Harnessing Liquiritigenin: A Flavonoid-Based Approach for the Prevention and Treatment of Cancer
Simple Summary
Abstract
1. Introduction
2. Sources of LIQ
3. Structure and Chemistry of LIQ
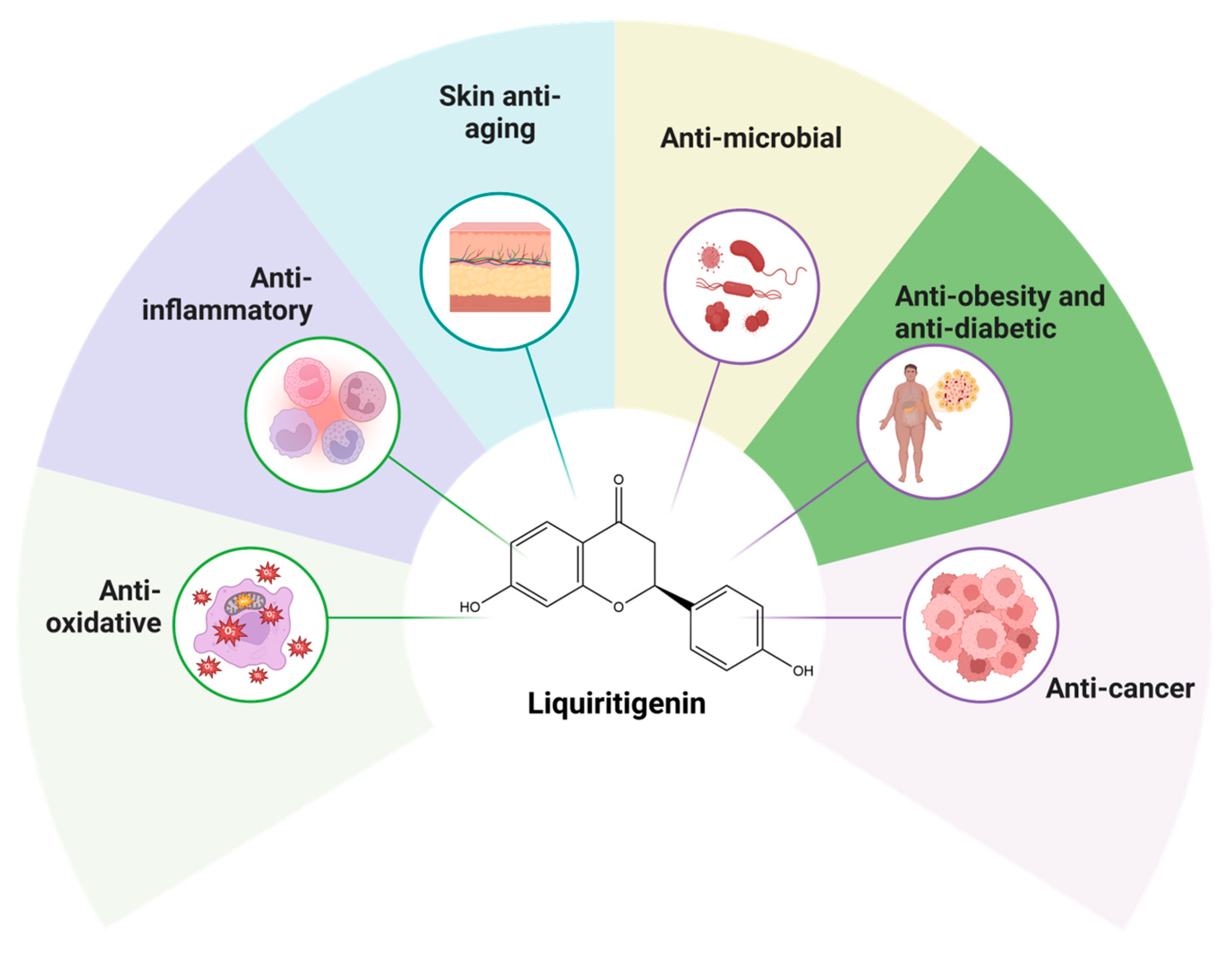
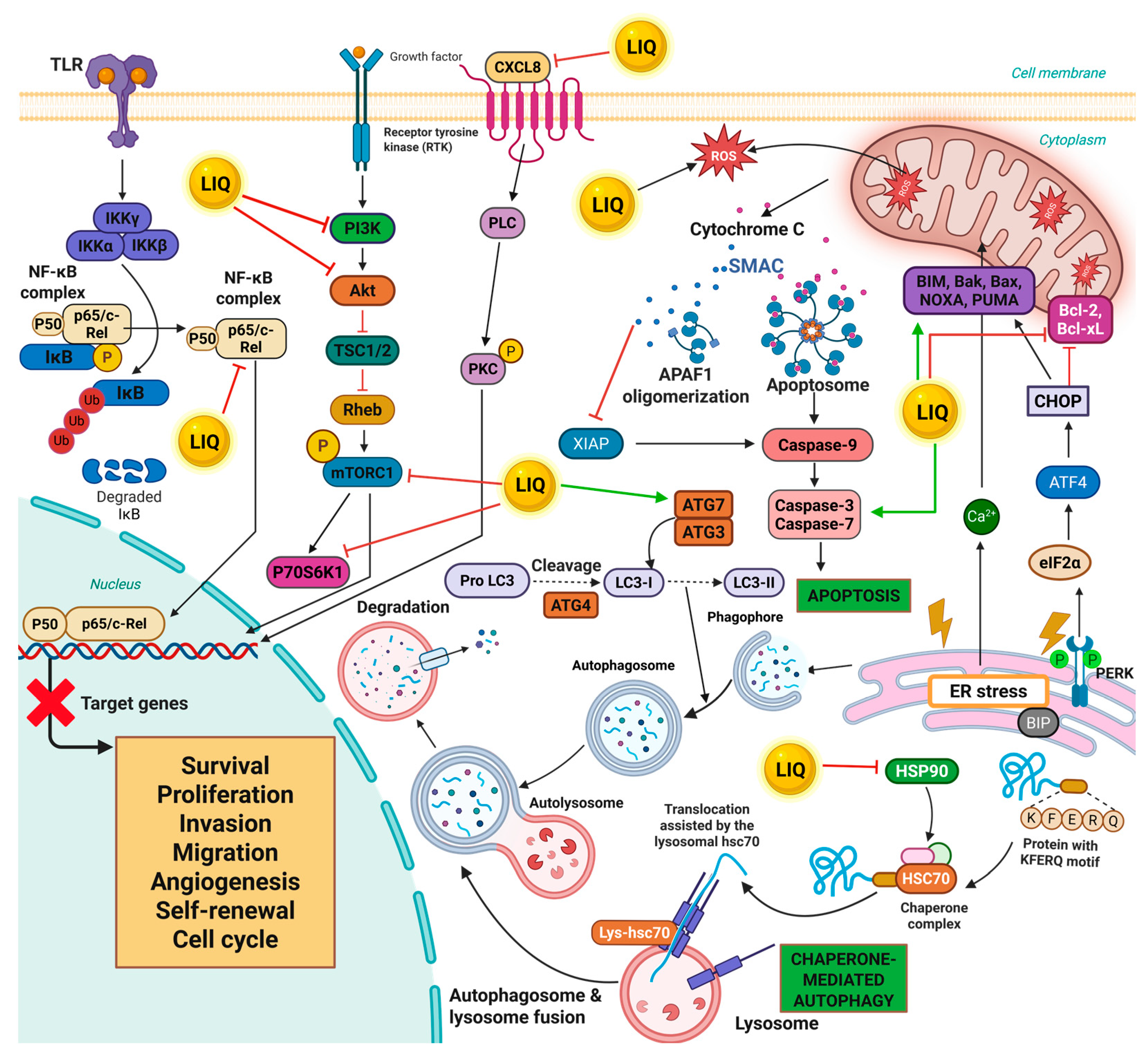
4. Biological Properties and Mechanism of Action of LIQ
5. Multifaceted Anticancer Effects of LIQ Across Diverse Cancer Types
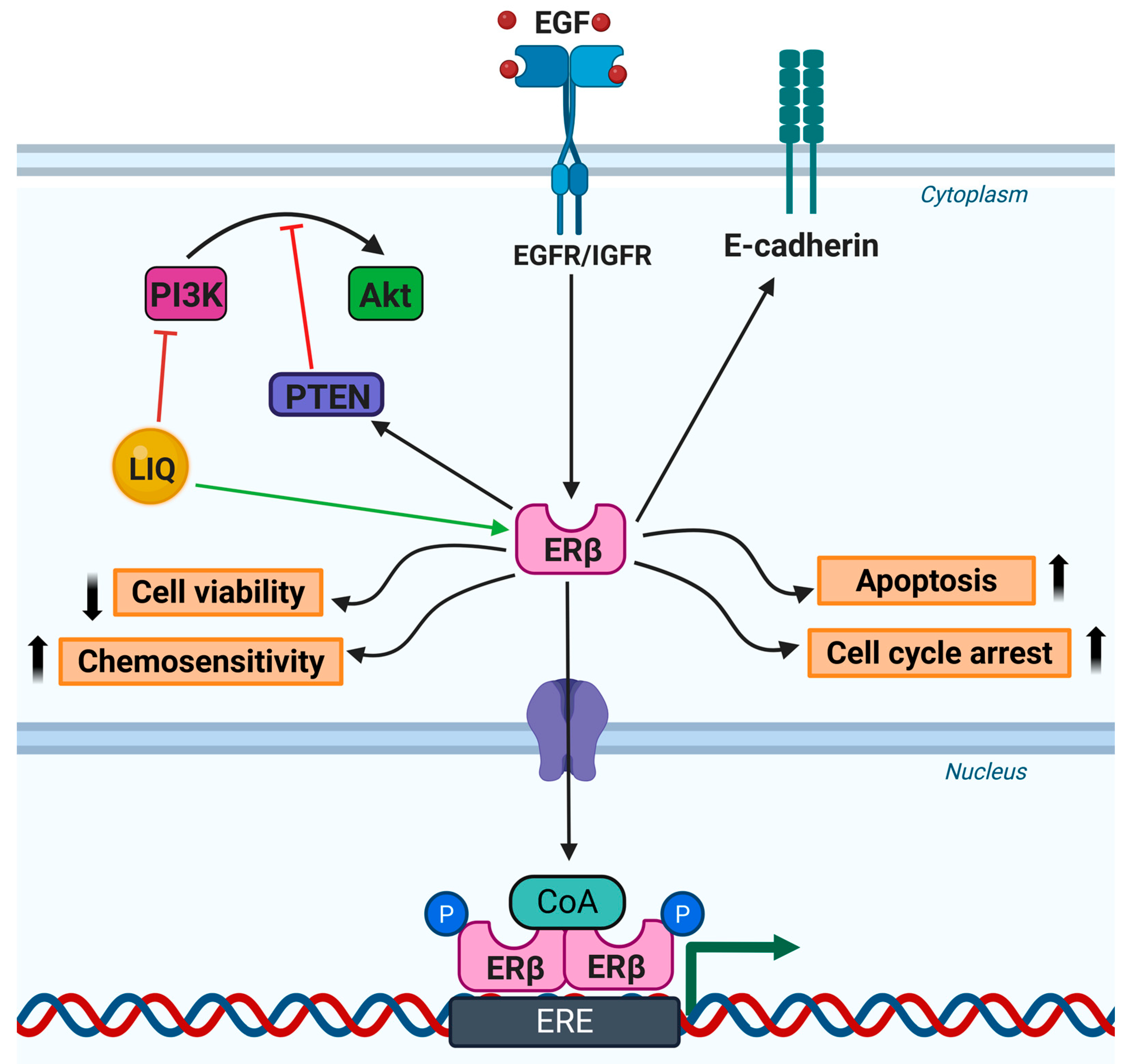
5.1. Breast Cancer
5.2. Brain Cancer
5.3. Colorectal Cancer
5.4. Liver Cancer
5.5. Lung Cancer
5.6. Ovarian Cancer
5.7. Prostate Cancer
5.8. Other Cancers
6. Pharmacokinetics of LIQ
7. Discussion
8. Conclusions
9. Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Behranvand, N.; Nasri, F.; Zolfaghari Emameh, R.; Khani, P.; Hosseini, A.; Garssen, J.; Falak, R. Chemotherapy: A double-edged sword in cancer treatment. Cancer Immunol. Immunother. 2022, 71, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Berkey, F.J. Managing the adverse effects of radiation therapy. Am. Fam. Physician 2010, 82, 381–388. [Google Scholar]
- Coates, A.; Abraham, S.; Kaye, S.B.; Sowerbutts, T.; Frewin, C.; Fox, R.M.; Tattersall, M.H. On the receiving end--patient perception of the side-effects of cancer chemotherapy. Eur. J. Cancer Clin. Oncol. 1983, 19, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Sajeev, A.; Hegde, M.; Daimary, U.D.; Kumar, A.; Girisa, S.; Sethi, G.; Kunnumakkara, A.B. Modulation of diverse oncogenic signaling pathways by oroxylin A: An important strategy for both cancer prevention and treatment. Phytomedicine 2022, 105, 154369. [Google Scholar] [CrossRef]
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and limitations in traditional anti-cancer therapies: A comprehensive review of surgery, chemotherapy, radiation therapy, and hormonal therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, H.; Tan, L.; Siu, K.T.H.; Guan, X.Y. Exploring treatment options in cancer: Tumor treatment strategies. Signal Transduct. Target. Ther. 2024, 9, 175. [Google Scholar] [CrossRef]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural Products/Bioactive Compounds as a Source of Anticancer Drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]
- Babu, B.; Jayram, H.; Nair, M.; Ajaikumar, K.; Padikkala, J. Free radical scavenging, antitumor and anticarcinogenic activity of gossypin. J. Exp. Clin. Cancer Res. CR 2003, 22, 581–589. [Google Scholar]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2019, 10, 1614. [Google Scholar] [CrossRef]
- Choudhury, B.; Kandimalla, R.; Bharali, R.; Monisha, J.; Kunnumakara, A.B.; Kalita, K.; Kotoky, J. Anticancer activity of Garcinia morella on T-cell murine lymphoma via apoptotic induction. Front. Pharmacol. 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.; Khan, W.R.; Yousaf, N.; Akram, S.; Murtaza, G.; Kudus, K.A.; Ditta, A.; Rosli, Z.; Rajpar, M.N.; Nazre, M. Exploring the Phytochemicals and Anti-Cancer Potential of the Members of Fabaceae Family: A Comprehensive Review. Molecules 2022, 27, 3863. [Google Scholar] [CrossRef] [PubMed]
- Girisa, S.; Saikia, Q.; Bordoloi, D.; Banik, K.; Monisha, J.; Daimary, U.D.; Verma, E.; Ahn, K.S.; Kunnumakkara, A.B. Xanthohumol from Hop: Hope for cancer prevention and treatment. IUBMB Life 2021, 73, 1016–1044. [Google Scholar] [CrossRef]
- Singh, Y.P.; Girisa, S.; Banik, K.; Ghosh, S.; Swathi, P.; Deka, M.; Padmavathi, G.; Kotoky, J.; Sethi, G.; Fan, L. Potential application of zerumbone in the prevention and therapy of chronic human diseases. J. Funct. Foods 2019, 53, 248–258. [Google Scholar] [CrossRef]
- Brockmueller, A.; Sajeev, A.; Koklesova, L.; Samuel, S.M.; Kubatka, P.; Busselberg, D.; Kunnumakkara, A.B.; Shakibaei, M. Resveratrol as sensitizer in colorectal cancer plasticity. Cancer Metastasis Rev. 2024, 43, 55–85. [Google Scholar] [CrossRef]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Kunnumakkara, A.B.; Aggarwal, B.B.; Shakibaei, M. Induction of the epithelial-to-mesenchymal transition of human colorectal cancer by human TNF-β (lymphotoxin) and its reversal by resveratrol. Nutrients 2019, 11, 704. [Google Scholar] [CrossRef]
- Hashem, S.; Ali, T.A.; Akhtar, S.; Nisar, S.; Sageena, G.; Ali, S.; Al-Mannai, S.; Therachiyil, L.; Mir, R.; Elfaki, I.; et al. Targeting cancer signaling pathways by natural products: Exploring promising anti-cancer agents. Biomed. Pharmacother. 2022, 150, 113054. [Google Scholar] [CrossRef]
- Manickasamy, M.K.; Kumar, A.; BharathwajChetty, B.; Alqahtani, M.S.; Abbas, M.; Alqahtani, A.; Unnikrishnan, J.; Bishayee, A.; Sethi, G.; Kunnumakkara, A.B. Synergistic enhancement: Exploring the potential of piperine in cancer therapeutics through chemosensitization and combination therapies. Life Sci. 2024, 354, 122943. [Google Scholar] [CrossRef]
- Monisha, J.; Padmavathi, G.; Roy, N.K.; Deka, A.; Bordoloi, D.; Anip, A.; Kunnumakkara, A.B. NF-κB blockers gifted by mother nature: Prospectives in cancer cell chemosensitization. Curr. Pharm. Des. 2016, 22, 4173–4200. [Google Scholar] [CrossRef]
- Muralimanoharan, S.B.; Kunnumakkara, A.; Shylesh, B.; Kulkarni, K.H.; Haiyan, X.; Ming, H.; Aggarwal, B.B.; Rita, G.; Kumar, A.P. Butanol fraction containing berberine or related compound from Nexrutine® inhibits NFκB signaling and induces apoptosis in prostate cancer cells. Prostate 2009, 69, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef] [PubMed]
- Babu, V.; Kapkoti, D.S.; Binwal, M.; Bhakuni, R.S.; Shanker, K.; Singh, M.; Tandon, S.; Mugale, M.N.; Kumar, N.; Bawankule, D.U. Liquiritigenin, isoliquiritigenin rich extract of glycyrrhiza glabra roots attenuates inflammation in macrophages and collagen-induced arthritis in rats. Inflammopharmacology 2023, 31, 983–996. [Google Scholar] [CrossRef]
- Erica, K.; Thabitha, A.; Ebenezar, K.K.; Kumar, S.S.A.; Abishek, V.; Priya, N.M.; Pazhani, G.P.; Ramachandran, S. Improved antioxidant and anti-tubercular potential of liquiritigenin grafted on low molecular weight chitosan from gladius of Sepioteuthis lessoniana. Int. J. Biol. Macromol. 2024, 268, 131728. [Google Scholar] [CrossRef]
- Gaur, R.; Yadav, K.S.; Verma, R.K.; Yadav, N.P.; Bhakuni, R.S. In vivo anti-diabetic activity of derivatives of isoliquiritigenin and liquiritigenin. Phytomedicine 2014, 21, 415–422. [Google Scholar] [CrossRef]
- Ning, X.; Ni, Y.; Cao, J.; Zhang, H. Liquiritigenin Attenuated Collagen-Induced Arthritis and Cardiac Complication via Inflammation and Fibrosis Inhibition in Mice. Chem. Pharm. Bull. 2023, 71, 269–276. [Google Scholar] [CrossRef]
- Zhai, Z.; Fu, J.; Ye, M.L.; Wang, J.Y.; Zhang, H.J.; Yu, H.; Yang, X.Y.; Xu, H.; Hu, J.C.; Lu, J.Y.; et al. The changes of intestinal microbiota and metabolomics during the inhibition of bladder cancer by liquiritigenin. J. Asian Nat. Prod. Res. 2024, 26, 1445–1454. [Google Scholar] [CrossRef]
- Kondo, K.; Shiba, M.; Nakamura, R.; Morota, T.; Shoyama, Y. Constituent properties of licorices derived from Glycyrrhiza uralensis, G. glabra, or G. inflata identified by genetic information. Biol. Pharm. Bull. 2007, 30, 1271–1277. [Google Scholar] [CrossRef]
- Yan, W.; Wang, L.; Cao, Y.; Chen, Y.; Lin, Y.; Qian, Y.; Wang, Y.; Dong, Z. Liquiritigenin regulates MAPK (p38/JNK) signaling through inhibition of IRAK4, attenuates inflammatory response, fibrosis and kidney dysfunction in a high-salt diet induced chronic kidney disease. Chem. Biol. Interact. 2025, 418, 111578. [Google Scholar] [CrossRef]
- Fiore, C.; Eisenhut, M.; Ragazzi, E.; Zanchin, G.; Armanini, D. A history of the therapeutic use of liquorice in Europe. J. Ethnopharmacol. 2005, 99, 317–324. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Herrera-Bravo, J.; Belen, L.H.; Kaur, R.; Kregiel, D.; Uprety, Y.; Beyatli, A.; Yeskaliyeva, B.; Kirkin, C.; et al. Glycyrrhiza Genus: Enlightening Phytochemical Components for Pharmacological and Health-Promoting Abilities. Oxid. Med. Cell Longev. 2021, 2021, 7571132. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Fan, P.; Wang, M.; Zhang, C.; Jiang, Y.; Huang, S.; Fang, M.; He, Z.; Wu, A. Elevated estrogen receptor beta expression in triple negative breast cancer cells is associated with sensitivity to doxorubicin by inhibiting the PI3K/AKT/mTOR signaling pathway. Exp. Ther. Med. 2020, 20, 1630–1636. [Google Scholar] [CrossRef] [PubMed]
- Sareddy, G.R.; Pratap, U.P.; Venkata, P.P.; Zhou, M.; Alejo, S.; Viswanadhapalli, S.; Tekmal, R.R.; Brenner, A.J.; Vadlamudi, R.K. Activation of estrogen receptor beta signaling reduces stemness of glioma stem cells. Stem Cells 2021, 39, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, S.; Liu, C.; Wu, Y.; Liu, Y.; Cai, Y. Inhibitory effect of liquiritigenin on migration via downregulation proMMP-2 and PI3K/Akt signaling pathway in human lung adenocarcinoma A549 cells. Nutr. Cancer 2012, 64, 627–634. [Google Scholar] [CrossRef]
- Ji, Y.; Hu, W.; Jin, Y.; Yu, H.; Fang, J. Liquiritigenin exerts the anti-cancer role in oral cancer via inducing autophagy-related apoptosis through PI3K/AKT/mTOR pathway inhibition in vitro and in vivo. Bioengineered 2021, 12, 6070–6082. [Google Scholar] [CrossRef]
- Wang, C.; Liu, B.; Dan, W.; Wei, Y.; Li, M.; Guo, C.; Zhang, Y.; Xie, H. Liquiritigenin inhibits the migration, invasion, and EMT of prostate cancer through activating ER stress. Arch. Biochem. Biophys. 2024, 761, 110184. [Google Scholar] [CrossRef]
- Wang, D.; Lu, J.; Liu, Y.; Meng, Q.; Xie, J.; Wang, Z.; Teng, L. Liquiritigenin induces tumor cell death through mitogen-activated protein kinase- (MPAKs-) mediated pathway in hepatocellular carcinoma cells. Biomed. Res. Int. 2014, 2014, 965316. [Google Scholar] [CrossRef]
- Ma, C.-J.; Li, G.-S.; Zhang, D.-L.; Liu, K.; Fan, X. One step isolation and purification of liquiritigenin and isoliquiritigenin from Glycyrrhiza uralensis Risch. using high-speed counter-current chromatography. J. Chromatogr. A 2005, 1078, 188–192. [Google Scholar] [CrossRef]
- Liu, R.-X.; Wang, Q.; Guo, H.-Z.; Li, L.; Bi, K.-S.; Guo, D.-A. Simultaneous determination of 10 major flavonoids in Dalbergia odorifera by high performance liquid chromatography. J. Pharm. Biomed. Anal. 2005, 39, 469–476. [Google Scholar] [CrossRef]
- Hong, Y.-H.; Wang, S.-C.; Hsu, C.; Lin, B.-F.; Kuo, Y.-H.; Huang, C.-J. Phytoestrogenic compounds in alfalfa sprout (Medicago sativa) beyond coumestrol. J. Agric. Food Chem. 2011, 59, 131–137. [Google Scholar] [CrossRef]
- Tarbeeva, D.V.; Pislyagin, E.A.; Menchinskaya, E.S.; Berdyshev, D.V.; Krylova, N.V.; Iunikhina, O.V.; Kalinovskiy, A.I.; Shchelkanov, M.Y.; Mishchenko, N.P.; Aminin, D.L.; et al. Polyphenols from Maackia amurensis Heartwood Protect Neuronal Cells from Oxidative Stress and Prevent Herpetic Infection. Int. J. Mol. Sci. 2024, 25, 4142. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Do, T.H.; Nguyen, T.H.; Duong, T.H.; Vo, H.C.; Do, V.M.; Nguyen, T.P.; Sichaem, J.; Nguyen, N.H.; Nguyen, H.T. Two new phenolic compounds from Boerhavia erecta collected in Vietnam. Nat. Prod. Res. 2023, 37, 3470–3477. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, S.; Alenzi, N.D. Evaluation of the antitrypanosomal activity, cytotoxicity and phytochemistry of red Brazilian propolis. PLoS ONE 2024, 19, e0313987. [Google Scholar] [CrossRef] [PubMed]
- Aldana-Mejia, J.A.; Ccana-Ccapatinta, G.V.; Ribeiro, V.P.; Arruda, C.; Veneziani, R.C.S.; Ambrosio, S.R.; Bastos, J.K. A validated HPLC-UV method for the analysis of phenolic compounds in Brazilian red propolis and Dalbergia ecastaphyllum. J. Pharm. Biomed. Anal. 2021, 198, 114029. [Google Scholar] [CrossRef]
- Kaszas, L.; Alshaal, T.; El-Ramady, H.; Kovacs, Z.; Koroknai, J.; Elhawat, N.; Nagy, E.; Cziaky, Z.; Fari, M.; Domokos-Szabolcsy, E. Identification of Bioactive Phytochemicals in Leaf Protein Concentrate of Jerusalem Artichoke (Helianthus tuberosus L.). Plants 2020, 9, 889. [Google Scholar] [CrossRef]
- Qiu, L.; Xiao, C.J.; Shen, Y.; Xu, W.; Liu, X.B.; Dong, X.; Jiang, B. Bioactive hydroxypropionylated glucose derivatives from Astragalus bhotanensis. Nat. Prod. Res. 2021, 35, 5066–5074. [Google Scholar] [CrossRef]
- Li, W.; Kim, T.I.; Kim, J.H.; Chung, H.S. Immune Checkpoint PD-1/PD-L1 CTLA-4/CD80 are Blocked by Rhus verniciflua Stokes and its Active Compounds. Molecules 2019, 24, 4062. [Google Scholar] [CrossRef]
- Yadav, V.K.; Mishra, A. In vitro & in silico study of hypoglycemic potential of Pterocarpus marsupium heartwood extract. Nat. Prod. Res. 2019, 33, 3298–3302. [Google Scholar] [CrossRef]
- Kil, Y.S.; Park, J.; Jafari, M.; Woo, H.A.; Seo, E.K. Minor phenolics from Angelica keiskei and their proliferative effects on Hep3B cells. Bioorganic Med. Chem. Lett. 2017, 27, 3065–3070. [Google Scholar] [CrossRef]
- de Sousa, L.M.; de Carvalho, J.L.; da Silva, H.C.; Lemos, T.L.; Arriaga, A.M.; Braz-Filho, R.; Militao, G.C.; Silva, T.D.; Ribeiro, P.R.; Santiago, G.M. New Cytotoxic Bibenzyl and Other Constituents from Bauhinia ungulata L. (Fabaceae). Chem. Biodivers. 2016, 13, 1630–1635. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Nguyen, N.T.; Nguyen, M.H.; Le, T.H.; Van Do, T.N.; Hung, T.M.; Nguyen, M.T. Tyrosinase inhibitory activity of flavonoids from Artocarpus heterophyllous. Chem. Cent. J. 2016, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Youn, I.S.; Han, A.R.; Roh, M.S.; Seo, E.K. Constituents of the leaves of Verbascum blattaria. Nat. Prod. Commun. 2015, 10, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Khamsan, S.; Liawruangrath, S.; Teerawutkulrag, A.; Pyne, S.G.; Garson, M.J.; Liawruangrath, B. The isolation of bioactive flavonoids from Jacaranda obtusifolia H. B. K. ssp. rhombifolia (G. F. W. Meijer) Gentry. Acta Pharm. 2012, 62, 181–190. [Google Scholar] [CrossRef]
- Valianou, L.; Stathopoulou, K.; Karapanagiotis, I.; Magiatis, P.; Pavlidou, E.; Skaltsounis, A.L.; Chryssoulakis, Y. Phytochemical analysis of young fustic (Cotinus coggygria heartwood) and identification of isolated colourants in historical textiles. Anal. Bioanal. Chem. 2009, 394, 871–882. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Sestili, P.; Ismail, T.; Neugart, S.; Qamar, M.; Esatbeyoglu, T. Toxicity, Antioxidant Activity, and Phytochemicals of Basil (Ocimum basilicum L.) Leaves Cultivated in Southern Punjab, Pakistan. Foods 2022, 11, 1239. [Google Scholar] [CrossRef]
- Sinan, K.I.; Chiavaroli, A.; Orlando, G.; Bene, K.; Zengin, G.; Cziaky, Z.; Jeko, J.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Menghini, L.; et al. Evaluation of Pharmacological and Phytochemical Profiles Piptadeniastrum africanum (Hook.f.) Brenan Stem Bark Extracts. Biomolecules 2020, 10, 516. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Wu, K.X.; Guo, X.R.; Tang, Z.H. A rapid method for sensitive profiling of bioactive triterpene and flavonoid from Astragalus mongholicus and Astragalus membranaceus by ultra-pressure liquid chromatography with tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1085, 110–118. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Harithpriya, K.; Jayasuriya, R.; Adhikari, T.; Rai, A.; Ramkumar, K.M. Modulation of transcription factors by small molecules in beta-cell development and differentiation. Eur. J. Pharmacol. 2023, 946, 175606. [Google Scholar] [CrossRef]
- Simmler, C.; Hajirahimkhan, A.; Lankin, D.C.; Bolton, J.L.; Jones, T.; Soejarto, D.D.; Chen, S.N.; Pauli, G.F. Dynamic residual complexity of the isoliquiritigenin-liquiritigenin interconversion during bioassay. J. Agric. Food Chem. 2013, 61, 2146–2157. [Google Scholar] [CrossRef]
- Zhang, M.; Qi, J.; He, Q.; Ma, D.; Li, J.; Chu, X.; Zuo, S.; Zhang, Y.; Li, L.; Chu, L. Liquiritigenin protects against myocardial ischemic by inhibiting oxidative stress, apoptosis, and L-type Ca(2+) channels. Phytother. Res. 2022, 36, 3619–3631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xue, Y.; Zheng, B.; Li, L.; Chu, X.; Zhao, Y.; Wu, Y.; Zhang, J.; Han, X.; Wu, Z.; et al. Liquiritigenin protects against arsenic trioxide-induced liver injury by inhibiting oxidative stress and enhancing mTOR-mediated autophagy. Biomed. Pharmacother. 2021, 143, 112167. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Dong, Y.; Su, Q.; Wang, H.; Chen, Y.; Xue, W.; Chen, C.; Xia, B.; Duan, J.; Chen, G. Liquiritigenin reverses depression-like behavior in unpredictable chronic mild stress-induced mice by regulating PI3K/Akt/mTOR mediated BDNF/TrkB pathway. Behav. Brain Res. 2016, 308, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Zhao, R.J.; Park, S.J.; Lee, J.R.; Cho, I.J.; Yang, C.H.; Kim, S.G.; Kim, S.C. Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-kappaB-dependent iNOS and proinflammatory cytokines production. Br. J. Pharmacol. 2008, 154, 165–173. [Google Scholar] [CrossRef]
- Bao, L.; Hao, P.; Jiang, M.; Chu, W. Liquiritigenin regulates insulin sensitivity and ameliorates inflammatory responses in the nonalcoholic fatty liver by activation PI3K/AKT pathway. Chem. Biol. Drug Des. 2023, 102, 793–804. [Google Scholar] [CrossRef]
- Qin, M.; Guo, A.; Li, F.; Zhang, F.; Bi, M.; Zhang, Y.; Zhu, W. Liquiritigenin enhances cyclic adenosine monophosphate production to mitigate inflammation in dendritic cells. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211038098. [Google Scholar] [CrossRef]
- Huang, Z.; Sheng, Y.; Chen, M.; Hao, Z.; Hu, F.; Ji, L. Liquiritigenin and liquiritin alleviated MCT-induced HSOS by activating Nrf2 antioxidative defense system. Toxicol. Appl. Pharmacol. 2018, 355, 18–27. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhao, Y.; Long, Z.; Song, A.; Huang, P.; Wang, K.; Xu, L.; Molloy, D.P.; He, G. Liquiritigenin promotes osteogenic differentiation and prevents bone loss via inducing auto-lysosomal degradation and inhibiting apoptosis. Genes Dis. 2023, 10, 284–300. [Google Scholar] [CrossRef]
- Carnovali, M.; Banfi, G.; Mariotti, M. Liquiritigenin reduces osteoclast activity in zebrafish model of glucocorticoid-induced osteoporosis. J. Pharmacol. Sci. 2020, 143, 300–306. [Google Scholar] [CrossRef]
- Uchino, K.; Okamoto, K.; Sakai, E.; Yoneshima, E.; Iwatake, M.; Fukuma, Y.; Nishishita, K.; Tsukuba, T. Dual Effects of Liquiritigenin on the Proliferation of Bone Cells: Promotion of Osteoblast Differentiation and Inhibition of Osteoclast Differentiation. Phytother. Res. 2015, 29, 1714–1721. [Google Scholar] [CrossRef]
- Liu, J.; Viswanadhapalli, S.; Garcia, L.; Zhou, M.; Nair, B.C.; Kost, E.; Rao Tekmal, R.; Li, R.; Rao, M.K.; Curiel, T.; et al. Therapeutic utility of natural estrogen receptor beta agonists on ovarian cancer. Oncotarget 2017, 8, 50002–50014. [Google Scholar] [CrossRef] [PubMed]
- Sajeev, A.; Manickasamy, M.K.; Vishwa, R.; Kunnumakkara, A.B. Signaling Pathways in Cancer Drug Resistance: Potential Targets for Therapeutic Intervention. In Molecular Targets in Cancer Therapy; Springer: Singapore, 2025; pp. 101–126. [Google Scholar]
- Liu, C.; Wang, Y.; Xie, S.; Zhou, Y.; Ren, X.; Li, X.; Cai, Y. Liquiritigenin induces mitochondria—Mediated apoptosis via cytochrome c release and caspases activation in heLa Cells. Phytother. Res. 2011, 25, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hirchaud, F.; Hermetet, F.; Ablise, M.; Fauconnet, S.; Vuitton, D.A.; Pretet, J.L.; Mougin, C. Isoliquiritigenin induces caspase-dependent apoptosis via downregulation of HPV16 E6 expression in cervical cancer Ca Ski cells. Planta Medica 2013, 79, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Seo, G.S.; Lee, S.H. Isoliquiritigenin-mediated p62/SQSTM1 induction regulates apoptotic potential through attenuation of caspase-8 activation in colorectal cancer cells. Eur. J. Pharmacol. 2018, 841, 90–97. [Google Scholar] [CrossRef]
- Qin, H.; Song, Z.; Zhao, C.; Yang, J.; Xia, F.; Wang, L.; Ali, A.; Zheng, W. Liquiritigenin inhibits lipid accumulation in 3T3-L1 cells via mTOR-mediated regulation of the autophagy mechanism. Nutrients 2022, 14, 1287. [Google Scholar] [CrossRef]
- Lu, Q.; Zou, L.F.; Gao, Y.Z.; Ye, T.; Li, M.J.; Zhang, Y.K.; Liang, B.; Sun, W.; Xing, D.M. Liquiritigenin reverses skin aging by inhibiting UV—Induced mitochondrial uncoupling and excessive energy consumption. J. Cosmet. Dermatol. 2023, 22, 1017–1030. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, J.; Zhang, J.; Yuan, Y.; Liu, Z.; Chen, S.; Chen, K.; Dong, L.; Cheng, Z.; Zhang, Y. Quantitation of global histone post-translational modifications reveal anti-inflammatory epigenetic mechanisms of liquiritigenin based on the optimized super-SILAC strategy. Front. Cell Dev. Biol. 2025, 13, 1566567. [Google Scholar] [CrossRef]
- Chi, J.-H.; Seo, G.S.; Cheon, J.H.; Lee, S.H. Isoliquiritigenin inhibits TNF-α-induced release of high-mobility group box 1 through activation of HDAC in human intestinal epithelial HT-29 cells. Eur. J. Pharmacol. 2017, 796, 101–109. [Google Scholar] [CrossRef]
- Liang, F.; Zhang, H.; Gao, H.; Cheng, D.; Zhang, N.; Du, J.; Yue, J.; Du, P.; Zhao, B.; Yin, L. Liquiritigenin decreases tumorigenesis by inhibiting DNMT activity and increasing BRCA1 transcriptional activity in triple-negative breast cancer. Exp. Biol. Med. 2021, 246, 459–466. [Google Scholar] [CrossRef]
- Hua, Q.; Ren, L. The SIRT1/Nrf2 signaling pathway mediates the anti-pulmonary fibrosis effect of liquiritigenin. Chin. Med. 2024, 19, 12. [Google Scholar] [CrossRef]
- Zhou, M.; Dai, Y.; Ma, Y.; Yan, Y.; Hua, M.; Gao, Q.; Geng, X.; Zhou, Q. Protective effects of liquiritigenin against cisplatin-induced nephrotoxicity via NRF2/SIRT3-mediated improvement of mitochondrial function. Molecules 2022, 27, 3823. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Gupta, V.K.; Singh, P.; Pal, A.; Darokar, M.P.; Bhakuni, R.S. Drug Resistance Reversal Potential of Isoliquiritigenin and Liquiritigenin Isolated from Glycyrrhiza glabra Against Methicillin-Resistant Staphylococcus aureus (MRSA). Phytother. Res. 2016, 30, 1708–1715. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Lee, J.H.; Park, J.H.; Kim, S.Y.; Choi, J.Y.; Lee, S.H.; Kim, Y.S.; Kang, S.S.; Jang, E.C.; Han, Y. Liquiritigenin, a licorice flavonoid, helps mice resist disseminated candidiasis due to Candida albicans by Th1 immune response, whereas liquiritin, its glycoside form, does not. Int. Immunopharmacol. 2009, 9, 632–638. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Zverev, Y.F.; Rykunova, A.Y. Modern nanocarriers as a factor in increasing the bioavailability and pharmacological activity of flavonoids. Appl. Biochem. Microbiol. 2022, 58, 1002–1020. [Google Scholar] [CrossRef]
- Charalabidis, A.; Sfouni, M.; Bergström, C.; Macheras, P. The biopharmaceutics classification system (BCS) and the biopharmaceutics drug disposition classification system (BDDCS): Beyond guidelines. Int. J. Pharm. 2019, 566, 264–281. [Google Scholar] [CrossRef]
- Taldaev, A.; Svotin, A.A.; Obukhov, S.I.; Terekhov, R.P.; Selivanova, I.A. Modification of biopharmaceutical parameters of flavonoids: A review. Front. Chem. 2025, 13, 1602967. [Google Scholar] [CrossRef]
- Shi, C.; Wu, H.; Xu, K.; Cai, T.; Qin, K.; Wu, L.; Cai, B. Liquiritigenin-loaded submicron emulsion protects against doxorubicin-induced cardiotoxicity via antioxidant, anti-inflammatory, and anti-apoptotic activity. Int. J. Nanomed. 2020, 15, 1101–1115. [Google Scholar] [CrossRef]
- Shi, C.C.; Qin, K.M.; Xu, K.; Chen, A.; Cai, T.; Cai, B.C. Development of liquiritigenin-phospholipid complex with the enhanced oral bioavailability. Chin. J. Nat. Med. 2020, 18, 916–921. [Google Scholar] [CrossRef]
- Zhang, X.; Qiao, H.; Zhang, T.; Shi, Y.; Ni, J. Enhancement of gastrointestinal absorption of isoliquiritigenin by nanostructured lipid carrier. Adv. Powder Technol. 2014, 25, 1060–1068. [Google Scholar] [CrossRef]
- Qiao, F.; Zhao, Y.; Mai, Y.; Guo, J.; Dong, L.; Zhang, W.; Yang, J. Isoliquiritigenin nanosuspension enhances cytostatic effects in A549 lung cancer cells. Planta Medica 2020, 86, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, X.; Zu, Y.; Wu, W.; Li, Y.; Guo, Z.; Wang, L.; Wang, L. Licorice flavonoids nanoparticles prepared by liquid antisolvent re-crystallization exhibit higher oral bioavailability and antioxidant activity in rat. J. Funct. Foods 2019, 57, 190–201. [Google Scholar] [CrossRef]
- Xu, S.; Ma, Z.; Xing, L.; Cheng, W. Polygonatum sibiricum component liquiritigenin restrains breast cancer cell invasion and migration by inhibiting HSP90 and chaperone-mediated autophagy. Korean J. Physiol. Pharmacol. 2024, 28, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.I.; Yu, J.S.; Zhang, Y.; Yoo, H.H. Evaluating flavonoids as potential aromatase inhibitors for breast cancer treatment: In vitro studies and in silico predictions. Chem. Biol. Interact. 2024, 392, 110927. [Google Scholar] [CrossRef]
- Hajirahimkhan, A.; Howell, C.; Bartom, E.T.; Dong, H.; Lantvit, D.D.; Xuei, X.; Chen, S.N.; Pauli, G.F.; Bolton, J.L.; Clare, S.E.; et al. Breast cancer prevention with liquiritigenin from licorice through the inhibition of aromatase and protein biosynthesis in high-risk women’s breast tissue. Sci. Rep. 2023, 13, 8734. [Google Scholar] [CrossRef]
- Zhang, Z.; Lin, J.; Hu, J.; Liu, L. Liquiritigenin Blocks Breast Cancer Progression by Inhibiting Connective Tissue Growth Factor Expression via Up-Regulating miR-383-5p. Int. J. Toxicol. 2022, 41, 5–15. [Google Scholar] [CrossRef]
- Liang, Y.; Besch-Williford, C.; Hyder, S.M. The estrogen receptor beta agonist liquiritigenin enhances the inhibitory effects of the cholesterol biosynthesis inhibitor RO 48-8071 on hormone-dependent breast-cancer growth. Breast Cancer Res. Treat. 2022, 192, 53–63. [Google Scholar] [CrossRef]
- Hao, Y.; Wei, Z.; Wang, Z.; Li, G.; Yao, Y.; Dun, B. Biotransformation of Flavonoids Improves Antimicrobial and Anti-Breast Cancer Activities In Vitro. Foods 2021, 10, 2367. [Google Scholar] [CrossRef]
- Hinsche, O.; Girgert, R.; Emons, G.; Grundker, C. Estrogen receptor beta selective agonists reduce invasiveness of triple-negative breast cancer cells. Int. J. Oncol. 2015, 46, 878–884. [Google Scholar] [CrossRef]
- Lecomte, S.; Lelong, M.; Bourgine, G.; Efstathiou, T.; Saligaut, C.; Pakdel, F. Assessment of the potential activity of major dietary compounds as selective estrogen receptor modulators in two distinct cell models for proliferation and differentiation. Toxicol. Appl. Pharmacol. 2017, 325, 61–70. [Google Scholar] [CrossRef]
- Schuler-Toprak, S.; Haring, J.; Inwald, E.C.; Moehle, C.; Ortmann, O.; Treeck, O. Agonists and knockdown of estrogen receptor beta differentially affect invasion of triple-negative breast cancer cells in vitro. BMC Cancer 2016, 16, 951. [Google Scholar] [CrossRef] [PubMed]
- Lattrich, C.; Stegerer, A.; Haring, J.; Schuler, S.; Ortmann, O.; Treeck, O. Estrogen receptor beta agonists affect growth and gene expression of human breast cancer cell lines. Steroids 2013, 78, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xie, S.; Wang, Y.; Luo, K.; Wang, Y.; Cai, Y. Liquiritigenin inhibits tumor growth and vascularization in a mouse model of HeLa cells. Molecules 2012, 17, 7206–7216. [Google Scholar] [CrossRef] [PubMed]
- Alrushaid, S.; Davies, N.M.; Martinez, S.E.; Sayre, C.L. Pharmacological characterization of liquiritigenin, a chiral flavonoid in licorice. Res. Pharm. Sci. 2016, 11, 355–365. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Chen, J.; Ling, Q.; Wang, H.; Li, S.; Li, L.; Yang, S.; Xia, M.; Jing, L. Estrogen receptor beta agonist enhances temozolomide sensitivity of glioma cells by inhibiting PI3K/AKT/mTOR pathway. Mol. Med. Rep. 2015, 11, 1516–1522. [Google Scholar] [CrossRef]
- Sareddy, G.R.; Nair, B.C.; Gonugunta, V.K.; Zhang, Q.G.; Brenner, A.; Brann, D.W.; Tekmal, R.R.; Vadlamudi, R.K. Therapeutic significance of estrogen receptor beta agonists in gliomas. Mol. Cancer Ther. 2012, 11, 1174–1182. [Google Scholar] [CrossRef]
- Zhou, M.; Higo, H.; Cai, Y. Inhibition of hepatoma 22 tumor by Liquiritigenin. Phytother. Res. 2010, 24, 827–833. [Google Scholar] [CrossRef]
- Frozza, C.; Santos, D.A.; Rufatto, L.C.; Minetto, L.; Scariot, F.J.; Echeverrigaray, S.; Pich, C.T.; Moura, S.; Padilha, F.F.; Borsuk, S.; et al. Antitumor activity of Brazilian red propolis fractions against Hep-2 cancer cell line. Biomed. Pharmacother. 2017, 91, 951–963. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Yang, Y.; Quan, Y.; Guo, M. Liquiritigenin Induces Cell Cycle Arrest and Apoptosis in Lung Squamous Cell Carcinoma. Cell Biochem. Biophys. 2024, 82, 1397–1407. [Google Scholar] [CrossRef]
- Shi, H.; Wu, Y.; Wang, Y.; Zhou, M.; Yan, S.; Chen, Z.; Gu, D.; Cai, Y. Liquiritigenin Potentiates the Inhibitory Effects of Cisplatin on Invasion and Metastasis Via Downregulation MMP-2/9 and PI3 K/AKT Signaling Pathway in B16F10 Melanoma Cells and Mice Model. Nutr. Cancer 2015, 67, 761–770. [Google Scholar] [CrossRef]
- Schuler-Toprak, S.; Moehle, C.; Skrzypczak, M.; Ortmann, O.; Treeck, O. Effect of estrogen receptor beta agonists on proliferation and gene expression of ovarian cancer cells. BMC Cancer 2017, 17, 319. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wong, H.K.; Feng, Y.B.; Zhang, Z.J. Liquiritigenin exhibits antitumour action in pituitary adenoma cells via Ras/ERKs and ROS-dependent mitochondrial signalling pathways. J. Pharm. Pharmacol. 2014, 66, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, L.; Gathani, T. Understanding breast cancer as a global health concern. Br. J. Radiol. 2022, 95, 20211033. [Google Scholar] [CrossRef]
- Aldape, K.; Brindle, K.M.; Chesler, L.; Chopra, R.; Gajjar, A.; Gilbert, M.R.; Gottardo, N.; Gutmann, D.H.; Hargrave, D.; Holland, E.C. Challenges to curing primary brain tumours. Nat. Rev. Clin. Oncol. 2019, 16, 509–520. [Google Scholar] [CrossRef]
- Czarnywojtek, A.; Borowska, M.; Dyrka, K.; Van Gool, S.; Sawicka-Gutaj, N.; Moskal, J.; Kościński, J.; Graczyk, P.; Hałas, T.; Lewandowska, A.M. Glioblastoma multiforme: The latest diagnostics and treatment techniques. Pharmacology 2023, 108, 423–431. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: Current state and future directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef]
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Slattery, M.; Levin, T.; Ma, K.; Goldgar, D.; Holubkov, R.; Edwards, S. Family history and colorectal cancer: Predictors of risk. Cancer Causes Control 2003, 14, 879–887. [Google Scholar] [CrossRef]
- Kraus, S.; Arber, N. Inflammation and colorectal cancer. Curr. Opin. Pharmacol. 2009, 9, 405–410. [Google Scholar] [CrossRef]
- Li, X.; Chang, Z.; Wang, J.; Ding, K.; Pan, S.; Hu, H.; Tang, Q. Unhealthy lifestyle factors and the risk of colorectal cancer: A Mendelian randomization study. Sci. Rep. 2024, 14, 13825. [Google Scholar] [CrossRef] [PubMed]
- Valle, L.; de Voer, R.M.; Goldberg, Y.; Sjursen, W.; Försti, A.; Ruiz-Ponte, C.; Caldés, T.; Garré, P.; Olsen, M.F.; Nordling, M. Update on genetic predisposition to colorectal cancer and polyposis. Mol. Asp. Med. 2019, 69, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Crooke, H.; Kobayashi, M.; Mitchell, B.; Nwokeji, E.; Laurie, M.; Kamble, S.; McKenna, M.; Masood, A.; Korytowsky, B. Estimating 1-and 5-year relative survival trends in colorectal cancer (CRC) in the United States: 2004 to 2014. J. Clin. Oncol. 2018, 36, 4. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Rudolph, K.L. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Girisa, S.; Aswani, B.S.; Manickasamy, M.K.; Hegde, M.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A.B. Restoring FXR expression as a novel treatment strategy in liver cancer and other liver disorders. Expert. Opin. Ther. Targets 2025, 29, 193–221. [Google Scholar] [CrossRef]
- Blandin Knight, S.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef]
- Ashrafi, A.; Akter, Z.; Modareszadeh, P.; Modareszadeh, P.; Berisha, E.; Alemi, P.S.; Chacon Castro, M.D.C.; Deese, A.R.; Zhang, L. Current Landscape of Therapeutic Resistance in Lung Cancer and Promising Strategies to Overcome Resistance. Cancers 2022, 14, 4562. [Google Scholar] [CrossRef]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef]
- Sajeev, A.; BharathwajChetty, B.; Manickasamy, M.K.; Alqahtani, M.S.; Abbas, M.; Shakibaei, M.; Sethi, G.; Ma, Z.; Kunnumakkara, A.B. Nuclear receptors in ovarian cancer: Changing paradigms in cancer therapeutics. Front. Oncol. 2024, 14, 1383939. [Google Scholar] [CrossRef]
- Gernier, F.; Ahmed-Lecheheb, D.; Pautier, P.; Floquet, A.; Nadeau, C.; Frank, S.; Alexandre, J.; Selle, F.; Berton-Rigaud, D.; Kalbacher, E. Chronic fatigue, quality of life and long-term side-effects of chemotherapy in patients treated for non-epithelial ovarian cancer: National case-control protocol study of the GINECO-Vivrovaire rare tumors INCa French network for rare malignant ovarian tumors. BMC Cancer 2021, 21, 1147. [Google Scholar]
- Kaler, J.; Hussain, A.; Haque, A.; Naveed, H.; Patel, S. A Comprehensive Review of Pharmaceutical and Surgical Interventions of Prostate Cancer. Cureus 2020, 12, e11617. [Google Scholar] [CrossRef] [PubMed]
- Gann, P.H. Risk factors for prostate cancer. Rev. Urol. 2002, 4, S3–S10. [Google Scholar] [PubMed]
- Shin, Y.W.; Bae, E.A.; Lee, B.; Lee, S.H.; Kim, J.A.; Kim, Y.S.; Kim, D.H. In vitro and in vivo antiallergic effects of Glycyrrhiza glabra and its components. Planta Medica 2007, 73, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Shi, J.; Li, H. Liquiritigenin attenuates high glucose-induced mesangial matrix accumulation, oxidative stress, and inflammation by suppression of the NF-kappaB and NLRP3 inflammasome pathways. Biomed. Pharmacother. 2018, 106, 976–982. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Li, H.Y.; Liu, F.Y.; Niu, J.; Wang, X.; Liang, C.; Sun, H. 7-hydroxy sulfonation of liquiritigenin by recombinant SULT1A3 enzyme and HEK-SULT1A3 cells. Zhongguo Zhong Yao Za Zhi 2019, 44, 4249–4256. [Google Scholar] [CrossRef]
- Kang, H.E.; Cho, Y.K.; Jung, H.Y.; Choi, K.Y.; Sohn, S.I.; Baek, S.R.; Lee, M.G. Pharmacokinetics and first-pass effects of liquiritigenin in rats: Low bioavailability is primarily due to extensive gastrointestinal first-pass effect. Xenobiotica 2009, 39, 465–475. [Google Scholar] [CrossRef]
- Shimamura, H.; Suzuki, H.; Hanano, M.; Suzuki, A.; Sugiyama, Y. Identification of tissues responsible for the conjugative metabolism of liquiritigenin in rats: An analysis based on metabolite kinetics. Biol. Pharm. Bull. 1993, 16, 899–907. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.W.; Kang, H.E.; Lee, M.G.; Hwang, S.J.; Kim, S.C.; Lee, C.H.; Kim, S.G. Liquiritigenin, a flavonoid aglycone from licorice, has a choleretic effect and the ability to induce hepatic transporters and phase-II enzymes. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G372-381. [Google Scholar] [CrossRef]
- Keranmu, A.; Pan, L.B.; Fu, J.; Han, P.; Yu, H.; Zhang, Z.W.; Xu, H.; Yang, X.Y.; Hu, J.C.; Zhang, H.J.; et al. Biotransformation of Liquiritigenin into Characteristic Metabolites by the Gut Microbiota. Molecules 2022, 27, 3057. [Google Scholar] [CrossRef]
- Alrushaid, S.; Davies, N.M.; Martinez, S.E.; Sayre, C.L. Stereospecific pharmacokinetic characterization of liquiritigenin in the rat. Res. Pharm. Sci. 2017, 12, 176–186. [Google Scholar] [CrossRef]
- Sayre, C.L.; Hopkins, M.; Takemoto, J.K.; Davies, N.M. Chiral analytical method development of liquiritigenin with application to a pharmacokinetic study. Biomed. Chromatogr. 2013, 27, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Ishihara, K.; Morota, T.; Takeda, S.; Aburada, M. Permeability of the flavonoids liquiritigenin and its glycosides in licorice roots and davidigenin, a hydrogenated metabolite of liquiritigenin, using human intestinal cell line Caco-2. J. Ethnopharmacol. 2003, 89, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Meng, X.S.; Bao, Y.R.; Wang, S. Pharmacokinetic study of four flavones of Glycyrrhiza in rat plasma using HPLC-MS. J. Ethnopharmacol. 2013, 148, 266–270. [Google Scholar] [CrossRef]
- Kang, H.E.; Jung, H.Y.; Cho, Y.K.; Kim, S.H.; Sohn, S.I.; Baek, S.R.; Lee, M.G. Pharmacokinetics of liquiritigenin in mice, rats, rabbits, and dogs, and animal scale-up. J. Pharm. Sci. 2009, 98, 4327–4342. [Google Scholar] [CrossRef]
- Kang, H.E.; Sohn, S.I.; Baek, S.R.; Lee, J.W.; Lee, M.G. Liquiritigenin pharmacokinetics in a rat model of diabetes mellitus induced by streptozotocin: Greater formation of glucuronides in the liver, especially M2, due to increased hepatic uridine 5′-diphosphoglucuronic acid level. Metabolism 2010, 59, 1472–1480. [Google Scholar] [CrossRef]
- Kang, H.E.; Kim, Y.W.; Sohn, S.I.; Baek, S.R.; Lee, J.W.; Kim, S.G.; Lee, I.; Lee, M.G. Pharmacokinetics of liquiritigenin and its two glucuronides, M1 and M2, in rats with acute hepatitis induced by d-galactosamine/lipopolysaccharide or CCl(4). Xenobiotica 2010, 40, 424–436. [Google Scholar] [CrossRef]
- Kang, H.E.; Sohn, S.I.; Baek, S.R.; Lee, J.W.; Lee, M.G. Effects of acute renal failure induced by uranyl nitrate on the pharmacokinetics of liquiritigenin and its two glucuronides, M1 and M2, in rats. J. Pharm. Pharmacol. 2011, 63, 49–57. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Zhang, H.; Zhu, L.; Zhong, J.; Zeng, J.; Meng, C.; Wu, J.; Wang, T.; Shi, R.; et al. Pharmacokinetics-based comprehensive strategy to identify multiple effective components in Huangqi decoction against liver fibrosis. Phytomedicine 2021, 84, 153513. [Google Scholar] [CrossRef]
- Mersereau, J.E.; Levy, N.; Staub, R.E.; Baggett, S.; Zogovic, T.; Chow, S.; Ricke, W.A.; Tagliaferri, M.; Cohen, I.; Bjeldanes, L.F.; et al. Liquiritigenin is a plant-derived highly selective estrogen receptor beta agonist. Mol. Cell. Endocrinol. 2008, 283, 49–57. [Google Scholar] [CrossRef]
- Yang, E.-J.; Park, G.H.; Song, K.-S. Neuroprotective effects of liquiritigenin isolated from licorice roots on glutamate-induced apoptosis in hippocampal neuronal cells. Neurotoxicology 2013, 39, 114–123. [Google Scholar] [CrossRef]
- Li, W.; Yin, Q.; Qiu, Y.; Liu, J.; Wang, J.; Li, C.; Zhang, D.; Zhang, P.; Lv, H.; Lv, Y. Mechanistic study of Liquiritigenin inhibiting bladder cancer cell proliferation and migration by regulating STING1. Cancer Genet. 2025, 294–295, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Homma, M.; Oka, K.; Taniguchi, C.; Niitsuma, T.; Hayashi, T. Systematic analysis of post-administrative saiboku-to urine by liquid chromatography to determine pharmacokinetics of traditional Chinese medicine. Biomed. Chromatogr. 1997, 11, 125–131. [Google Scholar] [CrossRef]
- Kitagawa, H.; Munekage, M.; Matsumoto, T.; Sadakane, C.; Fukutake, M.; Aoki, K.; Watanabe, J.; Maemura, K.; Hattori, T.; Kase, Y.; et al. Pharmacokinetic Profiles of Active Ingredients and Its Metabolites Derived from Rikkunshito, a Ghrelin Enhancer, in Healthy Japanese Volunteers: A Cross-Over, Randomized Study. PLoS ONE 2015, 10, e0133159. [Google Scholar] [CrossRef] [PubMed]
| Name of the Plant | Part Used | Amount of LG | References |
|---|---|---|---|
| Glycyrrhiza uralensis | Roots | 13.8 mg | [38] |
| Dalbergia odorifera | Heartwood | 2.70 mg/g | [39] |
| Medicago sativa | Sprouts | 2.1 mg | [40] |
| Maackia amurensis | Heartwood | - | [41] |
| Boerhavia erecta | Aerial parts | 3.7 mg | [42] |
| Brazilian red propolis | - | 30 mg | [43] |
| Brazilian red propolis extracts | - | - | [44] |
| Dalbergia ecastaphyllum | Leaves | 2.012 ± 0.025%/100 g | [44] |
| Helianthus tuberosus | Aerial parts | - | [45] |
| Astragalus bhotanensis | Roots | 7.2 mg | [46] |
| Rhus verniciflua | Bark | 15 mg | [47] |
| Pterocarpus marsupium | Heartwood | - | [48] |
| Angelica keiskei | Aerial parts | - | [49] |
| Bauhinia ungulata | Roots, stem | - | [50] |
| Artocarpus heterophyllous | Wood | 20.8 mg | [51] |
| Verbascum blattaria | Leaves | - | [52] |
| Jacaranda obtusifolia | Twigs | 2.2 mg | [53] |
| Cotinus coggygria | Heartwood | 2 mg | [54] |
| Ocimum basilicum | Seeds | - | [55] |
| Piptadeniastrum africanum | Stem bark | - | [56] |
| Astragalus mongholicus | Flowers | 0.34 μg/g | [57] |
| Intervention | In Vitro/In Vivo | Model | Mechanisms/Outcomes | References |
|---|---|---|---|---|
| Breast cancer | ||||
| LIQ | In vitro | MCF-7, BT20 cells | ↑ E-cadherin ↓ Cell viability, colony formation, invasion, migration, Snail, HSP90, LAMP-2A, HSC70, Chaperone-mediated autophagy | [94] |
| LIQ | - | Human CYP19A1 supersomes | ↓ Aromatase (CYP19A1) | [95] |
| LIQ | In vitro | Breast tissue microstructures of high-risk menopausal women | ↓ Aromatase (CYP19A1) | [96] |
| LIQ | In vitro | MCF-7 cells | ↓ Cell proliferation | [96] |
| LIQ | In vitro | BT483, AU565, BT20 cells | ↑ Apoptosis, miR-383-5p ↓ Cell viability, invasion, migration, CTGF | [97] |
| LIQ + RO | In vitro | BT474, MCF-7 cells | ↓ Cell viability | [98] |
| LIQ + RO | In vivo | Athymic nude mice (BT474 cells) xenograft | ↑ Tumor clearance, Apoptosis, ERβ ↓ Tumor volume, size, ERα, VEGF, CD31 | [98] |
| 7-methoxy-LIQ | In vitro | MCF-7 cells | ↓ Cell proliferation | [99] |
| LIQ | In vitro | HCC1806, HCC1937 cells (co-cultured with MG63 osteoblast-like cells) | ↓ Cell invasion, CXCR4 | [100] |
| LIQ | In vitro | MDA-MB-231, BT549 cells | ↑ Apoptosis, Caspase-3, E-cadherin, BRCA1, p21, GADD45A, %cells in G1 phase ↓ Cell viability, colony formation, N-cadherin, vimentin, MMP-9, invasion, migration, EMT, DNMT1, DNMT3a, DNMT3b | [80] |
| LIQ + DOX | In vitro | MDA-MB-231, BT549 cells | ↑ Sensitivity to DOX, ERβ ↓ Cell viability, | [32] |
| LIQ | In vitro | MDA-MB-231 cells | ↓ Number of colonies, PI3K/Akt/ mTOR signaling, p-Akt/Akt ratio, p-mTOR/mTOR ratio | [32] |
| LIQ | In vitro | MCF-7, T47D (ER-positive) cells | ↑ Cell number, CXCL12 | [101] |
| LIQ | In vitro | MDA-MB-231 cells | ↓ Invasion | [102] |
| LIQ | In vitro | MCF-7 cells | ↑ Cell number, Cyclin B1, PS2 | [103] |
| Cervical cancer | ||||
| LIQ | In vivo | BALB/c nude mice xenograft (HeLa cells) | ↓ Tumor weight, volume, VEGF, MVD, PCNA-positive cells | [104] |
| Colorectal cancer | ||||
| LIQ | In vitro | HT-29 cells | ↓ Cell survival | [105] |
| Brain cancer | ||||
| LIQ | In vitro | GSC10, GSC11 cells (Glioblastoma stem cells) | ↑ Apoptosis ↓ Cell viability, neurosphere formation, self-renewal ability, nestin, SOX2 | [33] |
| LIQ | In vivo | Athymic nude mice xenograft (U251-GSCs) | ↑ Mice survival ↓ Tumor growth | [33] |
| LIQ + TMZ | In vitro | U138 cells | ↑ Sensitivity to TMZ, ERβ ↓ Cell viability, p-Akt, p-P70SK6 | [106] |
| LIQ | In vitro | U87, LN229, T98G, U138 cells | ↓ Cell proliferation | [107] |
| LIQ | In vitro | U87, LN229 | ↑ G2/M phase arrest ↑ ERβ ↓ Number of colonies | [107] |
| LIQ | In vivo | Nude mice xenograft (U87 cells) | ↑ Apoptosis, ERβ ↓ Tumor growth, PCNA | [107] |
| Oral cancer | ||||
| LIQ | In vitro | SCC-9, CAL-27 cells | ↑ Apoptosis, cleaved Caspase-3&-9, autophagy, LC3II, ATG7, Beclin 1 ↓ Cell proliferation, Ki-67, PCNA, PI3K p85α, p-Akt, p-mTOR | [35] |
| LIQ | In vivo | BALB/c nude mice xenograft (CAL-27 cells) | ↑ Apoptosis, autophagy, Beclin 1+ cells ↓ Tumor growth, weight, volume, p-Akt, Ki-67+ cells | [35] |
| Liver cancer | ||||
| LIQ | In vivo | ICR mice allograft (Ascites H22 cells) | ↑ Body weight, thymus weight, necrosis ↓ Tumor volume | [108] |
| LIQ | In vitro | HepG2, PLC/PRF/5 cells | ↑ Intracellular LDH, Apoptosis, Caspase-3, cleaved PARP, JNK, p38, ROS ↓ p-ERK, Bcl-2, Bcl-xL | [37] |
| LIQ | In vivo | BALB/c athymic nude mice xenograft (PLC/PRF/5 cells) | ↓ Tumor size | [37] |
| Laryngeal cancer | ||||
| Red propolis fractions containing LIQ | In vitro | Hep2 cells | ↑ Apoptotic bodies, DNA fragmentation, chromatin condensation | [109] |
| Lung cancer | ||||
| LIQ | In vitro | A549 cells | ↑ p-ERK1/2 ↓ Cell adhesion, migration, proMMP-2, p-Akt | [34] |
| LIQ | In vitro | SK-MES-1, NCI-H520 cells | ↑ G2/M phase cells, p21, p27, Apoptosis, Bak, Bax, Cleaved caspase-3, cleaved PARP ↓ Cell viability, proliferation, Ki-67, Bcl-2, Bcl-xL, Mcl-1, PCNA, Cyclin B1, CDK1, p-PI3K, p-Akt, p-mTOR | [110] |
| In vivo | BALB/c nude mice xenograft (SK-MES-1 cells) | ↓ Tumor growth | [110] | |
| LIQ | In vitro | NCI-H187 cells | ↓ Cell viability | [53] |
| Melanoma | ||||
| LIQ | In vitro | B16F10 cells | ↓ Cell viability | [111] |
| LIQ + CDDP | In vitro | B16F10 cells | ↑PTEN ↓ Cell viability, invasion, migration, MMP-2&-9, PI3K, p-Akt, | [111] |
| LIQ | In vivo | C57BL/6 mice allograft (B16F10 cells) | ↑ PTEN ↓ Invasion, migration, p-Akt, PI3K metastatic nodules, MMP-2&-9 | [111] |
| Ovarian cancer | ||||
| LIQ | In vitro | SKOV3, ES-2 (cisplatin-resistant), BG-1, SKOV3 (taxol-resistant) cells | ↓ Cell viability | [71] |
| LIQ | In vitro | SKOV3, ES-2 cells | ↓ Cell viability, invasion, migration, colony formation | [71] |
| LIQ | In vitro | SKOV3, ES-2 (cisplatin-resistant), SKOV3 (taxol-resistant) cells | ↑ Caspase-3/-7 | [71] |
| LIQ + Paclitaxel, LIQ + Cisplatin | In vitro | ES-2, SKOV3 cells | ↑ Sensitivity to paclitaxel and cisplatin | [71] |
| LIQ | In vitro | ES-2, SKOV3 cells | ↓ NF-κB, IL-1β, CXCL8, PTGS2 | [71] |
| LIQ | In vivo | Nude mice xenograft (SKOV3 cells) | ↑ Apoptosis ↓ Tumor weight, volume, tumor nodules, Ki-67, IL-1β, COX-2 | [71] |
| LIQ | In vitro | OAW-42 cells | ↓ Cell viability, ND6 | [112] |
| LIQ | In vitro | OVCAR-3 cells | ↑ GAS2 ↓ Cell viability, CCNE2 | [112] |
| Pituitary adenocarcinoma | ||||
| LIQ | In vitro | MMQ, GH3 cells | ↑ Apoptosis, G1 phase arrest, ROS ↓ Cell viability, Bcl-2, Bcl-xL, Ras, p-ERK | [113] |
| LIQ | In vivo | BALB/c athymic nude mice xenograft (GH3 cells) | ↓ Tumor size | [113] |
| Prostate cancer | ||||
| LIQ | In vitro | C4-2, PC3 cells | ↑ E-cadherin, ER stress, IRE1, ATF6, BIP ↓ Cell proliferation, invasion, migration, N-cadherin, vimentin | [36] |
| LIQ + TUDCA (Stress inhibitor) | In vitro | C4-2, PC3 cells | ↑ E-cadherin, ER stress, IRE1, ATF6, BIP ↓ Invasion, migration, N-cadherin, vimentin | [36] |
| LIQ + shIRE1 | In vitro | C4-2, PC3 cells | ↑ Invasion, migration, N-cadherin, vimentin ↓ E-cadherin, IRE1 | [36] |
| LIQ | In vivo | Nude mice xenograft (PC3 cells) | ↑ E-cadherin, IRE1, BIP ↓ Tumor weight, volume, N-cadherin, lung metastasis | [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sajeev, A.; Aswani, B.S.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A.B. Harnessing Liquiritigenin: A Flavonoid-Based Approach for the Prevention and Treatment of Cancer. Cancers 2025, 17, 2328. https://doi.org/10.3390/cancers17142328
Sajeev A, Aswani BS, Alqahtani MS, Abbas M, Sethi G, Kunnumakkara AB. Harnessing Liquiritigenin: A Flavonoid-Based Approach for the Prevention and Treatment of Cancer. Cancers. 2025; 17(14):2328. https://doi.org/10.3390/cancers17142328
Chicago/Turabian StyleSajeev, Anjana, Babu Santha Aswani, Mohammed S. Alqahtani, Mohamed Abbas, Gautam Sethi, and Ajaikumar B. Kunnumakkara. 2025. "Harnessing Liquiritigenin: A Flavonoid-Based Approach for the Prevention and Treatment of Cancer" Cancers 17, no. 14: 2328. https://doi.org/10.3390/cancers17142328
APA StyleSajeev, A., Aswani, B. S., Alqahtani, M. S., Abbas, M., Sethi, G., & Kunnumakkara, A. B. (2025). Harnessing Liquiritigenin: A Flavonoid-Based Approach for the Prevention and Treatment of Cancer. Cancers, 17(14), 2328. https://doi.org/10.3390/cancers17142328







