Root Cause Analysis of Patients with Pancreatic Cancer Who Underwent Imaging Not Resulting in a Cancer Diagnosis in the 18 Months Prior to Diagnosis
Simple Summary
Abstract
1. Introduction
2. Methods
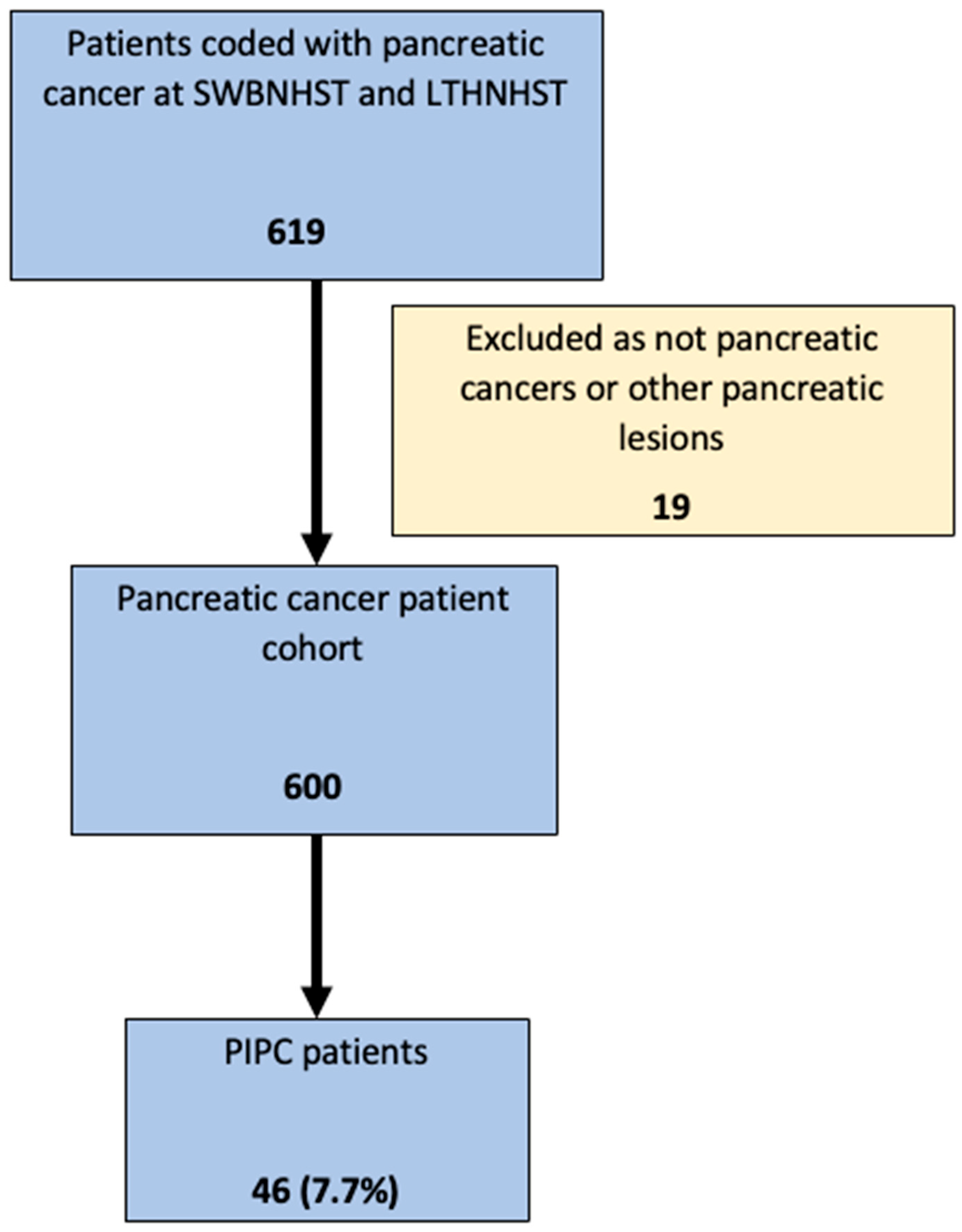
2.1. Study Subjects
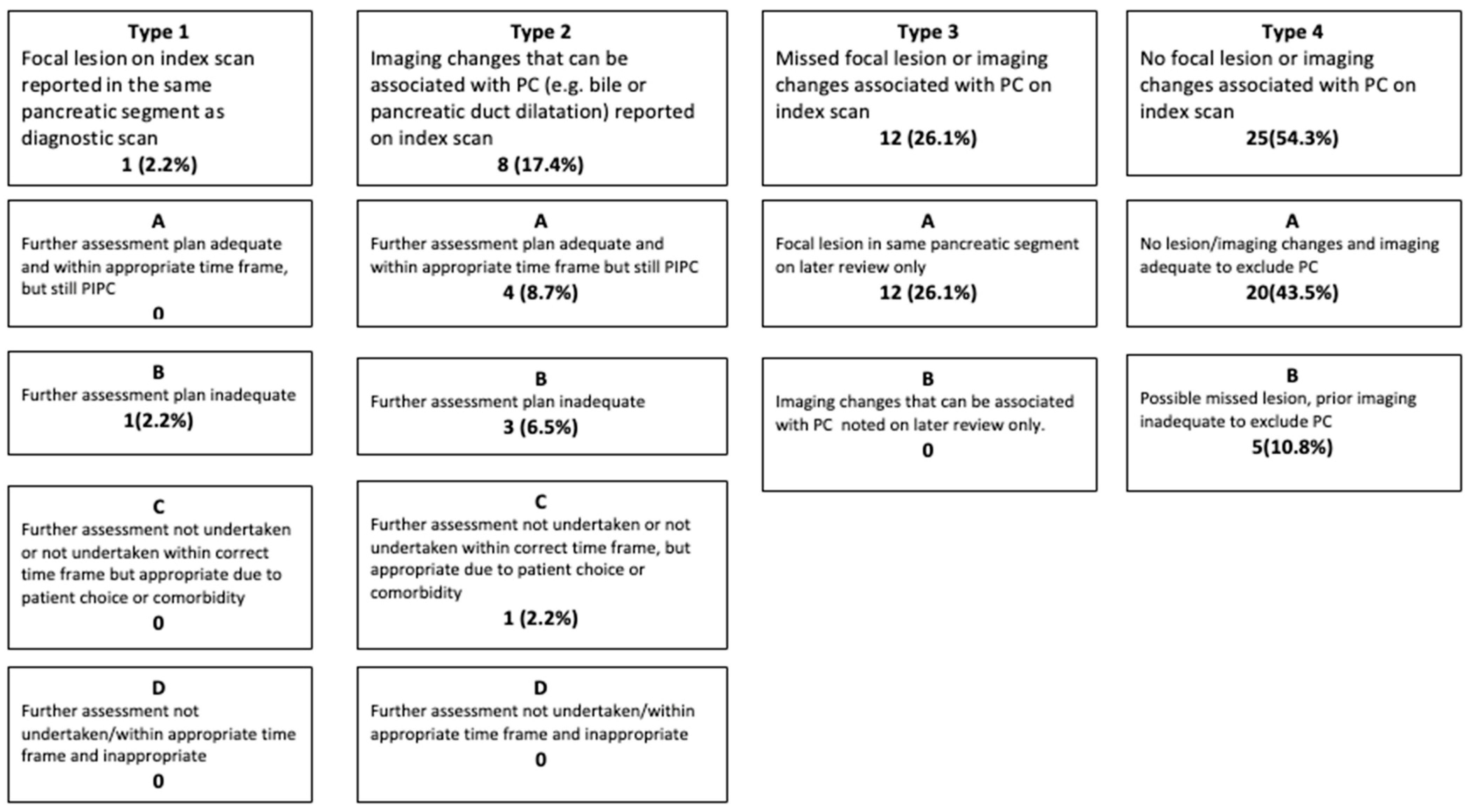
2.2. Root Cause Analysis of the Most Likely Explanation for PIPC
- Further assessment plan adequate and within appropriate time frame, but still PIPC.
- Further assessment plan inadequate.
- Further assessment not undertaken or not undertaken within correct time frame, but appropriate due to patient choice or comorbidity.
- Further assessment not undertaken/within an appropriate (urgent—less than two weeks) time frame, and inappropriate.
- Further assessment plan adequate and within appropriate time frame but still PIPC.
- Further assessment plan inadequate.
- Further assessment not undertaken or not undertaken within correct time frame but appropriate due to patient choice or comorbidity.
- Further assessment not undertaken/within appropriate time frame and inappropriate.
- Focal lesion in same pancreatic segment on later review only.
- Imaging changes (e.g., bile or pancreatic duct dilatation) that can be associated with pancreatic cancer noted on later review only.
- No lesion/imaging changes, and imaging adequate to exclude pancreatic cancer.
- Possible missed lesion, prior imaging inadequate to exclude pancreatic cancer.
2.3. Judgement on Whether PIPC Was Potentially Avoidable
2.4. Potential Impact of a Delay in Diagnosis on PIPC Clinical Outcomes
2.5. Statistical Analysis
2.6. Patient and Public Involvement
3. Results
3.1. Study Patients
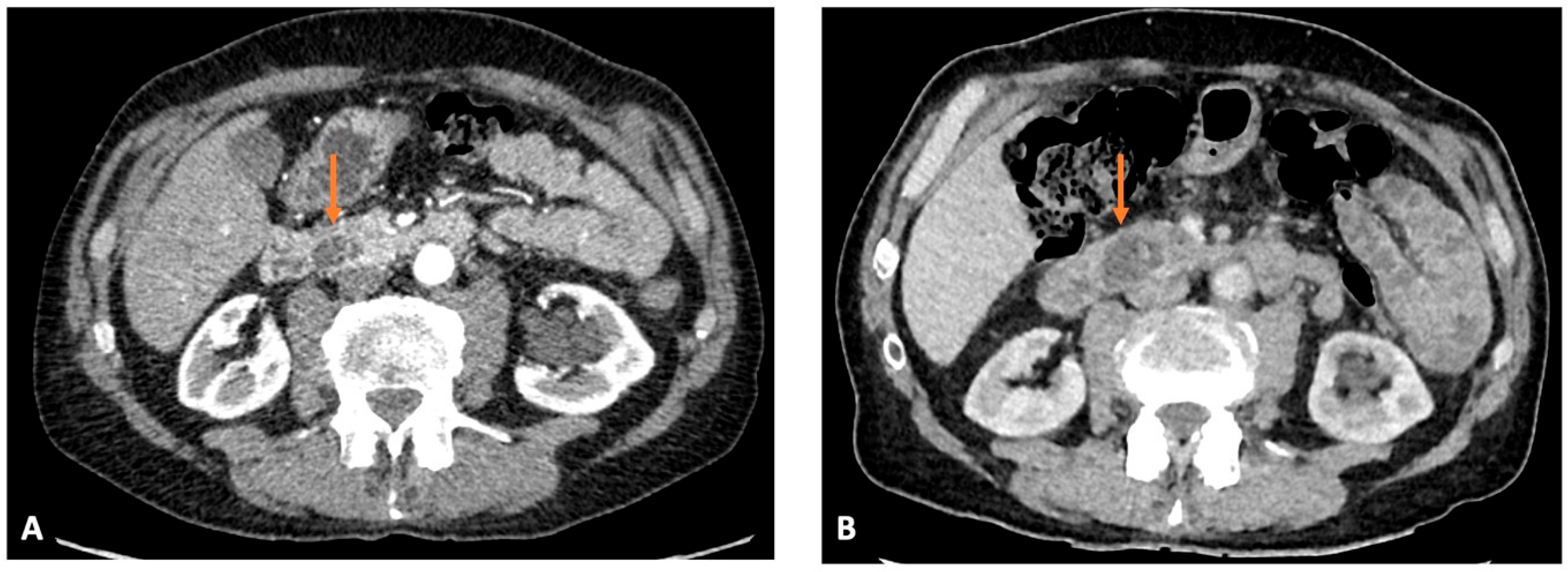
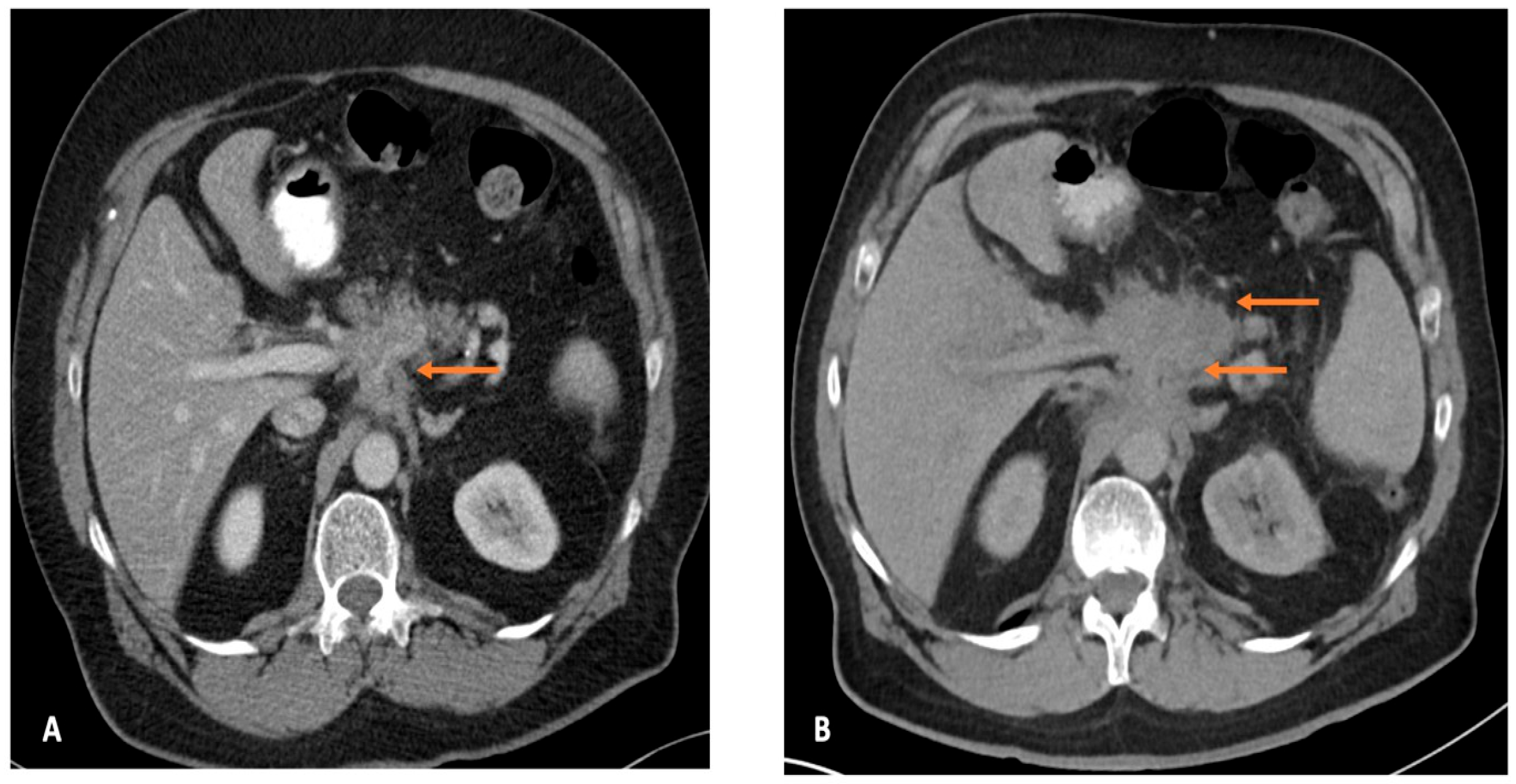
3.2. Root Cause Analysis of the Most Likely Explanation for PIPC
3.3. Potentially Avoidable PIPC
3.4. Potential Impact on Pancreatic Cancer Clinical Outcomes
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stoffel, E.M.; Brand, R.E.; Goggins, M. Pancreatic Cancer: Changing Epidemiology and New Approaches to Risk Assessment, Early Detection, and Prevention. Gastroenterology 2023, 164, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, G.; Ionescu, V.A.; Moldovan, H.; Diaconu, C.C. Clinical and Biological Data in Patients with Pancreatic Cancer vs. Chronic Pancreatitis—A Single Center Comparative Analysis. Diagnostics 2023, 13, 369. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.; Chapple, A.; Salisbury, H.; Corrie, P.; Ziebland, S. “It Can’t Be Very Important Because It Comes and Goes”—Patients’ Accounts of Intermittent Symptoms Preceding a Pancreatic Cancer Diagnosis: A Qualitative Study. BMJ Open 2014, 4, 4215. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, R.; Wang, C.; Qiu, J.; Ren, B.; You, L. Early Screening and Diagnosis Strategies of Pancreatic Cancer: A Comprehensive Review. Cancer Commun. 2021, 41, 1257–1274. [Google Scholar] [CrossRef]
- Park, W.; Chawla, A.; O’Reilly, E.M. Pancreatic Cancer: A Review. JAMA 2021, 326, 851–862. [Google Scholar] [CrossRef]
- Holly, E.A.; Chaliha, I.; Bracci, P.M.; Gautam, M. Signs and Symptoms of Pancreatic Cancer: A Population-Based Case-Control Study in the San Francisco Bay Area. Clin. Gastroenterol. Hepatol. 2004, 2, 510–517. [Google Scholar] [CrossRef]
- Walter, F.M.; Mills, K.; Mendonça, S.C.; Abel, G.A.; Basu, B.; Carroll, N.; Ballard, S.; Lancaster, J.; Hamilton, W.; Rubin, G.P.; et al. Symptoms and Patient Factors Associated with Diagnostic Intervals for Pancreatic Cancer (SYMPTOM Pancreatic Study): A Prospective Cohort Study. Lancet Gastroenterol. Hepatol. 2016, 1, 298–306. [Google Scholar] [CrossRef]
- National Pancreatic Cancer Audit State of the Nation Report 2024; National Cancer Audit Collaborating Centre: London, UK, 2024.
- Coveler, A.L.; Herman, J.M.; Simeone, D.M.; Chiorean, E.G. Localized Pancreatic Cancer: Multidisciplinary Management. Am. Soc. Clin. Oncol. Educ. Book 2016, 36, e217–e226. [Google Scholar] [CrossRef]
- Guarga, L.; Paco, N.; Manchon-Walsh, P.; Vela, E.; Delgadillo, J.; Pontes, C.; Borràs, J.M. Management, Survival, and Costs of Pancreatic Cancer: Population-Based Observational Study in Catalonia. Int. J. Environ. Res. Public Health 2023, 20, 5673. [Google Scholar] [CrossRef]
- Chu, L.C.; Park, S.; Kawamoto, S.; Yuille, A.L.; Hruban, R.H.; Fishman, E.K. Pancreatic Cancer Imaging: A New Look at an Old Problem. Curr. Probl. Diagn. Radiol. 2021, 50, 540–550. [Google Scholar] [CrossRef]
- Wong, J.C.; Lu, D.S.K. Staging of Pancreatic Adenocarcinoma by Imaging Studies. Clin. Gastroenterol. Hepatol. 2008, 6, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, A.J.; Chu, L.C.; Deig, C.R.; Fishman, E.K.; Hwang, W.L.; Maitra, A.; Marks, D.L.; Mehta, A.; Nabavizadeh, N.; Simeone, D.M.; et al. Multidisciplinary Standards of Care and Recent Progress in Pancreatic Ductal Adenocarcinoma. CA Cancer J. Clin. 2020, 70, 375–403. [Google Scholar] [CrossRef] [PubMed]
- Treadwell, J.R.; Zafar, H.M.; Mitchell, M.D.; Tipton, K.; Teitelbaum, U.; Jue, J. Imaging Tests for the Diagnosis and Staging of Pancreatic Adenocarcinoma: A Meta-Analysis. Pancreas 2016, 45, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.L.; Wu, T.; Chen, P.T.; Tsai, Y.M.; Roth, H.; Wu, M.S.; Liao, W.C.; Wang, W. Deep Learning to Distinguish Pancreatic Cancer Tissue from Non-Cancerous Pancreatic Tissue: A Retrospective Study with Cross-Racial External Validation. Lancet Digit. Health 2020, 2, e303–e313. [Google Scholar] [CrossRef]
- Kikuyama, M.; Kamisawa, T.; Kuruma, S.; Chiba, K.; Kawaguchi, S.; Terada, S.; Satoh, T. Early Diagnosis to Improve the Poor Prognosis of Pancreatic Cancer. Cancers 2018, 10, 48. [Google Scholar] [CrossRef]
- Ahn, S.S.; Kim, M.-J.; Choi, J.-Y.; Hong, H.-S.; Chung, Y.E.; Lim, J.S. Indicative Findings of Pancreatic Cancer in Prediagnostic CT. Eur. Radiol. 2009, 19, 2448–2455. [Google Scholar] [CrossRef]
- Gangi, S.; Fletcher, J.G.; Nathan, M.A.; Christensen, J.A.; Harmsen, W.S.; Crownhart, B.S.; Chari, S.T. Time Interval between Abnormalities Seen on CT and the Clinical Diagnosis of Pancreatic Cancer: Retrospective Review of CT Scans Obtained before Diagnosis. AJR Am. J. Roentgenol. 2004, 182, 897–903. [Google Scholar] [CrossRef]
- Kang, J.; Clarke, S.E.; Abdolell, M.; Ramjeesingh, R.; Payne, J.; Costa, A.F. The Implications of Missed or Misinterpreted Cases of Pancreatic Ductal Adenocarcinoma on Imaging: A Multi-Centered Population-Based Study. Eur. Radiol. 2021, 31, 212–221. [Google Scholar] [CrossRef]
- Kang, J.D.; Clarke, S.E.; Costa, A.F. Factors Associated with Missed and Misinterpreted Cases of Pancreatic Ductal Adenocarcinoma. Eur. Radiol. 2021, 31, 2422–2432. [Google Scholar] [CrossRef]
- Rees, C.J.; Thomas Gibson, S.; Rutter, M.D.; Baragwanath, P.; Pullan, R.; Feeney, M.; Haslam, N. UK Key Performance Indicators and Quality Assurance Standards for Colonoscopy. Gut 2016, 65, 1923–1929. [Google Scholar] [CrossRef]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic Effect of Weight Loss Prior to Chemotherapy in Cancer Patients. Eastern Cooperative Oncology Group. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Blunt, D.; Britton, I.; Chew, C.; Helbren, E.; Katz, J.; Lowe, A.; Morrison, I.; Plumb, A.; Stephenson, J.; Taylor, S.; et al. Standards of Practice for Computed Tomography Colonography (CTC); The Royal College of Radiologists. 2021. Available online: https://www.rcr.ac.uk/our-services/all-our-publications/clinical-radiology-publications/standards-of-practice-for-computed-tomography-colonography-ctc/ (accessed on 1 September 2025).
- Obaro, A.E.; Plumb, A.A.; Fanshawe, T.R.; Torres, U.S.; Baldwin-Cleland, R.; Taylor, S.A.; Halligan, S.; Burling, D.N. Post-Imaging Colorectal Cancer or Interval Cancer Rates after CT Colonography: A Systematic Review and Meta-Analysis. Lancet Gastroenterol. Hepatol. 2018, 3, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Rutter, M.D.; Beintaris, I.; Valori, R.; Chiu, H.M.; Corley, D.A.; Cuatrecasas, M.; Dekker, E.; Forsberg, A.; Gore-Booth, J.; Haug, U.; et al. World Endoscopy Organization Consensus Statements on Post-Colonoscopy and Post-Imaging Colorectal Cancer. Gastroenterology 2018, 155, 909–925.e3. [Google Scholar] [CrossRef] [PubMed]
- The Less Survivable Cancers—Less Survivable Cancers Taskforce. Available online: https://lesssurvivablecancers.org.uk/the-less-survivable-cancers/ (accessed on 7 May 2024).
- Grogan, A.; Loveday, B.; Michael, M.; Wong, H.L.; Gibbs, P.; Thomson, B.; Lee, B.; Ko, H.S. Real-World Staging Computed Tomography Scanning Technique and Important Reporting Discrepancies in Pancreatic Ductal Adenocarcinoma. ANZ J. Surg. 2022, 92, 1789–1796. [Google Scholar] [CrossRef]
- Cao, K.; Xia, Y.; Yao, J.; Han, X.; Lambert, L.; Zhang, T.; Tang, W.; Jin, G.; Jiang, H.; Fang, X.; et al. Large-Scale Pancreatic Cancer Detection via Non-Contrast CT and Deep Learning. Nat. Med. 2023, 29, 3033. [Google Scholar] [CrossRef]
- Pelaez-Luna, M.; Takahashi, N.; Fletcher, J.G.; Chari, S.T. Resectability of Presymptomatic Pancreatic Cancer and Its Relationship to Onset of Diabetes: A Retrospective Review of CT Scans and Fasting Glucose Values Prior to Diagnosis. Am. J. Gastroenterol. 2007, 102, 2157–2163. [Google Scholar] [CrossRef]
- Higashi, M.; Tanabe, M.; Onoda, H.; Nakao, S.; Miyoshi, K.; Iida, E.; Okada, M.; Furukawa, M.; Ito, K. Incidentally Detected Pancreatic Adenocarcinomas on Computed Tomography Obtained during the Follow-up for Other Diseases. Abdom. Radiol. 2020, 45, 774–781. [Google Scholar] [CrossRef]
| Variables | PIPC (n = 46) | % | |
|---|---|---|---|
| Time interval between scans (months) | 11 (IQR 7.4–14.9) | ||
| Age (years) | 75.9 (IQR 69.6–80.2) | ||
| Sex | Female | 27 | 58.7 |
| Male | 19 | 41.3 | |
| Location of pancreatic cancer | Body | 11 | 23.9% |
| Body/Tail | 5 | 10.9% | |
| Whole pancreas | 1 | 2.2% | |
| Head | 23 | 50.0% | |
| Head/Neck | 3 | 6.5% | |
| Tail | 3 | 6.5% | |
| Mass/duct abnormality at diagnosis | Mass | 26 | 56.5% |
| Duct abnormality | 11 | 23.9% | |
| Both | 8 | 17.4% | |
| Missing site information | 1 | 2.2% | |
| Basis of diagnosis | Histology | 21 | 45.7% |
| Imaging alone | 25 | 54.3% | |
| Mode of index imaging request | Outpatient routine | 9 | 19.6% |
| Outpatient urgent | 21 | 45.7% | |
| Outpatient surveillance | 2 | 4.3% | |
| Inpatient urgent | 14 | 30.4% | |
| Indication for index imaging | Alarm abdominal symptoms | 19 | 32.6% |
| Non-alarm abdominal symptoms | 10 | 17.4% | |
| Other indications for imaging | 17 | 50% | |
| Modality of index imaging | CT Abdomen and pelvis | 16 | 34.8% |
| CT Chest, Abdomen and Pelvis | 14 | 30.4% | |
| CT Pancreas | 3 | 6.5% | |
| CT Thorax | 6 | 13% | |
| CT Colon | 2 | 4.3% | |
| CT Urogram | 1 | 2.2% | |
| CT Renal (multiphase) | 1 | 2.2% | |
| MRI Abdomen | 1 | 2.2% | |
| MRI Liver | 1 | 2.2% | |
| MRI Renal | 1 | 2.2% | |
| Total scans | CT | 43 | 93.5% |
| MRI | 3 | 6.5% | |
| Reporting radiologist | Hepatobiliary or Gastrointestinal radiologist | 15 | 32.6% |
| Non-hepatobiliary or gastrointestinal radiologist | 31 | 67.4% |
| PIPC Patients | % | PC Patients Without PIPC | % | p-Value | |||
|---|---|---|---|---|---|---|---|
| Total | 23 | 263 | |||||
| Location of pancreatic cancer | Body | 7 | 30.4 | 62 | 23.6 | ||
| Body and Tail | 2 | 8.7 | 12 | 4.6 | |||
| Head | 13 | 56.5 | 117 | 44.1 | |||
| Neck | 1 | 4.4 | 7 | 2.7 | |||
| Tail | 0 | 0 | 42 | 15.9 | |||
| Uncinate process | 0 | 0 | 19 | 6.8 | |||
| Missing site information | 4 | 2.3 | 0.25 | ||||
| Sex | Male | 10 | 43.5 | 138 | 52.5 | ||
| Female | 13 | 56.5 | 125 | 47.5 | 0.41 | ||
| Age at index imaging or diagnosis for pancreatic cancer without PIPC | 75.9 | (63.4–80.2) | 75.3 | (65.1–81.4) | 0.94 | ||
| Indication for imaging | Alarm Abdominal symptoms | 12 | 72.7 | 177 | 67.3 | ||
| Non-alarm Abdominal symptoms | 5 | 13.6 | 36 | 13.7 | |||
| Other indications for imaging | 6 | 13.6 | 50 | 19.0 | 0.87 | ||
| Reporting radiologist | Hepatobiliary or Gastrointestinal radiologist | 5 | 21.7 | 63 | 23.9 | ||
| Non-hepatobiliary or gastrointestinal radiologist | 18 | 78.3 | 198 | 75.3 | |||
| Missing | 0 | 0.0 | 2 | 0.8 | 0.89 | ||
| Diagnosis | Pancreatic Ductal Adenocarcinoma | 14 | 60.9 | 75 | 28.5 | ||
| Pancreatic cancer diagnosed on imaging only | 9 | 39.1 | 176 | 66.9 | |||
| Neuroendocrine tumour | 0 | 0.0 | 11 | 4.2 | |||
| Small cell cancer | 0 | 0.0 | 1 | 0.4 | 0.01 | ||
| Basis of diagnosis | Histology | 14 | 60.9 | 87 | 33.1 | ||
| Imaging | 9 | 39.1 | 176 | 66.9 | 0.01 | ||
| Pancreatic Cancer Without PIPC | % | PIPC | % | p-Value | ||
|---|---|---|---|---|---|---|
| Total | 286 | 263 | 23 | |||
| Clinical stage at diagnosis | ||||||
| Early | I | 38 | 14.4 | 2 | 8.7 | 0.90 |
| II | 44 | 16.7 | 3 | 13.0 | ||
| Late | III | 37 | 14.1 | 4 | 17.4 | |
| IV | 143 | 54.4 | 14 | 60.9 | ||
| Not available | 1 | 0.4 | ||||
| Treatment intention | ||||||
| Curative | Surgical resection | 31 | 11.8 | 3 | 13.0 | |
| Adjuvant chemotherapy | 19 | 7.2 | 3 | 13.0 | 0.47 | |
| Palliative | Chemotherapy | 42 | 16.0 | 3 | 13.0 | |
| Best supportive care | 162 | 61.6 | 14 | 60.9 | 0.7 | |
| Died before further management | 23 | 8.7 | 1 | 4.3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, S.A.; Umar, N.; Sia, M.; Smyth, J.; Udumalagala, S.; Makki, M.U.; Roberts, K.; Mahon, B.; Albazaz, R.; Trudgill, N. Root Cause Analysis of Patients with Pancreatic Cancer Who Underwent Imaging Not Resulting in a Cancer Diagnosis in the 18 Months Prior to Diagnosis. Cancers 2025, 17, 3770. https://doi.org/10.3390/cancers17233770
Mohamed SA, Umar N, Sia M, Smyth J, Udumalagala S, Makki MU, Roberts K, Mahon B, Albazaz R, Trudgill N. Root Cause Analysis of Patients with Pancreatic Cancer Who Underwent Imaging Not Resulting in a Cancer Diagnosis in the 18 Months Prior to Diagnosis. Cancers. 2025; 17(23):3770. https://doi.org/10.3390/cancers17233770
Chicago/Turabian StyleMohamed, Shahd A., Nosheen Umar, Melisa Sia, Justin Smyth, Sumedha Udumalagala, Mujeeb Ullahj Makki, Keith Roberts, Brinder Mahon, Raneem Albazaz, and Nigel Trudgill. 2025. "Root Cause Analysis of Patients with Pancreatic Cancer Who Underwent Imaging Not Resulting in a Cancer Diagnosis in the 18 Months Prior to Diagnosis" Cancers 17, no. 23: 3770. https://doi.org/10.3390/cancers17233770
APA StyleMohamed, S. A., Umar, N., Sia, M., Smyth, J., Udumalagala, S., Makki, M. U., Roberts, K., Mahon, B., Albazaz, R., & Trudgill, N. (2025). Root Cause Analysis of Patients with Pancreatic Cancer Who Underwent Imaging Not Resulting in a Cancer Diagnosis in the 18 Months Prior to Diagnosis. Cancers, 17(23), 3770. https://doi.org/10.3390/cancers17233770






