KRAS-Wild Pancreatic Cancer—More Targets than Treatment Possibilities?
Simple Summary
Abstract
1. Introduction
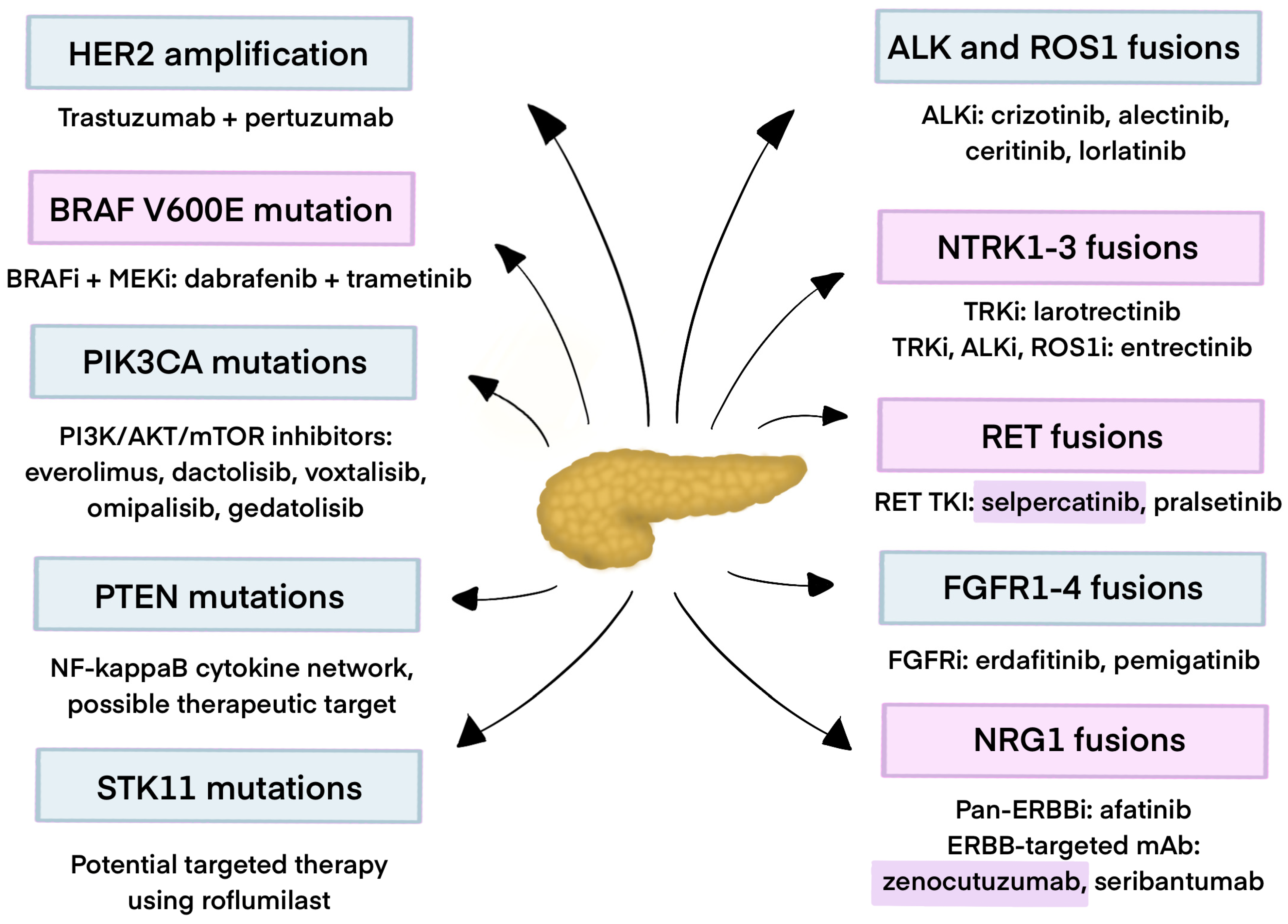
2. Fusion Genes in Pancreatic Cancer
2.1. ALK Fusions
2.2. ROS Fusions
2.3. NTRK Gene Fusions
2.4. RET Fusions
2.5. FGFR Fusions
2.6. NRG Fusions
3. Gene Amplifications in Pancreatic Cancer
4. Gene Mutations in PDAC
4.1. BRAF
- •
- The first one includes the most frequent V600E mutation, which can be targeted by BRAF inhibitors, optionally combined with MEK inhibitors.
- •
- The second class consists of in-frame deletions that can be targeted by MEK inhibitors.
- •
- The third class can be targeted by MEK inhibitors with RTK inhibitors [112].
4.2. HER2
4.3. PIK3CA
4.4. PTEN R130Q/STK11/TSC2
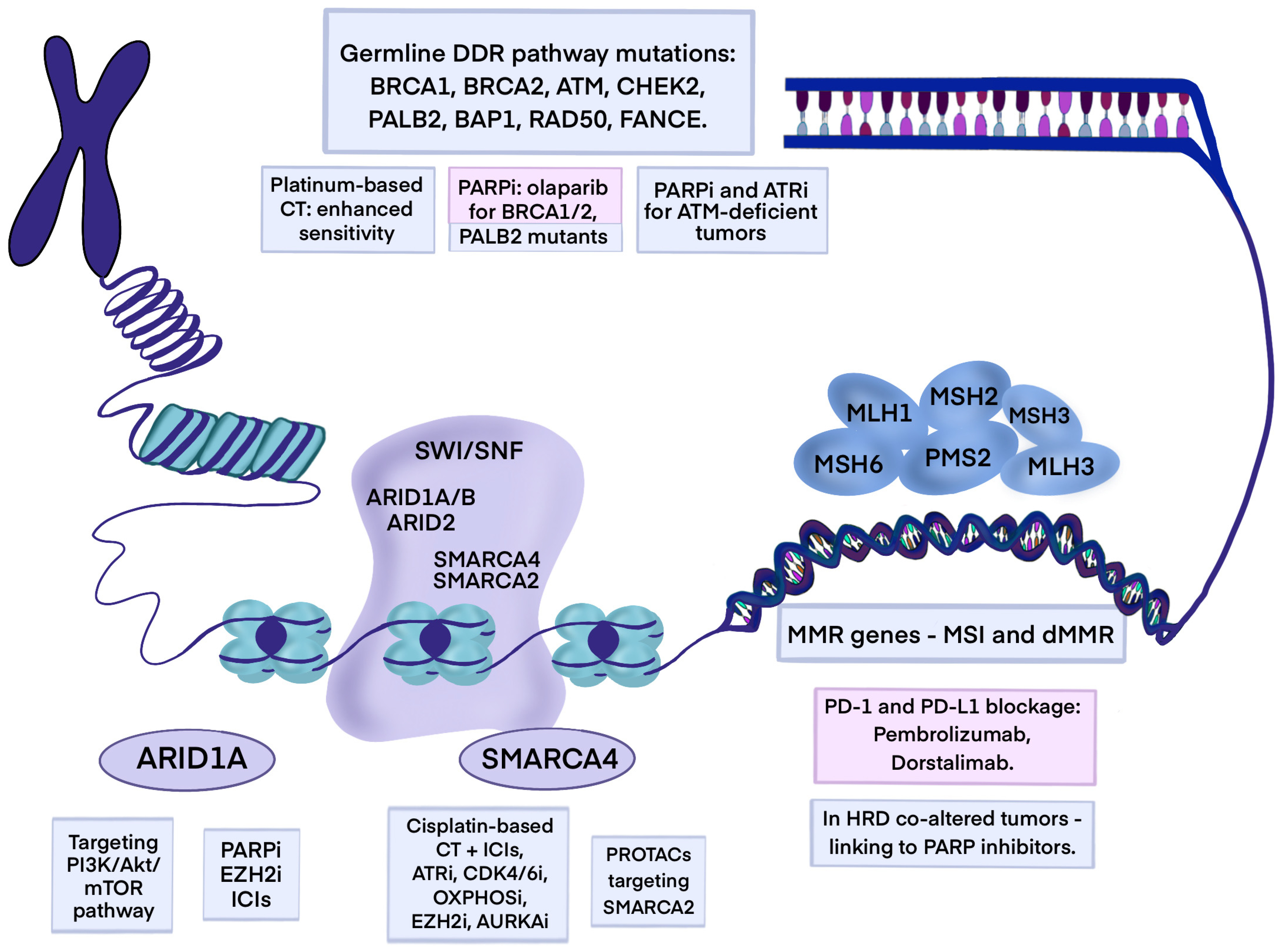
5. Chromatin Remodeling Genomic Alteration
5.1. ARID1A
5.2. SMARCA4
6. Mismatch Repair Deficiency and Microsatellite Instability
7. Germline Mutations in PDAC
7.1. BRCA1/2
7.2. PALB2 (HRD)
7.3. ATM
7.4. CHEK1/2
7.5. Summary
8. Conclusions
Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACMG | American College of Medical Genetics and Genomics |
| ADC | Antibody–Drug Conjugates |
| AEs | Adverse Effects |
| ALK | Anaplastic Lymphoma Kinase |
| ALT | Alanine Aminotransferase |
| AMP | Association for Molecular Pathology |
| AMPK | AMP-Activated Protein Kinase |
| APP | Amyloid Beta Precursor Protein |
| ARID1A/2 | AT-Rich Interaction Domain 1A/2 |
| ASCO | American Society of Clinical Oncology |
| AST | Aspartate Aminotransferase |
| ATM | Ataxia Telangiectasia Mutated |
| ATP1B1 | ATPase Na+/K+-Transporting Subunit Beta 1 |
| ATR inhibitors | Ataxia Telangiectasia and RAD3-Related Kinase Inhibitors |
| BMI | Body Mass Index |
| BRAF | B-Raf Proto-Oncogene/Serine/Threonine Kinase |
| BRCA1/2 | Breast Cancer Gene 1/2 |
| CAPS | Cancer of the Pancreas Screening Consortium |
| CD74 | Cluster of Differentiation 74 |
| CDC42 | Cell Division Cycle 42 |
| CDH6 | Cadherin 6 |
| CDKN2A | Cyclin-Dependent Kinase Inhibitor 2A |
| CENPW | Centromere Protein W |
| CHEK1/2 | Checkpoint Kinase 1/2 |
| CR | Complete Response |
| CRC | Colorectal Cancer |
| CtIP | CtBP-Interacting Protein |
| DCR | Disease Control Rate |
| DDR | DNA Damage Repair |
| DNA | Deoxyribonucleic Acid |
| DOR | Duration of Response |
| DSBs | DNA Double-Strand Breaks |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| EML4 | Echinoderm Microtubule-Associated Protein-Like 4 |
| EMT | Epithelial–Mesenchymal Transition |
| ERBB | Erythroblastic Leukemia Viral Oncogene |
| ERK | Extracellular Signal-Regulated Kinase (MAPK1/3) |
| EUS | Endoscopic Ultrasound |
| EUS-FNB | Endoscopic Ultrasound-Guided Fine-Needle Biopsy |
| EZH2 | Enhancer of Zeste Homolog 2 |
| FAMMM | Familial Atypical Multiple Mole Melanoma |
| FDA | Food and Drug Administration |
| FGF | Fibroblast Growth Factor |
| FGFR2/3 | Fibroblast Growth Factor Receptor 2/3 |
| FPC | Familial Pancreatic Cancer |
| G/GEJ | Gastric and Gastroesophageal Junction |
| GI | Gastrointestinal |
| GRB2 | Growth Factor Receptor-Bound Protein 2 |
| H2AX | H2A Histone Family Member X |
| HER2 | Human Epidermal Growth Factor Receptor 2 |
| HRD | Homologous Recombination Deficiency |
| HRR | Homologous Recombination Repair |
| ICB | Immune Checkpoint Blockade |
| IHC | Immunohistochemistry |
| IPMN | Intraductal Papillary Mucinous Neoplasm |
| JAK-STAT | Janus Kinase-Signal Transducer and Activator of Transcription |
| KANK4 | KN Motif and Ankyrin Repeat Domains 4 |
| KMT2D/2C | Lysine Methyltransferase 2D/2C |
| KRAS | Kirsten Rat Sarcoma Viral Oncogene |
| LAPC | Locally Advanced Pancreatic Cancer |
| LOH | Loss of Heterozygosity |
| MAPK | Mitogen-Activated Protein Kinase |
| MEK | Mitogen-Activated Protein Kinase Kinase (MAP2K1/2) |
| MMP | Matrix Metalloproteinase |
| mPFS | Median Progression-Free Survival |
| MRI | Magnetic Resonance Imaging |
| MRN complex | MRE11-RAD50-NBS1 Complex |
| MTD | Maximum Tolerated Dose |
| mTOR | Mammalian Target of Rapamycin |
| NCCN | National Comprehensive Cancer Network |
| NF-κB | Nuclear Factor Kappa B |
| NGS | Next-Generation Sequencing |
| NHEJ | Non-Homologous End Joining |
| NSCLC | Non-Small-Cell Lung Cancer |
| ORR | Objective Response Rate |
| OS | Overall Survival |
| PARP | Poly ADP Ribose Polymerase |
| PBRM1 | Polybromo 1 |
| PC | Pancreatic Cancer |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| PI3K | Phosphoinositide 3-Kinase |
| PI3K | Phosphatidylinositol 3-Kinase |
| PIP3 | Phosphatidylinositol (3,4,5)-Trisphosphate |
| PPFIBP1 | PPFIA-Binding Protein 1 |
| PR | Partial Response |
| PROTACs | Proteolysis Targeting Chimeras |
| PTEN | Phosphatase and Tensin Homolog Deleted on Chromosome 10 |
| RAD51 | RAD51 Recombinase |
| RAF | Rapid Fibrosarcoma |
| RET | Rearranged During Transfection Proto-Oncogene |
| RHOA | Ras Homolog Family Member A |
| ROCK1 | Rho-Associated Coiled-Coil-Containing Protein Kinase 1 |
| RP2D | Recommended Phase 2 Dose |
| RTK | Receptor Tyrosine Kinase |
| SARAF | Store-Operated Calcium Entry-Associated Regulatory Factor |
| SD | Stable Disease |
| SLC4A4 | Solute Carrier Family 4 Member 4 |
| SMAD4 | Mothers Against Decapentaplegic Homolog 4 |
| SMARCA4 | SWI/SNF-Related, Matrix-Associated, Actin-Dependent Regulator of Chromatin, Subfamily A, Member 4 |
| SRC | SRC Non-Receptor Tyrosine Kinase |
| STK11 | Serine/Threonine Kinase 11 |
| STRN | Striatin |
| SWI/SNF | Switch/Sucrose Non-Fermentable |
| T-DXd | Trastuzumab Deruxtecan |
| TERT | Telomerase Reverse Transcriptase |
| TGF-β | Transforming Growth Factor Beta |
| TKS5 | Tyrosine Kinase Substrate 5 |
| TME | Tumor Microenvironment |
| TP53 | Tumor Protein P53 |
| TPR | Translocated Promoter Region |
| TRAEs | Treatment-Related Adverse Events |
| TEAEs | Treatment-Emergent Adverse Events |
| VUS | Variants Of Uncertain Significance |
| WGS | Whole-Genome Sequencing |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Lin, Z.; Adeniran, E.A.; Cai, Y.; Qureshi, T.A.; Li, D.; Gong, J.; Li, J.; Pandol, S.J.; Jiang, Y. Early Detection of Pancreatic Cancer: Current Advances and Future Opportunities. Biomedicines 2025, 13, 1733. [Google Scholar] [CrossRef]
- Claridge, H.; Cooke, E.A.; Thomas, S.A.; Greenwood, N.; Lemanska, A. Pancreatic Cancer Risk Assessment Tools in Primary Care: A Mixed Methods Systematic Review. J. Gastrointest. Cancer 2025, 56, 128. [Google Scholar] [CrossRef]
- Zhou, K.; Liu, Y.; Tang, C.; Zhu, H. Pancreatic Cancer: Pathogenesis and Clinical Studies. MedComm 2025, 6, e70162. [Google Scholar] [CrossRef]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up☆. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Al-Hawary, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Peduzzi, G.; Archibugi, L.; Farinella, R.; de Leon Pisani, R.P.; Vodickova, L.; Vodicka, P.; Kraja, B.; Sainz, J.; Bars-Cortina, D.; Daniel, N.; et al. The exposome and pancreatic cancer, lifestyle and environmental risk factors for PDAC. Semin. Cancer Biol. 2025, 113, 100–129. [Google Scholar] [CrossRef] [PubMed]
- Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [CrossRef] [PubMed]
- Włoszek, E.; Krupa, K.; Fudalej, M.; Miski, H.; Badowska-Kozakiewicz, A.M.; Deptała, A. Unusual Manifestations of Primary Pancreatic Neoplasia. Cancers 2025, 17, 3240. [Google Scholar] [CrossRef]
- Dallavalle, S.; Campagnoli, G.; Pastena, P.; Martinino, A.; Schiliró, D.; Giovinazzo, F. New Frontiers in Pancreatic Cancer Management: Current Treatment Options and the Emerging Role of Neoadjuvant Therapy. Medicina 2024, 60, 1070. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Abdelghani, M.B.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Kokkinogoulis, K.; Kollas, A.; Simeonidis, D.; Papakostas, P.; Platoni, K.; Efstathopoulos, E.P.; Makropoulou, M. An overview of the feasibility of nanomedicine in pancreatic cancer theranostics. Explor. Target. Anti-Tumor Ther. 2025, 6, 1002326. [Google Scholar] [CrossRef] [PubMed]
- du Toit-Thompson, T.; Leck, L.; Gillson, J.; Pavlakis, N.; Gill, A.J.; Samra, J.S.; Mittal, A.; Sahni, S. Overcoming therapy resistance in pancreatic cancer: Challenges and emerging strategies. Adv. Drug Deliv. Rev. 2025, 224, 115647. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Q.; Castaneda, C.; Cook, S. Targeted Therapies in Pancreatic Cancer: A New Era of Precision Medicine. Biomedicines 2024, 12, 2175. [Google Scholar] [CrossRef]
- Buckley, C.W.; O’Reilly, E.M. Next-generation therapies for pancreatic cancer. Expert Rev. Gastroenterol. Hepatol. 2024, 18, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Krupa, K.; Fudalej, M.; Włoszek, E.; Miski, H.; Badowska-Kozakiewicz, A.M.; Mękal, D.; Budzik, M.P.; Czerw, A.; Deptała, A. Treatment of KRAS-Mutated Pancreatic Cancer: New Hope for the Patients? Cancers 2025, 17, 2453. [Google Scholar] [CrossRef] [PubMed]
- Fudalej, M.; Kwaśniewska, D.; Nurzyński, P.; Badowska-Kozakiewicz, A.; Mękal, D.; Czerw, A.; Sygit, K.; Deptała, A. New Treatment Options in Metastatic Pancreatic Cancer. Cancers 2023, 15, 2327. [Google Scholar] [CrossRef]
- Ben-Ammar, I.; Rousseau, A.; Nicolle, R.; Tarabay, A.; Boige, V.; Valery, M.; Pudlarz, T.; Malka, D.; Gelli, M.; Fernandez-De-Sevilla, E.; et al. Precision medicine for KRAS wild-type pancreatic adenocarcinomas. Eur. J. Cancer 2024, 197, 113497. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Blais, E.M.; Brody, J.R.; Lyons, E.; DeArbeloa, P.; Hendifar, A.; Mikhail, S.; Chung, V.; Sahai, V.; Sohal, D.P.S.; et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: A retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020, 21, 508–518. [Google Scholar] [CrossRef]
- Facciorusso, A.; Crinò, S.F.; Ramai, D.; Madhu, D.; Fugazza, A.; Carrara, S.; Spadaccini, M.; Mangiavillano, B.; Gkolfakis, P.; Mohan, B.P.; et al. Comparative diagnostic performance of different techniques for EUS-guided fine-needle biopsy sampling of solid pancreatic masses: A network meta-analysis. Gastrointest. Endosc. 2023, 97, 839–848.e835. [Google Scholar] [CrossRef]
- Facciorusso, A.; Mohan, B.P.; Crinò, S.F.; Ofosu, A.; Ramai, D.; Lisotti, A.; Chandan, S.; Fusaroli, P. Contrast-enhanced harmonic endoscopic ultrasound-guided fine-needle aspiration versus standard fine-needle aspiration in pancreatic masses: A meta-analysis. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 821–828. [Google Scholar] [CrossRef]
- Gkountakos, A.; Singhi, A.D.; Westphalen, C.B.; Scarpa, A.; Luchini, C. Fusion genes in pancreatic tumors. Trends Cancer 2024, 10, 430–443. [Google Scholar] [CrossRef]
- Yao, S.; Cheng, M.; Zhang, Q.; Wasik, M.; Kelsh, R.; Winkler, C. Anaplastic lymphoma kinase is required for neurogenesis in the developing central nervous system of zebrafish. PLoS ONE 2013, 8, e63757. [Google Scholar] [CrossRef]
- Camidge, D.R.; Doebele, R.C. Treating ALK-positive lung cancer--early successes and future challenges. Nat. Rev. Clin. Oncol. 2012, 9, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Ali, S.M.; Lacy, J.; Hendifar, A.; Nguyen, K.; Koo, J.; Chung, J.H.; Greenbowe, J.; Ross, J.S.; Nikiforova, M.N.; et al. Identification of Targetable ALK Rearrangements in Pancreatic Ductal Adenocarcinoma. J. Natl. Compr. Cancer Netw. 2017, 15, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.T.; Kim, D.-W.; Nakagawa, K.; Seto, T.; Crinó, L.; Ahn, M.-J.; Pas, T.D.; Besse, B.; Solomon, B.J.; Blackhall, F.; et al. Crizotinib versus Chemotherapy in Advanced ALK-Positive Lung Cancer. N. Engl. J. Med. 2013, 368, 2385–2394. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Liu, X.; Li, W.; Yang, Y.; Ying, J.; Yang, L. ALK Rearrangement-Positive Pancreatic Cancer with Brain Metastasis Has Remarkable Response to ALK Inhibitors: A Case Report. Front. Oncol. 2021, 11, 724815. [Google Scholar] [CrossRef]
- Gower, A.; Golestany, B.; Gong, J.; Singhi, A.D.; Hendifar, A.E. Novel ALK Fusion, PPFIBP1-ALK, in Pancreatic Ductal Adenocarcinoma Responsive to Alectinib and Lorlatinib. JCO Precis. Oncol. 2020, 4, 865–870. [Google Scholar] [CrossRef]
- Ambrosini, M.; Re, M.D.; Manca, P.; Hendifar, A.; Drilon, A.; Harada, G.; Ree, A.H.; Klempner, S.; Mælandsmo, G.M.; Flatmark, K.; et al. ALK Inhibitors in Patients with ALK Fusion–Positive GI Cancers: An International Data Set and a Molecular Case Series. JCO Precis. Oncol. 2022, 6, e2200015. [Google Scholar] [CrossRef]
- Luchini, C.; Paolino, G.; Mattiolo, P.; Piredda, M.L.; Cavaliere, A.; Gaule, M.; Melisi, D.; Salvia, R.; Malleo, G.; Shin, J.I.; et al. KRAS wild-type pancreatic ductal adenocarcinoma: Molecular pathology and therapeutic opportunities. J. Exp. Clin. Cancer Res. 2020, 39, 227. [Google Scholar] [CrossRef]
- Roswell Park Cancer Institute. Ceritinib and Combination Chemotherapy in Treating Patients with Advanced Solid Tumors or Locally Advanced or Metastatic Pancreatic Cancer; ClinicalTrials.gov: Bethesda, MD, USA, 2022. [Google Scholar]
- Davies, K.D.; Doebele, R.C. Molecular pathways: ROS1 fusion proteins in cancer. Clin. Cancer Res. 2013, 19, 4040–4045. [Google Scholar] [CrossRef]
- Yan, D.; Xu, M.; Wang, W.; Han, Y.; Zhu, J.; Ma, T. Analysis of ROS1 fusions in nonlung solid tumors. J. Clin. Oncol. 2022, 40, e15124. [Google Scholar] [CrossRef]
- Shaw, A.T.; Ou, S.-H.I.; Bang, Y.-J.; Camidge, D.R.; Solomon, B.J.; Salgia, R.; Riely, G.J.; Varella-Garcia, M.; Shapiro, G.I.; Costa, D.B.; et al. Crizotinib in ROS1-Rearranged Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2014, 371, 1963–1971. [Google Scholar] [CrossRef]
- Marinelli, D.; Siringo, M.; Metro, G.; Ricciuti, B.; Gelibter, A.J. Non-small-cell lung cancer: How to manage ALK-, ROS1- and NTRK-rearranged disease. Drugs Context 2022, 11, 2022-3-1. [Google Scholar] [CrossRef]
- FDA Approves Crizotinib Capsules. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-crizotinib-capsules (accessed on 13 August 2025).
- FDA Approves Entrectinib for NTRK Solid Tumors and ROS-1 NSCLC. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-entrectinib-ntrk-solid-tumors-and-ros-1-nsclc (accessed on 13 August 2025).
- Drilon, A.; Chiu, C.-H.; Fan, Y.; Cho, B.C.; Lu, S.; Ahn, M.-J.; Krebs, M.G.; Liu, S.V.; John, T.; Otterson, G.A.; et al. Long-Term Efficacy and Safety of Entrectinib in ROS1 Fusion–Positive NSCLC. JTO Clin. Res. Rep. 2022, 3, 100332. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Shaw, A.T.; Solomon, B.J.; Chiari, R.; Riely, G.J.; Besse, B.; Soo, R.A.; Kao, S.; Lin, C.-C.; Bauer, T.M.; Clancy, J.S.; et al. Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: A multicentre, open-label, single-arm, phase 1–2 trial. Lancet Oncol. 2019, 20, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Reutter, T.; Fassunke, J.; Püsken, M.; Weber, J.-P.; Binot, E.; Eisert, A.; Fischer, R.; Nogova, L.; Riedel, R.; Schaufler, D.; et al. Durable Response with Sequential Tyrosine Kinase Inhibitor Treatment in a Patient with ROS1 Fusion–Positive Pancreatic Adenocarcinoma: A Case Report. JCO Precis. Oncol. 2023, 7, e2200467. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Shen, Y.Y. Rare ROS1-CENPW gene in pancreatic acinar cell carcinoma and the effect of crizotinib plus AG chemotherapy: A case report. World J. Clin. Cases 2023, 11, 5823–5829. [Google Scholar] [CrossRef]
- Su, D.; Ruan, Y.; Shi, Y.; Cao, D.; Wu, T.; Dang, T.; Wang, H.; Xin, Y.; Ma, M.; Meng, H.; et al. Molecular Subtyping and Genomic Profiling Expand Precision Medicine in KRAS Wild-Type Pancreatic Cancer. Cancer Sci. 2025, 116, 1094–1106. [Google Scholar] [CrossRef] [PubMed]
- O’Haire, S.; Franchini, F.; Kang, Y.-J.; Steinberg, J.; Canfell, K.; Desai, J.; Fox, S.; Ijzerman, M. Systematic review of NTRK 1/2/3 fusion prevalence pan-cancer and across solid tumours. Sci. Rep. 2023, 13, 4116. [Google Scholar] [CrossRef]
- Brozos-Vázquez, E.; Toledano-Fonseca, M.; Costa-Fraga, N.; García-Ortiz, M.V.; Díaz-Lagares, Á.; Rodríguez-Ariza, A.; Aranda, E.; López-López, R. Pancreatic cancer biomarkers: A pathway to advance in personalized treatment selection. Cancer Treat. Rev. 2024, 125, 102719. [Google Scholar] [CrossRef]
- Amatu, A.; Sartore-Bianchi, A.; Bencardino, K.; Pizzutilo, E.G.; Tosi, F.; Siena, S. Tropomyosin receptor kinase (TRK) biology and the role of NTRK gene fusions in cancer. Ann. Oncol. 2019, 30, viii5–viii15. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Laetsch, T.W.; Kummar, S.; DuBois, S.G.; Lassen, U.N.; Demetri, G.D.; Nathenson, M.; Doebele, R.C.; Farago, A.F.; Pappo, A.S.; et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N. Engl. J. Med. 2018, 378, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; DuBois, S.G.; Kummar, S.; Farago, A.F.; Albert, C.M.; Rohrberg, K.S.; van Tilburg, C.M.; Nagasubramanian, R.; Berlin, J.D.; Federman, N.; et al. Larotrectinib in patients with TRK fusion-positive solid tumours: A pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020, 21, 531–540. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef]
- Pishvaian, M.J.; Rolfo, C.D.; Liu, S.V.; Multani, P.S.; Maneval, E.C.; Garrido-Laguna, I. Clinical benefit of entrectinib for patients with metastatic pancreatic cancer who harbor NTRK and ROS1 fusions. J. Clin. Oncol. 2018, 36, 521. [Google Scholar] [CrossRef]
- Zeng, Q.; Cheng, Y.; Zhu, Q.; Yu, Z.; Wu, X.; Huang, K.; Zhou, M.; Han, S.; Zhang, Q. The Relationship between Over-expression of Glial Cell-derived Neurotrophic Factor and Its RET Receptor with Progression and Prognosis of Human Pancreatic Cancer. J. Int. Med. Res. 2008, 36, 656–664. [Google Scholar] [CrossRef]
- Lian, E.Y.; Hyndman, B.D.; Moodley, S.; Maritan, S.M.; Mulligan, L.M. RET isoforms contribute differentially to invasive processes in pancreatic ductal adenocarcinoma. Oncogene 2020, 39, 6493–6510. [Google Scholar] [CrossRef]
- Ito, Y.; Okada, Y.; Sato, M.; Sawai, H.; Funahashi, H.; Murase, T.; Hayakawa, T.; Manabe, T. Expression of glial cell line-derived neurotrophic factor family members and their receptors in pancreatic cancers. Surgery 2005, 138, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Tahira, T.; Ishizaka, Y.; Itoh, F.; Sugimura, T.; Nagao, M. Characterization of ret proto-oncogene mRNAs encoding two isoforms of the protein product in a human neuroblastoma cell line. Oncogene 1990, 5, 97–102. [Google Scholar]
- Kato, S.; Subbiah, V.; Marchlik, E.; Elkin, S.K.; Carter, J.L.; Kurzrock, R. RET Aberrations in Diverse Cancers: Next-Generation Sequencing of 4,871 Patients. Clin. Cancer Res. 2017, 23, 1988–1997. [Google Scholar] [CrossRef] [PubMed]
- Hackshaw, A.; Fajardo, O.; Dafni, U.; Gelderblom, H.; Garrido, P.; Siena, S.; Taylor, M.H.; Bordogna, W.; Nikolaidis, C. Characteristics and Survival Outcomes of Patients with Metastatic RET Fusion–Positive Solid Tumors Receiving Non-RET Inhibitor Standards of Care in a Real-World Setting. JCO Precis. Oncol. 2024, 8, e2300334. [Google Scholar] [CrossRef] [PubMed]
- Duke, E.S.; Bradford, D.; Marcovitz, M.; Amatya, A.K.; Mishra-Kalyani, P.S.; Nguyen, E.; Price, L.S.L.; Fourie Zirkelbach, J.; Li, Y.; Bi, Y.; et al. FDA Approval Summary: Selpercatinib for the Treatment of Advanced RET Fusion-Positive Solid Tumors. Clin. Cancer Res. 2023, 29, 3573–3578. [Google Scholar] [CrossRef]
- Drilon, A.; Oxnard, G.R.; Tan, D.S.W.; Loong, H.H.F.; Johnson, M.; Gainor, J.; McCoach, C.E.; Gautschi, O.; Besse, B.; Cho, B.C.; et al. Efficacy of Selpercatinib in RET Fusion–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2020, 383, 813–824. [Google Scholar] [CrossRef]
- Subbiah, V.; Wolf, J.; Konda, B.; Kang, H.; Spira, A.; Weiss, J.; Takeda, M.; Ohe, Y.; Khan, S.; Ohashi, K.; et al. Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): A phase 1/2, open-label, basket trial. Lancet Oncol. 2022, 23, 1261–1273. [Google Scholar] [CrossRef]
- Wirth Lori, J.; Sherman, E.; Robinson, B.; Solomon, B.; Kang, H.; Lorch, J.; Worden, F.; Brose, M.; Patel, J.; Leboulleux, S.; et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N. Engl. J. Med. 2020, 383, 825–835. [Google Scholar] [CrossRef]
- Subbiah, V.; Drilon, A.E.; Sukrithan, V.; Spira, A.I.; Robinson, B.; Deschler-Baier, B.; Barker, S.; Lin, Y.; Szymczak, S.; Ohe, Y. Durable efficacy of selpercatinib in patients with RET fusion+ solid tumors, with a focus on GI tumors: LIBRETTO-001. J. Clin. Oncol. 2024, 42, 746. [Google Scholar] [CrossRef]
- Bhamidipati, D.; Yedururi, S.; Huse, J.; Chinapuvvula, S.V.; Wu, J.; Subbiah, V. Exceptional Responses to Selpercatinib in RET Fusion-Driven Metastatic Pancreatic Cancer. JCO Precis. Oncol. 2023, 7, e2300252. [Google Scholar] [CrossRef]
- Gainor, J.F.; Curigliano, G.; Kim, D.-W.; Lee, D.H.; Besse, B.; Baik, C.S.; Doebele, R.C.; Cassier, P.A.; Lopes, G.; Tan, D.S.W.; et al. Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): A multi-cohort, open-label, phase 1/2 study. Lancet Oncol. 2021, 22, 959–969. [Google Scholar] [CrossRef]
- Subbiah, V.; Hu, M.I.; Wirth, L.J.; Schuler, M.; Mansfield, A.S.; Curigliano, G.; Brose, M.S.; Zhu, V.W.; Leboulleux, S.; Bowles, D.W.; et al. Pralsetinib for patients with advanced or metastatic RET-altered thyroid cancer (ARROW): A multi-cohort, open-label, registrational, phase 1/2 study. Lancet Diabetes Endocrinol. 2021, 9, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Cassier, P.A.; Siena, S.; Garralda, E.; Paz-Ares, L.; Garrido, P.; Nadal, E.; Vuky, J.; Lopes, G.; Kalemkerian, G.P.; et al. Pan-cancer efficacy of pralsetinib in patients with RET fusion–positive solid tumors from the phase 1/2 ARROW trial. Nat. Med. 2022, 28, 1640–1645. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, H.; Cai, Z.; Zhang, S.; Jiang, C. RET rearrangement-positive pancreatic cancer has remarkable response to pralsetinib: A case report. Front. Oncol. 2023, 13, 1078076. [Google Scholar] [CrossRef]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef]
- Touat, M.; Ileana, E.; Postel-Vinay, S.; André, F.; Soria, J.-C. Targeting FGFR Signaling in Cancer. Clin. Cancer Res. 2015, 21, 2684–2694. [Google Scholar] [CrossRef]
- Gnatenko, D.A.; Kopantsev, E.P.; Sverdlov, E.D. The role of the signaling pathway FGF/FGFR in pancreatic cancer. Biochem. (Mosc.) Suppl. Ser. B Biomed. Chem. 2017, 11, 101–110. [Google Scholar] [CrossRef]
- Singh, D.; Chan, J.M.; Zoppoli, P.; Niola, F.; Sullivan, R.; Castano, A.; Liu, E.M.; Reichel, J.; Porrati, P.; Pellegatta, S.; et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science 2012, 337, 1231–1235. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, X.; Wang, S.; Moser, C.D.; Shaleh, H.M.; Mohamed, E.A.; Chaiteerakij, R.; Allotey, L.K.; Chen, G.; Miyabe, K.; et al. Antitumor effect of FGFR inhibitors on a novel cholangiocarcinoma patient derived xenograft mouse model endogenously expressing an FGFR2-CCDC6 fusion protein. Cancer Lett 2016, 380, 163–173. [Google Scholar] [CrossRef]
- Stein, L.; Murugesan, K.; Reeser, J.W.; Risch, Z.; Wing, M.R.; Paruchuri, A.; Samorodnitsky, E.; Hoskins, E.L.; Dao, T.; Smith, A.; et al. FGFR2-fusions define a clinically actionable molecular subset of pancreatic cancer. npj Precis. Oncol. 2024, 8, 207. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Necchi, A.; Park, S.H.; Garcia-Donas, J.; Huddart, R.; Burgess, E.; Fleming, M.; Rezazadeh, A.; Mellado, B.; Varlamov, S.; et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2019, 381, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Arnold, D.; Tabernero, J.; Loriot, Y.; Folprecht, G.; Haag, G.M.; Palmer, D.; Prenen, H.; Coward, J.; Lugowska, I.; et al. 1621P Efficacy and safety of erdafitinib in adults with pancreatic cancer and prespecified fibroblast growth factor receptor alterations (FGFRalt) in the phase II open-label: Single-arm RAGNAR trial. Ann. Oncol. 2023, 34, S898. [Google Scholar] [CrossRef]
- Pant, S.; Schuler, M.; Iyer, G.; Witt, O.; Doi, T.; Qin, S.; Tabernero, J.; Reardon, D.A.; Massard, C.; Minchom, A.; et al. Erdafitinib in patients with advanced solid tumours with FGFR alterations (RAGNAR): An international, single-arm, phase 2 study. Lancet Oncol. 2023, 24, 925–935. [Google Scholar] [CrossRef]
- Gong, J.; Mita, A.C.; Wei, Z.; Cheng, H.H.; Mitchell, E.P.; Wright, J.J.; Ivy, S.P.; Wang, V.; Gray, R.C.; McShane, L.M.; et al. Phase II Study of Erdafitinib in Patients with Tumors with FGFR Amplifications: Results From the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocol K1. JCO Precis. Oncol. 2024, 8, e2300406. [Google Scholar] [CrossRef]
- Gong, J.; Mita, A.C.; Wei, Z.; Cheng, H.H.; Mitchell, E.P.; Wright, J.J.; Ivy, S.P.; Wang, V.; Gray, R.C.; McShane, L.M.; et al. Phase II Study of Erdafitinib in Patients with Tumors with Fibroblast Growth Factor Receptor Mutations or Fusions: Results from the NCI-MATCH ECOG-ACRIN Trial (EAY131) Subprotocol K2. JCO Precis. Oncol. 2024, 8, e2300407. [Google Scholar] [CrossRef]
- Ng, C.F.; Glaspy, J.; Placencio-Hickok, V.R.; Thomassian, S.; Gong, J.; Osipov, A.; Hendifar, A.E.; Moshayedi, N. Exceptional Response to Erdafitinib in FGFR2-Mutated Metastatic Pancreatic Ductal Adenocarcinoma. J. Natl. Compr. Cancer Netw. 2022, 20, 1076–1079. [Google Scholar] [CrossRef]
- Poon, D.; Tan, M.H.; Khor, D. Stage 4 pancreatic adenocarcinoma harbouring an FGFR2-TACC2 fusion mutation with complete response to erdafitinib a pan-fibroblastic growth factor receptor inhibitor. BMJ Case Rep. 2021, 14, e244271. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Rodón, J.; Damian, S.; Furqan, M.; García-Donas, J.; Imai, H.; Italiano, A.; Spanggaard, I.; Ueno, M.; Yokota, T.; Veronese, M.L.; et al. Pemigatinib in previously treated solid tumors with activating FGFR1–FGFR3 alterations: Phase 2 FIGHT-207 basket trial. Nat. Med. 2024, 30, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Risch, Z.; Wing, M.; Reeser, J.; Stein, L.; Dao, T.; Smith, A.; Samorodnitsky, E.; Paruchuri, A.; Pan, X.J.; Roychowdhury, S. A phase II telemedicine study of pemigatinib in adult patients with unresectable or metastatic pancreas cancer with FGFR2 gene fusions or other FGFR genetic alterations. J. Clin. Oncol. 2024, 42, TPS717. [Google Scholar] [CrossRef]
- Helal, C.; Valéry, M.; Ducreux, M.; Hollebecque, A.; Smolenschi, C. FGFR2 fusion in metastatic pancreatic ductal adenocarcinoma: Is there hope? Eur. J. Cancer 2022, 176, 168–170. [Google Scholar] [CrossRef]
- Olayioye, M.A.; Neve, R.M.; Lane, H.A.; Hynes, N.E. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000, 19, 3159–3167. [Google Scholar] [CrossRef]
- Fernandez-Cuesta, L.; Thomas, R.K. Molecular Pathways: Targeting NRG1 Fusions in Lung Cancer. Clin. Cancer Res. 2015, 21, 1989–1994. [Google Scholar] [CrossRef]
- Alimandi, M.; Romano, A.; Curia, M.C.; Muraro, R.; Fedi, P.; Aaronson, S.A.; Di Fiore, P.P.; Kraus, M.H. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene 1995, 10, 1813–1821. [Google Scholar]
- Sheng, Q.; Liu, X.; Fleming, E.; Yuan, K.; Piao, H.; Chen, J.; Moustafa, Z.; Thomas, R.K.; Greulich, H.; Schinzel, A.; et al. An Activated ErbB3/NRG1 Autocrine Loop Supports In Vivo Proliferation in Ovarian Cancer Cells. Cancer Cell 2010, 17, 298–310. [Google Scholar] [CrossRef]
- Laskin, J.; Liu, S.V.; Tolba, K.; Heining, C.; Schlenk, R.F.; Cheema, P.; Cadranel, J.; Jones, M.R.; Drilon, A.; Cseh, A.; et al. NRG1 fusion-driven tumors: Biology, detection, and the therapeutic role of afatinib and other ErbB-targeting agents. Ann. Oncol. 2020, 31, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Drilon, A.; Somwar, R.; Mangatt, B.P.; Edgren, H.; Desmeules, P.; Ruusulehto, A.; Smith, R.S.; Delasos, L.; Vojnic, M.; Plodkowski, A.J.; et al. Response to ERBB3-Directed Targeted Therapy in NRG1-Rearranged Cancers. Cancer Discov. 2018, 8, 686–695. [Google Scholar] [CrossRef]
- Jonna, S.; Feldman, R.A.; Swensen, J.; Gatalica, Z.; Korn, W.M.; Borghaei, H.; Ma, P.C.; Nieva, J.J.; Spira, A.I.; Vanderwalde, A.M.; et al. Detection of NRG1 Gene Fusions in Solid Tumors. Clin. Cancer Res. 2019, 25, 4966–4972. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.J. Oncogenic NRG1 Fusions: A New Hope for Targeted Therapy in Pancreatic Cancer. Clin. Cancer Res. 2019, 25, 4589–4591. [Google Scholar] [CrossRef] [PubMed]
- Heining, C.; Horak, P.; Uhrig, S.; Codo, P.L.; Klink, B.; Hutter, B.; Fröhlich, M.; Bonekamp, D.; Richter, D.; Steiger, K.; et al. NRG1 Fusions in KRAS Wild-Type Pancreatic Cancer. Cancer Discov. 2018, 8, 1087–1095. [Google Scholar] [CrossRef]
- Fernandez-Cuesta, L.; Plenker, D.; Osada, H.; Sun, R.; Menon, R.; Leenders, F.; Ortiz-Cuaran, S.; Peifer, M.; Bos, M.; Daßler, J.; et al. CD74–NRG1 Fusions in Lung Adenocarcinoma. Cancer Discov. 2014, 4, 415–422. [Google Scholar] [CrossRef]
- Liu, S.V.; Frohn, C.; Minasi, L.; Fernamberg, K.; Klink, A.J.; Gajra, A.; Savill, K.M.Z.; Jonna, S. Real-world outcomes associated with afatinib use in patients with solid tumors harboring NRG1 gene fusions. Lung Cancer 2024, 188, 107469. [Google Scholar] [CrossRef]
- Cadranel, J.; Liu, S.V.; Duruisseaux, M.; Branden, E.; Goto, Y.; Weinberg, B.A.; Heining, C.; Schlenk, R.F.; Cheema, P.; Jones, M.R.; et al. Therapeutic Potential of Afatinib in NRG1 Fusion-Driven Solid Tumors: A Case Series. Oncologist 2021, 26, 7–16. [Google Scholar] [CrossRef]
- Huguet, F.; Fernet, M.; Giocanti, N.; Favaudon, V.; Larsen, A.K. Afatinib, an Irreversible EGFR Family Inhibitor, Shows Activity Toward Pancreatic Cancer Cells, Alone and in Combination with Radiotherapy, Independent of KRAS Status. Target. Oncol. 2016, 11, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.M.; Odintsov, I.; Espinosa-Cotton, M.; Khodos, I.; Sisso, W.J.; Mattar, M.S.; Lui, A.J.W.; Vojnic, M.; Shameem, S.H.; Chauhan, T.; et al. Zenocutuzumab, a HER2xHER3 Bispecific Antibody, Is Effective Therapy for Tumors Driven by NRG1 Gene Rearrangements. Cancer Discov. 2022, 12, 1233–1247. [Google Scholar] [CrossRef]
- Schram, A.M.; Goto, K.; Kim, D.-W.; Macarulla, T.; Hollebecque, A.; O’Reilly, E.M.; Ou, S.-H.I.; Rodon, J.; Rha, S.Y.; Nishino, K.; et al. Efficacy of Zenocutuzumab in NRG1 Fusion–Positive Cancer. N. Engl. J. Med. 2025, 392, 566–576. [Google Scholar] [CrossRef]
- FDA Grants Accelerated Approval to Zenocutuzumab-Zbco for Non-Small Cell Lung Cancer and Pancreatic Adenocarcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-zenocutuzumab-zbco-non-small-cell-lung-cancer-and-pancreatic (accessed on 13 August 2025).
- Odintsov, I.; Lui, A.J.W.; Sisso, W.J.; Gladstone, E.; Liu, Z.; Delasos, L.; Kurth, R.I.; Sisso, E.M.; Vojnic, M.; Khodos, I.; et al. The Anti-HER3 mAb Seribantumab Effectively Inhibits Growth of Patient-Derived and Isogenic Cell Line and Xenograft Models with Oncogenic NRG1 Fusions. Clin. Cancer Res. 2021, 27, 3154–3166. [Google Scholar] [CrossRef] [PubMed]
- Carrizosa, D.R.; Burkard, M.E.; Elamin, Y.Y.; Desai, J.; Gadgeel, S.M.; Lin, J.J.; Waqar, S.N.; Spigel, D.R.; Chae, Y.K.; Cheema, P.K.; et al. CRESTONE: Initial efficacy and safety of seribantumab in solid tumors harboring NRG1 fusions. J. Clin. Oncol. 2022, 40, 3006. [Google Scholar] [CrossRef]
- Patil, T.; Carrizosa, D.R.; Burkard, M.E.; Reckamp, K.L.; Desai, J.; Chae, Y.K.; Liu, S.V.; Konduri, K.; Gadgeel, S.M.; Lin, J.J.; et al. Abstract CT229: CRESTONE: A Phase 2 study of seribantumab in adult patients with neuregulin-1 (NRG1) fusion positive locally advanced or metastatic solid tumors. Cancer Res. 2023, 83, CT229. [Google Scholar] [CrossRef]
- Hughes, B.; Mileshkin, L.; Townley, P.; Gitlitz, B.; Eaton, K.; Mitchell, P.; Hicks, R.; Wood, K.; Amler, L.; Fine, B.M.; et al. Pertuzumab and Erlotinib in Patients with Relapsed Non-Small Cell Lung Cancer: A Phase II Study Using 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Imaging. Oncologist 2014, 19, 175–176. [Google Scholar] [CrossRef]
- Philip, P.A.; Azar, I.; Xiu, J.; Hall, M.J.; Hendifar, A.E.; Lou, E.; Hwang, J.J.; Gong, J.; Feldman, R.; Ellis, M.; et al. Molecular Characterization of KRAS Wild-type Tumors in Patients with Pancreatic Adenocarcinoma. Clin. Cancer Res. 2022, 28, 2704–2714. [Google Scholar] [CrossRef]
- Gupta, R.; Meric-Bernstam, F.; Rothe, M.; Garrett-Mayer, E.; Mangat, P.K.; D’Andre, S.; Ahn, E.R.; O’Lone, R.; Halabi, S.; Grantham, G.N.; et al. Pertuzumab Plus Trastuzumab in Patients with Colorectal Cancer with ERBB2 Amplification or ERBB2/3 Mutations: Results From the TAPUR Study. JCO Precis. Oncol. 2022, 6, e2200306. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Meric-Bernstam, F.; Swanton, C.; Hurwitz, H.; Spigel, D.R.; Sweeney, C.; Burris, H.A.; Bose, R.; Yoo, B.; Stein, A.; et al. Targeted Therapy for Advanced Solid Tumors on the Basis of Molecular Profiles: Results From MyPathway, an Open-Label, Phase IIa Multiple Basket Study. J. Clin. Oncol. 2018, 36, 536–542. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Bai, Y.; Xu, J.; Lonardi, S.; Metges, J.P.; Yanez Weber, P.E.; Wyrwicz, L.S.; Shen, L.; Ostapenko, Y.V.; et al. 1400O Final overall survival for the phase III, KEYNOTE-811 study of pembrolizumab plus trastuzumab and chemotherapy for HER2+ advanced, unresectable or metastatic G/GEJ adenocarcinoma. Ann. Oncol. 2024, 35, S877–S878. [Google Scholar] [CrossRef]
- King, D.A.; Smith, A.R.; Pineda, G.; Nakano, M.; Michelini, F.; Goedegebuure, S.P.; Thyparambil, S.; Liao, W.-L.; McCormick, A.; Ju, J.; et al. Complete Remission of Widely Metastatic Human Epidermal Growth Factor Receptor 2–Amplified Pancreatic Adenocarcinoma After Precision Immune and Targeted Therapy with Description of Sequencing and Organoid Correlates. JCO Precis. Oncol. 2023, 7, e2100489. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Ikeda, S.; Kim, K.H.; Lim, H.J.; Adashek, J.J.; Persha, H.E.; Okamura, R.; Lee, S.; Sicklick, J.K.; Kato, S.; et al. Targeting the FGF/FGFR axis and its co-alteration allies. ESMO Open 2022, 7, 100647. [Google Scholar] [CrossRef]
- Cocco, E.; Scaltriti, M.; Drilon, A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat. Rev. Clin. Oncol. 2018, 15, 731–747. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Sen, S.; Park, H.; Heist, R.S.; Gadgeel, S.M.; Zimmerman, Z.F.; Bazhenova, L. A phase I, open-label, multicenter, first-in-human study of the safety, tolerability, pharmacokinetics, and antitumor activity of TPX-0022, a novel MET/CSF1R/SRC inhibitor, in patients with advanced solid tumors harboring genetic alterations in MET. J. Clin. Oncol. 2020, 38, TPS3663. [Google Scholar] [CrossRef]
- Mouawad, A.; Habib, S.; Boutros, M.; Attieh, F.; Kourie, H.R. How to find a needle in a haystack: A systematic review on targeting KRAS wild-type pancreatic cancer. Future Oncol. 2024, 20, 3539–3547. [Google Scholar] [CrossRef]
- Del Curatolo, A.; Conciatori, F.; Cesta Incani, U.; Bazzichetto, C.; Falcone, I.; Corbo, V.; D’Agosto, S.; Eramo, A.; Sette, G.; Sperduti, I.; et al. Therapeutic potential of combined BRAF/MEK blockade in BRAF-wild type preclinical tumor models. J. Exp. Clin. Cancer Res. 2018, 37, 140. [Google Scholar] [CrossRef]
- Zaanan, A.; Dabout, V.; Garinet, S.; Giraud, D.; Perkins, G.; Taieb, J.; Gallois, C. Encorafenib, binimetinib and cetuximab in BRAF V600E-mutated advanced pancreatic adenocarcinoma. ESMO Open 2024, 9, 103975. [Google Scholar] [CrossRef] [PubMed]
- Reissig, T.M.; Tzianopoulos, I.; Liffers, S.T.; Rosery, V.K.; Guyot, M.; Ting, S.; Wiesweg, M.; Kasper, S.; Meister, P.; Herold, T.; et al. Smaller panel, similar results: Genomic profiling and molecularly informed therapy in pancreatic cancer. ESMO Open 2023, 8, 101539. [Google Scholar] [CrossRef]
- Lee, M.S.; Pant, S. Personalizing Medicine with Germline and Somatic Sequencing in Advanced Pancreatic Cancer: Current Treatments and Novel Opportunities. Am. Soc. Clin. Oncol. Educ. Book 2021, 41, e153–e165. [Google Scholar] [CrossRef]
- Singhi, A.D.; George, B.; Greenbowe, J.R.; Chung, J.; Suh, J.; Maitra, A.; Klempner, S.J.; Hendifar, A.; Milind, J.M.; Golan, T.; et al. Real-Time Targeted Genome Profile Analysis of Pancreatic Ductal Adenocarcinomas Identifies Genetic Alterations That Might Be Targeted with Existing Drugs or Used as Biomarkers. Gastroenterology 2019, 156, 2242–2253.e2244. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.T.; Yang, W.W.; Niu, Y.R.; Sun, Y.K. Recent advances in targeted therapy for pancreatic adenocarcinoma. World J. Gastrointest. Oncol. 2023, 15, 571–595. [Google Scholar] [CrossRef] [PubMed]
- Assenat, E.; Azria, D.; Mollevi, C.; Guimbaud, R.; Tubiana-Mathieu, N.; Smith, D.; Delord, J.-P.; Samalin, E.; Portales, F.; Larbouret, C.; et al. Dual targeting of HER1/EGFR and HER2 with cetuximab and trastuzumab in patients with metastatic pancreatic cancer after gemcitabine failure: Results of the “THERAPY”phase 1-2 trial. Oncotarget 2015, 6, 12796–12808. [Google Scholar] [CrossRef]
- Oaknin, A.; Lee, J.Y.; Makker, V.; Oh, D.Y.; Banerjee, S.; González-Martín, A.; Jung, K.H.; Ługowska, I.; Manso, L.; Manzano, A.; et al. Efficacy of Trastuzumab Deruxtecan in HER2-Expressing Solid Tumors by Enrollment HER2 IHC Status: Post Hoc Analysis of DESTINY-PanTumor02. Adv. Ther. 2024, 41, 4125–4139. [Google Scholar] [CrossRef]
- Harder, J.; Ihorst, G.; Heinemann, V.; Hofheinz, R.; Moehler, M.; Buechler, P.; Kloeppel, G.; Röcken, C.; Bitzer, M.; Boeck, S.; et al. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br. J. Cancer 2012, 106, 1033–1038. [Google Scholar] [CrossRef]
- Haas, M.; Waldschmidt, D.T.; Stahl, M.; Reinacher-Schick, A.; Freiberg-Richter, J.; Fischer von Weikersthal, L.; Kaiser, F.; Kanzler, S.; Frickhofen, N.; Seufferlein, T.; et al. Afatinib plus gemcitabine versus gemcitabine alone as first-line treatment of metastatic pancreatic cancer: The randomised, open-label phase II ACCEPT study of the Arbeitsgemeinschaft Internistische Onkologie with an integrated analysis of the ‘burden of therapy’ method. Eur. J Cancer 2021, 146, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Pitiyarachchi, O.; Sim, H.W. 256P PIK3CA mutations in pancreatic ductal adenocarcinoma (PDAC). Ann. Oncol. 2024, 35, S1499. [Google Scholar] [CrossRef]
- Stanciu, S.; Ionita-Radu, F.; Stefani, C.; Miricescu, D.; Stanescu, S., II; Greabu, M.; Ripszky Totan, A.; Jinga, M. Targeting PI3K/AKT/mTOR Signaling Pathway in Pancreatic Cancer: From Molecular to Clinical Aspects. Int. J. Mol. Sci. 2022, 23, 10132. [Google Scholar] [CrossRef]
- Kordes, S.; Klümpen, H.J.; Weterman, M.J.; Schellens, J.H.; Richel, D.J.; Wilmink, J.W. Phase II study of capecitabine and the oral mTOR inhibitor everolimus in patients with advanced pancreatic cancer. Cancer Chemother. Pharmacol. 2015, 75, 1135–1141. [Google Scholar] [CrossRef]
- Kordes, S.; Pollak, M.N.; Zwinderman, A.H.; Mathôt, R.A.; Weterman, M.J.; Beeker, A.; Punt, C.J.; Richel, D.J.; Wilmink, J.W. Metformin in patients with advanced pancreatic cancer: A double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2015, 16, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Bever, K.M.; Borazanci, E.H.; Thompson, E.A.; Durham, J.N.; Pinero, K.; Jameson, G.S.; Vrana, A.; Liu, M.; Wilt, C.; Wu, A.A.; et al. An exploratory study of metformin with or without rapamycin as maintenance therapy after induction chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncotarget 2020, 11, 1929–1941. [Google Scholar] [CrossRef] [PubMed]
- Wainberg, Z.A.; Alsina, M.; Soares, H.P.; Braña, I.; Britten, C.D.; Del Conte, G.; Ezeh, P.; Houk, B.; Kern, K.A.; Leong, S.; et al. A Multi-Arm Phase I Study of the PI3K/mTOR Inhibitors PF-04691502 and Gedatolisib (PF-05212384) plus Irinotecan or the MEK Inhibitor PD-0325901 in Advanced Cancer. Target Oncol. 2017, 12, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Schram, A.M.; Gandhi, L.; Mita, M.M.; Damstrup, L.; Campana, F.; Hidalgo, M.; Grande, E.; Hyman, D.M.; Heist, R.S. A phase Ib dose-escalation and expansion study of the oral MEK inhibitor pimasertib and PI3K/MTOR inhibitor voxtalisib in patients with advanced solid tumours. Br. J. Cancer 2018, 119, 1471–1476. [Google Scholar] [CrossRef]
- Awasthi, N.; Yen, P.L.; Schwarz, M.A.; Schwarz, R.E. The efficacy of a novel, dual PI3K/mTOR inhibitor NVP-BEZ235 to enhance chemotherapy and antiangiogenic response in pancreatic cancer. J. Cell. Biochem. 2012, 113, 784–791. [Google Scholar] [CrossRef]
- Munster, P.; Aggarwal, R.; Hong, D.; Schellens, J.H.; van der Noll, R.; Specht, J.; Witteveen, P.O.; Werner, T.L.; Dees, E.C.; Bergsland, E.; et al. First-in-Human Phase I Study of GSK2126458, an Oral Pan-Class I Phosphatidylinositol-3-Kinase Inhibitor, in Patients with Advanced Solid Tumor Malignancies. Clin. Cancer Res. 2016, 22, 1932–1939. [Google Scholar] [CrossRef]
- Maitra, A.; Hruban, R.H. A new mouse model of pancreatic cancer: PTEN gets its Akt together. Cancer Cell 2005, 8, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Elpek, K.G.; Vinjamoori, A.; Zimmerman, S.M.; Chu, G.C.; Yan, H.; Fletcher-Sananikone, E.; Zhang, H.; Liu, Y.; Wang, W.; et al. PTEN is a major tumor suppressor in pancreatic ductal adenocarcinoma and regulates an NF-κB-cytokine network. Cancer Discov. 2011, 1, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Rosty, C.; Jansen, M.; Fukushima, N.; Ueki, T.; Yeo, C.J.; Cameron, J.L.; Iacobuzio-Donahue, C.A.; Hruban, R.H.; Goggins, M. STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am. J. Pathol. 2001, 159, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yun, D.; Yang, H.; Eckstein, M.; Elbait, G.D.; Zhou, Y.; Lu, Y.; Yang, H.; Zhang, J.; Dörflein, I.; et al. Roflumilast inhibits tumor growth and migration in STK11/LKB1 deficient pancreatic cancer. Cell Death Discov. 2024, 10, 124. [Google Scholar] [CrossRef]
- Mullen, J.; Kato, S.; Sicklick, J.K.; Kurzrock, R. Targeting ARID1A mutations in cancer. Cancer Treat. Rev. 2021, 100, 102287. [Google Scholar] [CrossRef]
- Shen, J.; Peng, Y.; Wei, L.; Zhang, W.; Yang, L.; Lan, L.; Kapoor, P.; Ju, Z.; Mo, Q.; Shih Ie, M.; et al. ARID1A Deficiency Impairs the DNA Damage Checkpoint and Sensitizes Cells to PARP Inhibitors. Cancer Discov. 2015, 5, 752–767. [Google Scholar] [CrossRef]
- Bitler, B.G.; Aird, K.M.; Garipov, A.; Li, H.; Amatangelo, M.; Kossenkov, A.V.; Schultz, D.C.; Liu, Q.; Shih Ie, M.; Conejo-Garcia, J.R.; et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat. Med. 2015, 21, 231–238. [Google Scholar] [CrossRef]
- Li, R.; Xiong, G.; Zhao, J.; Yang, L. Targeting the alterations of ARID1A in pancreatic cancer: Tumorigenesis, prediction of treatment, and prognostic value. Am. J. Transl. Res. 2022, 14, 5952–5964. [Google Scholar]
- Okamura, R.; Kato, S.; Lee, S.; Jimenez, R.E.; Sicklick, J.K.; Kurzrock, R. ARID1A alterations function as a biomarker for longer progression-free survival after anti-PD-1/PD-L1 immunotherapy. J. Immunother. Cancer 2020, 8, e000438. [Google Scholar] [CrossRef]
- Botta, G.P.; Kato, S.; Patel, H.; Fanta, P.; Lee, S.; Okamura, R.; Kurzrock, R. SWI/SNF complex alterations as a biomarker of immunotherapy efficacy in pancreatic cancer. JCI Insight 2021, 6, e150453. [Google Scholar] [CrossRef]
- Mardinian, K.; Adashek, J.J.; Botta, G.P.; Kato, S.; Kurzrock, R. SMARCA4: Implications of an Altered Chromatin-Remodeling Gene for Cancer Development and Therapy. Mol. Cancer Ther. 2021, 20, 2341–2351. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Fan, Z.; Su, P.; Zhan, H. An unrecognized undifferentiated tumor of the pancreas: A case report. J. Pancreatol. 2023, 6, 225–227. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Bandlamudi, C.; Lavery, J.A.; Montecalvo, J.; Namakydoust, A.; Rizvi, H.; Egger, J.; Concepcion, C.P.; Paul, S.; Arcila, M.E.; et al. The Genomic Landscape of SMARCA4 Alterations and Associations with Outcomes in Patients with Lung Cancer. Clin. Cancer Res. 2020, 26, 5701–5708. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Xu, L.; Li, X.; Li, H.; Zhao, M. SMARCA4: Current status and future perspectives in non-small-cell lung cancer. Cancer Lett. 2023, 554, 216022. [Google Scholar] [CrossRef]
- Kotagiri, S.; Blazanin, N.; Xi, Y.; Han, Y.; Qudratullah, M.; Liang, X.; Wang, Y.; Pandey, P.; Mazhar, H.; Lam, T.N.; et al. Enhancer reprogramming underlies therapeutic utility of a SMARCA2 degrader in SMARCA4 mutant cancer. Cell Chem. Biol. 2024, 31, 2069–2084.e2069. [Google Scholar] [CrossRef]
- Cantley, J.; Ye, X.; Rousseau, E.; Januario, T.; Hamman, B.D.; Rose, C.M.; Cheung, T.K.; Hinkle, T.; Soto, L.; Quinn, C.; et al. Selective PROTAC-mediated degradation of SMARCA2 is efficacious in SMARCA4 mutant cancers. Nat. Commun. 2022, 13, 6814. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cowart, M.; Cote, J.; Kurian, J.; Rodgers, S.; Xu, C.; Basch, C.; Pitis, P.; Bersch, K.; Leal, R.; et al. Abstract 1646: Elucidating the molecular mechanism of action of the first in human SMARCA2 selective degrader PRT3789. Cancer Res. 2025, 85, 1646. [Google Scholar] [CrossRef]
- Guo, R.; Dowlati, A.; Dagogo-Jack, I.; Vibert, J.; Spira, A.I.; Moreno Garcia, V.; Punekar, S.; Calvo, E.; Sonpavde, G.P.; Awad, M.; et al. 603O First clinical results from a phase I trial of PRT3789: A first-in-class intravenous SMARCA2 degrader, in patients with advanced solid tumors with a SMARCA4 mutation. Ann. Oncol. 2024, 35, S483–S484. [Google Scholar] [CrossRef]
- Yang, L.; Tu, W.; Leng, L.; Huang, L.; Jiang, W.; Wang, M.; Wang, Y.; Meagher, J.L.; Chinnaswamy, K.; Stuckey, J.A.; et al. Discovery of SMD-3236: A Potent, Highly Selective and Efficacious SMARCA2 Degrader for the Treatment of SMARC4-Deficient Human Cancers. J. Med. Chem. 2025, 68, 1155–1178. [Google Scholar] [CrossRef]
- Modrich, P.; Lahue, R. Mismatch repair in replication fidelity, genetic recombination, and cancer biology. Annu. Rev. Biochem. 1996, 65, 101–133. [Google Scholar] [CrossRef]
- Marti, T.M.; Kunz, C.; Fleck, O. DNA mismatch repair and mutation avoidance pathways. J. Cell. Physiol. 2002, 191, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Räschle, M.; Marra, G.; Nyström-Lahti, M.; Schär, P.; Jiricny, J. Identification of hMutLβ, a Heterodimer of hMLH1 and hPMS1*. J. Biol. Chem. 1999, 274, 32368–32375. [Google Scholar] [CrossRef]
- Tomer, G.; Buermeyer, A.B.; Nguyen, M.M.; Liskay, R.M. Contribution of Human Mlh1 and Pms2 ATPase Activities to DNA Mismatch Repair*. J. Biol. Chem. 2002, 277, 21801–21809. [Google Scholar] [CrossRef]
- Li, G.M.; Modrich, P. Restoration of mismatch repair to nuclear extracts of H6 colorectal tumor cells by a heterodimer of human MutL homologs. Proc. Natl. Acad. Sci. USA 1995, 92, 1950–1954. [Google Scholar] [CrossRef]
- Modrich, P. Mechanisms in eukaryotic mismatch repair. J. Biol. Chem. 2006, 281, 30305–30309. [Google Scholar] [CrossRef]
- Li, G.-M. Mechanisms and functions of DNA mismatch repair. Cell Res. 2008, 18, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Lupinacci, R.M.; Goloudina, A.; Buhard, O.; Bachet, J.B.; Maréchal, R.; Demetter, P.; Cros, J.; Bardier-Dupas, A.; Collura, A.; Cervera, P.; et al. Prevalence of Microsatellite Instability in Intraductal Papillary Mucinous Neoplasms of the Pancreas. Gastroenterology 2018, 154, 1061–1065. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Brosens, L.A.A.; Wood, L.D.; Chatterjee, D.; Shin, J.I.; Sciammarella, C.; Fiadone, G.; Malleo, G.; Salvia, R.; Kryklyva, V.; et al. Comprehensive characterisation of pancreatic ductal adenocarcinoma with microsatellite instability: Histology, molecular pathology and clinical implications. Gut 2021, 70, 148–156. [Google Scholar] [CrossRef]
- Ogobuiro, I.; Baca, Y.; Ribeiro, J.R.; Walker, P.; Wilson, G.C.; Gulhati, P.; Marshall, J.L.; Shroff, R.T.; Spetzler, D.; Oberley, M.J.; et al. Multiomic Characterization Reveals a Distinct Molecular Landscape in Young-Onset Pancreatic Cancer. JCO Precis. Oncol. 2023, 7, e2300152. [Google Scholar] [CrossRef]
- Renouf, D.J.; Loree, J.M.; Knox, J.J.; Topham, J.T.; Kavan, P.; Jonker, D.; Welch, S.; Couture, F.; Lemay, F.; Tehfe, M.; et al. The CCTG PA.7 phase II trial of gemcitabine and nab-paclitaxel with or without durvalumab and tremelimumab as initial therapy in metastatic pancreatic ductal adenocarcinoma. Nat. Commun. 2022, 13, 5020. [Google Scholar] [CrossRef]
- Mucileanu, A.; Chira, R.; Mircea, P.A. PD-1/PD-L1 expression in pancreatic cancer and its implication in novel therapies. Med. Pharm. Rep. 2021, 94, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Howitt, B.E.; Shukla, S.A.; Sholl, L.M.; Ritterhouse, L.L.; Watkins, J.C.; Rodig, S.; Stover, E.; Strickland, K.C.; D’Andrea, A.D.; Wu, C.J.; et al. Association of Polymerase e–Mutated and Microsatellite-Instable Endometrial Cancers with Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015, 1, 1319–1323. [Google Scholar] [CrossRef]
- Nomi, T.; Sho, M.; Akahori, T.; Hamada, K.; Kubo, A.; Kanehiro, H.; Nakamura, S.; Enomoto, K.; Yagita, H.; Azuma, M.; et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin. Cancer Res. 2007, 13, 2151–2157. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012, 366, 2455–2465. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2020, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Cercek, A.; Lumish, M.; Sinopoli, J.; Weiss, J.; Shia, J.; Lamendola-Essel, M.; El Dika, I.H.; Segal, N.; Shcherba, M.; Sugarman, R.; et al. PD-1 Blockade in Mismatch Repair-Deficient, Locally Advanced Rectal Cancer. N. Engl. J. Med. 2022, 386, 2363–2376. [Google Scholar] [CrossRef]
- André, T.; Berton, D.; Curigliano, G.; Sabatier, R.; Tinker, A.V.; Oaknin, A.; Ellard, S.; de Braud, F.; Arkenau, H.-T.; Trigo, J.; et al. Antitumor Activity and Safety of Dostarlimab Monotherapy in Patients with Mismatch Repair Deficient Solid Tumors: A Nonrandomized Controlled Trial. JAMA Netw. Open 2023, 6, e2341165. [Google Scholar] [CrossRef]
- FDA Grants Accelerated Approval to Dostarlimab-Gxly for dMMR Advanced Solid Tumors. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dostarlimab-gxly-dmmr-advanced-solid-tumors (accessed on 14 October 2025).
- Yap, T.A.; Bessudo, A.; Hamilton, E.; Sachdev, J.; Patel, M.R.; Rodon, J.; Evilevitch, L.; Duncan, M.; Guo, W.; Kumar, S.; et al. IOLite: Phase 1b trial of doublet/triplet combinations of dostarlimab with niraparib, carboplatin-paclitaxel, with or without bevacizumab in patients with advanced cancer. J. Immunother. Cancer 2022, 10, e003924. [Google Scholar] [CrossRef]
- Incorvaia, L.; Bazan Russo, T.D.; Gristina, V.; Perez, A.; Brando, C.; Mujacic, C.; Di Giovanni, E.; Bono, M.; Contino, S.; Ferrante Bannera, C.; et al. The intersection of homologous recombination (HR) and mismatch repair (MMR) pathways in DNA repair-defective tumors. npj Precis. Oncol. 2024, 8, 190. [Google Scholar] [CrossRef]
- Reiss, K.A.; Mick, R.; Teitelbaum, U.; O’Hara, M.; Schneider, C.; Massa, R.; Karasic, T.; Tondon, R.; Onyiah, C.; Gosselin, M.K.; et al. Niraparib plus nivolumab or niraparib plus ipilimumab in patients with platinum-sensitive advanced pancreatic cancer: A randomised, phase 1b/2 trial. Lancet Oncol. 2022, 23, 1009–1020. [Google Scholar] [CrossRef]
- Eikenboom, E.L.; Nasar, N.; Seier, K.; Gönen, M.; Spaander, M.C.W.; O’Reilly, E.M.; Jarnagin, W.R.; Drebin, J.; D’Angelica, M.I.; Kingham, T.P.; et al. Survival of Patients with Resected Microsatellite Instability-High, Mismatch Repair Deficient, and Lynch Syndrome-Associated Pancreatic Ductal Adenocarcinomas. Ann. Surg. Oncol. 2025, 32, 3568–3577. [Google Scholar] [CrossRef] [PubMed]
- Casolino, R.; Corbo, V.; Beer, P.; Hwang, C.I.; Paiella, S.; Silvestri, V.; Ottini, L.; Biankin, A.V. Germline Aberrations in Pancreatic Cancer: Implications for Clinical Care. Cancers 2022, 14, 3239. [Google Scholar] [CrossRef]
- Astiazaran-Symonds, E.; Goldstein, A.M. A systematic review of the prevalence of germline pathogenic variants in patients with pancreatic cancer. J. Gastroenterol. 2021, 56, 713–721. [Google Scholar] [CrossRef]
- Pantaleo, A.; Forte, G.; Fasano, C.; Lepore Signorile, M.; Sanese, P.; De Marco, K.; Di Nicola, E.; Latrofa, M.; Grossi, V.; Disciglio, V.; et al. Understanding the Genetic Landscape of Pancreatic Ductal Adenocarcinoma to Support Personalized Medicine: A Systematic Review. Cancers 2023, 16, 56. [Google Scholar] [CrossRef]
- Rainone, M.; Singh, I.; Salo-Mullen, E.E.; Stadler, Z.K.; O’Reilly, E.M. An Emerging Paradigm for Germline Testing in Pancreatic Ductal Adenocarcinoma and Immediate Implications for Clinical Practice: A Review. JAMA Oncol. 2020, 6, 764–771. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017, 32, 185–203.e113. [Google Scholar] [CrossRef] [PubMed]
- Rosso, C.; Marciano, N.D.; Nathan, D.; Chen, W.P.; McLaren, C.E.; Osann, K.E.; Flodman, P.L.; Cho, M.T.; Lee, F.C.; Dayyani, F.; et al. Hereditary Cancer Clinics Improve Adherence to NCCN Germline Testing Guidelines for Pancreatic Cancer. J. Natl. Compr. Cancer Netw. 2024, 22, 299–305. [Google Scholar] [CrossRef]
- Tung, N.; Ricker, C.; Messersmith, H.; Balmaña, J.; Domchek, S.; Stoffel, E.M.; Almhanna, K.; Arun, B.; Chavarri-Guerra, Y.; Cohen, S.A.; et al. Selection of Germline Genetic Testing Panels in Patients with Cancer: ASCO Guideline. J. Clin. Oncol. 2024, 42, 2599–2615. [Google Scholar] [CrossRef]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef]
- Hu, C.; Hart, S.N.; Polley, E.C.; Gnanaolivu, R.; Shimelis, H.; Lee, K.Y.; Lilyquist, J.; Na, J.; Moore, R.; Antwi, S.O.; et al. Association Between Inherited Germline Mutations in Cancer Predisposition Genes and Risk of Pancreatic Cancer. JAMA 2018, 319, 2401–2409. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Roy, R.; Chun, J.; Powell, S.N. BRCA1 and BRCA2: Different roles in a common pathway of genome protection. Nat. Rev. Cancer 2011, 12, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Shindo, K.; Yu, J.; Suenaga, M.; Fesharakizadeh, S.; Cho, C.; Macgregor-Das, A.; Siddiqui, A.; Witmer, P.D.; Tamura, K.; Song, T.J.; et al. Deleterious Germline Mutations in Patients with Apparently Sporadic Pancreatic Adenocarcinoma. J. Clin. Oncol. 2017, 35, 3382–3390. [Google Scholar] [CrossRef] [PubMed]
- Holter, S.; Borgida, A.; Dodd, A.; Grant, R.; Semotiuk, K.; Hedley, D.; Dhani, N.; Narod, S.; Akbari, M.; Moore, M.; et al. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients with Pancreatic Adenocarcinoma. J. Clin. Oncol. 2015, 33, 3124–3129. [Google Scholar] [CrossRef] [PubMed]
- Yurgelun, M.B.; Chittenden, A.B.; Morales-Oyarvide, V.; Rubinson, D.A.; Dunne, R.F.; Kozak, M.M.; Qian, Z.R.; Welch, M.W.; Brais, L.K.; Da Silva, A.; et al. Germline cancer susceptibility gene variants, somatic second hits, and survival outcomes in patients with resected pancreatic cancer. Genet. Med. 2019, 21, 213–223. [Google Scholar] [CrossRef]
- Leibowitz, B.D.; Dougherty, B.V.; Bell, J.S.K.; Kapilivsky, J.; Michuda, J.; Sedgewick, A.J.; Munson, W.A.; Chandra, T.A.; Dry, J.R.; Beaubier, N.; et al. Validation of genomic and transcriptomic models of homologous recombination deficiency in a real-world pan-cancer cohort. BMC Cancer 2022, 22, 587. [Google Scholar] [CrossRef]
- Dodson, A.E.; Shenker, S.; Sullivan, P.; Nayak, S.U.; Middleton, C.; McGuire, M.; Chipumuro, E.; Mishina, Y.; Tobin, E.R.; Cadzow, L.; et al. Pan-Cancer Analysis of Homologous Recombination Deficiency in Cell Lines. Cancer Res. Commun. 2024, 4, 3084–3098. [Google Scholar] [CrossRef]
- Stossel, C.; Raitses-Gurevich, M.; Atias, D.; Beller, T.; Glick Gorman, Y.; Halperin, S.; Peer, E.; Denroche, R.E.; Zhang, A.; Notta, F.; et al. Spectrum of Response to Platinum and PARP Inhibitors in Germline BRCA-Associated Pancreatic Cancer in the Clinical and Preclinical Setting. Cancer Discov. 2023, 13, 1826–1843. [Google Scholar] [CrossRef]
- Wattenberg, M.M.; Asch, D.; Yu, S.; O’Dwyer, P.J.; Domchek, S.M.; Nathanson, K.L.; Rosen, M.A.; Beatty, G.L.; Siegelman, E.S.; Reiss, K.A. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline BRCA1, BRCA2 or PALB2 mutation. Br. J. Cancer 2020, 122, 333–339. [Google Scholar] [CrossRef]
- LaRose, M.; Manji, G.A.; Bates, S.E. Beyond BRCA: Diagnosis and management of homologous recombination repair deficient pancreatic cancer. Semin. Oncol. 2024, 51, 36–44. [Google Scholar] [CrossRef]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.O.; Hochhauser, D.; Arnold, D.; Oh, D.Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef]
- Boursi, B.; Wileyto, E.P.; Mamtani, R.; Domchek, S.M.; Golan, T.; Hood, R.; Reiss, K.A. Analysis of BRCA1- and BRCA2-Related Pancreatic Cancer and Survival. JAMA Netw. Open 2023, 6, e2345013. [Google Scholar] [CrossRef]
- Syngal, S.; Furniss, C.S. Germline Genetic Testing for Pancreatic Ductal Adenocarcinoma at Time of Diagnosis. JAMA 2018, 319, 2383–2385. [Google Scholar] [CrossRef] [PubMed]
- Pujol, P.; Barberis, M.; Beer, P.; Friedman, E.; Piulats, J.M.; Capoluongo, E.D.; Garcia Foncillas, J.; Ray-Coquard, I.; Penault-Llorca, F.; Foulkes, W.D.; et al. Clinical practice guidelines for B RCA1 and BRCA2 genetic testing. Eur. J. Cancer 2021, 146, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.; Castroviejo-Bermejo, M.; Gutiérrez-Enríquez, S.; Llop-Guevara, A.; Ibrahim, Y.H.; Gris-Oliver, A.; Bonache, S.; Morancho, B.; Bruna, A.; Rueda, O.M.; et al. RAD51 foci as a functional biomarker of homologous recombination repair and PARP inhibitor resistance in germline BRCA-mutated breast cancer. Ann. Oncol. 2018, 29, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, B.; Herencia-Ropero, A.; Llop-Guevara, A.; Pedretti, F.; Moles-Fernández, A.; Viaplana, C.; Villacampa, G.; Guzmán, M.; Rodríguez, O.; Grueso, J.; et al. Preclinical In Vivo Validation of the RAD51 Test for Identification of Homologous Recombination-Deficient Tumors and Patient Stratification. Cancer Res. 2022, 82, 1646–1657. [Google Scholar] [CrossRef]
- Witz, A.; Dardare, J.; Betz, M.; Michel, C.; Husson, M.; Gilson, P.; Merlin, J.-L.; Harlé, A. Homologous recombination deficiency (HRD) testing landscape: Clinical applications and technical validation for routine diagnostics. Biomark. Res. 2025, 13, 31. [Google Scholar] [CrossRef]
- Crim, A.; Rowland, M.; Ruskin, R.; Dvorak, J.; Greenwade, M.; Walter, A.; Gillen, J.; Ding, K.; Moore, K.; Gunderson, C. Evaluation of the efficacy and toxicity profile associated with intraperitoneal chemotherapy use in older women. Gynecol. Oncol. 2017, 146, 268–272. [Google Scholar] [CrossRef]
- Reiss, K.A.; Yu, S.; Judy, R.; Symecko, H.; Nathanson, K.L.; Domchek, S.M. Retrospective Survival Analysis of Patients with Advanced Pancreatic Ductal Adenocarcinoma and Germline BRCA or PALB2 Mutations. JCO Precis. Oncol. 2018, 2, 1–9. [Google Scholar] [CrossRef]
- Anbil, S.; Reiss, K.A. Targeting BRCA and PALB2 in Pancreatic Cancer. Curr. Treat. Options Oncol. 2024, 25, 346–363. [Google Scholar] [CrossRef]
- Kim, H.; Xu, H.; George, E.; Hallberg, D.; Kumar, S.; Jagannathan, V.; Medvedev, S.; Kinose, Y.; Devins, K.; Verma, P.; et al. Combining PARP with ATR inhibition overcomes PARP inhibitor and platinum resistance in ovarian cancer models. Nat. Commun. 2020, 11, 3726. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhou, J.; Zhang, K.; Chen, H.; Luo, M.; Lu, Y.; Sun, Y.; Chen, Y. Molecular Mechanisms of PALB2 Function and Its Role in Breast Cancer Management. Front. Oncol. 2020, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, O.; Nowak, K.M. Gene of the month: PALB2. J. Clin. Pathol. 2023, 76, 73–75. [Google Scholar] [CrossRef] [PubMed]
- GeneCards. PALB2 Gene. Available online: https://www.genecards.org/cgi-bin/carddisp.pl?gene=PALB2 (accessed on 7 August 2025).
- Buisson, R.; Masson, J.Y. PALB2 self-interaction controls homologous recombination. Nucleic Acids Res. 2012, 40, 10312–10323. [Google Scholar] [CrossRef]
- Ducy, M.; Sesma-Sanz, L.; Guitton-Sert, L.; Lashgari, A.; Gao, Y.; Brahiti, N.; Rodrigue, A.; Margaillan, G.; Caron, M.C.; Côté, J.; et al. The Tumor Suppressor PALB2: Inside Out. Trends Biochem. Sci. 2019, 44, 226–240. [Google Scholar] [CrossRef]
- The Atlas of Genetics and Cytogenetics in Oncology and Haematology. PALB2 (Partner and Localizer of BRCA2). Available online: https://atlasgeneticsoncology.org/gene/46402/palb2-(partner-and-localizer-of-brca2) (accessed on 7 August 2025).
- Institute, N.C. PALB2: Genetics of Breast and Other Cancers (PDQ®)–Health Professional Version. Available online: https://www.cancer.gov/publications/pdq/information-summaries/genetics/palb2-hp-pdq (accessed on 8 August 2025).
- Yin, L.; Wei, J.; Lu, Z.; Huang, S.; Gao, H.; Chen, J.; Guo, F.; Tu, M.; Xiao, B.; Xi, C.; et al. Prevalence of Germline Sequence Variations Among Patients with Pancreatic Cancer in China. JAMA Netw. Open 2022, 5, e2148721. [Google Scholar] [CrossRef]
- Tischkowitz, M.; Balmaña, J.; Foulkes, W.D.; James, P.; Ngeow, J.; Schmutzler, R.; Voian, N.; Wick, M.J.; Stewart, D.R.; Pal, T. Management of individuals with germline variants in PALB2: A clinical practice resource of the American College of Medical Genetics and Genomics (ACMG). Genet. Med. 2021, 23, 1416–1423. [Google Scholar] [CrossRef]
- Dal Buono, A.; Poliani, L.; Greco, L.; Bianchi, P.; Barile, M.; Giatti, V.; Bonifacio, C.; Carrara, S.; Malesci, A.; Laghi, L. Prevalence of Germline Mutations in Cancer Predisposition Genes in Patients with Pancreatic Cancer or Suspected Related Hereditary Syndromes: Historical Prospective Analysis. Cancers 2023, 15, 1852. [Google Scholar] [CrossRef]
- Chaffee, K.G.; Oberg, A.L.; McWilliams, R.R.; Majithia, N.; Allen, B.A.; Kidd, J.; Singh, N.; Hartman, A.-R.; Wenstrup, R.J.; Petersen, G.M. Prevalence of germ-line mutations in cancer genes among pancreatic cancer patients with a positive family history. Genet. Med. 2018, 20, 119–127. [Google Scholar] [CrossRef]
- eviCore Healthcare. PALB2 Genetic Testing for Cancer Risk. Clinical Guideline V2.0.2025. Available online: https://www.evicore.com/provider/clinical-guidelines (accessed on 23 November 2025).
- Principe, D.R. Precision Medicine for BRCA/PALB2-Mutated Pancreatic Cancer and Emerging Strategies to Improve Therapeutic Responses to PARP Inhibition. Cancers 2022, 14, 897. [Google Scholar] [CrossRef]
- Reiss, K.A.; Mick, R.; O’Hara, M.H.; Teitelbaum, U.; Karasic, T.B.; Schneider, C.; Cowden, S.; Southwell, T.; Romeo, J.; Izgur, N.; et al. Phase II Study of Maintenance Rucaparib in Patients with Platinum-Sensitive Advanced Pancreatic Cancer and a Pathogenic Germline or Somatic Variant in BRCA1, BRCA2, or PALB2. J. Clin. Oncol. 2021, 39, 2497–2505. [Google Scholar] [CrossRef]
- O’Reilly, E.M.; Lee, J.W.; Zalupski, M.; Capanu, M.; Park, J.; Golan, T.; Tahover, E.; Lowery, M.A.; Chou, J.F.; Sahai, V.; et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin With or Without Veliparib in Patients with Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J. Clin. Oncol. 2020, 38, 1378–1388. [Google Scholar] [CrossRef] [PubMed]
- Stiff, T.; O’Driscoll, M.; Rief, N.; Iwabuchi, K.; Löbrich, M.; Jeggo, P.A. ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res. 2004, 64, 2390–2396. [Google Scholar] [CrossRef]
- Bakkenist, C.J.; Kastan, M.B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 2003, 421, 499–506. [Google Scholar] [CrossRef]
- Perkhofer, L.; Schmitt, A.; Romero Carrasco, M.C.; Ihle, M.; Hampp, S.; Ruess, D.A.; Hessmann, E.; Russell, R.; Lechel, A.; Azoitei, N.; et al. ATM Deficiency Generating Genomic Instability Sensitizes Pancreatic Ductal Adenocarcinoma Cells to Therapy-Induced DNA Damage. Cancer Res. 2017, 77, 5576–5590. [Google Scholar] [CrossRef]
- Hsu, F.C.; Roberts, N.J.; Childs, E.; Porter, N.; Rabe, K.G.; Borgida, A.; Ukaegbu, C.; Goggins, M.G.; Hruban, R.H.; Zogopoulos, G.; et al. Risk of Pancreatic Cancer Among Individuals with Pathogenic Variants in the ATM Gene. JAMA Oncol. 2021, 7, 1664–1668. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.A.; Schultz, C.W.; Azimi-Sadjadi, A.; Brody, J.R.; Pishvaian, M.J. ATM Dysfunction in Pancreatic Adenocarcinoma and Associated Therapeutic Implications. Mol. Cancer Ther. 2019, 18, 1899–1908. [Google Scholar] [CrossRef] [PubMed]
- Astiazaran-Symonds, E.; Kim, J.; Haley, J.S.; Kim, S.Y.; Rao, H.S.; Genetics Center, R.; Carey, D.J.; Stewart, D.R.; Goldstein, A.M. A Genome-First Approach to Estimate Prevalence of Germline Pathogenic Variants and Risk of Pancreatic Cancer in Select Cancer Susceptibility Genes. Cancers 2022, 14, 3257. [Google Scholar] [CrossRef]
- Xun, J.; Ohtsuka, H.; Hirose, K.; Douchi, D.; Nakayama, S.; Ishida, M.; Miura, T.; Ariake, K.; Mizuma, M.; Nakagawa, K.; et al. Reduced expression of phosphorylated ataxia-telangiectasia mutated gene is related to poor prognosis and gemcitabine chemoresistance in pancreatic cancer. BMC Cancer 2023, 23, 835. [Google Scholar] [CrossRef]
- Kim, H.; Saka, B.; Knight, S.; Borges, M.; Childs, E.; Klein, A.; Wolfgang, C.; Herman, J.; Adsay, V.N.; Hruban, R.H.; et al. Having Pancreatic Cancer with Tumoral Loss of ATM and Normal TP53 Protein Expression Is Associated with a Poorer Prognosis. Clin. Cancer Res. 2014, 20, 1865–1872. [Google Scholar] [CrossRef]
- Hannan, Z.; Yu, S.; Domchek, S.; Mamtani, R.; Reiss, K.A. Clinical Characteristics of Patients with Pancreatic Cancer and Pathogenic ATM Alterations. JNCI Cancer Spectr. 2021, 5, pkaa121. [Google Scholar] [CrossRef] [PubMed]
- Gout, J.; Perkhofer, L.; Morawe, M.; Arnold, F.; Ihle, M.; Biber, S.; Lange, S.; Roger, E.; Kraus, J.M.; Stifter, K.; et al. Synergistic targeting and resistance to PARP inhibition in DNA damage repair-deficient pancreatic cancer. Gut 2021, 70, 743–760. [Google Scholar] [CrossRef] [PubMed]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Reaper, P.M.; Griffiths, M.R.; Long, J.M.; Charrier, J.D.; Maccormick, S.; Charlton, P.A.; Golec, J.M.; Pollard, J.R. Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nat. Chem. Biol. 2011, 7, 428–430. [Google Scholar] [CrossRef]
- Williamson, C.T.; Muzik, H.; Turhan, A.G.; Zamò, A.; O’Connor, M.J.; Bebb, D.G.; Lees-Miller, S.P. ATM deficiency sensitizes mantle cell lymphoma cells to poly(ADP-ribose) polymerase-1 inhibitors. Mol. Cancer Ther. 2010, 9, 347–357. [Google Scholar] [CrossRef]
- Fokas, E.; Prevo, R.; Pollard, J.R.; Reaper, P.M.; Charlton, P.A.; Cornelissen, B.; Vallis, K.A.; Hammond, E.M.; Olcina, M.M.; Gillies McKenna, W.; et al. Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis. 2012, 3, e441. [Google Scholar] [CrossRef]
- Ayars, M.; Eshleman, J.; Goggins, M. Susceptibility of ATM-deficient pancreatic cancer cells to radiation. Cell Cycle 2017, 16, 991–998. [Google Scholar] [CrossRef]
- Lloyd, R.L.; Wijnhoven, P.W.G.; Ramos-Montoya, A.; Wilson, Z.; Illuzzi, G.; Falenta, K.; Jones, G.N.; James, N.; Chabbert, C.D.; Stott, J.; et al. Combined PARP and ATR inhibition potentiates genome instability and cell death in ATM-deficient cancer cells. Oncogene 2020, 39, 4869–4883. [Google Scholar] [CrossRef]
- Yazinski, S.A.; Comaills, V.; Buisson, R.; Genois, M.M.; Nguyen, H.D.; Ho, C.K.; Todorova Kwan, T.; Morris, R.; Lauffer, S.; Nussenzweig, A.; et al. ATR inhibition disrupts rewired homologous recombination and fork protection pathways in PARP inhibitor-resistant BRCA-deficient cancer cells. Genes Dev. 2017, 31, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, H.-W.; Yang, C.-C.; Masai, H. Roles of Claspin in regulation of DNA replication, replication stress responses and oncogenesis in human cells. Genome Instab. Dis. 2021, 2, 263–280. [Google Scholar] [CrossRef]
- Jossen, R.; Bermejo, R. The DNA damage checkpoint response to replication stress: A Game of Forks. Front. Genet. 2013, 4, 26. [Google Scholar] [CrossRef]
- Ahn, J.; Urist, M.; Prives, C. The Chk2 protein kinase. DNA Repair 2004, 3, 1039–1047. [Google Scholar] [CrossRef]
- Falck, J.; Coates, J.; Jackson, S.P. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 2005, 434, 605–611. [Google Scholar] [CrossRef]
- Zannini, L.; Delia, D.; Buscemi, G. CHK2 kinase in the DNA damage response and beyond. J. Mol. Cell Biol. 2014, 6, 442–457. [Google Scholar] [CrossRef]
- Klomp, J.E.; Lee, Y.S.; Goodwin, C.M.; Papke, B.; Klomp, J.A.; Waters, A.M.; Stalnecker, C.A.; DeLiberty, J.M.; Drizyte-Miller, K.; Yang, R.; et al. CHK1 protects oncogenic KRAS-expressing cells from DNA damage and is a target for pancreatic cancer treatment. Cell Rep. 2021, 37, 110060. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.K.; Cimprich, K.A. Causes and consequences of replication stress. Nat. Cell Biol. 2014, 16, 2–9. [Google Scholar] [CrossRef]
- Veenstra, C.M.; Abrahamse, P.; Hamilton, A.S.; Ward, K.C.; Gomez, S.L.; Liu, L.; Katz, S.J.; Hofer, T.P.; Kurian, A.W. Breast, Colorectal, and Pancreatic Cancer Mortality with Pathogenic Variants in ATM, CHEK2, or PALB2. J. Clin. Oncol. 2025, 43, 1587–1596. [Google Scholar] [CrossRef]
- Sehdev, A.; Gbolahan, O.; Hancock, B.A.; Stanley, M.; Shahda, S.; Wan, J.; Wu, H.H.; Radovich, M.; O’Neil, B.H. Germline and Somatic DNA Damage Repair Gene Mutations and Overall Survival in Metastatic Pancreatic Adenocarcinoma Patients Treated with FOLFIRINOX. Clin. Cancer Res. 2018, 24, 6204–6211. [Google Scholar] [CrossRef]
- Vittal, A.; Saha, D.; Samanta, I.; Kasi, A. CHEK2 mutation in a patient with pancreatic adenocarcinoma-a rare case report. AME Case Rep. 2021, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Stoof, J.; Harrold, E.; Mariottino, S.; Lowery, M.A.; Walsh, N. DNA Damage Repair Deficiency in Pancreatic Ductal Adenocarcinoma: Preclinical Models and Clinical Perspectives. Front. Cell Dev. Biol. 2021, 9, 749490. [Google Scholar] [CrossRef] [PubMed]
- Kryklyva, V.; Pflüger, M.J.; Ouchene, H.; Volleberg-Gorissen, H.; Mensenkamp, A.R.; Jonker, M.A.; van de Water, C.; Nagtegaal, I.D.; Ligtenberg, M.J.L.; Brosens, L.A.A. Germline Pathogenic Variants in Patients with Pancreatic Ductal Adenocarcinoma and Extra-Pancreatic Malignancies: A Nationwide Database Analysis. Mod. Pathol. 2025, 38, 100709. [Google Scholar] [CrossRef] [PubMed]
- Farnes, I.; Lund-Iversen, M.; Aabakken, L.; Verbeke, C.; Labori, K.J. Molecular testing for personalized therapy is underutilized in patients with borderline resectable and locally advanced pancreatic cancer—Real world data from the NORPACT-2 study. Scand. J. Gastroenterol. 2024, 59, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
| Fusion Gene | Prevalence in PDAC | Common Fusion Partners | Targeted Therapies | Key Clinical Trials (NCT) |
|---|---|---|---|---|
| ALK | ~0.16% overall; ~1.3% in <50y | EML4, STRN, KANK4 | Crizotinib, Ceritinib, Alectinib, Lorlatinib | NCT02227940 NCT02568267 |
| ROS1 | ≤0.3% | SLC4A4, SLC34A2, CENPW | Crizotinib, Entrectinib, Lorlatinib | NCT02568267 |
| NTRK | ~0.3% | TPR, EML4, KANK1, THAP1, SEL1L, CTRC, NOS1AP, ERC1 | Larotrectinib, Entrectinib | NCT02122913, NCT02637687, NCT02576431, NCT02568267 |
| RET | ~0.6% | CCDC6, TRIM33, TRIM24, ERC1 | Selpercatinib, Pralsetinib, BOS172738 | NCT03157128, NCT03037385, NCT03780517 |
| FGFR | 1–1.5% | There are at least 114 unique FGFR fusion partner genes: FGFR2-BICC1, FGFR2-KIAA1217, FGFR2-SORBS1, FGFR2-AHCYL1, FGFR1-TACC1, FGFR3-TACC3 | Erdafitinib, Pemigatinib | NCT04083976, NCT03822117 |
| NRG1 | <1% | ATP1B1, APP, CD74, CDH6, SARAF, ROCK1 | Afatinib, Zenocutuzumab, Seribantumab | NCT02912949, NCT04383210 |
| Category | BRCA1 | BRCA2 | PALB2 | ATM | CHEK2 | CHEK1 |
|---|---|---|---|---|---|---|
| Primary DDR role | Early HRR: damage sensing, end-resection with MRN/CtIP; recruits PALB2, supports RAD51 loading | Late HRR: directly loads RAD52 via BRC repeats to ssDNA | HRR scaffold linings BRCA1 to BRCA1 and RAD51; stabilizes RAD52 filaments; DNA binding | DSB-response kinase; phosphorylates p53, BRCA2, H2AX | Checkpoint kinase downstream of ATM; enforces G1/s and G2/M checkpoints | Checkpoint kinase downstream of ATR; buffers replication stress; S/G2 checkpoints |
| Germline prevalence in PDAC | Part of combined BRCA1/2 ~3–7%; population background for BRCA1 ~0.2% | More frequent than BRCA1; ~3–7% combined BRCA1/2; ~2/3 of BRCA-related PDAC | ~0.5–1.5% | ~2–3% unselected PDAC, ~2.38% in genome-first population analysis | ~4.1% in a 298-patient series; ~11% of non-BRCA/ATM gPVs in a familial registry | Extremely rare in PDAC germline cohorts reported |
| Enrichment in high-risk groups | Enriched in FPC; HRR genes up to 20% in FPC cohorts | Enriched in FPC; BRCA2 dominant within BRCA cases | Present in high-risk settings (as the HRR gene) | Present in high-risk settings (as the HR gene) | Reported on multigene panels | No enrichment signal reported |
| Testing/diagnosis | Universal germline testing: RAD52 foci and HRD assays may complement selection | Universal germline testing: RAD52 foci and HRD assays may complement selection | Included on multigene panels; autosomal-dominant; cascade testing for relatives | Included in multigene panels; paired tumor-normal often used to confirm biallelic inactivation; IHC ATM loss can flag pathway disruption; CAPS-based MRI/EUS surveillance for carriers with family history; cascade testing | Detected as part of broad DDR panels; ACMG/AMP classification used | Included on panels, but detection is typically incidental due to rarity |
| Therapy sensitivity/strategies | Platinum sensitivity; PARP maintenance (olaparib) | Platinum sensitivity; PARP maintenance (olaparib); often a stronger signal for platinum response than BRCA1 | Platinum and PARP sensitivity; reports of rucaparib maintenance | Sensitivity to ATR inhibition, platinum, and radiation | Pan-DDR rationale for platinum; PARP use described anecdotally | Preclinical rationale: ATR-CHEK1 axis targeting under replication stress |
| Tumor behavior/survival | HRD with increased platinum responsiveness; different survival reported between BRCA2 and BRCA1 in one cohort | BRCA2 carriers showed better OS than BRCA1 | May mirror BRCA-type HRD responsiveness | Can be aggressive with tumoral ATM loss | Population analysis showed no significant mortality difference vs. non-carriers | No PDAC germline survival data reported |
| References | [182,183,184,185,186,188,189,190,196,198,199,207,208,209,210,211,212,213,214,215,216,217,222,223,224,225,226,227,228,229,230,231,232,239,240,241,242,243,244,245,246,247,248,249] | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupa, K.; Fudalej, M.; Miski, H.; Włoszek, E.; Szymczak, M.; Badowska-Kozakiewicz, A.; Czerw, A.; Deptała, A. KRAS-Wild Pancreatic Cancer—More Targets than Treatment Possibilities? Cancers 2025, 17, 3769. https://doi.org/10.3390/cancers17233769
Krupa K, Fudalej M, Miski H, Włoszek E, Szymczak M, Badowska-Kozakiewicz A, Czerw A, Deptała A. KRAS-Wild Pancreatic Cancer—More Targets than Treatment Possibilities? Cancers. 2025; 17(23):3769. https://doi.org/10.3390/cancers17233769
Chicago/Turabian StyleKrupa, Kamila, Marta Fudalej, Hanna Miski, Emilia Włoszek, Marta Szymczak, Anna Badowska-Kozakiewicz, Aleksandra Czerw, and Andrzej Deptała. 2025. "KRAS-Wild Pancreatic Cancer—More Targets than Treatment Possibilities?" Cancers 17, no. 23: 3769. https://doi.org/10.3390/cancers17233769
APA StyleKrupa, K., Fudalej, M., Miski, H., Włoszek, E., Szymczak, M., Badowska-Kozakiewicz, A., Czerw, A., & Deptała, A. (2025). KRAS-Wild Pancreatic Cancer—More Targets than Treatment Possibilities? Cancers, 17(23), 3769. https://doi.org/10.3390/cancers17233769






