SARS-CoV2 and Anti-COVID-19 mRNA Vaccines: Is There a Plausible Mechanistic Link with Cancer?
Simple Summary
Abstract
1. Introduction
2. The Virus, the Cancer, and the mRNA Vaccine: The Ugly, the Bad, and the Good?
2.1. The Cancer
2.2. The Virus
2.3. The Vaccine
3. The Complex Interplay Between COVID-19, Anti-COVID-19 mRNA Pro-Vaccine, and Cancer
4. Can SARS-CoV2 and/or Anti-COVID-19 mRNA Pro-Vaccine Cause Cancer? Putting the Puzzle Pieces Together
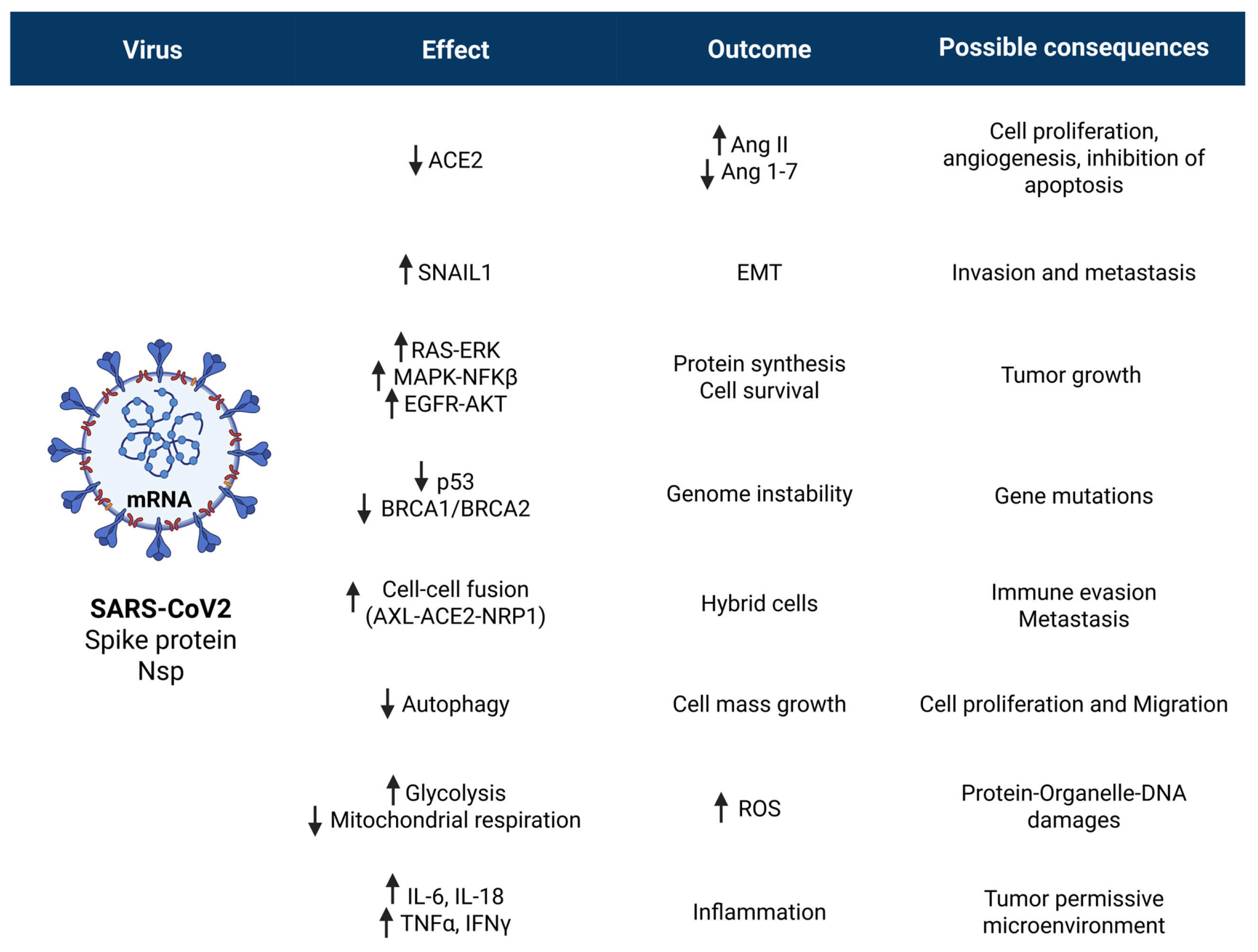
5. The SARS-CoV2 Virus and the Cancer
5.1. Oncogenic Potential of SARS-CoV2 Receptors ACE2 and AXL
5.2. SARS-CoV2 Spike Protein Can Trigger Oncogenic Signaling Pathways
5.3. SARS-CoV2 Spike Protein Can Inactivate Tumor Suppressor Signaling Pathways
5.4. Spike Protein Induces Cell–Cell Fusion: A Step Toward Cancer Transformation?
5.5. SARS-CoV2 Replication Dysregulates Autophagy: A Step Toward Carcinogenesis?
5.6. SARS-CoV2 Alters Mitochondrial Respiration and Induces Oxidative Stress
5.7. SARS-CoV2 Triggers the Inflammatory Cytokine Storm and Induces Immune Cell Depletion Leading to a Microenvironment Permissive for Relapses and Metastasis
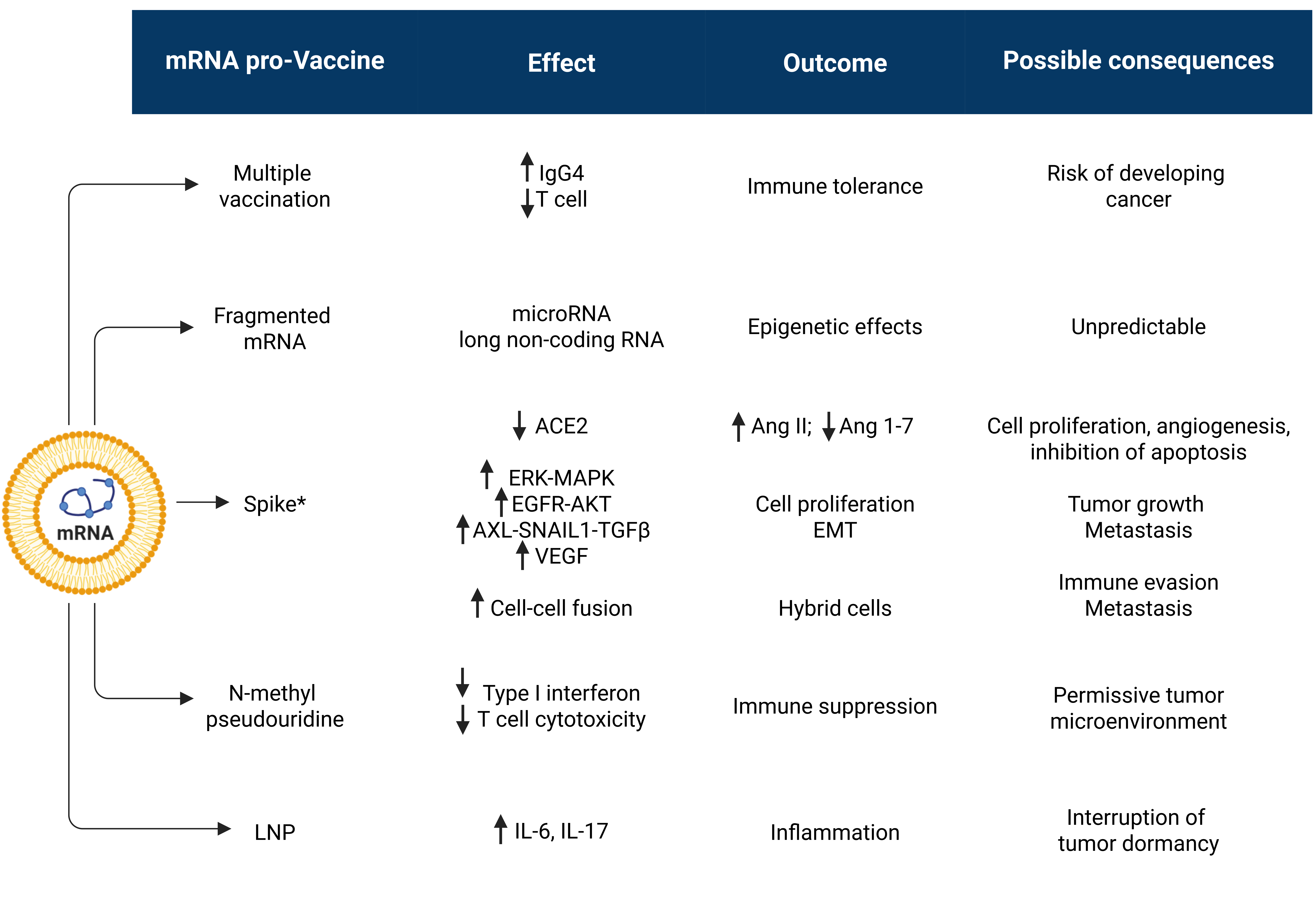
6. The Anti-COVID-19 mRNA Pro-Vaccine and the Cancer
6.1. The Vaccinal Spike Displays Pro-Carcinogenic Properties Like Viral Spike
6.2. Molecular, Biochemical, Genetic, and Epigenetic Effects of the mRNA Pro-Vaccine: Hypothesizing the Unpredictable
6.3. Disruption of the Immune Surveillance and Induction of Inflammation: Creating the Condition for Awakening the Dormant Tumor
7. Data from the Real World: Case Reports Linking Anti-COVID-19 mRNA Vaccination and Cancer
| Disease (Onset) | Clinical Features | Histological–Biological Features | Type of Vaccine | Ref. |
|---|---|---|---|---|
| Angio-immunoblastic T-lymphoma (onset 6 months after 2nd dose) | 66-year-old man presented with lymphadenopathies; increased number, size, and metabolic activity (of lymph nodes 8 days after 3rd dose) | Gene mutations: RHO, TET2, DNMT3A, IDH2 | BNT162b2 (March, April, September 2021) | [215] |
| Recurrence of axillary lymphoproliferative disorder (2 days after 1st dose) | 79-year-old man in remission from a primary cutaneous anaplastic large-cell lymphoma cured two years before; presented with ulcerated tumor with surrounding erythema | CD-30-positive lymphoproliferative disorder; TCR gene rearrangement matching the previous 2019 clone | BNT162b2 | [216] |
| Nodal Marginal zone B-cell lymphoma (sudden appearance of temporal mass the day after 1st dose) | 80-year-old woman presented with multiple (n. 12) lymphadenopathies at week 6 from 1st dose (week 3 from 2nd dose); increased number (>22) and size (2.5×) in ten weeks | Lymphoid cells positive for CD20, CD79a, and BCL-2; negative for CD3, BCL6 | BNT162b2 (2 doses, 3 weeks apart) | [217] |
| Diffuse large B-cell non-Hodgkin lymphoma (cervical mass appearance one week after 2nd dose) | 58-year-old woman presented with tumor mass at the angle of the left parotid gland progressively growing from June to September with multiple reactive lymph nodes, and finally operated in October 2021 | Confirmed DLBC NHL positive for CD20, PAX5 and negative for CD30, AE1/AE3; 85% Ki-67 positivity | BNT162b2 22 May; 12 June 2021 | [218] |
| Extranodal malignant non-HodgkinT/NK-cell lymphoma (ulcerative lesions appeared 3 days after 1st dose) | 53-year-old man presented (December 2021) with multiple ulcerative oral lesions appeared shortly after the 1st dose which worsened after the 2nd dose | Tumor proliferation with T cells positive for CD3 and CD7, granzyme B, CD30; negative for CD4, CD8, and CD20 | BNT162b2 6 November; 28 November 2021 | [218] |
| (A) Acute lymphoblastic leukemia (two days after 1st dose of mRNA vaccine); (B) recurrence of B-Acute lymphoblastic leukemia (after 1st dose of mRNA vaccine); (C) recurrence of acute myeloid leukemia (after the booster with BNT162b2) | (A) 49-year-old woman presenting with petechiae and bicytopenia, diagnosed with B-ALL; (B) 47-year-old woman; two years before diagnosed with B-cell lymphoma in remission in the last 14 months; (C) 67-year-old woman; diagnosed with AML in 2007 and in remission in the last 14 years after bone marrow transplant. She had two doses of inactivated SARS-CoV2 vaccine in July 2021 and mRNA BNT162b2 in September 2021 | (A) B-ALL: bone marrow showed 20–30% stained with CD19 diffuse positive TdT in blastic cells; (B) bicytopenia and blasts; (C) 90% blasts | BNT162b2 | [219] |
| Four cases of acute myeloid leukemias, one of which extramedullary | (A) 61-year-old man; 30 days after 3rd mRNA dose; (B) 28-year-old woman; 2 weeks after 2nd dose; (C) 72-year-old man; 5 weeks after the 5th dose; (D) 60-year-old man; 1 month after the 4th dose | (A) 80% blastic infiltration; (B) bicytopenia; blastic infiltration; (C) pancytopenia; 70% blastic infiltration; (D) occipital granulocytic sarcoma of CD34, CD123, and MPO positive immature cells; 30% myeloid blasts | BNT162b2 | [220] |
| Diffuse large B-cell lymphoma (lymphadenopathy was observed one day after the 1st dose) | 67-year-old man presented with 6 cm subcutaneous lymphadenopathies mass in the left axilla 2 weeks after the 2nd BNT162b2 vaccination | Large, atypical lymphocytes were positive for CD20, BCL2 and MUM-1/IRF4; negative for CD3; over 80% Ki-67 positivity | BNT162b2 (2 doses) | [221] |
| Diffuse large B-cell lymphoma (lymphadenopathy was observed two days after the 1st dose) | 80-year-old woman presented with enlarged 4.1 cm axillary nodule that developed 1 day after the 2nd dose; two months later the nodule increased to 6 cm and additional lesions appeared in the mesentery and the left cavernous sinus | Germinal center B-cell DLBC lymphoma positive for CD20, BCL6, BCL2; negative for CD3 and MUM-1/IRF4; over 90% Ki-67 positivity | BNT162b2 (2 doses) | [221] |
| Primary cutaneous anaplastic large cell lymphoma (10 days after the 3rd dose) | 76-year-old man presented a fast-growing lesion at the site of the injection 10 days after the 3rd dose. A large erythematous tumor of 6 cm diameter was diagnosed 1 month later. Spontaneous regression after 6 weeks | Anaplastic large cell lymphoma T1bN0M0; positive for CD30, CD4, CD2, CD5, MUM1, and negative for CD20, CD8, TIA1, ALK, EMA, CD56, CD123 and CD68 | BNT162b2 (1st and 2nd dose) Moderna mRNA-1273 (3rd dose) | [222] |
| High-grade sarcoma | 73-year-old woman; history of angiomyolipoma in 2019; presented with swelling 2–4 days after 2nd dose developed in 6 cm diameter soft mass in the right upper arm | Grade 3, stage IIIA undifferentiated, pleomorphic high-grade sarcoma | Moderna mRNA-1273 (2 doses) | [223] |
| Primary cutaneous lymphoproliferative disorders | Series of 14 cases, of which 6 classified as relapse and 8 as primary lesions; complete and partial remission within the 19 months follow-up | N.A. | BNT162b2 | [224] |
| Non-Hodgkin lymphoma (few weeks after the 3rd dose) | 66-year-old man presented with right axillary lymphadenopathy developed 10 days after the 3rd dose, which grew up to 7 cm in the following 3 months | Stage-II anaplastic large-cell lymphoma, ALK negative and CD30 positive, over 90% Ki-67 positivity | BNT162b2 (January, February, October 2021) | [225] |
| Conjunctival classic Kaposi sarcoma (few weeks after vaccine booster) | 75-year-old woman with complex ophthalmologic history that includes, among others, uveitic glaucoma OU, epiretinal membrane OU, and cystoid macular degeneration OS, presented with irritated conjunctival area | Conjunctival epithelium shows early squamous metaplasia and positive immunostaining with HHV8 within the CD34 positive vascular proliferation | BNT162b2 (three doses) | [226] |
| Basaloid carcinoma, wrongly cured as Bell’s palsy for almost 8 months (symptoms appeared 4 days after 1st dose) | 56-year-old man; no previous health problems; presented with a massive and aggressively infiltrating basaloid-featured cancer in the right side of his face that rapidly progressed and led the patient to death. CT scan (11 months after vaccination) revealed the presence of infiltrating tumor masses in the parotid gland, likely of cutaneous origin | D-dimer value was 1523 ng/mL (normal range is <500 ng/mL). Biopsy confirmed the diagnosis of basal cell carcinoma | BNT162b2 (one dose) | [227] |
| Philadelphia-positive B-cell acute lymphoblastic leukemia (five days after the booster vaccination with bi-valent mRNA vaccine) | 43-year-old woman; insignificant previous medical history; presented with splenomegaly, severe anemia and thrombocytopenia along with leukocytosis (1.0% neutrophil, 9.0% lymphocyte, 0% monocyte, eosinophil and basophil, and 90.0% blast) | Bone marrow shows 68% blastic infiltration; cells were positive for CD34 and TdT, negative for CD117 and MPO. The p190 BCR-ABL1 gene rearrangement was identified by RT-PCR | Five vaccinations as follows: two doses of Oxford/AstraZeneca (4 June and 31 August 2021); half-dose of Moderna mRNA-1273 (15 January 2022), NovaVax (15 July 2022), and booster dose of the bivalent (Omicron BA.4/BA.5–containing) mRNA-1273 COVID-19 vaccine (January 2023) plus SARS-CoV-2 infection on 19 August 2021 | [228] |
| Epstein–Barr virus-positive marginal zone lymphoma (EBV + MZL) at autopsy (17 days after 1st vaccination) | 71-year-old woman with history of methotrexate-treated rheumatoid arthritis; died due thrombosis and multi-organ failure 17 days after vaccination. The autopsy revealed systemic lymphadenopathy comprising atypical lymphocytes and scattered Hodgkin/Reed–Sternberg (H/RS)-like cells | Atypical lymphocytes were positive for CD79a, CD19, EBV-encoded small RNA and MUM-1 and negative for CD3, CD5, CD10, BCL6. H/RS-like cells were positive for CD3 | Unspecified the type of anti-COVID-19 vaccine | [229] |
| Intravascular large B-cell lymphoma at autopsy (105 days after the second dose) | 61-year-old woman affected by systemic lupus erythematosus recovered 1 month after vaccination for joint pain, clonic spasms, left-sided paralysis, and fever | Diagnosis of hemophagocytic lymphohistiocytosis with intra- and perivascular infiltration of CD20-positive atypical B lymphocytes in spleen, liver, and lungs | Pfizer BNT162b2 mRNA vaccine (2 doses one month apart) | [230] |
| Longitudinal melanonychia that progressed into subungual melanoma | 53-year-old woman affected by longitudinal melanonychia with no known risk factors for melanoma development | Malignant transformation into acral lentiginous melanoma within 2 years from vaccination | Pfizer BNT162b2 mRNA vaccine (3 doses) | [231] |
| Breast cancer skin metastasis that manifested 1 month after the 6th dose of mRNA vaccination | 85-year-old woman affected by breast cancer that was successfully removed by partial mastectomy with clear margins 2 years before | Metastatic cancer cells in the dermis and epidermis showed pagetoid atypical cells with ample cytoplasm features and were positive for spike protein, but not for nucleocapsid protein of SARS-CoV-2 | Pfizer-BioNtech BNT162b2 (six doses in 2 years) | [232] |
| Disease | Clinical Features | Type of Vaccine | Ref. |
|---|---|---|---|
| Pheochromocytoma | 63-year-old man; pheochromocytoma (very rare benign tumor) of 7 cm developed few days after the vaccination | Johnson and Johnson COVID-19 vaccine | [233] |
| Recurrence of cutaneous T-cell lymphoma | T-cell lymphoma has been reported in two patients, who were in remission since many years, after the 2nd | Vaxzevria (Oxford/AstraZeneca) | [234] |
| EBV-positive, diffuse large B-cell lymphoma | 51-year-old man; rapidly growing diffuse large B-cell lymphoma was reported in a heart post-transplanted (under immunosuppressant therapy since many years) 7 days after receiving the 1st dose | ChAdOx1 nCoV-19 vaccine | [235] |
| Primary cutaneous T-cell lymphoma | 28-year-old woman; primary cutaneous T-cell lymphoma (CD31, CD71, CD81 positive) mimicking a panniculitis has been reported in a few days after 1st vaccination | COVID-19 Janssen vaccine | [236] |
| Chronic myelomonocytic leukemia | 74-year-old woman; chronic myelomonocytic leukemia and scleroderma were diagnosed, with first signs manifesting two days after receiving the 1st dose, which then progressed to acute myeloid leukemia, severe anemia, and thrombocytopenia, and eventually died due to COVID-19-associated respiratory failure | Johnson and Johnson COVID-19 vaccine | [237] |
| Classic Kaposi sarcoma manifested 7 days after the 3rd dose of ChAdOx1 vaccine | 73-year-old man with a skin nodule of 2 × 3 × 1 cm HIV negative, positive for CD34 and HHV-8 | ChAdOx1 nCoV-19 vaccine | [238] |
8. Discussion and Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gremke, N.; Griewing, S.; Bausch, E.; Alymova, S.; Wagner, U.; Kostev, K.; Kalder, M. Therapy delay due to COVID-19 pandemic among European women with breast cancer: Prevalence and associated factors. J. Cancer Res. Clin. Oncol. 2023, 149, 11749–11757. [Google Scholar] [CrossRef] [PubMed]
- Roderburg, C.; Loosen, S.H.; Leyh, C.; Joerdens, M.S.; Mohr, R.; Luedde, T.; Alymova, S.; Klein, I.; Kostev, K. Prevalence of and factors associated with a treatment delay due to the COVID-19 pandemic in patients with gastrointestinal cancer in Europe. J. Cancer Res. Clin. Oncol. 2023, 149, 11849–11856. [Google Scholar] [CrossRef] [PubMed]
- Burus, T.; Lei, F.; Huang, B.; Christian, W.J.; Hull, P.C.; Ellis, A.R.; Slavova, S.; Tucker, T.C.; Kuhs, K.A.L. Undiagnosed Cancer Cases in the US During the First 10 Months of the COVID-19 Pandemic. JAMA Oncol. 2024, 10, 500–507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Castelo-Branco, L.; Lee, R.; Brandão, M.; Cortellini, A.; Freitas, A.; Garassino, M.; Geukens, T.; Grivas, P.; Halabi, S.; Oliveira, J.; et al. Learning lessons from the COVID-19 pandemic for real-world evidence research in oncology—Shared perspectives from international consortia. ESMO Open 2023, 8, 101596. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinato, D.J.; Scotti, L.; Gennari, A.; Colomba-Blameble, E.; Dolly, S.; Loizidou, A.; Chester, J.; Mukherjee, U.; Zambelli, A.; Aguilar-Company, J.; et al. Determinants of enhanced vulnerability to coronavirus disease 2019 in UK patients with cancer: A European study. Eur. J. Cancer 2021, 150, 190–202. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Meslé, M.M.; Brown, J.; Mook, P.; A Katz, M.; Pastore, R.; Benka, B.; Redlberger-Fritz, M.; Bossuyt, N.; Stouten, V.; Vernemmen, C.; et al. Estimated number of lives directly saved by COVID-19 vaccination programmes in the WHO European Region from December, 2020, to March, 2023: A retrospective surveillance study. Lancet Respir. Med. 2024, 12, 714–727, Correction in Lancet Respir Med. 2025, 13, e55. https://doi.org/10.1016/S2213-2600(25)00235-8. [Google Scholar] [CrossRef] [PubMed]
- Sarker, R.; Roknuzzaman, A.S.M.; Nazmunnahar; Shahriar, M.; Hossain, J.; Islam, R. The WHO has declared the end of pandemic phase of COVID-19: Way to come back in the normal life. Health Sci. Rep. 2023, 6, e1544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ugai, T.; Sasamoto, N.; Lee, H.-Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E.; et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49, Erratum in CA Cancer J. Clin. 2024, 74, 203. https://doi.org/10.3322/caac.21830. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; O’cOnnell, D.L.; Yu, X.Q.; Kahn, C.; Caruana, M.; Pesola, F.; Sasieni, P.; Grogan, P.B.; Aranda, S.; Cabasag, C.J.; et al. Cancer incidence and mortality in Australia from 2020 to 2044 and an exploratory analysis of the potential effect of treatment delays during the COVID-19 pandemic: A statistical modelling study. Lancet Public Health 2022, 7, e537–e548, Erratum in Lancet Public Health 2022, 7, e895. https://doi.org/10.1016/S2468-2667(22)00260-2. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenberg, P.S.; Miranda-Filho, A. Cancer Incidence Trends in Successive Social Generations in the US. JAMA Netw. Open 2024, 7, e2415731. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seegers, V.; Rousseau, G.; Zhou, K.; Blanc-Lapierre, A.; Bigot, F.; Mahammedi, H.; Lambert, A.; Moreau-Bachelard, C.; Campone, M.; Conroy, T.; et al. COVID-19 Infection despite Previous Vaccination in Cancer Patients and Healthcare Workers: Results from a French Prospective Multicenter Cohort (PAPESCO-19). Cancers 2023, 15, 4777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schmidt, A.; Labaki, C.; Hsu, C.-Y.; Bakouny, Z.; Balanchivadze, N.; Berg, S.; Blau, S.; Daher, A.; El Zarif, T.; Friese, C.; et al. COVID-19 vaccination and breakthrough infections in patients with cancer. Ann. Oncol. 2022, 33, 340–346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anand, S.T.; Vo, A.D.; La, J.; Do, N.V.; Fillmore, N.R.; Brophy, M.; Branch-Elliman, W.; Monach, P.A. Severe COVID-19 in Vaccinated Adults With Hematologic Cancers in the Veterans Health Administration. JAMA Netw. Open 2024, 7, e240288. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bellavite, P.; Donzelli, A.; Isidoro, C. The WHO Algorithm for Causality Assessment of Adverse Effects Following Immunization with Genetic-Based Anti-COVID-19 Vaccines: Pitfalls and Suggestions for Improvement. J. Clin. Med. 2024, 13, 7291. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ayuso, J.M.; Garrido, I.O. The Importance of the Tumor Microenvironment to Understand Tumor Origin, Evolution, and Treatment Response. Cancers 2022, 14, 1983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garavaglia, B.; Vallino, L.; Ferraresi, A.; Dhanasekaran, D.N.; Isidoro, C. Chapter 12—Tumor evolution during chemotherapy. In Peritoneal Tumor Microenvironment of Cancers on Cancer Hallmarks; Song, Y.S., Dhanasekaran, D.N., Tsang, B.K., Inazawa, J., Mirshahi, M., Pocard, M., Isidoro, C., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 285–305. ISBN 9780128240403. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Sinkala, M. Mutational landscape of cancer-driver genes across human cancers. Sci. Rep. 2023, 13, 12742. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Recillas-Targa, F. Cancer Epigenetics: An Overview. Arch. Med Res. 2022, 53, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Nohmi, T. Thresholds of Genotoxic and Non-Genotoxic Carcinogens. Toxicol. Res. 2018, 34, 281–290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Imyanitov, E.N.; Kuligina, E.S.; Sokolenko, A.P.; Suspitsin, E.N.; Yanus, G.A.; Iyevleva, A.G.; Ivantsov, A.O.; Aleksakhina, S.N. Hereditary cancer syndromes. World J. Clin. Oncol. 2023, 14, 40–68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stephens, P.J.; Greenman, C.D.; Fu, B.; Yang, F.; Bignell, G.R.; Mudie, L.J.; Pleasance, E.D.; Lau, K.W.; Beare, D.; Stebbings, L.A.; et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell 2011, 144, 27–40. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adashek, J.J.; Kato, S.; Lippman, S.M.; Kurzrock, R. The paradox of cancer genes in non-malignant conditions: Implications for precision medicine. Genome Med. 2020, 12, 16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fiala, C.; Diamandis, E.P. Mutations in normal tissues—Some diagnostic and clinical implications. BMC Med. 2020, 18, 283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baker, S.G. A Cancer Theory kerfuffle can lead to new lines of research. JNCI J. Natl. Cancer Inst. 2014, 107, dju405. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nazerian, Y.; Ghasemi, M.; Yassaghi, Y.; Nazerian, A.; Hashemi, S.M. Role of SARS-CoV-2-induced cytokine storm in multi-organ failure: Molecular pathways and potential therapeutic options. Int. Immunopharmacol. 2022, 113 Pt B, 109428. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, M.; Pushpakumar, S.; Zheng, Y.; Smolenkova, I.; Akinterinwa, O.E.; Luulay, B.; Tyagi, S.C. Novel mechanism of the COVID-19 associated coagulopathy (CAC) and vascular thromboembolism. NPJ Viruses 2023, 1, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brant, A.C.; Tian, W.; Majerciak, V.; Yang, W.; Zheng, Z.-M. SARS-CoV-2: From its discovery to genome structure, transcription, and replication. Cell Biosci. 2021, 11, 136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghosh, S.; Dellibovi-Ragheb, T.A.; Kerviel, A.; Pak, E.; Qiu, Q.; Fisher, M.; Takvorian, P.M.; Bleck, C.; Hsu, V.W.; Fehr, A.R.; et al. β-Coronaviruses Use Lysosomes for Egress Instead of the Biosynthetic Secretory Pathway. Cell 2020, 183, 1520–1535.e14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vidoni, C.; Fuzimoto, A.; Ferraresi, A.; Isidoro, C. Targeting autophagy with natural products to prevent SARS-CoV-2 infection. J. Tradit. Complement. Med. 2021, 12, 55–68. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, Y.; Bowman, J.W.; Jung, J.U. Autophagy during viral infection—A double-edged sword. Nat. Rev. Microbiol. 2018, 16, 341–354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Sun, H.; Pei, R.; Mao, B.; Zhao, Z.; Li, H.; Lin, Y.; Lu, K. The SARS-CoV-2 protein ORF3a inhibits fusion of autophagosomes with lysosomes. Cell Discov. 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tao, S.; Drexler, I. Targeting Autophagy in Innate Immune Cells: Angel or Demon During Infection and Vaccination? Front. Immunol. 2020, 11, 460. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, X.; Yu, J.; Wong, S.H.; Chan, M.T.V.; Zhang, L.; Wu, W.K.K. SARS-CoV-2 targets the lysosome to mediate airway inflammatory cell death. Autophagy 2022, 18, 2246–2248. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yong, Y.-Y.; Zhang, L.; Hu, Y.-J.; Wu, J.-M.; Yan, L.; Pan, Y.-R.; Tang, Y.; Yu, L.; Law, B.Y.-K.; Yu, C.-L.; et al. Targeting autophagy regulation in NLRP3 inflammasome-mediated lung inflammation in COVID-19. Clin. Immunol. 2022, 244, 109093. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Resnik, R.; Mingorance, F.L.; Rivera, F.; Mitchell, F.; Gonzalez, C.D.; Vaccaro, M.I. Autophagy in Inflammatory Response against SARS-CoV-2. Int. J. Mol. Sci. 2023, 24, 4928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Afshar, Z.M.; Dayani, M.; Naderi, M.; Ghanbarveisi, F.; Shiri, S.; Rajati, F. Fatality rate of COVID-19 in patients with malignancies: A systematic review and meta-analysis. J. Infect. 2020, 81, e114–e116. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gallo, O.; Locatello, L.G.; Orlando, P.; Martelli, F.; Piccica, M.; Lagi, F.; Trotta, M. Cancer population may be paradoxically protected from severe manifestations of COVID-19. J. Infect. 2020, 81, E156–E158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ravaioli, S.; Tebaldi, M.; Fonzi, E.; Angeli, D.; Mazza, M.; Nicolini, F.; Lucchesi, A.; Fanini, F.; Pirini, F.; Tumedei, M.M.; et al. ACE2 and TMPRSS2 Potential Involvement in Genetic Susceptibility to SARS-COV-2 in Cancer Patients. Cell Transplant. 2020, 29, 963689720968749. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montopoli, M.; Zumerle, S.; Vettor, R.; Rugge, M.; Zorzi, M.; Catapano, C.; Carbone, G.; Cavalli, A.; Pagano, F.; Ragazzi, E.; et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: A population-based study (N = 4532). Ann. Oncol. 2020, 31, 1040–1045. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, S.; Qiu, Z.; Hou, Y.; Deng, X.; Xu, W.; Zheng, T.; Wu, P.; Xie, S.; Bian, W.; Zhang, C.; et al. AXL is a candidate receptor for SARS-CoV-2 that promotes infection of pulmonary and bronchial epithelial cells. Cell Res. 2021, 31, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Cantuti-Castelvetri, L.; Ojha, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; Van Der Meer, F.; Kallio, K.; Kaya, T.; Anastasina, M.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 2020, 370, 856–860. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.-Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.-X.; Gong, L.; et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- You, J.; Huang, R.; Zhong, R.; Shen, J.; Huang, S.; Chen, J.; Chen, F.; Kang, Y.; Chen, L. Serum AXL is a potential molecular marker for predicting COVID-19 progression. Front. Immunol. 2024, 15, 1394429. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Xu, D.; Xiao, L.; Qin, W.; Liu, B.; Yuan, X. Characterization of the SARS-CoV-2 co-receptor NRP1 expression profiles in healthy people and cancer patients: Implication for susceptibility to COVID-19 disease and potential therapeutic strategy. Front. Genet. 2022, 13, 995736. [Google Scholar] [CrossRef]
- Xia, P.; Dubrovska, A. Tumor markers as an entry for SARS-CoV-2 infection? FEBS J. 2020, 287, 3677–3680. [Google Scholar] [CrossRef]
- Degenhardt, F.; Ellinghaus, D.; Juzenas, S.; Lerga-Jaso, J.; Wendorff, M.; Maya-Miles, D.; Uellendahl-Werth, F.; ElAbd, H.; Rühlemann, M.C.; Arora, J.; et al. Detailed stratified GWAS analysis for severe COVID-19 in four European populations. Hum. Mol. Genet. 2022, 31, 3945–3966. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corley, M.J.; Pang, A.P.; Dody, K.; A Mudd, P.; Patterson, B.K.; Seethamraju, H.; Bram, Y.; Peluso, M.J.; Torres, L.; Iyer, N.S.; et al. Genome-wide DNA methylation profiling of peripheral blood reveals an epigenetic signature associated with severe COVID-19. J. Leukoc. Biol. 2021, 110, 21–26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferraresi, A.; Isidoro, C. Will Omics Biotechnologies Save Us from Future Pandemics? Lessons from COVID-19 for Vaccinomics and Adversomics. Biomedicines 2022, 11, 52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ghosh, M.K.; Kumar, S.; Ganguly, K.K.; Ghosh, P.; Tabassum, S.; Basu, B.; Basu, M. COVID-19 and cancer: Insights into their association and influence on genetic and epigenetic landscape. Epigenomics 2023, 15, 227–248. [Google Scholar] [CrossRef] [PubMed Central]
- Qiu, S.; Hu, Y. Are COVID-19 susceptibility genes related to lung cancer? J. Infect. 2021, 83, 607–635. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Mao, Q.; Li, Y.; Cheng, J.; Xia, Q.; Chen, G.; Chen, P.; Jin, S.; Li, D.; Zhong, C.; et al. Cancer and COVID-19 Susceptibility and Severity: A Two-Sample Mendelian Randomization and Bioinformatic Analysis. Front. Cell Dev. Biol. 2022, 9, 759257. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gusakova, M.S.; Ivanov, M.V.; Kashtanova, D.A.; Taraskina, A.N.; Erema, V.V.; Mikova, V.M.; Loshkarev, R.I.; Ignatyeva, O.A.; Akinshina, A.I.; Mitrofanov, S.I.; et al. GWAS reveals genetic basis of a predisposition to severe COVID-19 through in silico modeling of the FYCO1 protein. Front. Med. 2023, 10, 1178939. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Beukenhorst, A.L.; Koch, C.M.; Hadjichrysanthou, C.; Alter, G.; de Wolf, F.; Anderson, R.M.; Goudsmit, J. SARS-CoV-2 elicits non-sterilizing immunity and evades vaccine-induced immunity: Implications for future vaccination strategies. Eur. J. Epidemiology 2023, 38, 237–242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoshimura, M.; Sakamoto, A.; Ozuru, R.; Kurihara, Y.; Itoh, R.; Ishii, K.; Shimizu, A.; Chou, B.; Sechi, Y.; Fujikane, A.; et al. Insufficient anti-spike RBD IgA responses after triple vaccination with intramuscular mRNA BNT162b2 vaccine against SARS-CoV-2. Heliyon 2023, 10, e23595. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Soeder, E.; Toro-Pape, F.W.; Lampen-Sachar, K. Isolated breast parenchymal changes following COVID-19 vaccine booster. Radiol. Case Rep. 2022, 17, 4556–4560. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andresciani, F.; Ricci, M.; Grasso, R.F.; Zobel, B.B.; Quattrocchi, C.C. COVID-19 vaccination simulating lymph node progression in a patient with prostate cancer. Radiol. Case Rep. 2022, 17, 2996–2999. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; et al. Class switch toward noninflammatory, spike-specific IgG4 antibodies after repeated SARS-CoV-2 mRNA vaccination. Sci. Immunol. 2023, 8, eade2798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kiszel, P.; Sík, P.; Miklós, J.; Kajdácsi, E.; Sinkovits, G.; Cervenak, L.; Prohászka, Z. Class switch towards spike protein-specific IgG4 antibodies after SARS-CoV-2 mRNA vaccination depends on prior infection history. Sci. Rep. 2023, 13, 13166. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hwang, J.K.; Zhang, T.; Wang, A.Z.; Li, Z. COVID-19 vaccines for patients with cancer: Benefits likely outweigh risks. J. Hematol. Oncol. 2021, 14, 38. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with Cancer Appear More Vulnerable to SARS-COV-2: A Multicenter Study During the COVID-19 Outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Corti, C.; Crimini, E.; Tarantino, P.; Pravettoni, G.; Eggermont, A.M.; Delaloge, S.; Curigliano, G. SARS-CoV-2 vaccines for cancer patients: A call to action. Eur. J. Cancer 2021, 148, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Ljungman, P.; Nahi, H.; Linde, A. Vaccination of patients with haematological malignancies with one or two doses of influenza vaccine: A randomised study. Br. J. Haematol. 2005, 130, 96–98. [Google Scholar] [CrossRef] [PubMed]
- Mackay, H.J.; McGee, J.; Villa, D.; Gubbay, J.B.; Tinker, L.M.; Shi, L.; Kuruvilla, J.; Wang, L.; MacAlpine, K.; Oza, A.M. Evaluation of pandemic H1N1 (2009) influenza vaccine in adults with solid tumor and hematological malignancies on active systemic treatment. J. Clin. Virol. 2011, 50, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Sommer, A.L.; Wachel, B.K.; A Smith, J. Evaluation of vaccine dosing in patients with solid tumors receiving myelosuppressive chemotherapy. J. Oncol. Pharm. Pract. 2006, 12, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Shumilov, E.; Aperdannier, L.; Schmidt, N.; Szuszies, C.; Neesse, A.; Hoffknecht, P.; Khandanpour, C.; Mikesch, J.-H.; Stelljes, M.; Boeckel, G.R.; et al. Clinical Post-SARS-CoV-2 Infection Scenarios in Vaccinated and Non-Vaccinated Cancer Patients in Three German Cancer Centers: A Retrospective Analysis. Cancers 2022, 14, 3746. [Google Scholar] [CrossRef]
- Song, Q.; Bates, B.; Shao, Y.R.; Hsu, F.-C.; Liu, F.; Madhira, V.; Mitra, A.K.; Bergquist, T.; Kavuluru, R.; Li, X.; et al. Risk and Outcome of Breakthrough COVID-19 Infections in Vaccinated Patients with Cancer: Real-World Evidence From the National COVID Cohort Collaborative. J. Clin. Oncol. 2022, 40, 1414–1427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campagna, R.; Dominelli, F.; Zingaropoli, M.A.; Ciurluini, F.; Grilli, G.; Amoroso, A.; De Domenico, A.; Amatore, D.; Lia, M.S.; Cortesi, E.; et al. COVID-19 vaccination in cancer patients: Immune responses one year after the third dose. Vaccine 2024, 42, 2687–2694. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Fan, R.; Fan, Y.; Chen, F. Immune response of COVID-19 vaccines in solid cancer patients: A meta-analysis. Hum. Vaccines Immunother. 2024, 20, 2357424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gong, I.Y.; Vijenthira, A.; Powis, M.; Calzavara, A.; Patrikar, A.; Sutradhar, R.; Hicks, L.K.; Wilton, D.; Singh, S.; Krzyzanowska, M.K.; et al. Association of COVID-19 Vaccination with Breakthrough Infections and Complications in Patients with Cancer. JAMA Oncol. 2023, 9, 386–394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, L.; Kaelber, D.C.; Xu, R.; Berger, N.A. COVID-19 breakthrough infections, hospitalizations and mortality in fully vaccinated patients with hematologic malignancies: A clarion call for maintaining mitigation and ramping-up research. Blood Rev. 2022, 54, 100931. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Amatu, A.; Pani, A.; Patelli, G.; Gagliardi, O.M.; Loparco, M.; Piscazzi, D.; Cassingena, A.; Tosi, F.; Ghezzi, S.; Campisi, D.; et al. Impaired seroconversion after SARS-CoV-2 mRNA vaccines in patients with solid tumours receiving anticancer treatment. Eur. J. Cancer 2021, 163, 16–25. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Webber, T.B.; Provinciali, N.; Musso, M.; Ugolini, M.; Boitano, M.; Clavarezza, M.; D’AMico, M.; Defferrari, C.; Gozza, A.; Briata, I.M.; et al. Predictors of poor seroconversion and adverse events to SARS-CoV-2 mRNA BNT162b2 vaccine in cancer patients on active treatment. Eur. J. Cancer 2021, 159, 105–112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herishanu, Y.; Avivi, I.; Aharon, A.; Shefer, G.; Levi, S.; Bronstein, Y.; Morales, M.; Ziv, T.; Arbel, Y.S.; Scarfò, L.; et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021, 137, 3165–3173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vormehr, M.; Lehar, S.; Kranz, L.M.; Tahtinen, S.; Oei, Y.; Javinal, V.; Delamarre, L.; Walzer, K.C.; Diken, M.; Kreiter, S.; et al. Dexamethasone premedication suppresses vaccine-induced immune responses against cancer. OncoImmunology 2020, 9, 1758004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Verma, V.; Shrimali, R.K.; Ahmad, S.; Dai, W.; Wang, H.; Lu, S.; Nandre, R.; Gaur, P.; Lopez, J.; Sade-Feldman, M.; et al. PD-1 blockade in subprimed CD8 cells induces dysfunctional PD-1+CD38hi cells and anti-PD-1 resistance. Nat. Immunol. 2019, 20, 1231–1243. [Google Scholar] [CrossRef]
- Spiliopoulou, P.; van Rensburg, H.J.J.; Avery, L.; Kulasingam, V.; Razak, A.; Bedard, P.; Hansen, A.; Chruscinski, A.; Wang, B.; Kulikova, M.; et al. Longitudinal efficacy and toxicity of SARS-CoV-2 vaccination in cancer patients treated with immunotherapy. Cell Death Dis. 2023, 14, 49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nelli, F.; Giannarelli, D.; Fabbri, A.; Virtuoso, A.; Berrios, J.R.G.; Marrucci, E.; Fiore, C.; Schirripa, M.; Signorelli, C.; Chilelli, M.G.; et al. Immune-related adverse events and disease outcomes after the third dose of SARS-CoV-2 mRNA-BNT162b2 vaccine in cancer patients receiving immune checkpoint inhibitors. Cancer Immunol. Immunother. 2023, 72, 3217–3228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Echaide, M.; Labiano, I.; Delgado, M.; de Lascoiti, A.F.; Ochoa, P.; Garnica, M.; Ramos, P.; Chocarro, L.; Fernández, L.; Arasanz, H.; et al. Immune Profiling Uncovers Memory T-Cell Responses with a Th17 Signature in Cancer Patients with Previous SARS-CoV-2 Infection Followed by mRNA Vaccination. Cancers 2022, 14, 4464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alshehri, S.; Almutawif, Y.A.; Khan, N.U. Impact of COVID-19 vaccination on cancer patients: Safety, efficacy, and long-term effects. Support. Care Cancer 2025, 33, 753. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.D.B.; Mohamed, K.M.; Aguilar, A.d.L.; García, C.J.; Guevara-Hoyer, K.; Fernandez-Arquero, M.; de la Peña, M.A.R.; Bravo, L.G.; Ortega, A.F.J.; Navarro, P.F.; et al. Evidence of exhausted lymphocytes after the third anti-SARS-CoV-2 vaccine dose in cancer patients. Front. Oncol. 2022, 12, 975980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raineri, D.; Mazzucca, C.B.; Moia, R.; Bruna, R.; Kustrimovic, N.; Cappellano, G.; Bellan, M.; Perazzi, M.; Gaidano, G.; Chiocchetti, A. Impairment of the T cell memory response in chronic lymphocytic leukemia patients after SARS-CoV-2 vaccination. Vaccine 2025, 48, 126723. [Google Scholar] [CrossRef] [PubMed]
- Nelli, F.; Signorelli, C.; Fabbri, A.; Giannarelli, D.; Virtuoso, A.; Berrios, J.R.G.; Marrucci, E.; Fiore, C.; Schirripa, M.; Chilelli, M.G.; et al. Changes in Peripheral Immune Cells after the Third Dose of SARS-CoV-2 mRNA-BNT162b2 Vaccine and Disease Outcomes in Cancer Patients Receiving Immune Checkpoint Inhibitors: A Prospective Analysis of the Vax-on-Third-Profile Study. Cancers 2023, 15, 3625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grippin, A.J.; Marconi, C.; Copling, S.; Li, N.; Braun, C.; Woody, C.; Young, E.; Gupta, P.; Wang, M.; Wu, A.; et al. SARS-CoV-2 mRNA vaccines sensitize tumours to immune checkpoint blockade. Nature 2025, 647, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Meo, C.; Palma, G.; Bruzzese, F.; Budillon, A.; Napoli, C.; de Nigris, F. Spontaneous cancer remission after COVID-19: Insights from the pandemic and their relevance for cancer treatment. J. Transl. Med. 2023, 21, 273. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Sousa, L.G.; McGrail, D.J.; Li, K.; Marques-Piubelli, M.L.; Gonzalez, C.; Dai, H.; Ferri-Borgogno, S.; Godoy, M.; Burks, J.; Lin, S.-Y.; et al. Spontaneous tumor regression following COVID-19 vaccination. J. Immunother. Cancer 2022, 10, e004371. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gambichler, T.; Boms, S.; Hessam, S.; Tischoff, I.; Tannapfel, A.; Lüttringhaus, T.; Beckman, J.; Stranzenbach, R. Primary cutaneous anaplastic large-cell lymphoma with marked spontaneous regression of organ manifestation after SARS-CoV-2 vaccination. Br. J. Dermatol. 2021, 185, 1259–1262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wesselmann, U.; Kvasnicka, H.M.; Bozkurt, A.; Wieland, U.; Hofmann, S.C. Long lasting complete regression of a metastatic polyomavirus-positive Merkel cell carcinoma after COVID-19 booster vaccination. EJC Skin Cancer 2014, 2, 100275. [Google Scholar] [CrossRef]
- Eslinger, C.; Jr, P.L.S.U.; Nagalo, B.M.; Borad, M.J. Spontaneous regression of advanced hepatocellular carcinoma following COVID-19 infection and vaccination: A case report and review of literature. J. Gastrointest. Oncol. 2024, 15, 1933–1938. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Neophytou, C.M.; Kyriakou, T.-C.; Papageorgis, P. Mechanisms of Metastatic Tumor Dormancy and Implications for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 6158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Wang, L.; Wei, Y.; Wei, C.; Yang, H.; Chen, Q.; Zhang, R.; Shen, H. Advances in the molecular regulation mechanism of tumor dormancy and its therapeutic strategy. Discov. Oncol. 2024, 15, 184. [Google Scholar] [CrossRef]
- Gunes, D.; Ustal, A.; Ertem, Y.E.; Akkoc, Y.; Gozuacik, D. Autophagy in the regulation of cancer dormancy. FEBS Lett. 2025, 599, 2272–2300. [Google Scholar] [CrossRef] [PubMed]
- Esposito, A.; Ferraresi, A.; Salwa, A.; Vidoni, C.; Dhanasekaran, D.N.; Isidoro, C. Resveratrol Contrasts IL-6 Pro-Growth Effects and Promotes Autophagy-Mediated Cancer Cell Dormancy in 3D Ovarian Cancer: Role of miR-1305 and of Its Target ARH-I. Cancers 2022, 14, 2142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manjili, S.H.; Isbell, M.; Ghochaghi, N.; Perkinson, T.; Manjili, M.H. Multifaceted functions of chronic inflammation in regulating tumor dormancy and relapse. Semin. Cancer Biol. 2021, 78, 17–22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferraresi, A.; Girone, C.; Esposito, A.; Vidoni, C.; Vallino, L.; Secomandi, E.; Dhanasekaran, D.N.; Isidoro, C. How Autophagy Shapes the Tumor Microenvironment in Ovarian Cancer. Front. Oncol. 2020, 10, 599915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Langley, R.R.; Fidler, I.J. The seed and soil hypothesis revisited—The role of tumor-stroma interactions in metastasis to different organs. Int. J. Cancer 2011, 128, 2527–2535. [Google Scholar] [CrossRef]
- Unterlass, J.E.; Curtin, N.J. Warburg and Krebs and related effects in cancer. Expert Rev. Mol. Med. 2019, 21, e4. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; De Giglio, M.A.R.; Roviello, G.N. Deciphering the Relationship between SARS-CoV-2 and Cancer. Int. J. Mol. Sci. 2023, 24, 7803. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rapti, V.; Tsaganos, T.; Vathiotis, I.A.; Syrigos, N.K.; Li, P.; Poulakou, G. New Insights into SARS-CoV-2 and Cancer Cross-Talk: Does a Novel Oncogenesis Driver Emerge? Vaccines 2022, 10, 1607. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Venuti, A.; Donzelli, S.; Nisticò, P.; Blandino, G.; Ciliberto, G. Does Interleukin-6 Bridge SARS-CoV-2 With Virus-Associated Cancers? J. Immunother. Precis. Oncol. 2021, 4, 79–85. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vieira, C.; Nery, L.; Martins, L.; Jabour, L.; Dias, R.; e Silva, A.C.S. Downregulation of Membrane-bound Angiotensin Converting Enzyme 2 (ACE2) Receptor has a Pivotal Role in COVID-19 Immunopathology. Curr. Drug Targets 2021, 22, 254–281. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein Impairs Endothelial Function via Downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bellavite, P.; Ferraresi, A.; Isidoro, C. Immune Response and Molecular Mechanisms of Cardiovascular Adverse Effects of Spike Proteins from SARS-CoV-2 and mRNA Vaccines. Biomedicines 2023, 11, 451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hassani, B.; Attar, Z.; Firouzabadi, N. The renin-angiotensin-aldosterone system (RAAS) signaling pathways and cancer: Foes versus allies. Cancer Cell Int. 2023, 23, 451. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Menon, J.; Soto-Pantoja, D.R.; Callahan, M.F.; Cline, J.M.; Ferrario, C.M.; Tallant, E.A.; Gallagher, P.E. Angiotensin-(1-7) inhibits growth of human lung adenocarcinoma xenografts in nude mice through a reduction in cyclooxygenase-2. Cancer Res. 2007, 67, 2809–2815. [Google Scholar] [CrossRef] [PubMed]
- Soto-Pantoja, D.R.; Menon, J.; Gallagher, P.E.; Tallant, E.A. Angiotensin-(1-7) inhibits tumor angiogenesis in human lung cancer xenografts with a reduction in vascular endothelial growth factor. Mol. Cancer Ther. 2009, 8, 1676–1683. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Q.; Lu, S.; Li, T.; Yu, L.; Zhang, Y.; Zeng, H.; Qian, X.; Bi, J.; Lin, Y. ACE2 inhibits breast cancer angiogenesis via suppressing the VEGFa/VEGFR2/ERK pathway. J. Exp. Clin. Cancer Res. 2019, 38, 173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feng, Y.; Wan, H.; Liu, J.; Zhang, R.; Ma, Q.; Han, B.; Xiang, Y.; Che, J.; Cao, H.; Fei, X.; et al. The angiotensin-converting enzyme 2 in tumor growth and tumor-associated angiogenesis in non-small cell lung cancer. Oncol. Rep. 2010, 23, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Guo, L.; Yu, Q.; Zhao, Y.; Yu, M.; Wang, H.; Wu, M.; Xu, W.; Xu, M.; Zhu, X.-D.; et al. ACE2 Enhances Sensitivity to PD-L1 Blockade by Inhibiting Macrophage-Induced Immunosuppression and Angiogenesis. Cancer Res. 2024, 85, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Gay, C.M.; Ramkumar, K.; Cargill, K.R.; Cardnell, R.J.; Nilsson, M.B.; Heeke, S.; Park, E.M.; Kundu, S.T.; Diao, L.; et al. Lung Cancer Models Reveal Severe Acute Respiratory Syndrome Coronavirus 2–Induced Epithelial-to-Mesenchymal Transition Contributes to Coronavirus Disease 2019 Pathophysiology. J. Thorac. Oncol. 2021, 16, 1821–1839. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lai, Y.-J.; Chao, C.-H.; Liao, C.-C.; Lee, T.-A.; Hsu, J.-M.; Chou, W.-C.; Wang, J.; Huang, H.-C.; Chang, S.-J.; Lin, Y.-L.; et al. Epithelial-mesenchymal transition induced by SARS-CoV-2 required transcriptional upregulation of Snail. Am. J. Cancer Res. 2021, 11, 2278–2290. [Google Scholar] [PubMed] [PubMed Central]
- Huang, H.-C.; Liao, C.-C.; Wang, S.-H.; Lee, I.-J.; Lee, T.-A.; Hsu, J.-M.; Kuo, C.-T.; Wang, J.; Hsieh, W.-C.; Chang, S.-J.; et al. Hyperglycosylated spike of SARS-CoV-2 gamma variant induces breast cancer metastasis. Am. J. Cancer Res. 2021, 11, 4994–5005. [Google Scholar]
- Yu, C.; Tang, W.; Wang, Y.; Shen, Q.; Wang, B.; Cai, C.; Meng, X.; Zou, F. Downregulation of ACE2/Ang-(1–7)/Mas axis promotes breast cancer metastasis by enhancing store-operated calcium entry. Cancer Lett. 2016, 376, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Catarata, M.J.; Ribeiro, R.; Oliveira, M.J.; Cordeiro, C.R.; Medeiros, R. Renin-Angiotensin System in Lung Tumor and Microenvironment Interactions. Cancers 2020, 12, 1457. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, Z.; Yao, T.; Wang, Z.; Liu, B.; Wu, N.; Lu, M.; Shen, N. Association between angiotensin-converting enzyme inhibitors and the risk of lung cancer: A systematic review and meta-analysis. Br. J. Cancer 2022, 128, 168–176. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Emslander, Q.; Krey, K.; Hamad, S.; Maidl, S.; Oubraham, L.; Hesse, J.; Henrici, A.; Austen, K.; Mergner, J.; Grass, V.; et al. MDM2 Influences ACE2 Stability and SARS-CoV-2 Uptake. Viruses 2023, 15, 1763. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goyette, M.-A.; Duhamel, S.; Aubert, L.; Pelletier, A.; Savage, P.; Thibault, M.-P.; Johnson, R.M.; Carmeliet, P.; Basik, M.; Gaboury, L.; et al. The Receptor Tyrosine Kinase AXL Is Required at Multiple Steps of the Metastatic Cascade during HER2-Positive Breast Cancer Progression. Cell Rep. 2018, 23, 1476–1490, Erratum in Cell Rep. 2023, 42, 113604. https://doi.org/10.1016/j.celrep.2023.113604. [Google Scholar] [CrossRef]
- Chen, I.-Y.; Chang, S.C.; Wu, H.-Y.; Yu, T.-C.; Wei, W.-C.; Lin, S.; Chien, C.-L.; Chang, M.-F. Upregulation of the chemokine (c-c motif) ligand 2 via a severe acute respiratory syndrome coronavirus spike-ace2 signaling pathway. J. Virol. 2010, 84, 7703–7712. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, Y.J.; Nikolaienko, S.I.; Dibrova, V.A.; Dibrova, Y.V.; Vasylyk, V.M.; Novikov, M.Y.; Shults, N.V.; Gychka, S.G. SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells. Vasc. Pharmacol. 2021, 137, 106823. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Patra, T.; Meyer, K.; Geerling, L.; Isbell, T.S.; Hoft, D.F.; Brien, J.; Pinto, A.K.; Ray, R.B.; Ray, R. SARS-CoV-2 spike protein promotes IL-6 trans-signaling by activation of angiotensin II receptor signaling in epithelial cells. PLOS Pathog. 2020, 16, e1009128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shin, H.J.; Lee, W.; Ku, K.B.; Yoon, G.Y.; Moon, H.-W.; Kim, C.; Kim, M.-H.; Yi, Y.-S.; Jun, S.; Kim, B.-T.; et al. SARS-CoV-2 aberrantly elevates mitochondrial bioenergetics to induce robust virus propagation. Signal Transduct. Target. Ther. 2024, 9, e1009128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCubrey, J.A.; Steelman, L.S.; Abrams, S.L.; Lee, J.T.; Chang, F.; Bertrand, F.E.; Navolanic, P.M.; Terrian, D.M.; Franklin, R.A.; D’assoro, A.B.; et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv. Enzym. Regul. 2006, 46, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Solis, O.; Beccari, A.R.; Iaconis, D.; Talarico, C.; Ruiz-Bedoya, C.A.; Nwachukwu, J.C.; Cimini, A.; Castelli, V.; Bertini, R.; Montopoli, M.; et al. The SARS-CoV-2 spike protein binds and modulates estrogen receptors. Sci. Adv. 2022, 8, eadd4150. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Hatiboglu, M.A. Can COVID-19 induce glioma tumorogenesis through binding cell receptors? Med Hypotheses 2020, 144, 110009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Raimundo, L.; Ramos, H.; Loureiro, J.B.; Calheiros, J.; Saraiva, L. BRCA1/P53: Two strengths in cancer chemoprevention. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188339. [Google Scholar] [CrossRef] [PubMed]
- Salwa, A.; Ferraresi, A.; Chinthakindi, M.; Vallino, L.; Vidoni, C.; Dhanasekaran, D.N.; Isidoro, C. BECN1 and BRCA1 Deficiency Sensitizes Ovarian Cancer to Platinum Therapy and Confers Better Prognosis. Biomedicines 2021, 9, 207. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kastenhuber, E.R.; Lowe, S.W. Putting p53 in Context. Cell 2017, 170, 1062–1078. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shim, D.; Duan, L.; Maki, C.G. P53-regulated autophagy and its impact on drug resistance and cell fate. Cancer Drug Resist. 2021, 4, 85–95. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma-Lauer, Y.; Carbajo-Lozoya, J.; Hein, M.Y.; Müller, M.A.; Deng, W.; Lei, J.; Meyer, B.; Kusov, Y.; von Brunn, B.; Bairad, D.R.; et al. p53 down-regulates SARS coronavirus replication and is targeted by the SARS-unique domain and PLpro via E3 ubiquitin ligase RCHY1. Proc. Natl. Acad. Sci. USA 2016, 113, E5192–E5201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Singh, N.; Singh, A.B. S2 subunit of SARS-nCoV-2 interacts with tumor suppressor protein p53 and BRCA: An in silico study. Transl. Oncol. 2020, 13, 100814. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, S.; El-Deiry, W.S. Transfected SARS-CoV-2 spike DNA for mammalian cell expression inhibits p53 activation of p21(WAF1), TRAIL Death Receptor DR5 and MDM2 proteins in cancer cells and increases cancer cell viability after chemotherapy exposure. Oncotarget 2024, 15, 275–284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.-F.; Xiang, W.; Xue, B.-Z.; Wang, Y.-H.; Yi, D.-Y.; Jiang, X.-B.; Zhao, H.-Y.; Fu, P. Cell fusion in cancer hallmarks: Current research status and future indications (Review). Oncol. Lett. 2021, 22, 530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shultes, P.V.; Weaver, D.T.; Tadele, D.S.; Barker-Clarke, R.J.; Scott, J.G. Cell-cell fusion in cancer: The next cancer hallmark? Int. J. Biochem. Cell Biol. 2024, 175, 106649. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; Zheng, Y.; Niu, Z.; Zhang, B.; Wang, C.; Yao, X.; Peng, H.; Franca, D.N.; Wang, Y.; Zhu, Y.; et al. SARS-CoV-2 spike protein dictates syncytium-mediated lymphocyte elimination. Cell Death Differ. 2021, 28, 2765–2777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rajah, M.M.; Hubert, M.; Bishop, E.; Saunders, N.; Robinot, R.; Grzelak, L.; Planas, D.; Dufloo, J.; Gellenoncourt, S.; Bongers, A.; et al. SARS-CoV-2 Alpha, Beta, and Delta variants display enhanced Spike-mediated syncytia formation. EMBO J. 2021, 40, e108944. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, Q.; He, X.; Zheng, X.; Fu, Y.; Fu, T.; Luo, J.; Du, Y.; Lan, J.; Yang, J.; Luo, Y.; et al. Verifying AXL and putative proteins as SARS-CoV-2 receptors by DnaE intein-based rapid cell–cell fusion assay. J. Med Virol. 2023, 95, e28953. [Google Scholar] [CrossRef] [PubMed]
- Schilling, W.H.K.; Mukaka, M.; Callery, J.J.; Llewelyn, M.J.; Cruz, C.V.; Dhorda, M.; Ngernseng, T.; Waithira, N.; Ekkapongpisit, M.; Watson, J.A.; et al. Evaluation of hydroxychloroquine or chloroquine for the prevention of COVID-19 (COPCOV): A double-blind, randomised, placebo-controlled trial. PLOS Med. 2024, 21, e1004428. [Google Scholar] [CrossRef]
- Tretyakova, M.S.; Subbalakshmi, A.R.; Menyailo, M.E.; Jolly, M.K.; Denisov, E.V. Tumor Hybrid Cells: Nature and Biological Significance. Front. Cell Dev. Biol. 2022, 10, 814714. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Melzer, C.; von der Ohe, J.; Hass, R. Altered Tumor Plasticity after Different Cancer Cell Fusions with MSC. Int. J. Mol. Sci. 2020, 21, 8347. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Debnath, J.; Gammoh, N.; Ryan, K.M. Autophagy and autophagy-related pathways in cancer. Nat. Rev. Mol. Cell Biol. 2023, 24, 560–575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferraresi, A.; Esposito, A.; Girone, C.; Vallino, L.; Salwa, A.; Ghezzi, I.; Thongchot, S.; Vidoni, C.; Dhanasekaran, D.N.; Isidoro, C. Resveratrol Contrasts LPA-Induced Ovarian Cancer Cell Migration and Platinum Resistance by Rescuing Hedgehog-Mediated Autophagy. Cells 2021, 10, 3213, Correction in Cells 2025, 14, 1020. https://doi.org/10.3390/cells14131020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Koepke, L.; Hirschenberger, M.; Hayn, M.; Kirchhoff, F.; Sparrer, K.M. Manipulation of autophagy by SARS-CoV-2 proteins. Autophagy 2021, 17, 2659–2661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, D.; Zheng, Q.; Sun, L.; Ji, M.; Li, Y.; Deng, H.; Zhang, H. ORF3a of SARS-CoV-2 promotes lysosomal exocytosis-mediated viral egress. Dev. Cell 2021, 56, 3250–3263.e5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Hou, P.; Ma, W.; Wang, X.; Wang, H.; Yu, Z.; Chang, H.; Wang, T.; Jin, S.; Wang, X.; et al. SARS-CoV-2 ORF10 suppresses the antiviral innate immune response by degrading MAVS through mitophagy. Cell. Mol. Immunol. 2021, 19, 67–78, Correction in Cell Mol Immunol. 2023, 20, 686. https://doi.org/10.1038/s41423-023-01023-y. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.; Zhang, Z.; Wang, Z.; Gutiérrez-Castrellón, P.; Shi, H. Cell deaths: Involvement in the pathogenesis and intervention therapy of COVID-19. Signal Transduct. Target. Ther. 2022, 7, 186. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Eissa, S.; Matboli, M.; Awad, N.; Kotb, Y. Identification and validation of a novel autophagy gene expression signature for human bladder cancer patients. Tumor Biol. 2017, 39, 1010428317698360. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhou, L.; Wang, X.; Li, Y.; Liu, X.; Chen, Y.; Zhong, Z.; Chen, J. FYCO1 regulates migration, invasion, and invadopodia formation in HeLa cells through CDC42/N-WASP/Arp2/3 signaling pathway. Biochem. Cell Biol. 2022, 100, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; She, Z.-G.; Cheng, X.; Qin, J.-J.; Zhang, X.-J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077.e3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218, Erratum in Trends Biochem Sci. 2016, 41, 287. https://doi.org/10.1016/j.tibs.2016.01.004. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balamurugan, K. HIF-1 at the crossroads of hypoxia, inflammation, and cancer. Int. J. Cancer 2015, 138, 1058–1066. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Codo, A.C.; Davanzo, G.G.; de Brito Monteiro, L.; de Souza, G.F.; Muraro, S.P.; Virgilio-Da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; de Biagi Junior, C.A.O.; Crunfli, F.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 437–446.e5, Erratum in Cell Metab. 2020, 32, 498–499. https://doi.org/10.1016/j.cmet.2020.07.015. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tian, M.; Liu, W.; Li, X.; Zhao, P.; Shereen, M.A.; Zhu, C.; Huang, S.; Liu, S.; Yu, X.; Yue, M.; et al. HIF-1α promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19. Signal Transduct. Target. Ther. 2021, 6, 308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vidoni, C.; Ferraresi, A.; Vallino, L.; Salwa, A.; Ha, J.H.; Seca, C.; Garavaglia, B.; Dhanasekaran, D.N.; Isidoro, C. Glycolysis Inhibition of Autophagy Drives Malignancy in Ovarian Cancer: Exacerbation by IL-6 and Attenuation by Resveratrol. Int. J. Mol. Sci. 2023, 24, 1723. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferraresi, A.; Girone, C.; Maheshwari, C.; Vallino, L.; Dhanasekaran, D.N.; Isidoro, C. Ovarian Cancer Cell-Conditioning Medium Induces Cancer-Associated Fibroblast Phenoconversion through Glucose-Dependent Inhibition of Autophagy. Int. J. Mol. Sci. 2024, 25, 5691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hasan, A.; Rizvi, S.F.; Parveen, S.; Pathak, N.; Nazir, A.; Mir, S.S. Crosstalk Between ROS and Autophagy in Tumorigenesis: Understanding the Multifaceted Paradox. Front. Oncol. 2022, 12, 852424. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Molnar, T.; Lehoczki, A.; Fekete, M.; Varnai, R.; Zavori, L.; Erdo-Bonyar, S.; Simon, D.; Berki, T.; Csecsei, P.; Ezer, E. Mitochondrial dysfunction in long COVID: Mechanisms, consequences, and potential therapeutic approaches. GeroScience 2024, 46, 5267–5286. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Sharma, B.R.; Tuladhar, S.; Williams, E.P.; Zalduondo, L.; Samir, P.; Zheng, M.; Sundaram, B.; Banoth, B.; Malireddi, R.K.S.; et al. Synergism of TNF-α and IFN-γ Triggers Inflammatory Cell Death, Tissue Damage, and Mortality in SARS-CoV-2 Infection and Cytokine Shock Syndromes. Cell 2021, 184, 149–168.e17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, M.; Gao, Y.; Wang, G.; Song, G.; Liu, S.; Sun, D.; Xu, Y.; Tian, Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell. Mol. Immunol. 2020, 17, 533–535. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iwasaki, M.; Saito, J.; Zhao, H.; Sakamoto, A.; Hirota, K.; Ma, D. Inflammation Triggered by SARS-CoV-2 and ACE2 Augment Drives Multiple Organ Failure of Severe COVID-19: Molecular Mechanisms and Implications. Inflammation 2020, 44, 13–34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Francescangeli, F.; De Angelis, M.L.; Baiocchi, M.; Rossi, R.; Biffoni, M.; Zeuner, A. COVID-19–Induced Modifications in the Tumor Microenvironment: Do They Affect Cancer Reawakening and Metastatic Relapse? Front. Oncol. 2020, 10, 592891. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Francescangeli, F.; De Angelis, M.L.; Zeuner, A. COVID-19: A potential driver of immune-mediated breast cancer recurrence? Breast Cancer Res. 2020, 22, 117. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yumoto, K.; Eber, M.R.; Wang, J.; Cackowski, F.C.; Decker, A.M.; Lee, E.; Nobre, A.R.; Aguirre-Ghiso, J.A.; Jung, Y.; Taichman, R.S. Axl is required for TGF-β2-induced dormancy of prostate cancer cells in the bone marrow. Sci. Rep. 2016, 6, 36520. [Google Scholar] [CrossRef]
- Han, J.; Bae, J.; Choi, C.-Y.; Choi, S.-P.; Kang, H.-S.; Jo, E.-K.; Park, J.; Lee, Y.S.; Moon, H.-S.; Park, C.-G.; et al. Autophagy induced by AXL receptor tyrosine kinase alleviates acute liver injury via inhibition of NLRP3 inflammasome activation in mice. Autophagy 2016, 12, 2326–2343. [Google Scholar] [CrossRef]
- Copaescu, A.; Smibert, O.; Gibson, A.; Phillips, E.J.; Trubiano, J.A. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J. Allergy Clin. Immunol. 2020, 146, 518–534.e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thongchot, S.; Vidoni, C.; Ferraresi, A.; Loilome, W.; Khuntikeo, N.; Sangkhamanon, S.; Titapun, A.; Isidoro, C.; Namwat, N. Cancer-Associated Fibroblast-Derived IL-6 Determines Unfavorable Prognosis in Cholangiocarcinoma by Affecting Autophagy-Associated Chemoresponse. Cancers 2021, 13, 2134. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chia, S.B.; Johnson, B.J.; Hu, J.; Valença-Pereira, F.; Chadeau-Hyam, M.; Guntoro, F.; Montgomery, H.; Boorgula, M.P.; Sreekanth, V.; Goodspeed, A.; et al. Respiratory viral infections awaken metastatic breast cancer cells in lungs. Nature 2025, 645, 496–506. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xia, X. Detailed Dissection and Critical Evaluation of the Pfizer/BioNTech and Moderna mRNA Vaccines. Vaccines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Riley, T.P.; Chou, H.-T.; Hu, R.; Bzymek, K.P.; Correia, A.R.; Partin, A.C.; Li, D.; Gong, D.; Wang, Z.; Yu, X.; et al. Enhancing the Prefusion Conformational Stability of SARS-CoV-2 Spike Protein Through Structure-Guided Design. Front. Immunol. 2021, 12, 660198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ogata, A.F.; Cheng, C.-A.; Desjardins, M.; Senussi, Y.; Sherman, A.C.; Powell, M.; Novack, L.; Von, S.; Li, X.; Baden, L.R.; et al. Circulating Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Vaccine Antigen Detected in the Plasma of mRNA-1273 Vaccine Recipients. Clin. Infect. Dis. 2022, 74, 715–718. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brogna, C.; Cristoni, S.; Marino, G.; Montano, L.; Viduto, V.; Fabrowski, M.; Lettieri, G.; Piscopo, M. Detection of recombinant Spike protein in the blood of individuals vaccinated against SARS-CoV-2: Possible molecular mechanisms. Proteom. Clin. Appl. 2023, 17, e2300048. [Google Scholar] [CrossRef] [PubMed]
- Castruita, J.A.S.; Vest Schneider, U.; Mollerup, S.; Leineweber, T.D.; Weis, N.; Bukh, J.; Pedersen, M.S.; Westh, H. SARS-CoV-2 spike mRNA vaccine sequences circulate in blood up to 28 days after COVID-19 vaccination. APMIS 2023, 131, 128–132. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boros, L.G.; Kyriakopoulos, A.M.; Brogna, C.; Piscopo, M.; McCullough, P.A.; Seneff, S. Long-lasting, biochemically modified mRNA, and its frameshifted recombinant spike proteins in human tissues and circulation after COVID-19 vaccination. Pharmacol. Res. Perspect. 2024, 12, e1218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bansal, S.; Perincheri, S.; Fleming, T.; Poulson, C.; Tiffany, B.; Bremner, R.M.; Mohanakumar, T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer–BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J. Immunol. 2021, 207, 2405–2410. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yasmin, F.; Najeeb, H.; Naeem, U.; Moeed, A.; Atif, A.R.; Asghar, M.S.; Nimri, N.; Saleem, M.; Bandyopadhyay, D.; Krittanawong, C.; et al. Adverse events following COVID-19 mRNA vaccines: A systematic review of cardiovascular complication, thrombosis, and thrombocytopenia. Immun. Inflamm. Dis. 2023, 11, e807. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, J.Y.; Lee, Y.; Park, N.G.; Kim, M.S.; Rhie, S.J. Serious Safety Signals and Prediction Features Following COVID-19 mRNA Vaccines Using the Vaccine Adverse Event Reporting System. Pharmaceuticals 2024, 17, 356. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; Shen, Q.; Chang, H. Vaccines for COVID-19: A Systematic Review of Immunogenicity, Current Development, and Future Prospects. Front. Immunol. 2022, 13, 843928. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hajjo, R.; Sabbah, D.A.; Tropsha, A. Analyzing the Systems Biology Effects of COVID-19 mRNA Vaccines to Assess Their Safety and Putative Side Effects. Pathogens 2022, 11, 743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine 2024, 42, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.B.; Taraban, M.B.; Briggs, K.T. All vials are not the same: Potential role of vaccine quality in vaccine adverse reactions. Vaccine 2021, 39, 6565–6569. [Google Scholar] [CrossRef] [PubMed]
- Tinari, S. The EMA covid-19 data leak, and what it tells us about mRNA instability. BMJ 2021, 372, n627. [Google Scholar] [CrossRef] [PubMed]
- Mulroney, T.E.; Pöyry, T.; Yam-Puc, J.C.; Rust, M.; Harvey, R.F.; Kalmar, L.; Horner, E.; Booth, L.; Ferreira, A.P.; Stoneley, M.; et al. N1-methylpseudouridylation of mRNA causes +1 ribosomal frameshifting. Nature 2024, 625, 189–194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guillen-Angel, M.; Roignant, J.-Y. Exploring pseudouridylation: Dysregulation in disease and therapeutic potential. Curr. Opin. Genet. Dev. 2024, 87, 102210. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Q.; Burgute, B.D.; Tzeng, S.-C.; Jing, C.; Jungers, C.; Zhang, J.; Yan, L.L.; Vierstra, R.D.; Djuranovic, S.; Evans, B.S.; et al. N1-methylpseudouridine found within COVID-19 mRNA vaccines produces faithful protein products. Cell Rep. 2022, 40, 111300. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rubio-Casillas, A.; Cowley, D.; Raszek, M.; Uversky, V.N.; Redwan, E.M. Review: N1-methyl-pseudouridine (m1Ψ): Friend or foe of cancer? Int. J. Biol. Macromol. 2024, 267 Pt 1, 131427, Erratum in Int. J. Biol. Macromol. 2024, 270 Pt 2, 132447. https://doi.org/10.1016/j.ijbiomac.2024.132447. [Google Scholar] [CrossRef] [PubMed]
- Föhse, K.; Geckin, B.; Zoodsma, M.; Kilic, G.; Liu, Z.; Röring, R.J.; Overheul, G.J.; van de Maat, J.; Bulut, O.; Hoogerwerf, J.J.; et al. The impact of BNT162b2 mRNA vaccine on adaptive and innate immune responses. Clin. Immunol. 2023, 255, 109762. [Google Scholar] [CrossRef] [PubMed]
- Sittplangkoon, C.; Alameh, M.-G.; Weissman, D.; Lin, P.J.C.; Tam, Y.K.; Prompetchara, E.; Palaga, T. mRNA vaccine with unmodified uridine induces robust type I interferon-dependent anti-tumor immunity in a melanoma model. Front. Immunol. 2022, 13, 983000. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Yu, J.; Wong, C.C. Adenosine-to-inosine RNA editing in cancer: Molecular mechanisms and downstream targets. Protein Cell 2025, 16, 391–417, Erratum in Protein Cell 2024, pwae062. https://doi.org/10.1093/procel/pwae062. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, Y.-Y.; Liang, Y.-P.; Pan, J.-Q.; Huang, W.-H.; Feng, Y.-M.; Sui, W.-J.; Yu, H.; Tang, X.-D.; Zhu, L.; Chen, J.-H. RNA editing in response to COVID-19 vaccines: Unveiling dynamic epigenetic regulation of host immunity. Front. Immunol. 2024, 15, 1413704. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Y.; Kuramitsu, Y.; Kitagawa, T.; Baron, B.; Yoshino, S.; Maehara, S.-I.; Maehara, Y.; Oka, M.; Nakamura, K. Cofilin-phosphatase slingshot-1L (SSH1L) is over-expressed in pancreatic cancer (PC) and contributes to tumor cell migration. Cancer Lett. 2015, 360, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food Chem. Toxicol. 2022, 164, 113008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miglietta, G.; Russo, M.; Capranico, G. G-quadruplex–R-loop interactions and the mechanism of anticancer G-quadruplex binders. Nucleic Acids Res. 2020, 48, 11942–11957, Erratum in Nucleic Acids Res. 2021, 49, 6000–6001. https://doi.org/10.1093/nar/gkab483. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- König, B.; Kirchner, J.O. Methodological Considerations Regarding the Quantification of DNA Impurities in the COVID-19 mRNA Vaccine Comirnaty®. Methods Protoc. 2024, 7, 41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kobbe, R.; Rau, C.M.; Schulze-Sturm, U.; Stahl, F.; Fonseca-Brito, L.; Diemert, A.; Lütgehetmann, M.; Addo, M.M.; Arck, P.; Weskamm, L.M. Delayed Induction of Noninflammatory SARS-CoV-2 Spike-Specific IgG4 Antibodies Detected 1 Year After BNT162b2 Vaccination in Children. Pediatr. Infect. Dis. J. 2024, 43, 1200–1203. [Google Scholar] [CrossRef]
- Gao, F.-X.; Wu, R.-X.; Shen, M.-Y.; Huang, J.-J.; Li, T.-T.; Hu, C.; Luo, F.-Y.; Song, S.-Y.; Mu, S.; Hao, Y.-N.; et al. Extended SARS-CoV-2 RBD booster vaccination induces humoral and cellular immune tolerance in mice. iScience 2022, 25, 105479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, T.; Wu, Y.; Liu, J.; Zhuang, Y.; Jin, X.; Wang, L. The risk of malignancy in patients with IgG4-related disease: A systematic review and meta-analysis. Arthritis Res. Ther. 2022, 24, 1–9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, H.; Xu, Q.; Zhao, C.; Zhu, Z.; Zhu, X.; Zhou, J.; Zhang, S.; Yang, T.; Zhang, B.; Li, J.; et al. An immune evasion mechanism with IgG4 playing an essential role in cancer and implication for immunotherapy. J. Immunother. Cancer 2020, 8, e000661. [Google Scholar] [CrossRef]
- Shrestha, P.; Ghoreyshi, Z.S.; George, J.T. How modulation of the tumor microenvironment drives cancer immune escape dynamics. Sci. Rep. 2025, 15, 7308. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mulligan, M.J.; Lyke, K.E.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Raabe, V.; Bailey, R.; Swanson, K.A.; et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature 2020, 586, 589–593, Erratum in Nature 2021, 590, E26. https://doi.org/10.1038/s41586-020-03098-3. [Google Scholar] [CrossRef] [PubMed]
- Gandolfo, C.; Anichini, G.; Mugnaini, M.; Bocchia, M.; Terrosi, C.; Sicuranza, A.; Savellini, G.G.; Gozzetti, A.; Franchi, F.; Cusi, M.G. Overview of Anti-SARS-CoV-2 Immune Response Six Months after BNT162b2 mRNA Vaccine. Vaccines 2022, 10, 171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, X.; Li, B.; Lan, T.; Chiari, C.; Ye, X.; Wang, K.; Chen, J. The role of interleukin-17 in inflammation-related cancers. Front. Immunol. 2025, 15, 1479505. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alghamdi, A.; Hussain, S.D.; Wani, K.; Sabico, S.; Alnaami, A.M.; Amer, O.E.; Al-Daghri, N.M. Altered Circulating Cytokine Profile Among mRNA-Vaccinated Young Adults: A Year-Long Follow-Up Study. Immun. Inflamm. Dis. 2025, 13, e70194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Indraccolo, S.; Stievano, L.; Minuzzo, S.; Tosello, V.; Esposito, G.; Piovan, E.; Zamarchi, R.; Chieco-Bianchi, L.; Amadori, A. Interruption of tumor dormancy by a transient angiogenic burst within the tumor microenvironment. Proc. Natl. Acad. Sci. USA 2006, 103, 4216–4221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bakos, T.; Mészáros, T.; Kozma, G.T.; Berényi, P.; Facskó, R.; Farkas, H.; Dézsi, L.; Heirman, C.; de Koker, S.; Schiffelers, R.; et al. mRNA-LNP COVID-19 Vaccine Lipids Induce Complement Activation and Production of Proinflammatory Cytokines: Mechanisms, Effects of Complement Inhibitors, and Relevance to Adverse Reactions. Int. J. Mol. Sci. 2024, 25, 3595. [Google Scholar] [CrossRef]
- Soyfer, V.; Gutfeld, O.; Shamai, S.; Schlocker, A.; Merimsky, O. COVID-19 Vaccine-Induced Radiation Recall Phenomenon. Int. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 957–961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, H.J.; Kim, M.-H.; Choi, M.G.; Chun, E.M. 1-year risks of cancers associated with COVID-19 vaccination: A large population-based cohort study in South Korea. Biomark. Res. 2025, 13, 114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goldman, S.; Bron, D.; Tousseyn, T.; Vierasu, I.; Dewispelaere, L.; Heimann, P.; Cogan, E.; Goldman, M. Rapid Progression of Angioimmunoblastic T Cell Lymphoma Following BNT162b2 mRNA Vaccine Booster Shot: A Case Report. Front. Med. 2021, 8, 798095. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brumfiel, C.M.; Patel, M.H.; DiCaudo, D.J.; Rosenthal, A.C.; Pittelkow, M.R.; Mangold, A.R. Recurrence of primary cutaneous CD30-positive lymphoproliferative disorder following COVID-19 vaccination. Leuk. Lymphoma 2021, 62, 2554–2555. [Google Scholar] [CrossRef]
- Sekizawa, A.; Hashimoto, K.; Kobayashi, S.; Kozono, S.; Kobayashi, T.; Kawamura, Y.; Kimata, M.; Fujita, N.; Ono, Y.; Obuchi, Y.; et al. Rapid progression of marginal zone B-cell lymphoma after COVID-19 vaccination (BNT162b2): A case report. Front. Med. 2022, 9, 963393. [Google Scholar] [CrossRef] [PubMed]
- Zamfir, M.-A.; Moraru, L.; Dobrea, C.; Scheau, A.-E.; Iacob, S.; Moldovan, C.; Scheau, C.; Caruntu, C.; Caruntu, A. Hematologic Malignancies Diagnosed in the Context of the mRNA COVID-19 Vaccination Campaign: A Report of Two Cases. Medicina 2022, 58, 874. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erdogdu, B.; Erdogdu, B.; Cinar, O.E.; Malkan, U.Y.; Aksu, S.; Demiroglu, H.; Buyukasik, Y.; Goker, H.; Sayinalp, N.; Haznedaroglu, I.C. Hematopoietic Adverse Events Associated with BNT162b2 mRNA Covid-19 Vaccine. Int. J. Hematol. Oncol. 2022, 32, 65–67. [Google Scholar] [CrossRef]
- Çınar, O.E.; Erdoğdu, B.; Karadeniz, M.; Ünal, S.; Malkan, Ü.Y.; Göker, H.; Haznedaroğlu, İ.C. Comment on Zamfir et al. Hematologic Malignancies Diagnosed in the Context of the mRNA COVID-19 Vaccination Campaign: A Report of Two Cases. Medicina 2022, 58, 874, Medicina2022, 58, 1575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mizutani, M.; Mitsui, H.; Amano, T.; Ogawa, Y.; Deguchi, N.; Shimada, S.; Miwa, A.; Kawamura, T.; Ogido, Y. Two cases of axillary lymphadenopathy diagnosed as diffuse large B-cell lymphoma developed shortly after BNT162b2 COVID-19 vaccination. J. Eur. Acad. Dermatol. Venereol. 2022, 36, E613–E615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Revenga-Porcel, L.; Peñate, Y.; Granados-Pacheco, F. Anaplastic large cell lymphoma at the SARS-CoV2 vaccine injection site. J. Eur. Acad. Dermatol. Venereol. 2023, 37, E32–E34. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.; Bae, S.; Vays, M.; Abdelwahed, M.; Sarkar, K.; Bae, S.; Vaysblat, M. Development of High-Grade Sarcoma After Second Dose of Moderna Vaccine. Cureus 2023, 15, e37612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avallone, G.; Maronese, C.A.; Conforti, C.; Fava, P.; Gargiulo, L.; Marzano, A.V.; Massone, C.; Mastorino, L.; Paradisi, A.; Pileri, A.; et al. Real-world data on primary cutaneous lymphoproliferative disorders following SARS-CoV-2 vaccination: A multicentre experience from tertiary referral hospitals. J. Eur. Acad. Dermatol. Venereol. 2023, 37, E451–E455. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cavanna, L.; Grassi, S.O.; Ruffini, L.; Michieletti, E.; Carella, E.; Palli, D.; Zangrandi, A.; Inzerilli, N.; Bernuzzi, P.; Di Nunzio, C.; et al. Non-Hodgkin Lymphoma Developed Shortly after mRNA COVID-19 Vaccination: Report of a Case and Review of the Literature. Medicina 2023, 59, 157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- White, E.; Fazio, N.; Tourmouzis, K.; Ryu, S.; Finger, P.T.; Sassoon, J.; Keresztes, R.; Chou, T.; Kaplowitz, K.; Honkanen, R. Unilateral conjunctival Classic Kaposi Sarcoma following a COVID 19 booster. Am. J. Ophthalmol. Case Rep. 2023, 34, 101986. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kyriakopoulos, A.M.; Nigh, G.; A McCullough, P.; Olivier, M.D.; Seneff, S. Bell’s palsy or an aggressive infiltrating basaloid carcinoma post-mRNA vaccination for COVID-19? A case report and review of the literature. EXCLI J. 2023, 22, 992–1011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ang, S.-Y.; Huang, Y.-F.; Chang, C.-T. Ph-Positive B-Cell Acute Lymphoblastic Leukemia Occurring after Receipt of Bivalent SARS-CoV-2 mRNA Vaccine Booster: A Case Report. Medicina 2023, 59, 627. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.; Muto, R.; Miyoshi, H.; Aoki, M.; Uesugi, N.; Murayama, H.; Masutani, K.; Hamasaki, M. The first autopsy case of Epstein–Barr virus-positive marginal zone lymphoma that deteriorated after COVID-19 vaccination. Pathol. Int. 2024, 74, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Ueda, Y.; Sakai, T.; Yamada, K.; Arita, K.; Ishige, Y.; Hoshi, D.; Yanagisawa, H.; Iwao-Kawanami, H.; Kawanami, T.; Mizuta, S.; et al. Fatal hemophagocytic lymphohistiocytosis with intravascular large B-cell lymphoma following coronavirus disease 2019 vaccination in a patient with systemic lupus erythematosus: An intertwined case. Immunol. Med. 2024, 47, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M.; Zouein, J.; Khater, J.A.; Sarkis, A.-S.; Helou, J. A Case of Rapid Transformation of a Nail Matrix Nevi to Melanoma After Messenger RNA COVID-19 Vaccine: A Cause or a Coincidence? Cureus 2024, 16, e76312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sano, S. A case of metastatic breast carcinoma to the skin expressing SARS-CoV-2 spike protein possibly derived from mRNA vaccine. J. Dermatol. Sci. 2025, 120, 71–73. [Google Scholar] [CrossRef] [PubMed]
- Haji, N.; Ali, S.; A Wahashi, E.; Khalid, M.; Ramamurthi, K. Johnson and Johnson COVID-19 Vaccination Triggering Pheochromocytoma Multisystem Crisis. Cureus 2021, 13, e18196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panou, E.; Nikolaou, V.; Marinos, L.; Kallambou, S.; Sidiropoulou, P.; Gerochristou, M.; Stratigos, A. Recurrence of cutaneous T-cell lymphoma post viral vector COVID-19 vaccination. J. Eur. Acad. Dermatol. Venereol. 2022, 36, E91–E93. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tang, W.-R.; Hsu, C.-W.; Lee, C.-C.; Huang, W.-L.; Lin, C.-Y.; Hsu, Y.-T.; Chang, C.; Tsai, M.-T.; Hu, Y.-N.; Hsu, C.-H.; et al. A Case Report of Posttransplant Lymphoproliferative Disorder After AstraZeneca Coronavirus Disease 2019 Vaccine in a Heart Transplant Recipient. Transplant. Proc. 2022, 54, 1575–1578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kreher, M.A.; Ahn, J.; Werbel, T.; Motaparthi, K. Subcutaneous panniculitis-like T-cell lymphoma after COVID-19 vaccination. JAAD Case Rep. 2022, 28, 18–20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veeraballi, S.; Patel, A.; Are, G.; Ramahi, A.; Chittamuri, S.; Shaaban, H. A Case of Chronic Myelomonocytic Leukemia Unmasked After Receiving J&J COVID-19 Vaccine. Cureus 2022, 14, e26070. [Google Scholar] [CrossRef]
- Martínez-Ortega, J.I.; Cibrian, A.G.R.; Martinez-Jaramillo, E.; Silva, M.d.C.G. Sporadic Kaposi Sarcoma Following a COVID-19 Vaccine: Mere Coincidence or Something More? Cureus 2024, 16, e53925. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tenforde, M.W.; Self, W.H.; Adams, K.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Association Between mRNA Vaccination and COVID-19 Hospitalization and Disease Severity. JAMA 2021, 326, 2043–2054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Bernal, J.L. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adhikari, B.; Bednash, J.S.; Horowitz, J.C.; Rubinstein, M.P.; Vlasova, A.N. Brief research report: Impact of vaccination on antibody responses and mortality from severe COVID-19. Front. Immunol. 2024, 15, 1325243, Correction in Front Immunol. 2024, 15, 1384209. https://doi.org/10.3389/fimmu.2024.1384209. [Google Scholar] [CrossRef] [PubMed]
- Berrino, F.; Donzelli, A.; Bellavite, P.; Malatesta, G. COVID-19 vaccination and all-cause and non-COVID-19 mortality. A revaluation of a study carried out in an Italian Province. Epidemiol. Prev. 2023, 47, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Teodori, L.; Osimani, B.; Isidoro, C.; Ramakrishna, S. Mass versus personalized medicine againstCOVID-19 in the “system sciences” era. Cytom. Part A 2022, 101, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Boeckel, G.R.; Hölscher, S.D.; Bürger, C.; Jacob, T.; Krekeler, C.; Shumilov, E.; Reicherts, C.; Bleckmann, A.; Lenz, G.; Vollenberg, R.; et al. Comprehensive Treatment of Hematological Patients with SARS-CoV-2 Infection Including Anti-SARS-CoV-2 Monoclonal Antibodies: A Single-Center Experience Case Series. Curr. Oncol. 2022, 29, 2312–2325. [Google Scholar] [CrossRef]
- Janssen, M.; Leo, A.; Wolf, C.; Stenzinger, M.; Bartenschlager, M.; Brandt, J.; Sauer, S.; Schmitt, M.; Dreger, P.; Schlenk, R.F.; et al. Treatment of chronic COVID-19 with convalescent/postvaccination plasma in patients with hematologic malignancies. Int. J. Cancer 2024, 155, 618–626. [Google Scholar] [CrossRef]
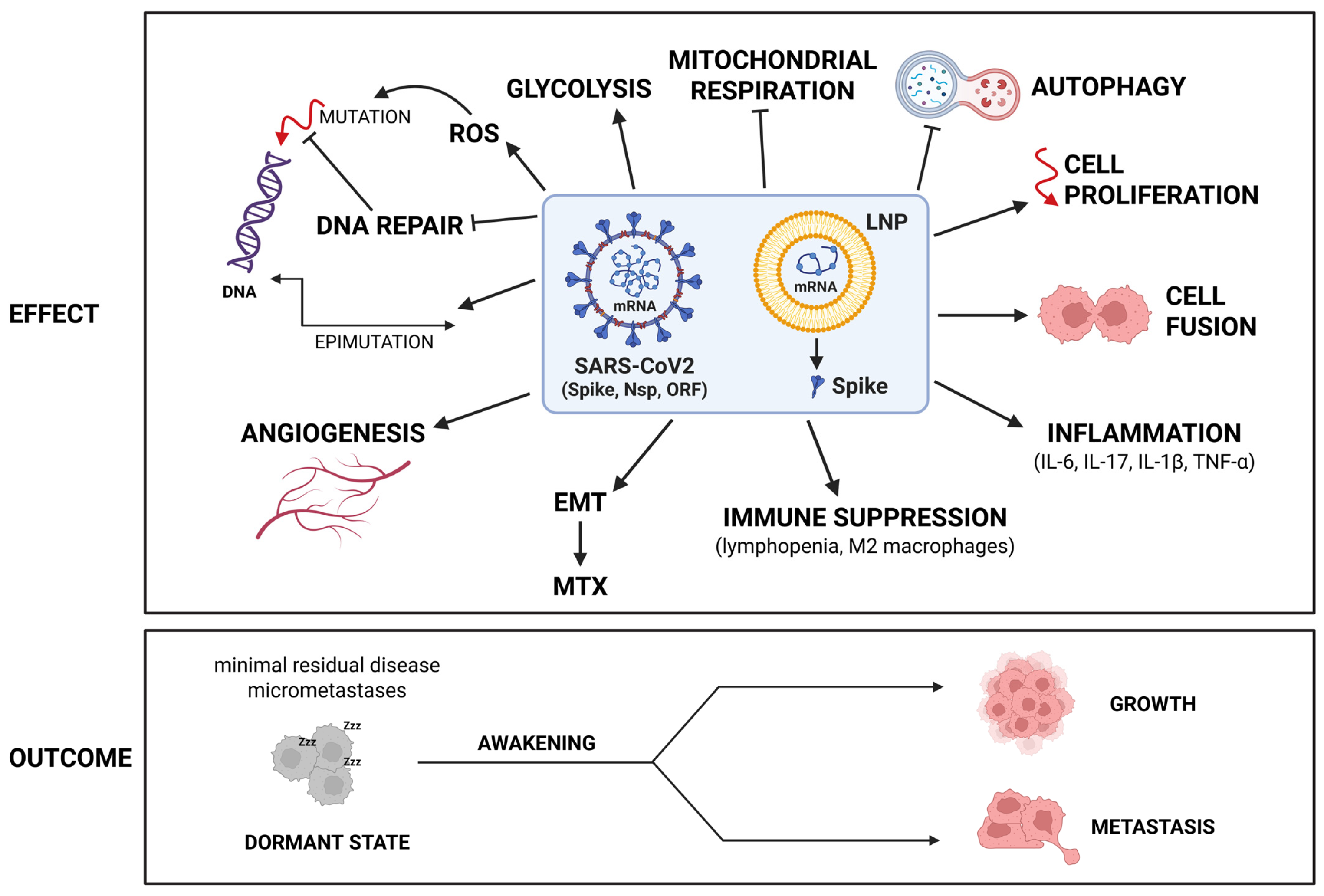
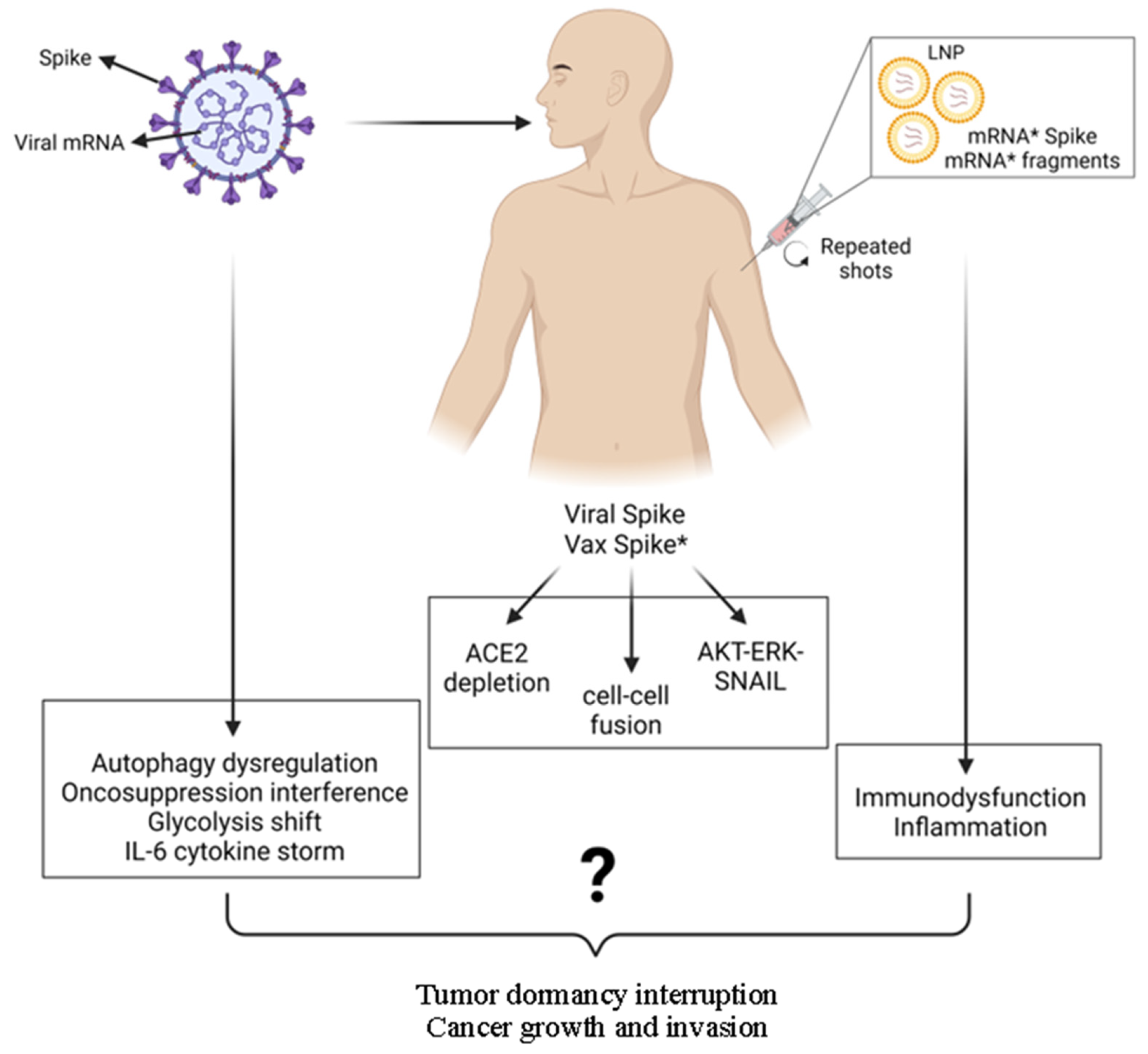
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isidoro, C. SARS-CoV2 and Anti-COVID-19 mRNA Vaccines: Is There a Plausible Mechanistic Link with Cancer? Cancers 2025, 17, 3867. https://doi.org/10.3390/cancers17233867
Isidoro C. SARS-CoV2 and Anti-COVID-19 mRNA Vaccines: Is There a Plausible Mechanistic Link with Cancer? Cancers. 2025; 17(23):3867. https://doi.org/10.3390/cancers17233867
Chicago/Turabian StyleIsidoro, Ciro. 2025. "SARS-CoV2 and Anti-COVID-19 mRNA Vaccines: Is There a Plausible Mechanistic Link with Cancer?" Cancers 17, no. 23: 3867. https://doi.org/10.3390/cancers17233867
APA StyleIsidoro, C. (2025). SARS-CoV2 and Anti-COVID-19 mRNA Vaccines: Is There a Plausible Mechanistic Link with Cancer? Cancers, 17(23), 3867. https://doi.org/10.3390/cancers17233867






