Occupational, Lifestyle, and Medical Risk Factors for Non-Hodgkin Lymphoma: A Case–Control Study in Ethiopia
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design, Setting and Population
2.2. Study Procedures
2.3. Sample Size
2.4. Statistical Analysis
3. Results
3.1. Exploratory Subgroup Analysis
3.2. Sensitivity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Exposures | Category | Crude OR (95% CI) | Adjusted ** OR (95% CI) |
|---|---|---|---|
| Education | Uneducated (ref: Educated) | 2.08 (1.21–3.77) | - |
| Residence | Rural (ref: Urban) | 1.95 (1.19–3.21) | - |
| Alcohol consumption | Yes (ref: No) | 0.52 (0.30–0.90) | 0.55 (0.32–0.96) |
| Smoking status | Ever smoker (ref: Never smoker) | 0.27 (0.14–0.54) | 0.30 (0.15–0.60) |
| Fruit intake >once per week | Yes (ref: No) | 0.80 (0.48–1.33) | 0.96 (0.57–1.62) |
| Gardening/backyard farming *** | Yes (ref: No) | 1.95 (1.17–3.28) | 1.68 (0.99–2.88) |
| Agriculture employment *** | Yes (ref: No) | 1.99 (1.19–3.32) | 1.73 (1.01–2.93) |
| Pesticide use *** | Yes (ref: No) | 2.50 (1.45–4.35) | 1.99 (1.1–3.56) |
| Presence of farm animals within the household premises | Yes (ref: No) | 2.67 (1.58–4.5) | 2.07 (1.15–3.72) |
| Birth order | First-born (ref: ≥Fourth-born) | 1.27 (0.73–2.20) | 1.07 (0.62–1.88) |
| BMI | Continuous | 0.88 (0.81–0.96) | 0.90 (0.82–0.99) |
| Tonsillectomy/uvulectomy | Yes (ref: No) | 3.03 (1.17–7.85) | 3.0 (1.11–8.1) |
References
- Baissa, O.T.; Ben-Shushan, T.; Paltiel, O. Lymphoma in Sub-Saharan Africa: A scoping review of the epidemiology, treatment challenges, and patient pathways. Cancer Causes Control 2025, 36, 199–230. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer. GLOBOCAN 2020: World Fact Sheet. Lyon: IARC. 2020. Available online: https://gco.iarc.who.int/media/globocan/factsheets/populations/900-world-fact-sheet.pdf (accessed on 9 January 2025).
- Sharma, R.; Aashima; Nanda, M.; Fronterre, C.; Sewagudde, P.; Ssentongo, A.E.; Yenney, K.; Arhin, N.D.; Oh, J.; Amponsah-Manu, F.; et al. Mapping cancer in Africa: A comprehensive and comparable characterization of 34 cancer types using estimates from GLOBOCAN 2020. Front. Public Health 2022, 10, 744. [Google Scholar] [CrossRef]
- Mezger, N.C.S.S.; Feuchtner, J.; Griesel, M.; Hämmerl, L.; Seraphin, T.P.; Zietsman, A.; Péko, J.F.; Tadesse, F.; Buziba, N.G.; Wabinga, H.; et al. Clinical presentation and diagnosis of adult patients with non-Hodgkin lymphoma in Sub-Saharan Africa. Br. J. Haematol. 2020, 190, 209–221. [Google Scholar] [CrossRef]
- Feuchtner, J.; Mathewos, A.; Solomon, A.; Timotewos, G.; Aynalem, A.; Wondemagegnehu, T.; Gebremedhin, A.; Adugna, F.; Griesel, M.; Wienke, A.; et al. Addis Ababa population-based pattern of cancer therapy, Ethiopia. PLoS ONE 2019, 14, e0219519. [Google Scholar] [CrossRef]
- National Cancer Institute. International Lymphoma Epidemiology Consortium (InterLymph). Available online: https://epi.grants.cancer.gov/interlymph/ (accessed on 8 November 2024).
- Cerhan, J.R.; Kricker, A.; Paltiel, O.; Flowers, C.R.; Wang, S.S.; Monnereau, A.; Blair, A.; Da Maso, L.; Kane, E.V.; Nieters, A.; et al. Medical history, lifestyle, family history, and occupational risk factors for diffuse large B-cell lymphoma: The InterLymph non-Hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Padala, S.A.; Barsouk, A.; Rawla, P. Epidemiology of non-Hodgkin’s lymphoma. Med. Sci. 2021, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Craver, A.; Bahl, K.; Stepniak, L.; Moore, K.; King, J.; Zhang, Y.; Aschebrook-Kilfoy, B. Etiology of non-Hodgkin lymphoma: A review from epidemiologic studies. J. Natl. Cancer Cent. 2022, 2, 226–234. [Google Scholar] [CrossRef]
- Becker, N.; Falster, M.O.; Vajdic, C.M.; de Sanjosé, S.; Martínez-Maza, O.; Bracci, P.M.; Melbye, M.; Smedby, K.E.; Engels, E.A.; Turner, J.; et al. Self-reported history of infections and the risk of non-Hodgkin lymphoma: An InterLymph pooled analysis. Int. J. Cancer 2012, 131, 2342–2348. [Google Scholar] [CrossRef]
- Parkin, D.M.; Hämmerl, L.; Ferlay, J.; Kantelhardt, E.J. Cancer in Africa 2018: The role of infections. Int. J. Cancer 2020, 146, 2089–2103. [Google Scholar] [CrossRef]
- Ugwu, N.; Okoye, A.; Ugwu, C.; Iyare, F.; Edegbe, F.; Ugwu, G. Distribution pattern and prevalence of haematological cancers among adults in Abakaliki, South-Eastern Nigeria. Niger. Postgrad. Med. J. 2021, 28, 266–272. [Google Scholar] [CrossRef]
- Inamasu, T.; Patel, M.; Espina, C.; Pentz, A.; Joffe, M.; Winde, F.; Schüz, J. Retrospective case-series analysis of haematological malignancies in gold-mining areas of South Africa. S. Afr. Med. J. 2018, 108, 858–864. [Google Scholar] [CrossRef]
- Chekol, D.; Bedimo, M.; Mulugeta, Y.; Bantie, G.M. Determinants of non-Hodgkin’s lymphoma at Felegehiwot Specialized Hospital, North West Ethiopia: A case-control study. PLoS ONE 2021, 15, e0243561. [Google Scholar] [CrossRef]
- Anderson, B.O. NCCN Harmonized Guidelines for Sub-Saharan Africa: A collaborative methodology for translating resource-adapted guidelines into actionable in-country cancer control plans. JCO Glob. Oncol. 2020, 6, 1419–1421. [Google Scholar] [CrossRef]
- Besson, H.; Brennan, P.; Becker, N.; de Sanjosé, S.; Nieters, A.; Font, R.; Maynadié, M.; Foretova, L.; Cocco, P.L.; Staines, A.; et al. Tobacco smoking, alcohol drinking and Hodgkin’s lymphoma: A European multicentre case–control study (EPILYMPH). Br. J. Cancer 2006, 95, 378–384. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Memirie, S.T.; Habtemariam, M.K.; Asefa, M.; Deressa, B.T.; Abayneh, G.; Tsegaye, B.; Abraha, M.W.; Ababi, G.; Jemal, A.; Rebbeck, T.R.; et al. Estimates of cancer incidence in Ethiopia in 2015 using population-based registry data. J. Glob. Oncol. 2018, 4, 1–11. [Google Scholar] [CrossRef]
- Perry, A.M.; Perner, Y.; Diebold, J.; Nathwani, B.N.; MacLennan, K.A.; Müller-Hermelink, H.K.; Bast, M.; Boilesen, E.; Armitage, J.O.; Weisenburger, D.D. Non-Hodgkin lymphoma in Southern Africa: Review of 487 cases from the International Non-Hodgkin Lymphoma Classification Project. Br. J. Haematol. 2016, 172, 716–723. [Google Scholar] [CrossRef]
- Minayehu, T.M.; Begna, K.; Tola, M.A.; Tabor, B.B.; Mekonen, Y.F.; Hilawi, H.G. Patterns of lymphoproliferative disorders in Ethiopia, based on single-center experience. Blood 2022, 140 (Suppl. 1), 12137–12138. [Google Scholar] [CrossRef]
- UNAIDS Ethiopia Country Profile. Geneva: UNAIDS. Available online: https://www.unaids.org/en/regionscountries/countries/ethiopia (accessed on 18 December 2024).
- De Roos, A.J.; Fritschi, L.; Ward, M.H.; Monnereau, A.; Hofmann, J.; Bernstein, L.; Bhatti, P.; Benavente Moreno, Y.; Benke, G.; Casabonne, D.; et al. Herbicide use in farming and other jobs in relation to non-Hodgkin’s lymphoma (NHL) risk. Occup. Environ. Med. 2022, 79, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Mannetje, A.; de Roos, A.J.; Boffetta, P.; Vermeulen, R.; Benke, G.; Fritschi, L.; Brennan, P.; Foretova, L.; Maynadié, M.; Becker, N.; et al. Occupation and risk of non-Hodgkin lymphoma and its subtypes: A pooled analysis from the InterLymph Consortium. Environ. Health Perspect. 2016, 124, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Cocco, P.; Satta, G.; Dubois, S.; Pili, C.; Pilleri, M.; Zucca, M.; ’t Mannetje, A.; Becker, N.; Benavente, Y.; de Sanjosé, S.; et al. Lymphoma risk and occupational exposure to pesticides: Results of the EPILYMPH study. Occup. Environ. Med. 2013, 70, 91–98. [Google Scholar] [CrossRef]
- Negatu, B.; Dugassa, S.; Mekonnen, Y. Environmental and health risks of pesticide use in Ethiopia. J. Health Pollut. 2021, 11, 210601. [Google Scholar] [CrossRef] [PubMed]
- Mojo, D.; Zemedu, L. Pesticide use practices and effects on crop yield, human health and the environment in selected areas of Ethiopia. Ethiop. J. Agric. Sci. 2022, 32, 17–36. [Google Scholar]
- Kleinstern, G.; Seir, R.A.; Perlman, R.; Khatib, A.; Abdeen, Z.; Elyan, H.; Nirel, R.; Amir, G.; Ramlawi, A.; Sabatin, F.; et al. Ethnic variation in medical and lifestyle risk factors for B-cell non-Hodgkin lymphoma: A case-control study among Israelis and Palestinians. PLoS ONE 2017, 12, e0171709. [Google Scholar] [CrossRef]
- El-Zaemey, S.; Schinasi, L.H.; Ferro, G.; Tual, S.; Lebailly, P.; Baldi, I.; Nordby, K.C.; Kjærheim, K.; Schüz, J.; Monnereau, A.; et al. Animal farming and the risk of lymphohaematopoietic cancers: A meta-analysis of three cohort studies within the AGRICOH consortium. Occup. Environ. Med. 2019, 76, 827–837. [Google Scholar] [CrossRef]
- Cocco, P.; Satta, G.; D’Andrea, I.; Nonne, T.; Udas, G.; Zucca, M.; Mannetje, A.; Becker, N.; de Sanjosé, S.; Foretova, L.; et al. Lymphoma risk in livestock farmers: Results of the EPILYMPH study. Int. J. Cancer 2013, 132, 2613–2618. [Google Scholar] [CrossRef]
- Daba, C.; Debela, S.A.; Gasheya, K.A.; Endawkie, A.; Gebrehiwot, M. Spatial variation and determinant factors of alcohol consumption in Ethiopia: Spatial and multilevel analysis of Ethiopian Demographic and Health Survey. PLoS ONE 2025, 20, e0309943. [Google Scholar] [CrossRef]
- Teferra, S.; Medhin, G.; Selamu, M.; Bhana, A.; Hanlon, C.; Fekadu, A. Hazardous alcohol use and associated factors in a rural Ethiopian district: A cross-sectional community survey. BMC Public Health 2016, 16, 218. [Google Scholar] [CrossRef]
- Besson, H.; Brennan, P.; Becker, N.; Nieters, A.; de Sanjosé, S.; Font, R.; Maynadié, M.; Foretova, L.; Cocco, P.L.; Staines, A.; et al. Tobacco smoking, alcohol drinking and non-Hodgkin’s lymphoma: A European multicenter case-control study (EPILYMPH). Int. J. Cancer 2006, 119, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Morton, L.M.; Hartge, P.; Holford, T.R.; Holly, E.A.; Chiu, B.C.H.; Vincis, P.; Siagnaro, E.; Willett, E.V.; Franceschi, S.; La Vecchia, C.; et al. Cigarette smoking and risk of non-Hodgkin lymphoma: A pooled analysis from the International Lymphoma Epidemiology Consortium (InterLymph). Cancer Epidemiol. Biomarkers Prev. 2005, 14, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Linet, M.S.; Vajdic, C.M.; Morton, L.M.; de Roos, A.J.; Skibola, C.F.; Boffetta, P.; Cerhan, J.R.; Flowers, C.R.; de Sanjosé, S.; Monnereau, A.; et al. Medical history, lifestyle, family history, and occupational risk factors for follicular lymphoma: The InterLymph non-Hodgkin lymphoma subtypes project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 26–40. [Google Scholar] [CrossRef]
- Daba, C.; Atamo, A.; Debela, S.A.; Dagne, M.; Desye, B.; Gebrehiwot, M. Prevalence of tobacco smoking and associated factors among adults in Ethiopia: A systematic review and meta-analysis. Front. Public Health 2024, 12, 1353033. [Google Scholar] [CrossRef]
- Paltiel, O.; Laniado, D.E.; Yanetz, R.; Deutsch, L.; Calderon-Margalit, R.; Harlap, S.; Friedlander, Y. The risk of cancer following hospitalization for infection in infancy: A population-based cohort study. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1964–1968. [Google Scholar] [CrossRef]
- Goldin, L.R.; Landgren, O.; Kristinsson, S.Y.; Björkholm, M.; Paltiel, O. Infection in infancy and subsequent risk of developing lymphoma in children and young adults. Blood 2011, 117, 1670–1672. [Google Scholar] [CrossRef]
- Getachew, T.; Negash, A.; Eyeberu, A.; Abdurahman, D.; Jibro, U.; Deressa, A.; Birhanu, A.; Regassa, L.D.; Debella, A.; Mohammed, F.; et al. The burdens, associated factors, and reasons for traditional uvulectomy in Ethiopia: A systematic review and meta-analysis. Int. J. Pediatr. Otorhinolaryngol. 2024, 176, 111835. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, Y.; Yin, L.; Sadeghi, F.; Yang, Y.; Xiao, X.; Adami, H.O.; Ye, W.; Zhang, Z.; Fang, F. Cancer risk following surgical removal of tonsils and adenoids—A population-based, sibling-controlled cohort study in Sweden. BMC Med. 2023, 21, 194. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Crosignani, P.; Sacerdote, C.; Fontana, A.; Masala, G.; Miligi, L.; Nanni, O.; Ramazzotti, V.; Rodella, S.; Stagnaro, E.; et al. Haematopoietic cancer and medical history: A multicentre case-control study. J. Epidemiol. Community Health 2000, 54, 431–436. [Google Scholar] [CrossRef] [PubMed]
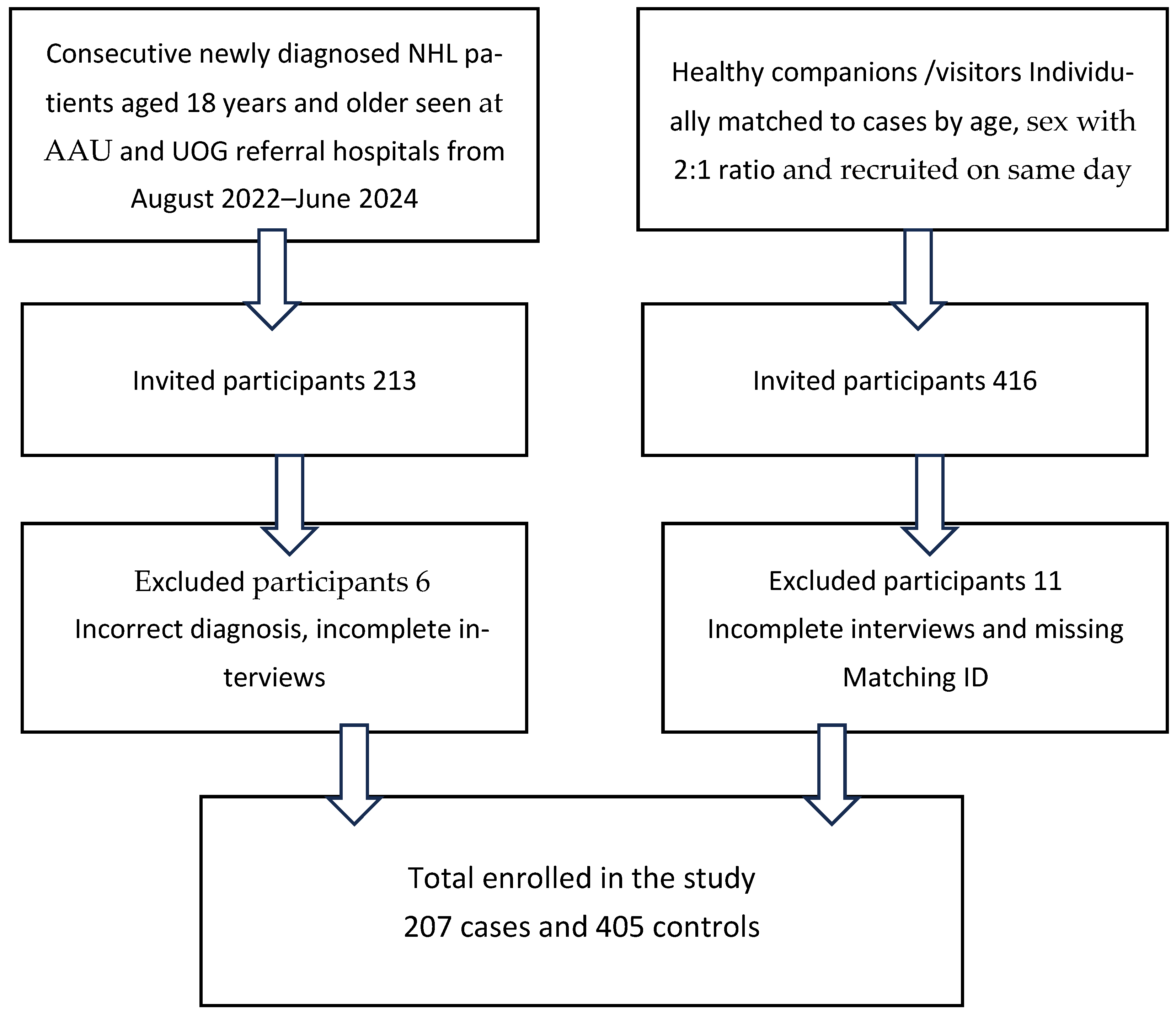
| Sociodemographic Characteristics of NHL Cases and Age- and Sex-Matched Controls | |||||
| Variables | Cases (n = 207) | % Cases | Controls (n = 405) | % Controls | |
| Study site | Addis Ababa | 165 | 79.6 | 329 | 81.2 |
| Gondar | 42 | 20.3 | 76 | 18.8 | |
| Sex | Male | 133 | 64.3 | 275 | 67.9 |
| Female | 74 | 35.7 | 128 | 31.6 | |
| Age (years) | 18–35 | 27 | 13 | 70 | 17.4 |
| 36–50 | 71 | 34.3 | 135 | 33.6 | |
| 51–65 | 77 | 37.2 | 155 | 38.6 | |
| ≥66 | 32 | 15.5 | 41 | 10.2 | |
| Marital status | Married | 141 | 68.1 | 289 | 71.4 |
| Divorced | 10 | 4.8 | 17 | 4.2 | |
| Widowed | 17 | 8.2 | 19 | 4.7 | |
| Single | 38 | 18.4 | 77 | 19 | |
| Educational status | ≤Primary | 140 | 67.6 | 223 | 55.5 |
| ≥High school | 66 | 31.9 | 177 | 44 | |
| Religion | Orthodox Christian | 142 | 68.6 | 289 | 72 |
| Muslim | 32 | 15.5 | 65 | 16.2 | |
| Protestant | 31 | 15 | 43 | 10.8 | |
| Others | 2 | 1 | 4 | 1 | |
| Birth order | First-born | 61 | 29.5 | 129 | 32.1 |
| Second-born | 63 | 30.4 | 104 | 25.9 | |
| Third-born or later | 81 | 39.1 | 165 | 41.1 | |
| Residence | Urban | 110 | 53.1 | 283 | 70.9 |
| Rural | 96 | 46.4 | 116 | 29.1 | |
| Lifestyle and Occupational Exposures among NHL Cases and Controls | |||||
| Variables | Cases (n = 207) | % Cases | Controls (n = 405) | % Controls | |
| Smoking status | Never smoker | 175 | 84.5 | 288 | 73.1 |
| Former/current smoker | 24 | 11.6 | 106 | 26.9 | |
| Alcohol consumption | Yes | 53 | 25.6 | 137 | 34.1 |
| Fruit intake | ≤Once/week | 128 | 61.8 | 227 | 57.6 |
| >Once/week | 75 | 36.2 | 167 | 42.4 | |
| History of agricultural employment | Yes | 65 | 31.4 | 80 | 19.8 |
| Gardening/backyard farming | Yes | 58 | 28 | 68 | 16.9 |
| Self-reported pesticide use | Yes | 67 | 31.9 | 74 | 18.4 |
| Presence of farm animals in home premise | Yes | 66 | 31.9 | 71 | 17.7 |
| Self-Reported Medical History among NHL Cases and Controls | |||||
| Variables | Cases (n = 207) | % Cases | Controls (n = 405) | % Controls | |
| Overweight (BMI ≥ 25 g/m2) | Yes | 14 | 6.7% | 52 | 12.9% |
| History of hospitalization for infection during childhood | Yes | 33 | 15.9 | 46 | 11.4 |
| History of tonsillectomy/uvulectomy | Yes | 16 | 8.2 | 14 | 3.5 |
| Diabetes/Prediabetes | Yes | 5 | 2.4 | 14 | 3.5 |
| History of dental extraction(s) | Yes | 83 | 40.1 | 182 | 45.3 |
| No | 112 | 54.1 | 210 | 53.4 | |
| Exposures | Crude OR (95 % CI) | OR (Adjusted) ** | |
|---|---|---|---|
| Education | Uneducated (ref: Educated) | 2.08 (1.33–3.15) | |
| Residence | Rural (ref: Urban) | 2.62 (1.71–4.00) | |
| Birth order | First-born (ref: Fourth or later) | 1.11 (0.69–2.43) | 1.14 (0.66–1.91) |
| Second-born | 1.24 (0.72–2.14) | 1.25 (0.69–2.11) | |
| Third-born | 1.65 (0.92–2.95) | 1.58 (0.82–2.75) | |
| Alcohol consumption | Yes (ref: No) | 0.53 (0.34–0.84) | 0.55 (0.35–0.87) |
| Smoking status | Ever smoker (ref: Never smoker) | 0.29 (0.17–0.51) | 0.31 (0.17–0.55) |
| Fruit intake >once per week | Yes (ref: No) | 0.74 (0.48–1.15) | 0.86 (0.55–1.35) |
| Gardening/backyard farming | Yes (ref: No) | 2.01 (1.31–3.11) | 1.68 (1.04–2.20) |
| Agricultural employment *** | Yes (ref: No) | 2.15 (1.38–3.34) | 1.76 (1.12–2.83) |
| Pesticide use *** | Yes (ref: No) | 2.43 (1.56–3.81) | 2.14 (1.34–3.40) |
| Presence of farm animals within the household premises *** | Yes (ref: No) | 2.77 (1.76–4.36) | 2.41 (1.51–3.84) |
| Previous hospitalization for infection during childhood | Yes (ref: No) | 1.55 (1.30–1.97) | 2.12 (1.15–3.96) |
| Tonsillectomy/uvulectomy | Yes (ref: No) | 2.61 (1.18–5.20) | 2.61 (1.19–5.71) |
| BMI (Kg/m2) | Continuous | 0.86 (0.80–0.92) | 0.88 (0.81–0.95) |
| High-Grade NHL (n = 81) | Low-Grade NHL (n = 123) | ||||
|---|---|---|---|---|---|
| Exposures | Category (Reference) | Crude OR (95 % CI) | OR (Adjusted) ** | Crude OR (95% CI) | Adjusted OR (95% CI) |
| Education | Uneducated (ref: Educated) | 2.27 (1.02–4.76) | -- | 2.26 (1.33–4.16) | -- |
| Residence | Rural (ref: Urban) | 4.42 (1.95–8.95) | -- | 2.04 (1.20–3.47) | -- |
| Birth order | First-born (ref: ≥Fourth-born) | 1.42 (0.65–3.09) | 1.22 (0.55–2.78) | 1.37 (0.74–2.54) | 1.13 (0.60–2.13) |
| Second-born | 1.08 (0.54–1.91) | 1.03 (0.68–2.45) | 1.33 (0.97–2.65) | 1.7 (0.89–2.54) | |
| Third-born | 1.1 (0.69–1.87) | 1.17 (0.75–2.01) | 1.29 (0.85–2.01) | 1.23 (0.95–2.16) | |
| Alcohol consumption | Yes (ref: No) | 0.70 (0.33–1.52) | 0.70 (0.31–1.62) | 0.45 (0.25–0.79) | 0.46 (0.26–0.83) |
| Smoking status | Ever smoker (ref: Never smoker) | 0.16 (0.05–0.47) | 0.18 (0.06–0.55) | 0.40 (0.21–0.78) | 0.40 (0.26–0.89) |
| Fruit intake >once per week | Yes (ref: No) | 0.86 (0.41–1.76) | 0.86 (0.41–1.76) | 0.65 (0.38–1.12) | 0.71 (0.40–1.25) |
| Gardening/backyard farming | Yes (ref: No) | 4.47 (1.96–10.20) | 4.08 (1.77–9.40) | 1.49 (0.87–2.54) | 1.19 (0.68–2.09) |
| Agricultural employment *** | Yes (ref: No) | 2.57 (1.20–5.52) | 2.19 (0.99–4.80) | 2.04 (1.17–3.56) | 1.60 (0.89–2.88) |
| Pesticide use *** | Yes (ref: No) | 3.56 (1.60–7.92) | 2.85 (1.20–6.68) | 2.10 (1.21–3.64) | 1.74 (0.99–3.11) |
| Presence of farm animals within the household premises *** | Yes (ref: No) | 3.32 (1.55–7.10) | 3.04 (1.41–6.36) | 2.40 (1.36–4.25) | 1.91 (1.04–3.49) |
| Tonsillectomy/uvulectomy | Yes (ref: No) | 1.59 (0.54–4.66) | 1.34 (0.44–4.06) | 3.45 (1.15–10.31) | 3.95 (1.23–12.65) |
| BMI (Kg/m2) | Continuous | 0.85 (0.76–0.95) | 0.87 (0.77–0.98) | 0.87 (0.78–0.95) | 0.89 (0.81–0.99) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baissa, O.T.; Abdela, F.; Tadesse, F.; Damie, A.; Sori, M.; Hailu, W.; Bizuneh, S.; Abebe, B.; Adamu, B.; Amir, G.; et al. Occupational, Lifestyle, and Medical Risk Factors for Non-Hodgkin Lymphoma: A Case–Control Study in Ethiopia. Cancers 2025, 17, 3745. https://doi.org/10.3390/cancers17233745
Baissa OT, Abdela F, Tadesse F, Damie A, Sori M, Hailu W, Bizuneh S, Abebe B, Adamu B, Amir G, et al. Occupational, Lifestyle, and Medical Risk Factors for Non-Hodgkin Lymphoma: A Case–Control Study in Ethiopia. Cancers. 2025; 17(23):3745. https://doi.org/10.3390/cancers17233745
Chicago/Turabian StyleBaissa, Obsie T., Fozia Abdela, Fissehatsion Tadesse, Amanuel Damie, Moti Sori, Workagegnehu Hailu, Segenet Bizuneh, Bewketu Abebe, Begashaw Adamu, Gail Amir, and et al. 2025. "Occupational, Lifestyle, and Medical Risk Factors for Non-Hodgkin Lymphoma: A Case–Control Study in Ethiopia" Cancers 17, no. 23: 3745. https://doi.org/10.3390/cancers17233745
APA StyleBaissa, O. T., Abdela, F., Tadesse, F., Damie, A., Sori, M., Hailu, W., Bizuneh, S., Abebe, B., Adamu, B., Amir, G., & Paltiel, O. (2025). Occupational, Lifestyle, and Medical Risk Factors for Non-Hodgkin Lymphoma: A Case–Control Study in Ethiopia. Cancers, 17(23), 3745. https://doi.org/10.3390/cancers17233745






