The Impact of Growth Hormone Deficiency on Endothelial Function in Childhood Brain Cancer Survivors
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical, Biochemical and Biophysical Endothelial Evaluation
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics
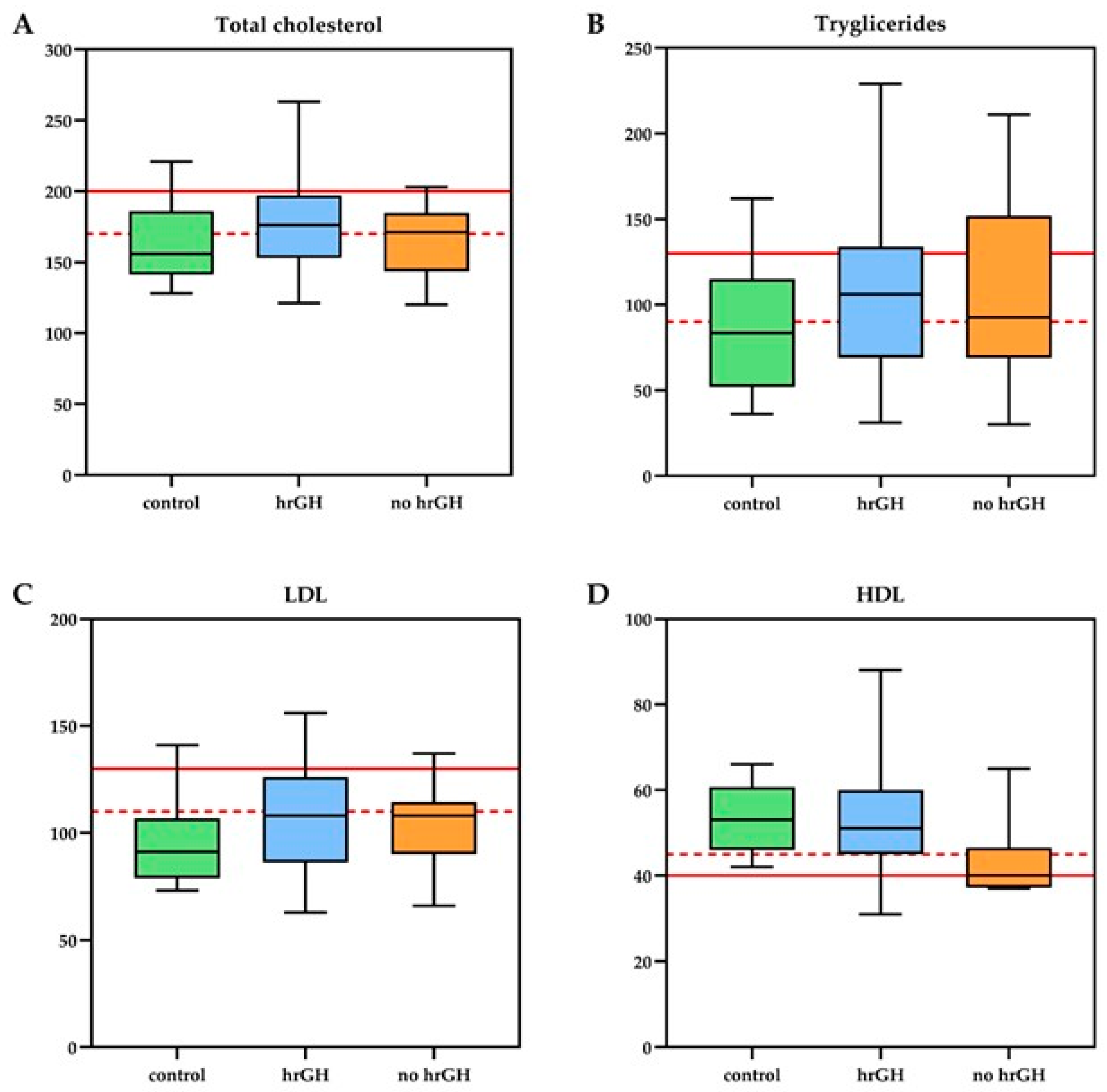
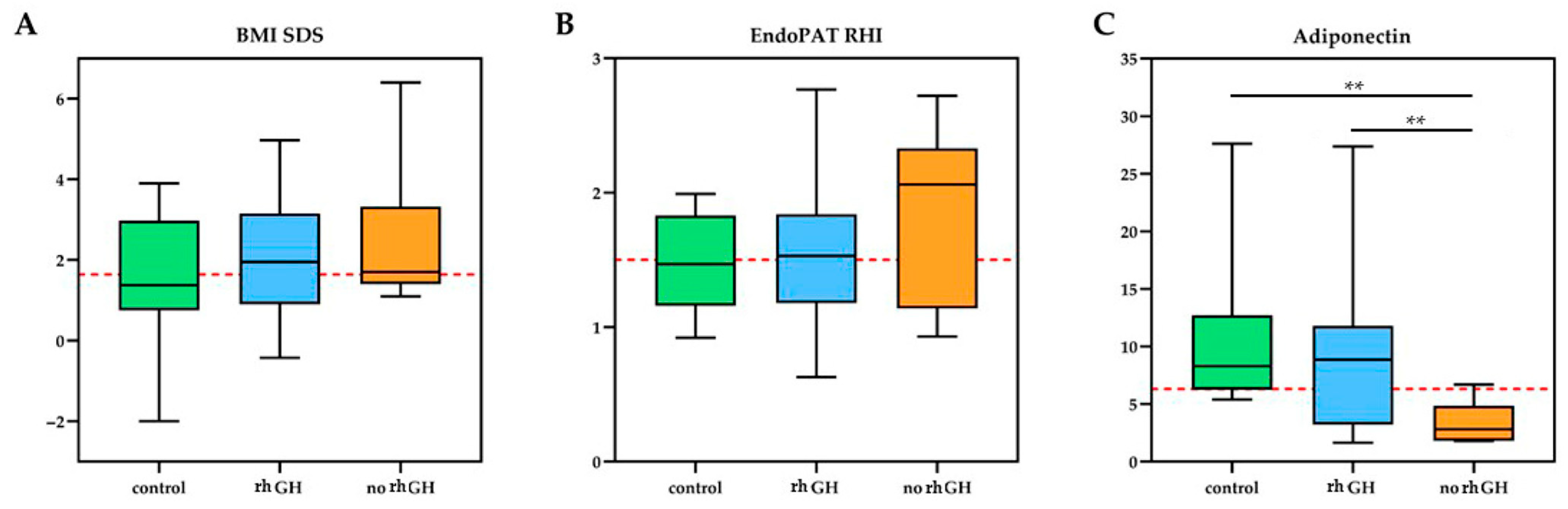
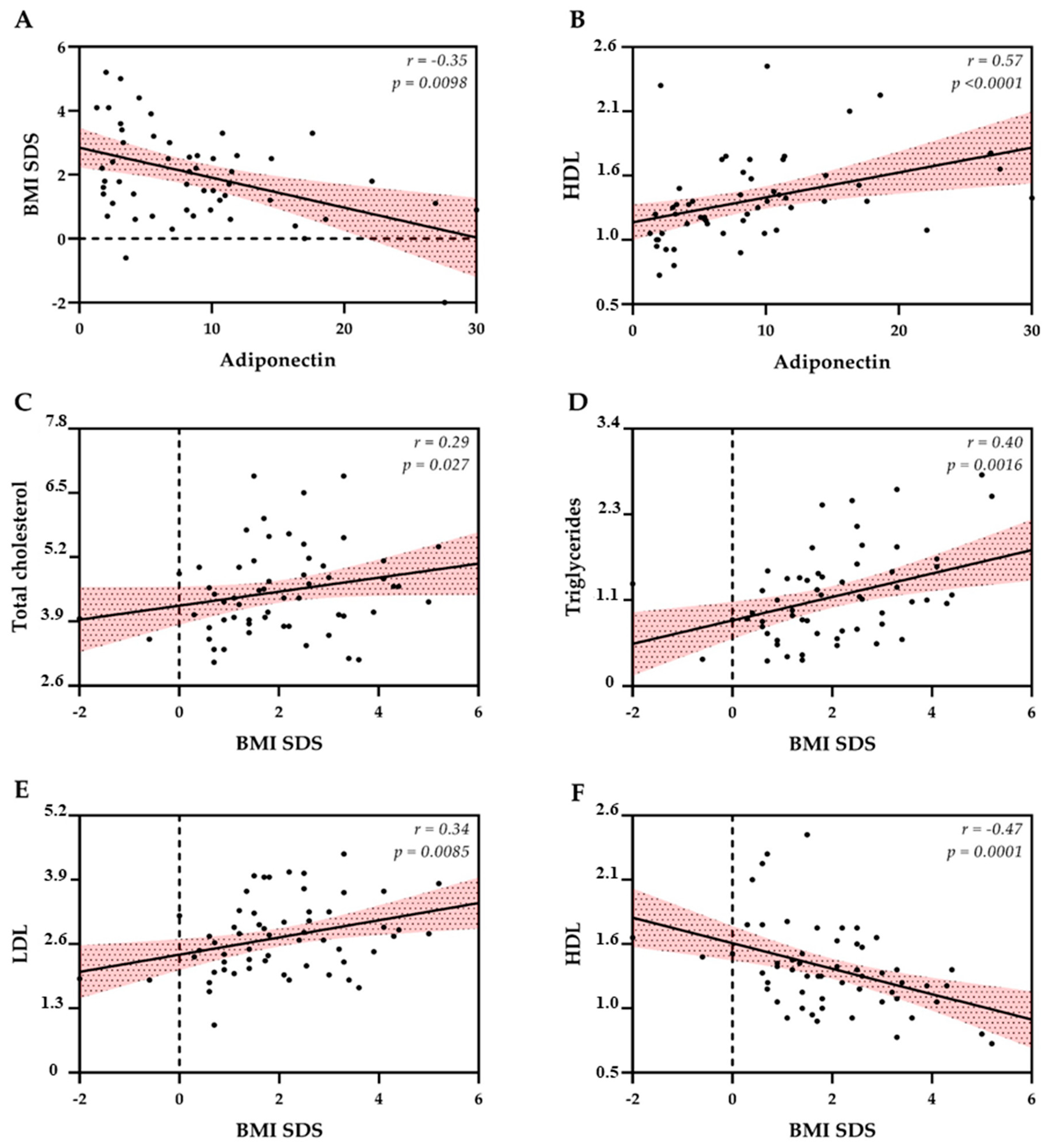
3.2. Biochemical and Biophysical Endothelial Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Paula Silva, N.; Gini, A.; Dolya, A.; Colombet, M.; Soerjomataram, I.; Youlden, D.; Tiller, C.; Steliarova-Foucher, E.; CRICCS consortium; Aitken, J.; et al. Prevalence of Childhood Cancer Survivors in Europe: A Scoping Review. EJC Paediatr. Oncol. 2024, 3, 100155. [Google Scholar] [CrossRef]
- Haupt, R.; Jankovic, M.; Hjorth, L.; Skinner, R. Late Effects in Childhood Cancer Survivors and Survivorship Issues. Epidemiol. Prev. 2013, 37 (Suppl. S1), 266–273. [Google Scholar]
- Wheeler, G.; Grassberger, C.; Samers, J.; Dwyer, M.; Wiltshire, K.; Daly, P.; Alvarez, B.; Campbell, B.A.; Kerr, A.J.; Kron, T.; et al. Central Endocrine Complications Among Childhood Cancer Survivors Treated With Radiation Therapy: A PENTEC Comprehensive Review. Int. J. Radiat. Oncol. Biol. Phys. 2024, 119, 457–466. [Google Scholar] [CrossRef]
- González Briceño, L.G.; Kariyawasam, D.; Samara-Boustani, D.; Giani, E.; Beltrand, J.; Bolle, S.; Fresneau, B.; Puget, S.; Sainte-Rose, C.; Alapetite, C.; et al. High Prevalence of Early Endocrine Disorders After Childhood Brain Tumors in a Large Cohort. J. Clin. Endocrinol. Metab. 2022, 107, e2156–e2166. [Google Scholar] [CrossRef]
- van Iersel, L.; Mulder, R.L.; Denzer, C.; Cohen, L.E.; Spoudeas, H.A.; Meacham, L.R.; Sugden, E.; Schouten-van Meeteren, A.Y.N.; Hoving, E.W.; Packer, R.J.; et al. Hypothalamic–Pituitary and Other Endocrine Surveillance Among Childhood Cancer Survivors. Endocr. Rev. 2022, 43, 794–823. [Google Scholar] [CrossRef]
- Zucchini, S.; Di Iorgi, N.; Pozzobon, G.; Pedicelli, S.; Parpagnoli, M.; Driul, D.; Matarazzo, P.; Baronio, F.; Crocco, M.; Iudica, G.; et al. Management of Childhood-Onset Craniopharyngioma in Italy: A Multicenter, 7-Year Follow-Up Study of 145 Patients. J. Clin. Endocrinol. Metab. 2022, 107, e1020–e1031. [Google Scholar] [CrossRef]
- Feola, T.; Pirchio, R.S.; Puliani, G.; Pofi, R.; Crocco, M.; Sada, V.; Sesti, F.; Verdecchia, F.; Gianfrilli, D.; Appetecchia, M.; et al. Sellar and Parasellar Lesions in the Transition Age: A Retrospective Italian Multi-Centre Study. J. Endocrinol. Investig. 2023, 46, 181–188. [Google Scholar] [CrossRef]
- Wellbrock, M.; Voigt, M.; Ronckers, C.; Grabow, D.; Spix, C.; Erdmann, F. Registration, Incidence Patterns, and Survival Trends of Central Nervous System Tumors Among Children in Germany 1980–2019: An Analysis of 40 Years Based on Data from the German Childhood Cancer Registry. Pediatr. Blood Cancer 2024, 71, e30954. [Google Scholar] [CrossRef]
- Tonorezos, E.S.; Cohn, R.J.; Glaser, A.W.; Lewin, J.; Poon, E.; Wakefield, C.E.; Oeffinger, K.C. Long-Term Care for People Treated for Cancer During Childhood and Adolescence. Lancet 2022, 399, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Renzi, S.; Michaeli, O.; Ramaswamy, V.; Huang, A.; Stephens, D.; Maguire, B.; Tabori, U.; Bouffet, E.; Bartels, U. Causes of Death in Pediatric Neuro-Oncology: The SickKids Experience from 2000 to 2017. J. Neurooncol. 2020, 149, 181–189. [Google Scholar] [CrossRef]
- Bottinor, W.; Im, C.; Doody, D.R.; Armenian, S.H.; Arynchyn, A.; Hong, B.; Howell, R.M.; Jacobs, D.R., Jr.; Ness, K.K.; Oeffinger, K.C.; et al. Mortality After Major Cardiovascular Events in Survivors of Childhood Cancer. J. Am. Coll. Cardiol. 2024, 83, 827–838. [Google Scholar] [CrossRef]
- Dixon, S.B.; Liu, Q.; Chow, E.J.; Oeffinger, K.C.; Nathan, P.C.; Howell, R.M.; Leisenring, W.M.; Ehrhardt, M.J.; Ness, K.K.; Krull, K.R.; et al. Specific Causes of Excess Late Mortality and Association with Modifiable Risk Factors Among Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study Cohort. Lancet 2023, 401, 1447–1457. [Google Scholar] [CrossRef]
- Zaorsky, N.G.; Zhang, Y.; Tchelebi, L.T.; Mackley, H.B.; Chinchilli, V.M.; Zacharia, B.E. Stroke Among Cancer Patients. Nat. Commun. 2019, 10, 5172. [Google Scholar] [CrossRef] [PubMed]
- Maciel, J.; Dias, D.; Cavaco, D.; Donato, S.; Pereira, M.C.; Simões-Pereira, J. Growth Hormone Deficiency and Other Endocrinopathies After Childhood Brain Tumors: Results from a Close Follow-Up in a Cohort of 242 Patients. J. Endocrinol. Investig. 2021, 44, 2367–2374. [Google Scholar] [CrossRef]
- Sbardella, E.; Crocco, M.; Feola, T.; Papa, F.; Puliani, G.; Gianfrilli, D.; Isidori, A.M.; Grossman, A.B. GH Deficiency in Cancer Survivors in the Transition Age: Diagnosis and Therapy. Pituitary 2020, 23, 432–456. [Google Scholar] [CrossRef]
- Higashi, Y. Roles of Oxidative Stress and Inflammation in Vascular Endothelial Dysfunction-Related Disease. Antioxidants 2022, 11, 1958. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.R.; Borges-Canha, M.; Cardoso, R.; Neves, J.S.; Castro-Ferreira, R.; Leite-Moreira, A. Novel Biomarkers for Evaluation of Endothelial Dysfunction. Angiology 2020, 71, 397–410. [Google Scholar] [CrossRef]
- Wilk, G.; Osmenda, G.; Matusik, P.; Nowakowski, D.; Jasiewicz-Honkisz, B.; Ignacak, A.; Cześnikiewicz-Guzik, M.; Guzik, T.J. Endothelial Function Assessment in Atherosclerosis: Comparison of Brachial Artery Flow-Mediated Vasodilation and Peripheral Arterial Tonometry. Pol. Arch. Med. Wewn. 2013, 123, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Merchant, T.E.; Rose, S.R.; Bosley, C.; Wu, S.; Xiong, X.; Lustig, R.H. Growth Hormone Secretion After Conformal Radiation Therapy in Pediatric Patients with Localized Brain Tumors. J. Clin. Oncol. 2011, 29, 4776–4780. [Google Scholar] [CrossRef]
- Brignardello, E.; Felicetti, F.; Castiglione, A.; Chiabotto, P.; Corrias, A.; Fagioli, F.; Ciccone, G.; Boccuzzi, G. Endocrine Health Conditions in Adult Survivors of Childhood Cancer: The Need for Specialized Adult-Focused Follow-Up Clinics. Eur. J. Endocrinol. 2013, 168, 465–472. [Google Scholar] [CrossRef]
- Chemaitilly, W.; Li, Z.; Huang, S.; Ness, K.K.; Clark, K.L.; Green, D.M.; Barnes, N.; Armstrong, G.T.; Krasin, M.J.; Srivastava, D.K.; et al. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: A report from the St Jude Lifetime Cohort study. J. Clin. Oncol. 2015, 33, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Follin, C.; Thilén, U.; Ahrén, B.; Erfurth, E.M. Improvement in cardiac systolic function and reduced prevalence of metabolic syndrome after two years of growth hormone (GH) treatment in GH-deficient adult survivors of childhood-onset acute lymphoblastic leukemia. J. Clin. Endocrinol. Metab. 2006, 91, 1872–1875. [Google Scholar] [CrossRef] [PubMed]
- Deepak, D.; Daousi, C.; Javadpour, M.; Clark, D.; Perry, Y.; Pinkney, J.; Macfarlane, I.A. The influence of growth hormone replacement on peripheral inflammatory and cardiovascular risk markers in adults with severe growth hormone deficiency. Growth Horm. IGF Res. 2010, 20, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Gazzaruso, C.; Gola, M.; Karamouzis, I.; Giubbini, R.; Giustina, A. Cardiovascular risk in adult patients with growth hormone (GH) deficiency and following substitution with GH—An update. J. Clin. Endocrinol. Metab. 2014, 99, 18–29. [Google Scholar] [CrossRef]
- Crocco, M.; d’Annunzio, G.; La Valle, A.; Piccolo, G.; Chiarenza, D.S.; Bigatti, C.; Molteni, M.; Milanaccio, C.; Garrè, M.L.; Di Iorgi, N.; et al. Endothelial dysfunction in childhood cancer survivors: A narrative review. Life 2021, 12, 45. [Google Scholar] [CrossRef]
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004, 114 (Suppl. S2), 555–576. [Google Scholar] [CrossRef]
- Sklar, C.A.; Antal, Z.; Chemaitilly, W.; Cohen, L.E.; Follin, C.; Meacham, L.R.; Murad, M.H. Hypothalamic-pituitary and growth disorders in survivors of childhood cancer: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2018, 103, 2761–2784. [Google Scholar] [CrossRef]
- Agenzia Italiana del Farmaco (AIFA). Nota 39. Available online: https://www.aifa.gov.it/nota-39 (accessed on 1 November 2025).
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents; National Heart, Lung, and Blood Institute. Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics 2011, 128 (Suppl. S5), S213–S256. [Google Scholar] [CrossRef]
- Kuvin, J.T.; Patel, A.R.; Sliney, K.A.; Pandian, N.G.; Sheffy, J.; Schnall, R.P.; Karas, R.H.; Udelson, J.E. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am. Heart J. 2003, 146, 168–174. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R., Jr.; Kuvin, J.T.; Lerman, A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef] [PubMed]
- Fatihoglu, S.G.; Jam, F.; Okutucu, S.; Oto, A. Noninvasive investigation of the presence and extent of coronary artery disease by the evaluation of fingertip-reactive hyperemia. Med. Princ. Pract. 2022, 31, 262–268. [Google Scholar] [CrossRef]
- Suwaidi, J.A.; Hamasaki, S.; Higano, S.T.; Nishimura, R.A.; Holmes, D.R., Jr.; Lerman, A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation 2000, 101, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Hayden, J.; O’Donnell, G.; deLaunois, I.; O’Gorman, C. Endothelial peripheral arterial tonometry (Endo-PAT 2000) use in paediatric patients: A systematic review. BMJ Open 2023, 13, e062098. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; pp. 1–17. [Google Scholar] [CrossRef]
- Kyriakakis, N.; Giannoudi, M.; Kumar, S.S.; Seejore, K.; Dimitriadis, G.K.; Randeva, H.; Glaser, A.; Kwok-Williams, M.; Gerrard, G.; Loughrey, C.; et al. Evaluation of cardiovascular risk factors in long-term survivors of adult- and childhood-onset brain tumours: A pilot study. Endocr. Connect. 2023, 12, e220491. [Google Scholar] [CrossRef]
- Romano, A.; Sollazzo, F.; Rivetti, S.; Morra, L.; Servidei, T.; Lucchetti, D.; Attinà, G.; Maurizi, P.; Mastrangelo, S.; Zovatto, I.C.; et al. Evaluation of metabolic and cardiovascular risk measured by laboratory biomarkers and cardiopulmonary exercise test in children and adolescents recovered from brain tumors: The CARMEP study. Cancers 2024, 16, 324. [Google Scholar] [CrossRef] [PubMed]
- Zelcer, S.; Chen, B.; Mangel, J.; Vujovic, O.; Thiessen-Philbrook, H.R.; Reider, M.; Mahmud, F.H. Impaired vascular function in asymptomatic young adult survivors of Hodgkin Lymphoma following mediastinal radiation. J. Cancer Surviv. 2010, 4, 218–224. [Google Scholar] [CrossRef]
- Ruble, K.; Davis, C.L.; Han, H.R. Endothelial health in childhood acute lymphoid leukemia survivors: Pilot evaluation with peripheral artery tonometry. J. Pediatr. Hematol. Oncol. 2015, 37, 117–120. [Google Scholar] [CrossRef]
- Odanaka, Y.; Takitani, K.; Katayama, H.; Fujiwara, H.; Kishi, K.; Ozaki, N.; Ashida, A.; Takaya, R.; Tamai, H. Microvascular endothelial function in Japanese early adolescents. J. Clin. Biochem. Nutr. 2017, 61, 228–232. [Google Scholar] [CrossRef][Green Version]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018, 20 (Suppl. S4), iv1–iv86. [Google Scholar] [CrossRef] [PubMed]
- Gurney, J.G.; Kadan-Lottick, N.S.; Packer, R.J.; Neglia, J.P.; Sklar, C.A.; Punyko, J.A.; Stovall, M.; Yasui, Y.; Nicholson, H.S.; Wolden, S.; et al. Endocrine and cardiovascular late effects among adult survivors of childhood brain tumors: Childhood Cancer Survivor Study. Cancer 2003, 97, 663–673. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Li, T.; Zhao, Y.; Yang, X.; Feng, Y.; Li, Y.; Wu, Y.; Zhang, M.; Li, X.; Hu, H.; Zhang, J.; et al. Association between insulin-like growth factor-1 and cardiovascular events: A systematic review and dose-response meta-analysis of cohort studies. J. Endocrinol. Investig. 2022, 45, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Pivonello, R.; Grasso, L.F.; Auriemma, R.S.; Galdiero, M.; Savastano, S.; Lombardi, G. Determinants of cardiac disease in newly diagnosed patients with acromegaly: Results of a 10-year survey study. Eur. J. Endocrinol. 2011, 165, 713–721. [Google Scholar] [CrossRef]
- Pappachan, J.M.; Raskauskiene, D.; Kutty, V.R.; Clayton, R.N. Excess mortality associated with hypopituitarism in adults: A meta-analysis of observational studies. J. Clin. Endocrinol. Metab. 2015, 100, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Casirati, A.; Somaschini, A.; Muraca, M.; Cereda, E.; Morsellino, V.; Di Iorgi, N.; Caccialanza, R.; Haupt, R. Fat-to-lean mass ratio as a tool to detect the dysmetabolic profile in childhood cancer survivors. Nutrition 2023, 113, 112129. [Google Scholar] [CrossRef]
- Sharma, V.M.; Vestergaard, E.T.; Jessen, N.; Kolind-Thomsen, P.; Nellemann, B.; Nielsen, T.S.; Vendelbo, M.H.; Møller, N.; Sharma, R.; Lee, K.Y.; et al. Growth hormone acts along the PPARγ–FSP27 axis to stimulate lipolysis in human adipocytes. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E34–E42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kopchick, J.J.; Berryman, D.E.; Puri, V.; Lee, K.Y.; Jorgensen, J.O.L. The effects of growth hormone on adipose tissue: Old observations, new mechanisms. Nat. Rev. Endocrinol. 2020, 16, 135–146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ashwell, M.; Gunn, P.; Gibson, S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: Systematic review and meta-analysis. Obes. Rev. 2012, 13, 275–286. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, F.; Zhang, Y.; Xue, J.; Kuang, J.; Jin, Z.; Zhang, T.; Jiang, C.; Wang, D.; Liang, S. Effect of One-Year Growth Hormone Therapy on Cardiometabolic Risk Factors in Boys with Obesity. Biomed. Res. Int. 2020, 2020, 2308124. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferruzzi, A.; Vrech, M.; Pietrobelli, A.; Cavarzere, P.; Zerman, N.; Guzzo, A.; Flodmark, C.E.; Piacentini, G.; Antoniazzi, F. The influence of growth hormone on pediatric body composition: A systematic review. Front. Endocrinol. 2023, 14, 1093691. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Noubiap, J.J.; Nansseu, J.R.; Lontchi-Yimagou, E.; Nkeck, J.R.; Nyaga, U.F.; Ngouo, A.T.; Tounouga, D.N.; Tianyi, F.L.; Foka, A.J.; Ndoadoumgue, A.L.; et al. Global, regional, and country estimates of metabolic syndrome burden in children and adolescents in 2020: A systematic review and modelling analysis. Lancet Child Adolesc. Health 2022, 6, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Codazzi, V.; Frontino, G.; Galimberti, L.; Giustina, A.; Petrelli, A. Mechanisms and risk factors of metabolic syndrome in children and adolescents. Endocrine 2024, 84, 16–28. [Google Scholar] [CrossRef]
- Okui, H.; Hamasaki, S.; Ishida, S.; Kataoka, T.; Orihara, K.; Fukudome, T.; Ogawa, M.; Oketani, N.; Saihara, K.; Shinsato, T.; et al. Adiponectin is a better predictor of endothelial function of the coronary artery than HOMA-R, body mass index, immunoreactive insulin, or triglycerides. Int. J. Cardiol. 2008, 126, 53–61. [Google Scholar] [CrossRef]
- Deng, G.; Long, Y.; Yu, Y.R.; Li, M.R. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK–eNOS pathway. Int. J. Obes. 2010, 34, 165–171. [Google Scholar] [CrossRef]
- Lanes, R.; Soros, A.; Gunczler, P.; Paoli, M.; Carrillo, E.; Villaroel, O.; Palacios, A. Growth hormone deficiency, low levels of adiponectin, and unfavorable plasma lipid and lipoproteins. J. Pediatr. 2006, 149, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Sumner, A.D.; Sardi, G.L.; Reed, J.F., III. Components of the metabolic syndrome differ between young and old adults in the US population. J. Clin. Hypertens. 2012, 14, 502–506. [Google Scholar] [CrossRef]
- Johnson, W.D.; Kroon, J.J.; Greenway, F.L.; Bouchard, C.; Ryan, D.; Katzmarzyk, P.T. Prevalence of risk factors for metabolic syndrome in adolescents: National Health and Nutrition Examination Survey (NHANES), 2001–2006. Arch. Pediatr. Adolesc. Med. 2009, 163, 371–377. [Google Scholar] [CrossRef]
- Crocco, M.; Verrico, A.; Milanaccio, C.; Piccolo, G.; De Marco, P.; Gaggero, G.; Iurilli, V.; Di Profio, S.; Malerba, F.; Panciroli, M.; et al. Dyslipidemia in children treated with a BRAF inhibitor for low-grade gliomas: A new side effect? Cancers 2022, 14, 2693. [Google Scholar] [CrossRef]
- Jakubowski, M.; Turek-Jakubowska, A.; Szahidewicz-Krupska, E.; Gawrys, K.; Gawrys, J.; Doroszko, A. Profiling the endothelial function using both peripheral artery tonometry (EndoPAT) and laser Doppler flowmetry (LD)—Complementary studies or waste of time? Microvasc. Res. 2020, 130, 104008. [Google Scholar] [CrossRef] [PubMed]
- Reneau, J.; Goldblatt, M.; Gould, J.; Kindel, T.; Kastenmeier, A.; Higgins, R.; Rengel, L.R.; Schoyer, K.; James, R.; Obi, B.; et al. Effect of adiposity on tissue-specific adiponectin secretion. PLoS ONE 2018, 13, e0198889. [Google Scholar] [CrossRef]
- Lubkowska, A.; Radecka, A.; Bryczkowska, I.; Rotter, I.; Laszczyńska, M.; Dudzińska, W. Serum adiponectin and leptin concentrations in relation to body fat distribution, hematological indices and lipid profile in humans. Int. J. Environ. Res. Public Health 2015, 12, 11528–11548. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, N.; Mantzoros, C.; Mikhailidis, D.P. Adiponectin, lipids and atherosclerosis. Curr. Opin. Lipidol. 2017, 28, 347–354. [Google Scholar] [CrossRef]
- Polak-Szczybyło, E.; Tabarkiewicz, J. The influence of body composition, lifestyle, and dietary components on adiponectin and resistin levels and AR index in obese individuals. Int. J. Mol. Sci. 2025, 26, 393. [Google Scholar] [CrossRef] [PubMed]
- Lind, L. Relationships between three different tests to evaluate endothelium-dependent vasodilation and cardiovascular risk in a middle-aged sample. J. Hypertens. 2013, 31, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Yeboah, J.; Folsom, A.R.; Burke, G.L.; Johnson, C.; Polak, J.F.; Post, W.; Lima, J.A.; Crouse, J.R.; Herrington, D.M. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: The multi-ethnic study of atherosclerosis. Circulation 2009, 120, 502–509. [Google Scholar] [CrossRef]
- Lind, L.; Berglund, L.; Larsson, A.; Sundström, J. Endothelial function in resistance and conduit arteries and 5-year risk of cardiovascular disease. Circulation 2011, 123, 1545–1551. [Google Scholar] [CrossRef]
- Anderson, T.J.; Charbonneau, F.; Title, L.M.; Buithieu, J.; Rose, M.S.; Conradson, H.; Hildebrand, K.; Fung, M.; Verma, S.; Lonn, E.M. Microvascular function predicts cardiovascular events in primary prevention: Long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation 2011, 123, 163–169. [Google Scholar] [CrossRef]
- Park, K.H.; Park, W.J. Endothelial dysfunction: Clinical implications in cardiovascular disease and therapeutic approaches. J. Korean Med. Sci. 2015, 30, 1213–1225. [Google Scholar] [CrossRef]
- Crocco, M. Cardio-Metabolic Risk Factors and Quality of Life in Childhood-Onset Brain Tumors with Growth Hormone Deficiency. PhD Thesis, Università Degli Studi di Genova, Genova, Italy, 2023. Available online: https://hdl.handle.net/20.500.14242/170764 (accessed on 1 November 2025).
- Urbina, E.M.; Williams, R.V.; Alpert, B.S.; Collins, R.T.; Daniels, S.R.; Hayman, L.; Jacobson, M.; Mahoney, L.; Mietus-Snyder, M.; Rocchini, A.; et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: Recommendations for standard assessment for clinical research—A scientific statement from the American Heart Association. Hypertension 2009, 54, 919–950. [Google Scholar] [CrossRef] [PubMed]
- Dalla Pozza, R.; Ehringer-Schetitska, D.; Fritsch, P.; Jokinen, E.; Petropoulos, A.; Oberhoffer, R.; Association for European Paediatric Cardiology Working Group Cardiovascular Prevention. Intima media thickness measurement in children: A statement from the Association for European Paediatric Cardiology (AEPC) Working Group on Cardiovascular Prevention endorsed by the Association for European Paediatric Cardiology. Atherosclerosis 2015, 238, 380–387. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | rhGH Group (n = 40) | No rhGH Group (n = 8) | CG (n = 12) | p Value rhGH vs. No rhGH | p Value rhGH vs. CG | p Value No rhGH vs. CG | Mean Difference [95% CI]; Cohen’s d |
|---|---|---|---|---|---|---|---|
| Age at diagnosis: | |||||||
| mean years ± SD | 7.6 ± 4.5 | 6.9 ± 5 | 5 ± 4.9 | 0.69 | 0.20 | 0.79 | |
| Age at the evaluation: | |||||||
| mean years ± SD | 16.2 ± 4.8 | 18.2 ± 5.4 | 14 ± 4.7 | 0.33 | 0.15 | 0.11 | |
| Time cancer diagnosis–evaluation: | |||||||
| mean years ± SD | 8.7 ± 4.6 | 11.4 ± 5.1 | 9 ± 4.9 | 0.16 | 0.95 | 0.38 | |
| Time cancer diagnosis–end of treatments: | |||||||
| mean years ± SD | 7.2 ± 4.5 | 7.8 ± 6.4 | 6.9 ± 5.6 | 0.94 | 0.62 | 0.91 | |
| Cranial radiotherapy: | |||||||
| mean Gy ± SD | 54.1 ± 8.2 | 54.5 ± 9.8 | 55.5 ± 9 | 0.89 | 0.23 | 0.44 | |
| Pituitary deficiencies: | |||||||
| mean numbers ± SD | 3.2 ± 1.2 | 2.9 ± 1.5 | 0.5 ± 0.5 | 0.52 | 0.0001 | 0.0001 | rhGH vs. CG: 2.7 [2.1–3.3]; d = 2.40 No rhGH vs. CG: 2.4 [1.5–3.3]; d = 1.95 |
| Tanner stage: | |||||||
| mean ± SD | 3.5 ± 1.5 | 4 ± 1.6 | 3.1 ± 1.4 | 0.29 | 0.34 | 0.13 | |
| Weight: | |||||||
| mean kg ± SD | 61.3 ± 22.1 | 66.8 ± 24.8 | 57.2 ± 19.6 | 0.56 | 0.66 | 0.34 | |
| Height: | |||||||
| mean cm ± SD | 153.5 ± 16.7 | 156.5 ± 19.8 | 152.6 ± 11.8 | 0.78 | 0.75 | 0.85 | |
| mean SDS ± SD | −0.9 ± 1.6 | −1.1 ± 1.8 | 0.2 ± 1.4 | 0.80 | 0.06 | 0.16 | |
| genetic target SDS ± SD | −0.4 ± 1 | −0.6 ± 0.7 | −0.0 ± 1 | 0.65 | 0.29 | 0.18 | |
| BMI: | |||||||
| mean kg/m2 ± SD | 25.2 ± 5.6 | 26.5 ± 6.4 | 23.8 ± 5 | 0.60 | 0.38 | 0.21 | |
| mean SDS ± SD | 2.1 ± 1.4 | 2.5 ± 1.8 | 1.6 ± 1.7 | 0.75 | 0.51 | 0.21 | |
| Waist to Hip Ratio | |||||||
| mean ± SD | 0.92 ± 0.2 | 0.90 ± 0.1 | 0.95 ± 0.1 | 0.92 | 0.05 | 0.21 | rhGH vs. CG: −0.03 [−0.06–0.00]; d = 0.18 |
| Body area surface: | |||||||
| mean m2 ± SD | 1.6 ± 0.4 | 1.7 ± 0.4 | 1.5 ± 0.3 | 0.56 | 0.47 | 0.27 | |
| Fasting glucose: | |||||||
| mean mmol/L ± SD | 4.8 ± 0.4 | 4.8 ± 0.4 | 4.9 ± 0.8 | 0.82 | 0.35 | 0.49 | |
| Fasting insulin: | |||||||
| mean uU/mL | 16.3 ± 8.8 | 16.9 ± 11.5 | 18.7 ± 12.1 | 0.94 | 0.71 | 0.78 |
| Characteristic | rhGH Group (n = 40) | No rhGH Group (n = 8) | CG (n = 12) | p Value rhGH vs. No rhGH | p Value rhGH vs. CG | p Value No rhGH vs. CG | Mean Difference [95% CI]; Cohen’s d |
|---|---|---|---|---|---|---|---|
| Lipid metabolism: | |||||||
| triglycerides mean mmol/L ± SD | 1.24 ± 0.6 | 1.21 ± 0.6 | 0.99 ± 0.4 | 0.86 | 0.23 | 0.62 | |
| cholesterol mean mmol/L ± SD | 4.61 ± 0.9 | 4.29 ± 0.7 | 4.25 ± 0.8 | 0.43 | 0.21 | 0.73 | |
| HDL mean mmol/L ± SD | 1.39 ± 0.4 | 1.13 ± 0.2 | 1.36 ± 0.2 | 0.02 | 0.89 | 0.02 | rhGH vs. No rhGH: 0.26 [0.04–0.48]; d = 0.83 No rhGH vs. CG: −0.23 [−0.42–−0.04]; d = 1.39 |
| LDL mean mmol/L ± SD | 2.82 ± 0.8 | 2.69 ± 0.5 | 2.5 ± 0.6 | 0.73 | 0.17 | 0.30 | |
| adiponectin mg/L ± SD | 9.6 ± 7.2 | 3.4 ± 1.8 | 10.5 ± 6.7 | 0.007 | 0.72 | 0.0001 | rhGH vs. No rhGH: 6.2 [1.8–10.6]; d = 1.19 No rhGH vs. CG: −7.1 [−10.5–−3.7]; d = 1.74 |
| Prothrombotic factors: | |||||||
| SBP mean Hgmm ± SD | 112.6 ± 12.1 | 113.6 ± 14.2 | 105.8 ± 7.2 | 0.84 | 0.07 | 0.47 | |
| DBP mean Hgmm ± SD | 66.6 ± 10.2 | 65 ± 8.5 | 63.17 ± 7.00 | 0.67 | 0.23 | 0.85 | |
| fibrinogen mean mg/dL ± SD | 277.5 ± 50 | 297.4 ± 88.1 | 290.8 ± 72.1 | 0.69 | 0.48 | 0.90 | |
| homocysteine mean umol/L ± SD | 14.3 ± 21.4 | 19.4 ± 14.7 | 13.3 ± 5.8 | 0.10 | 0.09 | 0.36 | |
| Endothelial function: | |||||||
| EndoPAT RHI mean ± SD | 1.6 ± 0.6 | 1.8 ± 0.7 | 1.4 ± 0.5 | 0.53 | 0.57 | 0.14 | |
| EndoPAT ln RHI mean ± SD | 0.4 ± 0.4 | 0.5 ± 0.4 | 0.3 ± 0.5 | 0.51 | 0.39 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crocco, M.; Malerba, F.; Zampatti, N.; Nosratian, V.; Bigatti, C.; Panciroli, M.; Casirati, A.; Calevo, M.G.; Maghnie, M.; Di Iorgi, N. The Impact of Growth Hormone Deficiency on Endothelial Function in Childhood Brain Cancer Survivors. Cancers 2025, 17, 3746. https://doi.org/10.3390/cancers17233746
Crocco M, Malerba F, Zampatti N, Nosratian V, Bigatti C, Panciroli M, Casirati A, Calevo MG, Maghnie M, Di Iorgi N. The Impact of Growth Hormone Deficiency on Endothelial Function in Childhood Brain Cancer Survivors. Cancers. 2025; 17(23):3746. https://doi.org/10.3390/cancers17233746
Chicago/Turabian StyleCrocco, Marco, Federica Malerba, Noemi Zampatti, Valentina Nosratian, Carolina Bigatti, Marta Panciroli, Amanda Casirati, Maria Grazia Calevo, Mohamad Maghnie, and Natascia Di Iorgi. 2025. "The Impact of Growth Hormone Deficiency on Endothelial Function in Childhood Brain Cancer Survivors" Cancers 17, no. 23: 3746. https://doi.org/10.3390/cancers17233746
APA StyleCrocco, M., Malerba, F., Zampatti, N., Nosratian, V., Bigatti, C., Panciroli, M., Casirati, A., Calevo, M. G., Maghnie, M., & Di Iorgi, N. (2025). The Impact of Growth Hormone Deficiency on Endothelial Function in Childhood Brain Cancer Survivors. Cancers, 17(23), 3746. https://doi.org/10.3390/cancers17233746







