| BL | Basal lamina |
| KPs | Keratin pearls |
| SD | Squamous dysplasia |
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Rastogi, V.; Puri, N.; Misra, S.; Arora, S.; Kaur, G.; Yadav, L. An Insight to Oral Epithelial Dysplasia. Int. J. Head Neck Surg. 2013, 4, 74–82. [Google Scholar] [CrossRef]
- Barnes, L.; Eveson, J.W.; Reichart, P.; Sidransky, D. Classification of Tumours: Pathology and Genetics of Tumours of the Head and Neck; World Health Organization, Ed.; IARC Press: Lyon, France, 2005. [Google Scholar]
- Zygogianni, A.G.; Kyrgias, G.; Karakitsos, P.; Psyrri, A.; Kouvaris, J.; Kelekis, N.; Kouloulias, V. Oral squamous cell cancer: Early detection and the role of alcohol and smoking. Head Neck Oncol. 2011, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Ramqvist, T.; Dalianis, T. An epidemic of oropharyngeal squamous cell carcinoma (OSCC) due to human papillomavirus (HPV) infection and aspects of treatment and prevention. Anticancer Res. 2011, 31, 1515–1519. [Google Scholar] [PubMed]
- Stahl, S.; Meskin, L.H.; Brown, L.J. The American Dental Association’s oral cancer campaign: The impact on consumers and dentists. J. Am. Dent. Assoc. 2004, 135, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Baumann, E.; Koller, M.; Wenz, H.-J.; Wiltfang, J.; Hertrampf, K. Oral cancer awareness campaign in Northern Germany: Successful steps to raise awareness for early detection. J. Cancer Res. Clin. Oncol. 2023, 149, 8779–8789. [Google Scholar] [CrossRef]
- Maybury, C.; Horowitz, A.M.; Goodman, H.S. Outcomes of oral cancer early detection and prevention statewide model in Maryland. J. Public Health Dent. 2012, 72, S34–S38. [Google Scholar] [CrossRef]
- Mauceri, R.; Bazzano, M.; Coppini, M.; Tozzo, P.; Panzarella, V.; Campisi, G. Diagnostic delay of oral squamous cell carcinoma and the fear of diagnosis: A scoping review. Front. Psychol. 2022, 13, 1009080. [Google Scholar] [CrossRef]
- Varela-Centelles, P.; Lopez-Cedrun, J.L.; Fernandez-Santroman, J.; Alvarez-Novoa, P.; Luaces-Rey, R.; Pombo-Castro, M.J.; Lopez-Jornet, M.P.; Seoane, J. Assessment of time intervals in the pathway to oral cancer diagnosis in north-westerm Spain. Relative contribution of patient interval. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e478–e483. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Xiong, L.; Wang, H.; Liu, C.; Wang, C.; Feng, Z. The impact of comorbidity on the diagnosis delay, treatment options and prognosis for advanced oral cancer: A retrospective result of the POROMS database. J. Cranio-Maxillofac. Surg. 2024, 52, 260–268. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, Z.; Xu, M.; Li, B.; Huang, Z.; Qin, S.; Nice, E.C.; Tang, J.; Huang, C. Oral squamous cell carcinomas: State of the field and emerging directions. Int. J. Oral Sci. 2023, 15, 44. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Nauta, J.M.; Roodenburg, J.L.; Nikkels, P.G.; Witjes, M.J.; Vermey, A. Comparison of epithelial dysplasia--the 4NQO rat palate model and human oral mucosa. Int. J. Oral Maxillofac. Surg. 1995, 24, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Khayatan, D.; Hussain, A.; Tebyaniyan, H. Exploring animal models in oral cancer research and clinical intervention: A critical review. Vet. Med. Sci. 2023, 9, 1833–1847. [Google Scholar] [CrossRef]
- Mognetti, B.; Di Carlo, F.; Berta, G.N. Animal models in oral cancer research. Oral Oncol. 2006, 42, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Tomita, H.; Nakashima, T.; Hirata, A.; Tanaka, T.; Shibata, T.; Hara, A. Current mouse models of oral squamous cell carcinoma: Genetic and chemically induced models. Oral Oncol. 2017, 73, 16–20. [Google Scholar] [CrossRef]
- Opitz, O.G.; Harada, H.; Suliman, Y.; Rhoades, B.; Sharpless, N.E.; Kent, R.; Kopelovich, L.; Nakagawa, H.; Rustgi, A.K. A mouse model of human oral-esophageal cancer. J. Clin. Investig. 2002, 110, 761–769. [Google Scholar] [CrossRef]
- Caulin, C.; Nguyen, T.; Longley, M.A.; Zhou, Z.; Wang, X.J.; Roop, D.R. Inducible activation of oncogenic K-ras results in tumor formation in the oral cavity. Cancer Res. 2004, 64, 5054–5058. [Google Scholar] [CrossRef]
- Frijhoff, A.F.; Conti, C.J.; Senderowicz, A.M. Advances in molecular carcinogenesis: Current and future use of mouse models to screen and validate molecularly targeted anticancer drugs. Mol. Carcinog. 2004, 39, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.L. Factors influencing experimental carcinogensis in the hamster cheek pouch. J. Dent. Res. 1961, 40, 3–15. [Google Scholar] [CrossRef]
- Salley, J.J. Experimental carcinogenesis in the cheek pouch of the Syrian hamster. J. Dent. Res. 1954, 33, 253–262. [Google Scholar] [CrossRef]
- MacDonald, D.G. Comparison of epithelial dysplasia in hamster cheek pouch carcinogenesis and human oral mucosa. J. Oral Pathol. 1981, 10, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Wallenius, K.; Lekholm, U. Oral cancer in rats induced by the water-soluble carcinogen 4-nitrochinoline N-oxide. Odontol. Revy 1973, 24, 39–48. [Google Scholar] [PubMed]
- Fujino, H.; Chino, T.; Imai, T. Experimental production of labial and lingual carcinoma by local application of 4-nitroquinoline N-oxide. J. Natl. Cancer Inst. 1965, 35, 907–918. [Google Scholar]
- Kanojia, D.; Vaidya, M.M. 4-nitroquinoline-1-oxide induced experimental oral carcinogenesis. Oral Oncol. 2006, 42, 655–667. [Google Scholar] [CrossRef]
- Nunoshiba, T.; Demple, B. Potent intracellular oxidative stress exerted by the carcinogen 4-nitroquinoline-N-oxide. Cancer Res. 1993, 53, 3250–3252. [Google Scholar]
- Sequeira, I.; Rashid, M.; Tomas, I.M.; Williams, M.J.; Graham, T.A.; Adams, D.J.; Vigilante, A.; Watt, F.M. Genomic landscape and clonal architecture of mouse oral squamous cell carcinomas dictate tumour ecology. Nat. Commun. 2020, 11, 5671. [Google Scholar] [CrossRef]
- Vered, M.; Yarom, N.; Dayan, D. 4NQO oral carcinogenesis: Animal models, molecular markers and future expectations. Oral Oncol. 2005, 41, 337–339. [Google Scholar] [CrossRef]
- Essat, M.; Cooper, K.; Bessey, A.; Clowes, M.; Chilcott, J.B.; Hunter, K.D. Diagnostic accuracy of conventional oral examination for detecting oral cavity cancer and potentially malignant disorders in patients with clinically evident oral lesions: Systematic review and meta-analysis. Head Neck 2022, 44, 998–1013. [Google Scholar] [CrossRef]
- Machiels, J.P.; Leemans, C.R.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Ng, G.T.E.; Phang, S.C.; Yu, K.S.; Tiwari, L.; Khurram, S.A.; Sloan, P.; Kujan, O. Understanding interobserver variability of pathologists to improve oral epithelial dysplasia grading. Oral Dis. 2025, 31, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Hahn, W.C.; Weinberg, R.A. Rules for making human tumor cells. N. Engl. J. Med. 2002, 347, 1593–1603. [Google Scholar] [CrossRef] [PubMed]
- Rivera Martínez, C.A. 4NQO Carcinogenesis: A Model of Oral Squamous Cell Carcinoma. Int. J. Morphol. 2012, 30, 309–314. [Google Scholar] [CrossRef]
- Zigmundo, G.C.O.; Schuch, L.F.; Schmidt, T.R.; Silveira, F.M.; Martins, M.A.T.; Carrard, V.C.; Martins, M.D.; Wagner, V.P. 4-nitroquinoline-1-oxide (4NQO) induced oral carcinogenesis: A systematic literature review. Pathol. Res. Pract. 2022, 236, 153970. [Google Scholar] [CrossRef]
- Tang, X.H.; Knudsen, B.; Bemis, D.; Tickoo, S.; Gudas, L.J. Oral cavity and esophageal carcinogenesis modeled in carcinogen-treated mice. Clin. Cancer Res. 2004, 10, 301–313. [Google Scholar] [CrossRef]
- Nauta, J.M.; Roodenburg, J.L.; Nikkels, P.G.; Witjes, M.J.; Vermey, A. Epithelial dysplasia and squamous cell carcinoma of the Wistar rat palatal mucosa: 4NQO model. Head Neck 1996, 18, 441–449. [Google Scholar] [CrossRef]
- Bakker Schut, T.C.; Witjes, M.J.; Sterenborg, H.J.; Speelman, O.C.; Roodenburg, J.L.; Marple, E.T.; Bruining, H.A.; Puppels, G.J. In vivo detection of dysplastic tissue by Raman spectroscopy. Anal. Chem. 2000, 72, 6010–6018. [Google Scholar] [CrossRef]
- Braams, J.W.; Witjes, M.J.; Nooren, C.A.; Nikkels, P.G.; Vaalburg, W.; Vermey, A.; Roodenburg, J.L. Detection of oral dysplasia in animals with fluorine-18-FDG and carbon-11-tyrosine. J. Nucl. Med. 1998, 39, 1476–1480. [Google Scholar]
- Harada, H.; Kikuchi, M.; Asato, R.; Hamaguchi, K.; Tamaki, H.; Mizuta, M.; Hori, R.; Kojima, T.; Honda, K.; Tsujimura, T.; et al. Characteristics of oral squamous cell carcinoma focusing on cases unaffected by smoking and drinking: A multicenter retrospective study. Head Neck 2023, 45, 1812–1822. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.A.; Salvadori, D.M. Gingival changes in wistar rats after oral treatment with 4-nitroquinoline 1-oxide. Eur. J. Dent. 2007, 1, 152–157. [Google Scholar] [CrossRef]
- Barcessat, A.R.; Huang, I.; Rabelo, G.D.; Rosin, F.C.; Ferreira, L.G.; de Cerqueira Luz, J.G.; Correa, L. Systemic toxic effects during early phases of topical 4-NQO-induced oral carcinogenesis in rats. J. Oral Pathol. Med. 2014, 43, 770–777. [Google Scholar] [CrossRef]
- Czerninski, R.; Amornphimoltham, P.; Patel, V.; Molinolo, A.A.; Gutkind, J.S. Targeting mammalian target of rapamycin by rapamycin prevents tumor progression in an oral-specific chemical carcinogenesis model. Cancer Prev. Res. 2009, 2, 27–36. [Google Scholar] [CrossRef] [PubMed]
- El-Rouby, D.H. Histological and immunohistochemical evaluation of the chemopreventive role of lycopene in tongue carcinogenesis induced by 4-nitroquinoline-1-oxide. Arch. Oral Biol. 2011, 56, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Vered, M.; Daniel, N.; Hirshberg, A.; Dayan, D. Histomorphologic and morphometric changes in minor salivary glands of the rat tongue during 4-nitroquinoline-1-oxide-induced carcinogenesis. Oral Oncol. 2003, 39, 491–496. [Google Scholar] [CrossRef]
- Vered, M.; Allon, I.; Buchner, A.; Dayan, D. Stromal myofibroblasts and malignant transformation in a 4NQO rat tongue carcinogenesis model. Oral Oncol. 2007, 43, 999–1006. [Google Scholar] [CrossRef]
- Dayan, D.; Hirshberg, A.; Kaplan, I.; Rotem, N.; Bodner, L. Experimental tongue cancer in desalivated rats. Oral Oncol. 1997, 33, 105–109. [Google Scholar] [CrossRef]
- Cecilio, H.P.; Valente, V.B.; Pereira, K.M.; Kayahara, G.M.; Furuse, C.; Biasoli, E.R.; Miyahara, G.I.; Oliveira, S.H.P.; Bernabe, D.G. Beta-adrenergic blocker inhibits oral carcinogenesis and reduces tumor invasion. Cancer Chemother. Pharmacol. 2020, 86, 681–686. [Google Scholar] [CrossRef]
- Ge, S.; Zhou, H.; Zhou, Z.; Liu, L.; Lou, J. Serum metabolite profiling of a 4-Nitroquinoline-1-oxide-induced experimental oral carcinogenesis model using gas chromatography-mass spectrometry. PeerJ 2021, 9, e10619. [Google Scholar] [CrossRef]
- Lim, W.; Choi, H.; Kim, J.; Kim, S.; Jeon, S.; Ni, K.; Song, S.Y.; Oh, H.K.; Im, Y.; Lee, G.; et al. Expression of cancer stem cell marker during 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. J. Mol. Histol. 2014, 45, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.R.; Thakur, S.; Peroumal, D.; Utkalaja, B.G.; Dutta, A.; Kumari, P.; Subhadarsini, I.; Acharya, N. 4-nitroquinoline 1-oxide induces immune cells death to onset early immunosuppression during oral squamous cell carcinoma development. Front. Immunol. 2023, 14, 1274519. [Google Scholar] [CrossRef] [PubMed]
- Pitstick, L.D.; Goral, J.; Schmelter, R.A.; Fuja, C.M.; Ciancio, M.J.; Pytynia, M.; Meyer, A.; Green, J.M. Fat and exposure to 4-nitroquinoline-1-oxide causes histologic and inflammatory changes in murine livers. PLoS ONE 2022, 17, e0268891. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L. Immunology and Immunotherapy of Head and Neck Cancer. J. Clin. Oncol. 2015, 33, 3293–3304. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Ho, H.-C.; Lee, M.-R.; Lai, K.-C.; Yeh, C.-M.; Lin, Y.-M.; Ho, T.-Y.; Hsiang, C.-Y.; Chung, J.-G. Early induction of cytokines/cytokine receptors and Cox2, and activation of NF-κB in 4-nitroquinoline 1-oxide-induced murine oral cancer model. Toxicol. Appl. Pharmacol. 2012, 262, 107–116. [Google Scholar] [CrossRef]
- Sato, J.; Yamazaki, Y.; Satoh, A.; Notani, K.; Kitagawa, Y. Pain is associated with an endophytic cancer growth pattern in patients with oral squamous cell carcinoma before treatment. Odontology 2010, 98, 60–64. [Google Scholar] [CrossRef]
- Moore, C.; Kuhns, J.G.; Greenberg, R.A. Thickness as prognostic aid in upper aerodigestive tract cancer. Arch. Surg. 1986, 121, 1410–1414. [Google Scholar] [CrossRef]
- Alves, A.M.; Correa, M.B.; Silva, K.D.D.; Araujo, L.M.A.; Vasconcelos, A.C.U.; Gomes, A.P.N.; Etges, A.; Tarquinio, S.B.C. Demographic and Clinical Profile of Oral Squamous Cell Carcinoma from a Service-Based Population. Braz. Dent. J. 2017, 28, 301–306. [Google Scholar] [CrossRef]
- Mishra, R. Oral tumor heterogeneity, its implications for patient monitoring and designing anti-cancer strategies. Pathol. Res. Pract. 2024, 253, 154953. [Google Scholar] [CrossRef]
- Mroz, E.A.; Patel, K.B.; Rocco, J.W. Intratumor heterogeneity could inform the use and type of postoperative adjuvant therapy in patients with head and neck squamous cell carcinoma. Cancer 2020, 126, 1895–1904. [Google Scholar] [CrossRef]
- Sarrion Perez, M.G.; Bagan, J.V.; Jimenez, Y.; Margaix, M.; Marzal, C. Utility of imaging techniques in the diagnosis of oral cancer. J. Craniomaxillofac. Surg. 2015, 43, 1880–1894. [Google Scholar] [CrossRef] [PubMed]
- Hoek, K.S.; Eichhoff, O.M.; Schlegel, N.C.; Dobbeling, U.; Kobert, N.; Schaerer, L.; Hemmi, S.; Dummer, R. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res. 2008, 68, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Ramalingam, K.; Yasothkumar, D.; Debnath, D.; Sundar, V. Ki-67 Expression as a Prognostic Marker: A Comparative Immunohistochemical Analysis of Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. Cureus 2023, 15, e38941. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Liu, Y.; Qiao, X.; Hua, R.X.; Wang, K.; Shan, X.F.; Cai, Z.G. What is the Prognostic Significance of Ki-67 Positivity in Oral Squamous Cell Carcinoma? J. Cancer 2016, 7, 758–767. [Google Scholar] [CrossRef]
- Shih Ie, M. Trophogram, an immunohistochemistry-based algorithmic approach, in the differential diagnosis of trophoblastic tumors and tumorlike lesions. Ann. Diagn. Pathol. 2007, 11, 228–234. [Google Scholar] [CrossRef]
- Bouaoud, J.; De Souza, G.; Darido, C.; Tortereau, A.; Elkabets, M.; Bertolus, C.; Saintigny, P. The 4-NQO mouse model: An update on a well-established in vivo model of oral carcinogenesis. Methods Cell Biol. 2021, 163, 197–229. [Google Scholar] [CrossRef]
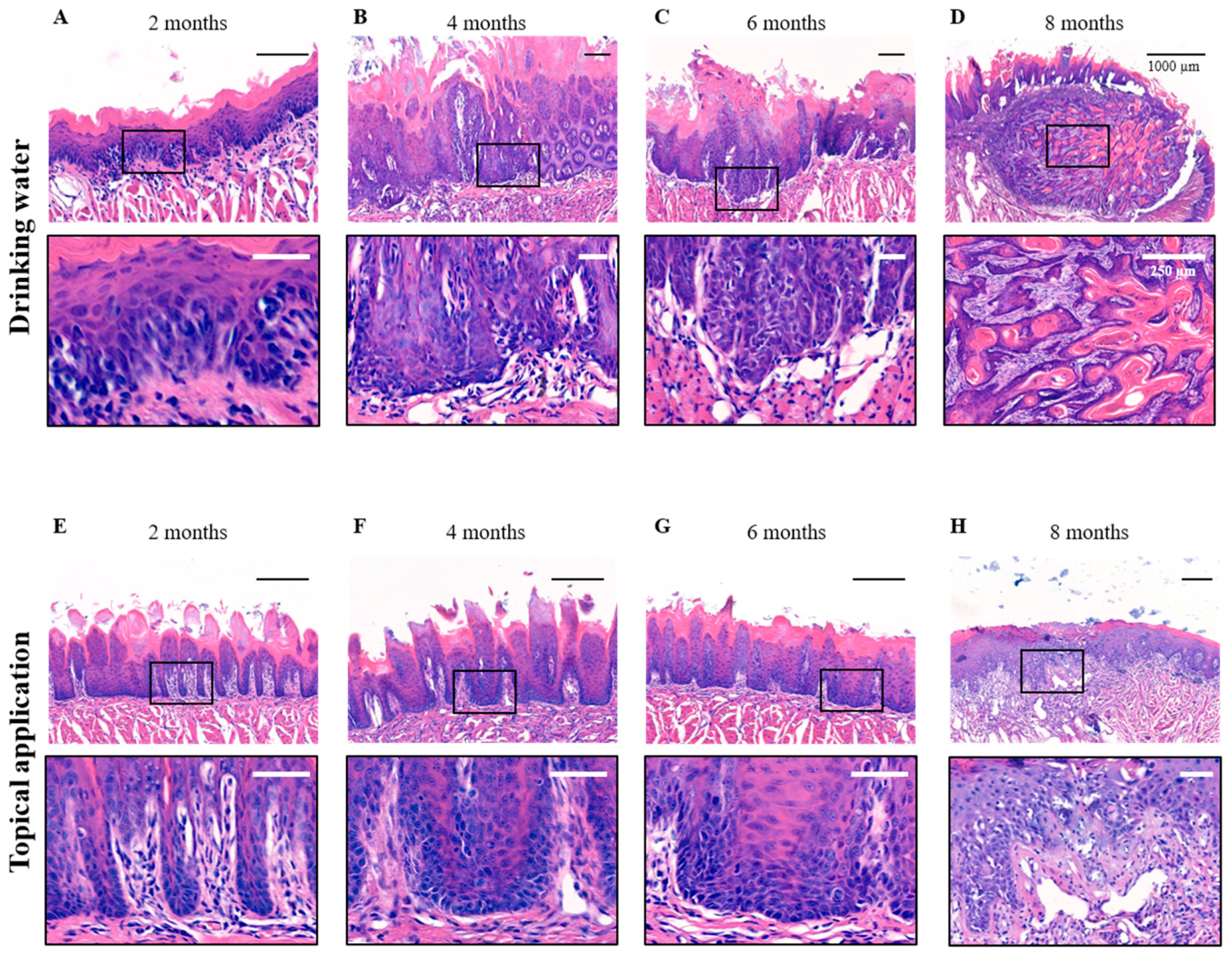
| A | 2 Months | 3 Months | 4 Months | 5 Months | 6 Months | 7 Months | 8 Months |
|---|---|---|---|---|---|---|---|
| Drinking water | Reactive | ||||||
| Low-grade dysplasia | |||||||
| Medium-grade dysplasia | |||||||
| Carcinoma in situ | |||||||
| Invasive carcinoma | |||||||
| Apex | Flat SD | Flat SD | Flat and papillary SD | Flat SD | Flat and papillary SD | Well- differentiated, exophytic and endophytic (pushing border) invasion | Well- differentiated, exophytic and endophytic (pushing border) invasion |
| Body | Papillary SD | Papillary SD | Papillary SD | Papillary SD | Well-differentiated, endophytic (pushing border and infiltrative) invasion | Well-differentiated, endophytic (pushing border and infiltrative) invasion | Well-differentiated, endophytic (pushing border and infiltrative) invasion |
| Root | Flat SD | Flat and papillary SD | Flat SD | Flat SD | Papillary and endophytic SD | Well-differentiated, endophytic (infiltrative) invasion | |
| B | 2 Months | 3 Months | 4 Months | 5 Months | 6 Months | 7 Months | 8 Months |
| Topical application | Reactive | ||||||
| Low-grade dysplasia | |||||||
| Medium-grade dysplasia | |||||||
| Carcinoma in situ | |||||||
| Apex | No hyperplasia | Flat SD | Flat SD | Papillary SD | Papillary SD | Flat SD | |
| Body | Flat SD | Flat SD | Papillary SD | Flat SD | Papillary SD | Flat SD | Papillary and endophytic SD |
| Root | Flat SD | Flat hyperplasia | Papillary SD | Flat SD | Flat SD | Flat SD | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van den Bosch, J.; Caz, N.; Martens, S.; Erens, C.; Rasking, L.; Gervois, P.; Nijsten, K.; Himmelreich, U.; Van Cauter, S.; Hillen, L.M.; et al. Optimizing Preclinical Models for Oral Cancer: The Influence of 4NQO Administration Routes on Tumor Development. Cancers 2025, 17, 2108. https://doi.org/10.3390/cancers17132108
Van den Bosch J, Caz N, Martens S, Erens C, Rasking L, Gervois P, Nijsten K, Himmelreich U, Van Cauter S, Hillen LM, et al. Optimizing Preclinical Models for Oral Cancer: The Influence of 4NQO Administration Routes on Tumor Development. Cancers. 2025; 17(13):2108. https://doi.org/10.3390/cancers17132108
Chicago/Turabian StyleVan den Bosch, Jolien, Nuran Caz, Sandrina Martens, Céline Erens, Leen Rasking, Pascal Gervois, Kim Nijsten, Uwe Himmelreich, Sofie Van Cauter, Lisa M. Hillen, and et al. 2025. "Optimizing Preclinical Models for Oral Cancer: The Influence of 4NQO Administration Routes on Tumor Development" Cancers 17, no. 13: 2108. https://doi.org/10.3390/cancers17132108
APA StyleVan den Bosch, J., Caz, N., Martens, S., Erens, C., Rasking, L., Gervois, P., Nijsten, K., Himmelreich, U., Van Cauter, S., Hillen, L. M., Plasschaert, H., Lambrichts, I., & Wolfs, E. (2025). Optimizing Preclinical Models for Oral Cancer: The Influence of 4NQO Administration Routes on Tumor Development. Cancers, 17(13), 2108. https://doi.org/10.3390/cancers17132108







