Role of Geriatric Assessment Scores as Predictors of Intensive Therapy Feasibility and Survival in Elderly Patients with Primary CNS Lymphoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. GA Scores
2.3. Endpoints
2.4. Statistical Analysis
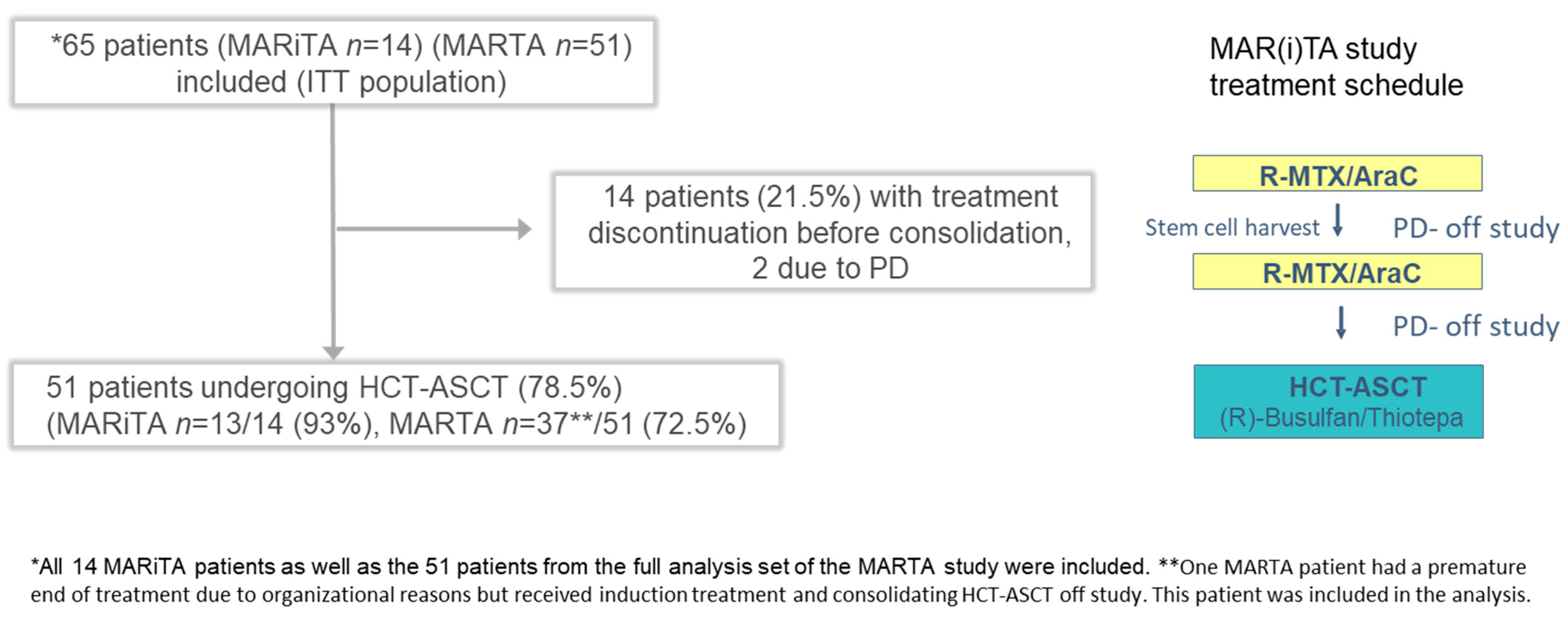
3. Results
3.1. Patient Characteristics
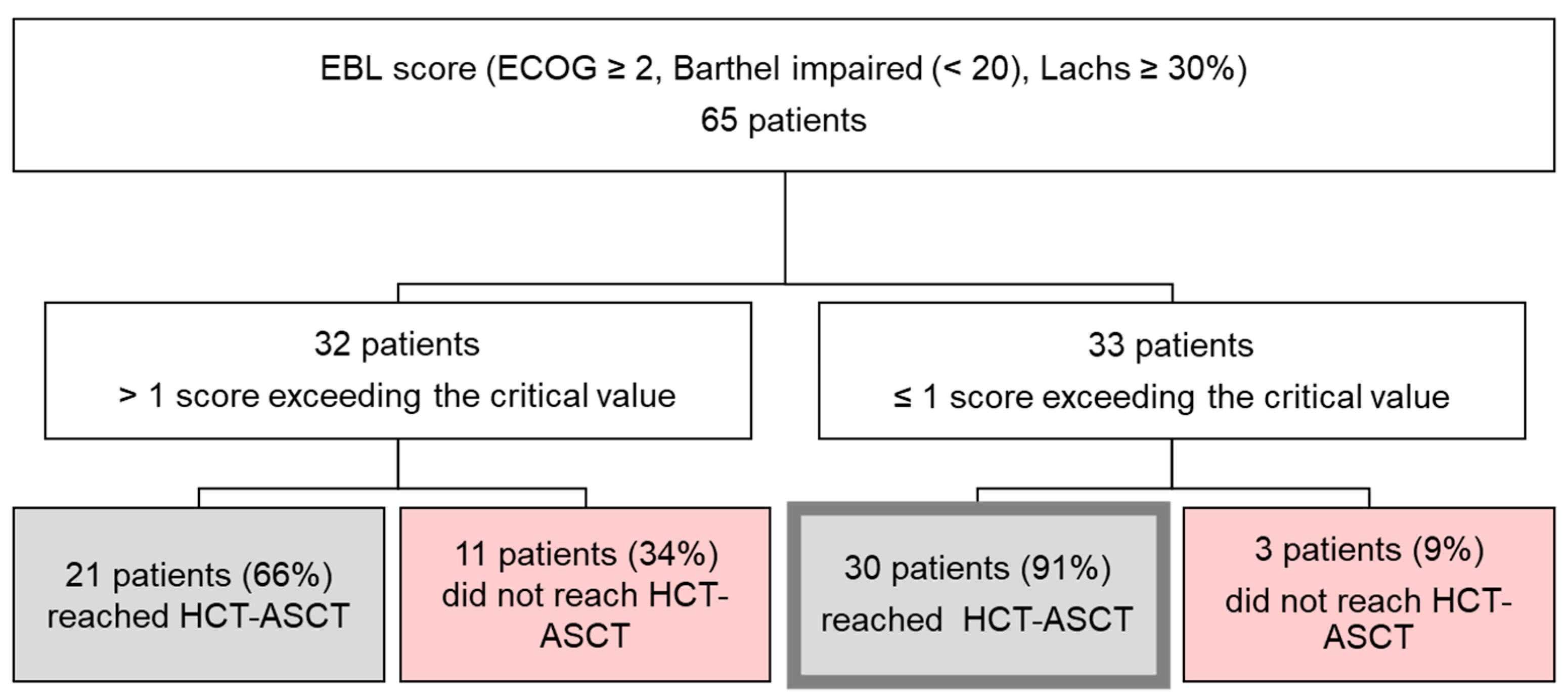
3.2. Evaluation of Geriatric Assessment Scores Including a Composite ECOG–Barthel–Lachs (EBL) Score with Regard to Premature End of Treatment
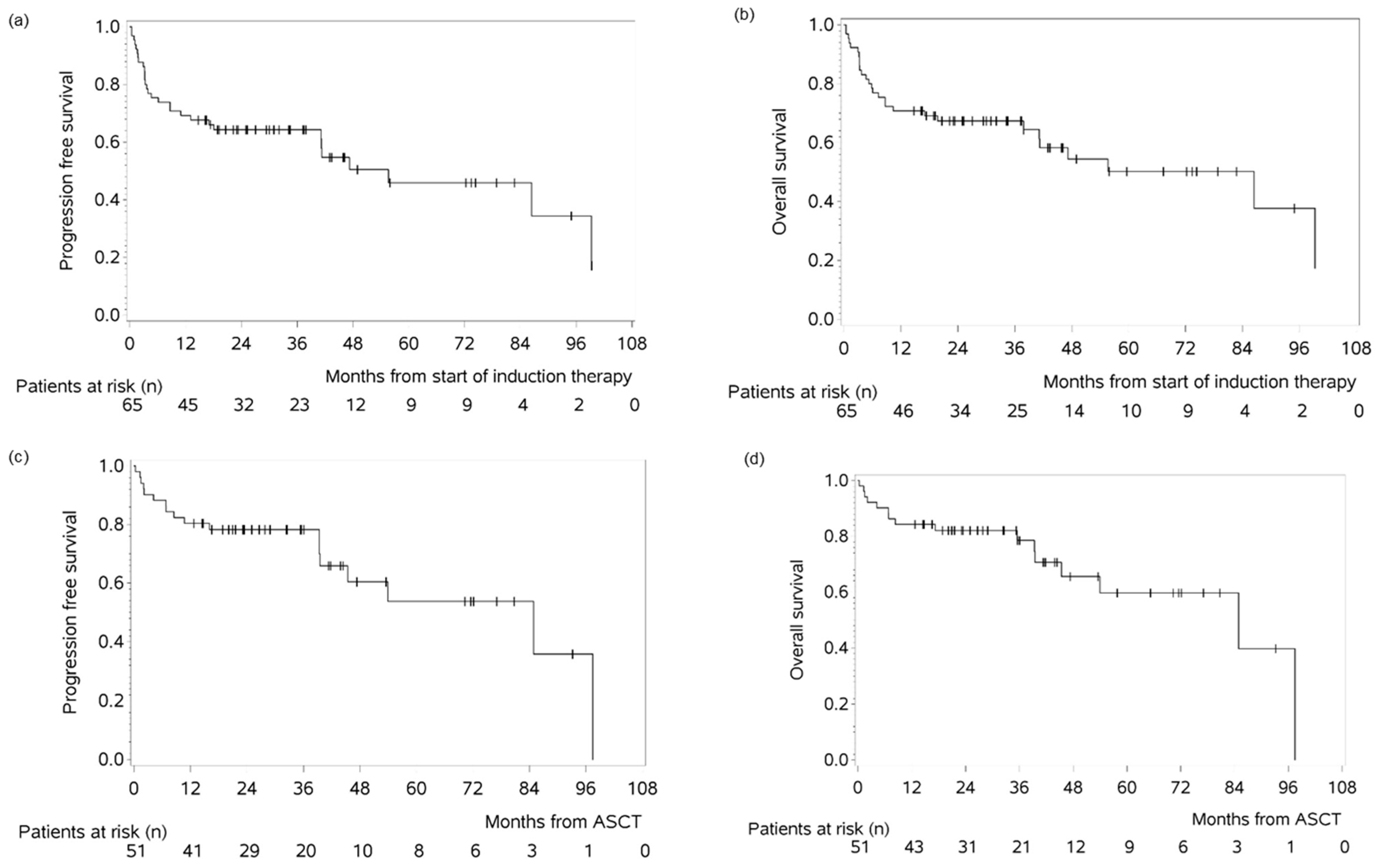
3.3. Survival Outcomes in the MAR(i)TA Cohort
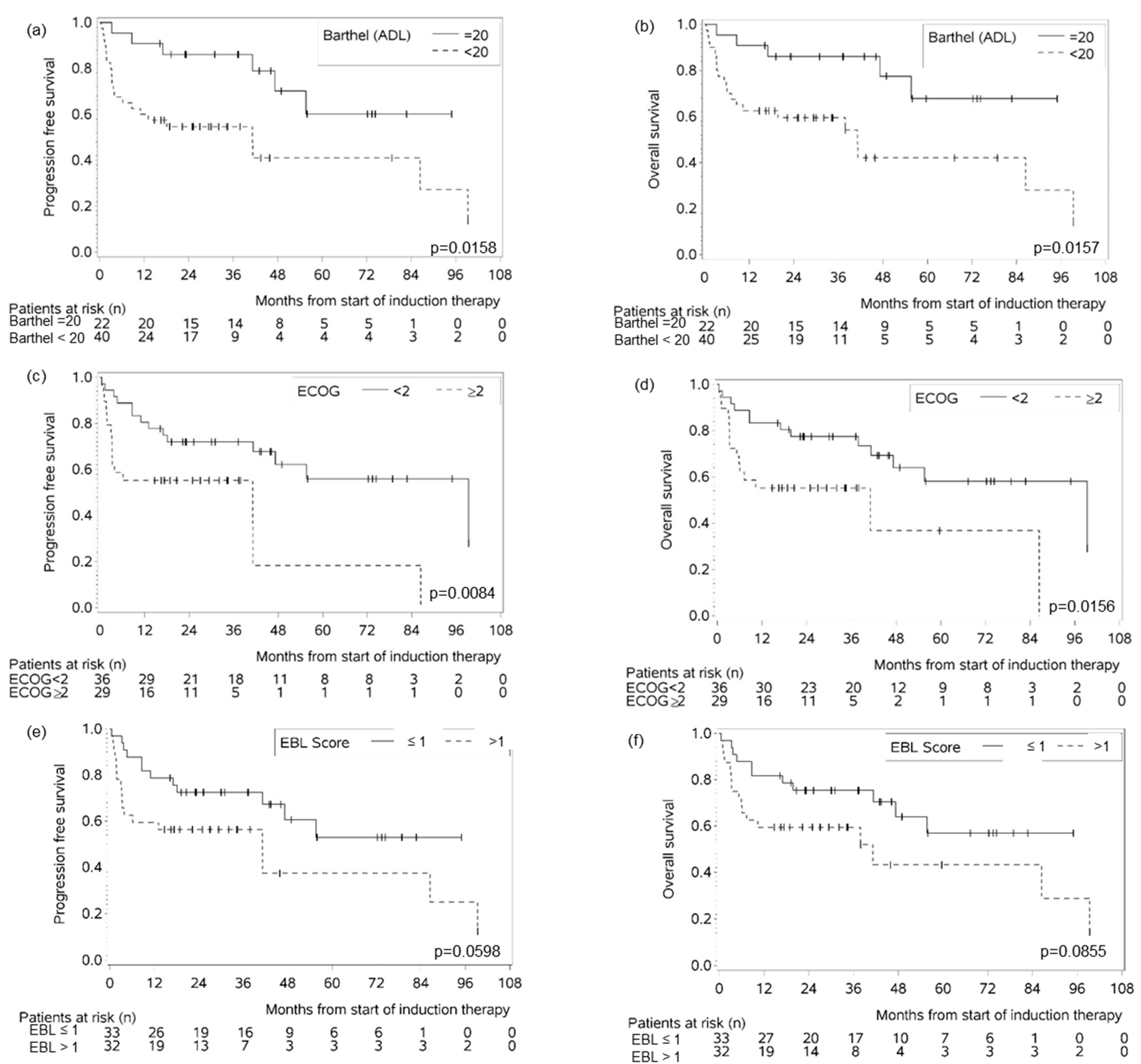
3.4. Geriatric Assessment Scores as Prognostic Factors for Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADL | Activity of daily living |
| AraC | cytarabine |
| CCI | Charlson comorbidity index |
| CI | Confidence interval |
| CIRS-G | Cumulative Illness Rating Scale-Geriatric |
| DLBCL | Diffuse large B-cell lymphoma |
| EBL | ECOG–Barthel–Lachs |
| ECOG PS | Eastern Cooperative Oncology Group Performance Status |
| eCRF | Electronic case report form |
| GA | Geriatric assessment |
| G8 | Geriatric 8 |
| HCT-ASCT | High-dose chemotherapy and autologous stem cell transplantation |
| HCT-CI | Hematopoietic cell transplantation–comorbidity index |
| (HD-)MTX | (High-dose) methotrexate |
| IADL | Instrumental activity of daily living |
| IELSG | International Extranodal Lymphoma Study Group |
| LOC | French Oculo-Cerebral Lymphoma Network |
| MMSE | Mini-Mental State Examination |
| MSKCC | Memorial Sloan Kettering Cancer Center |
| MVA | Multivariate analysis |
| NHL | Non-Hodgkin lymphoma |
| OS | Overall survival |
| PCNSL | Primary central nervous system lymphoma |
| PD | Progressive disease |
| pEOT | Premature end of treatment |
| PFS | Progression-free survival |
| R | Rituximab |
| R-MPV | Rituximab, MTX, procarbazine, vincristine |
| R-MP | Rituximab, MTX, procarbazine |
| UVA | Univariate analysis |
| WBRT | Whole brain radiotherapy |
References
- Roth, P.; Martus, P.; Kiewe, P.; Mohle, R.; Klasen, H.; Rauch, M.; Roth, A.; Kaun, S.; Thiel, E.; Korfel, A.; et al. Outcome of elderly patients with primary CNS lymphoma in the G-PCNSL-SG-1 trial. Neurology 2012, 79, 890–896. [Google Scholar] [CrossRef]
- Olivier, G.; Clavert, A.; Lacotte-Thierry, L.; Gardembas, M.; Escoffre-Barbe, M.; Brion, A.; Cumin, I.; Legouffe, E.; Solal-Celigny, P.; Chabin, M.; et al. A phase 1 dose escalation study of idarubicin combined with methotrexate, vindesine, and prednisolone for untreated elderly patients with primary central nervous system lymphoma. The GOELAMS LCP 99 trial. Am. J. Hematol. 2014, 89, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Illerhaus, G.; Doorduijn, J.K.; Auer, D.P.; Bromberg, J.E.C.; Calimeri, T.; Cwynarski, K.; Fox, C.P.; Hoang-Xuan, K.; Malaise, D.; et al. Primary central nervous system lymphomas: EHA-ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 491–507. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Cwynarski, K.; Pulczynski, E.; Fox, C.P.; Schorb, E.; La Rosee, P.; Binder, M.; Fabbri, A.; Torri, V.; Minacapelli, E.; et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: Results of the second randomisation of the International Extranodal Lymphoma Study Group-32 phase 2 trial. Lancet Haematol. 2017, 4, e510–e523. [Google Scholar] [CrossRef]
- Liu, J.; Guo, J.; Sun, X.; Liu, Y.; Gao, C. Efficacy and Safety of Autologous Stem-Cell Transplantation as Part of First-Line Treatment for Newly Diagnosed Primary Central Nervous System Lymphoma: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 799721. [Google Scholar] [CrossRef]
- Carrabba, M.G.; Reni, M.; Foppoli, M.; Chiara, A.; Franzin, A.; Politi, L.S.; Villa, E.; Ciceri, F.; Ferreri, A.J. Treatment approaches for primary CNS lymphomas. Expert Opin. Pharmacother. 2010, 11, 1263–1276. [Google Scholar] [CrossRef]
- Kasenda, B.; Ferreri, A.J.; Marturano, E.; Forst, D.; Bromberg, J.; Ghesquieres, H.; Ferlay, C.; Blay, J.Y.; Hoang-Xuan, K.; Pulczynski, E.J.; et al. First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL)—A systematic review and individual patient data meta-analysis. Ann. Oncol. 2015, 26, 1305–1313. [Google Scholar] [CrossRef]
- Villano, J.L.; Koshy, M.; Shaikh, H.; Dolecek, T.A.; McCarthy, B.J. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br. J. Cancer 2011, 105, 1414–1418. [Google Scholar] [CrossRef]
- Mendez, J.S.; Ostrom, Q.T.; Gittleman, H.; Kruchko, C.; DeAngelis, L.M.; Barnholtz-Sloan, J.S.; Grommes, C. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol. 2018, 20, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Siegal, T.; Bairey, O. Primary CNS Lymphoma in the Elderly: The Challenge. Acta Haematol. 2019, 141, 138–145. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Q.; Zhang, F. Diagnosis, prognosis and treatment of primary central nervous system lymphoma in the elderly population (Review). Int. J. Oncol. 2021, 58, 371–387. [Google Scholar] [CrossRef]
- Houillier, C.; Soussain, C.; Ghesquieres, H.; Soubeyran, P.; Chinot, O.; Taillandier, L.; Lamy, T.; Choquet, S.; Ahle, G.; Damaj, G.; et al. Management and outcome of primary CNS lymphoma in the modern era: An LOC network study. Neurology 2020, 94, e1027–e1039. [Google Scholar] [CrossRef]
- Burns, E.A.; Sanchez, C.G.; Mathur, S.; Guerrero, C.; Muhsen, I.N.; Sarfraz, H.; Hu, C.A.; Tang, C.A.; Shah, S.S.; Tremont, I.W.; et al. Long term outcomes in older patients with primary central nervous system lymphoma: An analysis of the Texas Cancer Registry. Ann. Hematol. 2023, 102, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Schorb, E.; Kasenda, B.; Ihorst, G.; Scherer, F.; Wendler, J.; Isbell, L.; Fricker, H.; Finke, J.; Illerhaus, G. High-dose chemotherapy and autologous stem cell transplant in elderly patients with primary CNS lymphoma: A pilot study. Blood Adv. 2020, 4, 3378–3381. [Google Scholar] [CrossRef] [PubMed]
- Schorb, E.; Isbell, L.K.; Kerkhoff, A.; Mathas, S.; Braulke, F.; Egerer, G.; Roth, A.; Schliffke, S.; Borchmann, P.; Brunnberg, U.; et al. High-dose chemotherapy and autologous haematopoietic stem-cell transplantation in older, fit patients with primary diffuse large B-cell CNS lymphoma (MARTA): A single-arm, phase 2 trial. Lancet Haematol. 2024, 11, e196–e205. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, O.S.; Arshad, S.; Lian, Q.; Ahn, K.W.; D’Souza, A.; Dhakal, B.; Mohan, M.; Pasquini, M.; Longo, W.; Shah, N.N.; et al. Comparison of Thiotepa-based Conditioning Regimens for Older Adults with Primary Diffuse Large B-cell Lymphoma of the Central Nervous System Undergoing Autologous Hematopoietic Cell Transplantation. Transpl. Cell. Ther. 2024, 30, 1191.e1–1191.e8. [Google Scholar] [CrossRef]
- Martinez-Calle, N.; Poynton, E.; Alchawaf, A.; Kassam, S.; Horan, M.; Rafferty, M.; Kelsey, P.; Scott, G.; Culligan, D.J.; Buckley, H.; et al. Outcomes of older patients with primary central nervous system lymphoma treated in routine clinical practice in the UK: Methotrexate dose intensity correlates with response and survival. Br. J. Haematol. 2020, 190, 394–404. [Google Scholar] [CrossRef]
- Kassam, S.; Chernucha, E.; O’Neill, A.; Hemmaway, C.; Cummins, T.; Montoto, S.; Lennard, A.; Adams, G.; Linton, K.; McKay, P.; et al. High-dose chemotherapy and autologous stem cell transplantation for primary central nervous system lymphoma: A multi-centre retrospective analysis from the United Kingdom. Bone Marrow Transpl. 2017, 52, 1268–1272. [Google Scholar] [CrossRef]
- Hurria, A.; Cirrincione, C.T.; Muss, H.B.; Kornblith, A.B.; Barry, W.; Artz, A.S.; Schmieder, L.; Ansari, R.; Tew, W.P.; Weckstein, D.; et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J. Clin. Oncol. 2011, 29, 1290–1296. [Google Scholar] [CrossRef]
- Scheepers, E.R.M.; Vondeling, A.M.; Thielen, N.; van der Griend, R.; Stauder, R.; Hamaker, M.E. Geriatric assessment in older patients with a hematologic malignancy: A systematic review. Haematologica 2020, 105, 1484–1493. [Google Scholar] [CrossRef]
- Mohile, S.G.; Dale, W.; Somerfield, M.R.; Hurria, A. Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology Summary. J. Oncol. Pract. 2018, 14, 442–446. [Google Scholar] [CrossRef]
- Martinez-Calle, N.; Isbell, L.K.; Cwynarski, K.; Schorb, E. Advances in treatment of elderly primary central nervous system lymphoma. Br. J. Haematol. 2022, 196, 473–487. [Google Scholar] [CrossRef]
- Plattel, W.J.; Kluin-Nelemans, H.C.; de Bock, G.H.; van Imhoff, G.W. Prognostic value of comorbidity for auto-SCT eligibility and outcome in relapsed or refractory aggressive non-Hodgkin’s lymphoma. Bone Marrow Transpl. 2011, 46, 827–834. [Google Scholar] [CrossRef]
- Merli, F.; Luminari, S.; Tucci, A.; Arcari, A.; Rigacci, L.; Hawkes, E.; Chiattone, C.S.; Cavallo, F.; Cabras, G.; Alvarez, I.; et al. Simplified Geriatric Assessment in Older Patients with Diffuse Large B-Cell Lymphoma: The Prospective Elderly Project of the Fondazione Italiana Linfomi. J. Clin. Oncol. 2021, 39, 1214–1222. [Google Scholar] [CrossRef]
- Eren, R.; Serin, I.; Atak, S.; Pirdal, B.Z.; Nizam, N.; Gemici, A.; Aydin, D.; Demirel, N.; Dogan, E.E.; Yokus, O. Charlson Comorbidity Index (CCI) in Diffuse Large B-cell Lymphoma: A New Approach in a Multicenter Study. Indian J. Hematol. Blood Transfus. 2023, 39, 191–199. [Google Scholar] [CrossRef]
- Isbell, L.K.; Uibeleisen, R.; Friedl, A.; Burger, E.; Dopatka, T.; Scherer, F.; Orban, A.; Lauer, E.; Malenica, N.; Semenova, I.; et al. Age-adjusted high-dose chemotherapy followed by autologous stem cell transplantation or conventional chemotherapy with R-MP as first-line treatment in elderly primary CNS lymphoma patients—The randomized phase III PRIMA-CNS trial. BMC Cancer 2023, 23, 767. [Google Scholar] [CrossRef] [PubMed]
- Farhi, J.; Laribi, K.; Orvain, C.; Hamel, J.F.; Mercier, M.; Sutra Del Galy, A.; Clavert, A.; Rousselet, M.C.; Tanguy-Schmidt, A.; Hunault-Berger, M.; et al. Impact of front line relative dose intensity for methotrexate and comorbidities in immunocompetent elderly patients with primary central nervous system lymphoma. Ann. Hematol. 2018, 97, 2391–2401. [Google Scholar] [CrossRef] [PubMed]
- David, K.A.; Sundaram, S.; Kim, S.H.; Vaca, R.; Lin, Y.; Singer, S.; Malecek, M.K.; Carter, J.; Zayac, A.; Kim, M.S.; et al. Older patients with primary central nervous system lymphoma: Survival and prognostication across 20 U.S. cancer centers. Am. J. Hematol. 2023, 98, 900–912. [Google Scholar] [CrossRef]
- Yamasaki, F.; Fudaba, H.; Asano, K.; Sasayama, T.; Natsumeda, M.; Shimabukuro, T.; Taguchi, K.; Koizumi, S.; Nakayama, N.; Fujii, K.; et al. Multidrug chemotherapy, whole-brain radiation and cytarabine therapy for primary central nervous system lymphoma in elderly patients with dose modification based on geriatric assessment: Study protocol for a phase II, multicentre, non-randomised study. BMJ Open 2023, 13, e071350. [Google Scholar] [CrossRef] [PubMed]
- Lachs, M.S.; Feinstein, A.R.; Cooney, L.M., Jr.; Drickamer, M.A.; Marottoli, R.A.; Pannill, F.C.; Tinetti, M.E. A simple procedure for general screening for functional disability in elderly patients. Ann. Intern. Med. 1990, 112, 699–706. [Google Scholar] [CrossRef]
- Ferreri, A.J.; Blay, J.Y.; Reni, M.; Pasini, F.; Spina, M.; Ambrosetti, A.; Calderoni, A.; Rossi, A.; Vavassori, V.; Conconi, A.; et al. Prognostic scoring system for primary CNS lymphomas: The International Extranodal Lymphoma Study Group experience. J. Clin. Oncol. 2003, 21, 266–272. [Google Scholar] [CrossRef]
- Abrey, L.E.; Ben-Porat, L.; Panageas, K.S.; Yahalom, J.; Berkey, B.; Curran, W.; Schultz, C.; Leibel, S.; Nelson, D.; Mehta, M.; et al. Primary central nervous system lymphoma: The Memorial Sloan-Kettering Cancer Center prognostic model. J. Clin. Oncol. 2006, 24, 5711–5715. [Google Scholar] [CrossRef]
- Collin, C.; Wade, D.T.; Davies, S.; Horne, V. The Barthel ADL Index: A reliability study. Int. Disabil. Stud. 1988, 10, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Krupp, S. Screening according to Lachs as common thread in geriatric assessment. MMW Fortschr. Med. 2023, 165, 40–42. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.J.; Kaempf, A.; Sitlinger, A.; Shouse, G.; Mei, M.; Brander, D.M.; Salous, T.; Hill, B.T.; Alqahtani, H.; Choi, M.; et al. The Chronic Lymphocytic Leukemia Comorbidity Index (CLL-CI): A Three-Factor Comorbidity Model. Clin. Cancer Res. 2021, 27, 4814–4824. [Google Scholar] [CrossRef] [PubMed]
| MAR(i)TA n = 65 | |
|---|---|
| Variable | Number (%) |
| Median age (range), years | 73 (65–80) |
| 65–69 | 19 (29%) |
| 70–74 | 27 (42%) |
| 75–80 | 19 (29%) |
| Sex | |
| Female | 37 (57%) |
| Male | 28 (43%) |
| ECOG Performance Status | |
| Grade 0 | 7 (10.8%) |
| Grade 1 | 29 (44.6%) |
| Grade 2 | 25 (38.5%) |
| Grade 3 | 4 (6.1%) |
| Serum lactate dehydrogenase | |
| Elevated | 28 (43.1%) |
| Normal | 36 (55.4%) |
| Not done | 1 (1.5%) |
| Lymphoma in deep brain structures | |
| Yes | 35 (53.8%) |
| No | 26 (40%) |
| Not done | 4 (6.2%) |
| CSF involvement | |
| Yes | 5 (7.7%) |
| Suspicious | 6 (9.2%) |
| No | 47 (72.3%) |
| Not done | 7 (10.8%) |
| Ocular involvement | |
| Yes or suspicious | 4 (6.2%) |
| No | 52 (80%) |
| Not done | 9 (13.8%) |
| Histology | |
| DLBCL | 65 (100%) |
| IELSG score | |
| Low 0–1 | 5 (7.7%) |
| Intermediate 2–3 | 34 (52.3%) |
| High 4–5 | 12 (18.5%) |
| Missing | 14 (21.5%) |
| MSKCC score | |
| class 2 | 39 (60%) |
| class 3 | 26 (40%) |
| Univariate Analysis | |||
|---|---|---|---|
| Variables | Premature End of Treatment n = 14/65 | ||
| OR | 95% CI | p-Value | |
| CIRS-G ≥6/<6 n = 32/33 | 4.832 | 1.202–19.431 | 0.027 |
| CIRS-G ≥7/<7 n = 25/40 | 3.937 | 1.136–13.647 | 0.031 |
| CIRS-G ≥8/<8 n = 19/46 | 4.848 | 1.387–16.944 | 0.013 |
| ECOG PS ≥2/<2 n = 29/36 | 4.210 | 1.158–15.311 | 0.029 |
| Barthel <20/=20 n = 40/22 missing n = 3 | 3.792 | 0.758–18.983 | 0.105 |
| Lachs ≥30%/<30% n = 31/34 | 5.683 | 1.409–22.929 | 0.015 |
| MMSE <24/≥24 n = 23/29 missing n = 13 | 1.065 | 0.280–4.057 | 0.927 |
| CCI ≥2/<2 n = 18/47 | 2.438 | 0.705–8.428 | 0.159 |
| Geriatric syndrome present n = 18/46 missing n = 1 | 0.720 | 0.173–2.990 | 0.651 |
| Age ≥75/<75 years n = 19/46 | 0.597 | 0.146–2.436 | 0.472 |
| Multivariate Analysis | |||
| Variable | Premature end of treatment n = 14/65 | ||
| OR | 95% CI | p-Value | |
| CIRS-G (≥7 vs.<7) | 7.295 | 1.447–36.781 | 0.016 |
| ECOG PS (≥2 vs.<2) | 5.794 | 0.997–33.676 | 0.050 |
| Barthel (<20 vs. =20) | 1.372 | 0.176–10.709 | 0.763 |
| Lachs (≥30% vs. <30%) | 3.803 | 0.603–24.004 | 0.155 |
| Progression-Free Survival | Overall Survival | |||||
|---|---|---|---|---|---|---|
| Variable | Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value |
| Barthel < 20 | 2.968 | 1.179–7.472 | 0.0209 | 3.203 | 1.186–8.648 | 0.0216 |
| ECOG PS ≥ 2 | 2.723 | 1.257–5.898 | 0.0111 | 2.563 | 1.165–5.638 | 0.0193 |
| LACHS ≥ 30% | 1.279 | 0.608–2.688 | 0.5164 | 1.249 | 0.578–2.699 | 0.5713 |
| CIRS-G < 6 | 0.717 | 0.344–1.493 | 0.3737 | 0.854 | 0.401–1.818 | 0.6824 |
| CIRS-G < 7 | 0.812 | 0.389–1.695 | 0.5784 | 1.024 | 0.472–2.224 | 0.9517 |
| CIRS-G < 8 | 0.935 | 0.426–2.051 | 0.8673 | 1.025 | 0.450–2.335 | 0.9539 |
| MMSE < 24 | 2.019 | 0.854–4.771 | 0.1094 | 1.925 | 0.796–4.652 | 0.1459 |
| CCI ≥ 2 | 0.897 | 0.396–2.030 | 0.7941 | 0.784 | 0.331–1.857 | 0.5796 |
| Geriatric syndrome present | 1.464 | 0.675–3.173 | 0.3344 | 1.325 | 0.590–2.976 | 0.4951 |
| EBL score (Lachs ≥ 30, Barthel < 20, ECOG ≥ 2) > 1 | 2.062 | 0.971–4.379 | 0.0598 | 1.983 | 0.909–4.327 | 0.0855 |
| Variable | Progression-Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| ECOG PS (≥2 vs.<2) | 3.161 | 1.209–8.269 | 0.0190 | 3.006 | 1.121–8.063 | 0.0288 |
| Barthel (<20 vs. =20) | 2.222 | 0.785–6.287 | 0.1324 | 2.448 | 0.825–7.261 | 0.1066 |
| Lachs (≥30% vs. <30%) | 0.636 | 0.258–1.569 | 0.3260 | 0.649 | 0.257–1.643 | 0.3617 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isbell, L.K.; Vreden, A.; Ihorst, G.; Uibeleisen, R.; Friedl, A.; Neumaier, S.; Wendler, J.; Orban, A.; Lauer, E.M.; Fricker, H.; et al. Role of Geriatric Assessment Scores as Predictors of Intensive Therapy Feasibility and Survival in Elderly Patients with Primary CNS Lymphoma. Cancers 2025, 17, 3759. https://doi.org/10.3390/cancers17233759
Isbell LK, Vreden A, Ihorst G, Uibeleisen R, Friedl A, Neumaier S, Wendler J, Orban A, Lauer EM, Fricker H, et al. Role of Geriatric Assessment Scores as Predictors of Intensive Therapy Feasibility and Survival in Elderly Patients with Primary CNS Lymphoma. Cancers. 2025; 17(23):3759. https://doi.org/10.3390/cancers17233759
Chicago/Turabian StyleIsbell, Lisa K., Annika Vreden, Gabriele Ihorst, Roswitha Uibeleisen, Alexander Friedl, Simone Neumaier, Julia Wendler, Andras Orban, Eliza M. Lauer, Heidi Fricker, and et al. 2025. "Role of Geriatric Assessment Scores as Predictors of Intensive Therapy Feasibility and Survival in Elderly Patients with Primary CNS Lymphoma" Cancers 17, no. 23: 3759. https://doi.org/10.3390/cancers17233759
APA StyleIsbell, L. K., Vreden, A., Ihorst, G., Uibeleisen, R., Friedl, A., Neumaier, S., Wendler, J., Orban, A., Lauer, E. M., Fricker, H., Malenica, N., Illerhaus, G., & Schorb, E. (2025). Role of Geriatric Assessment Scores as Predictors of Intensive Therapy Feasibility and Survival in Elderly Patients with Primary CNS Lymphoma. Cancers, 17(23), 3759. https://doi.org/10.3390/cancers17233759







