Unveiling the Interplay Between the Human Microbiome and Gastric Cancer: A Review of the Complex Relationships and Therapeutic Avenues
Simple Summary
Abstract
1. Introduction
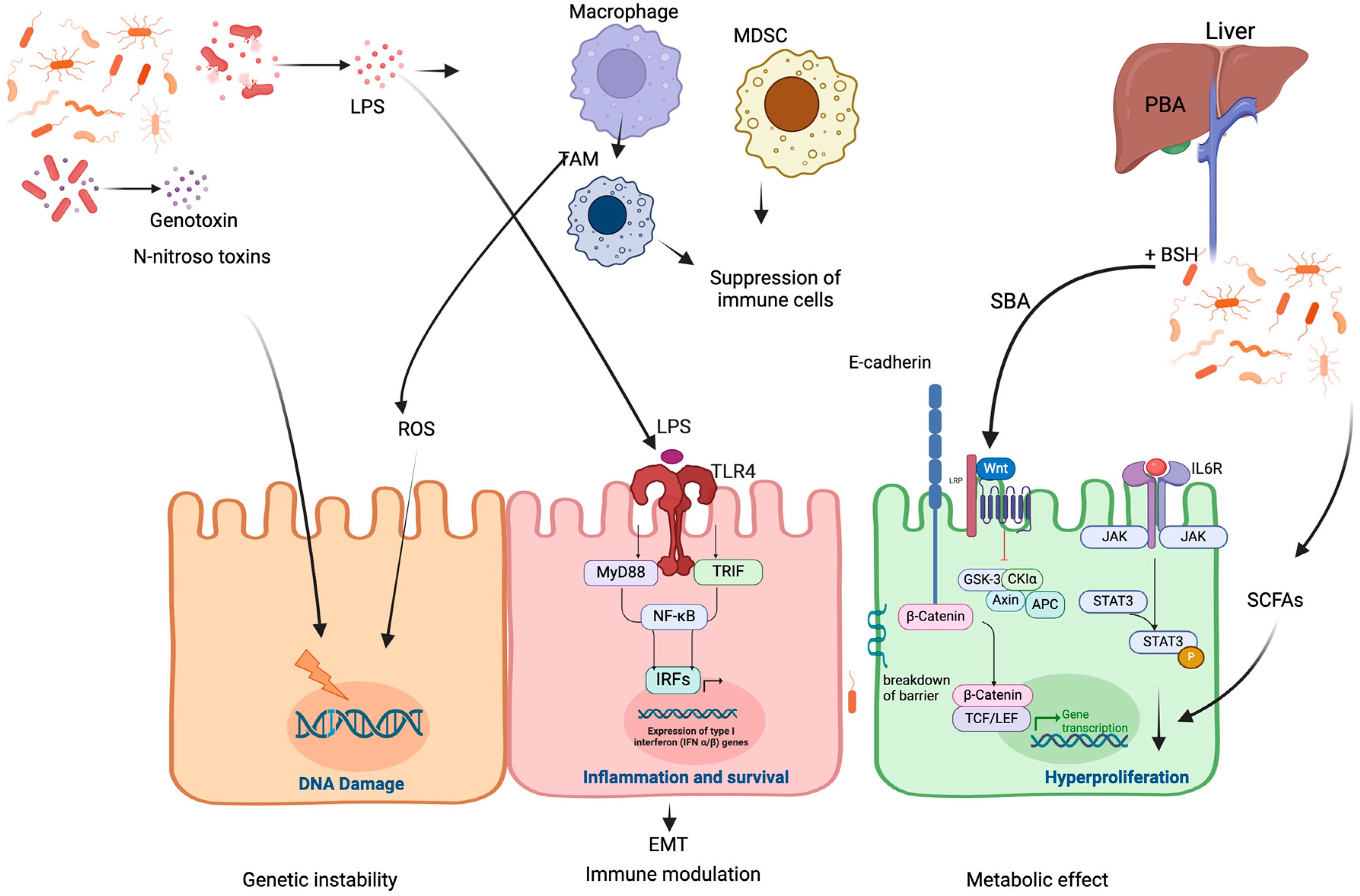
2. Mechanisms of Microbiome-Induced Cancer Development
2.1. Microbiota-Induced Genetic Instability
2.2. Microbiota-Induced Immune Modulation
2.3. Microbiota-Induced Metabolic Effects
3. Microbiota and Gastric Cancer
4. Microbiome as a Predictive Biomarker for Gastric Cancer
5. The Impact of the Microbiome on Cancer Treatment
5.1. Microbiota Affects the Efficacy of Chemotherapy
5.2. Gut Microbiota Reduces Chemotherapy-Induced Toxicity
5.3. Role of the Microbiome in Gastric Cancer Immunotherapy
6. Positive Impact of Gastric Microbiome on Cancer Treatment Through Immune Modulation
6.1. Immune Enhancement
6.2. Direct Tumor Cell Toxicity
6.3. Oncolytic Microorganisms (Bacteria and Virus)
6.4. Synergistic Effects with Conventional Therapies
7. Microbiota Modulation Approaches
7.1. Antibiotics and Microbiome Disruption
7.2. Dietary Interventions
7.3. Probiotics, Prebiotics, and Synbiotics
7.3.1. Microbiome Modulation Attempts in Cancer
7.3.2. Potential Limitations of Microbiome Modulation Using Probiotics
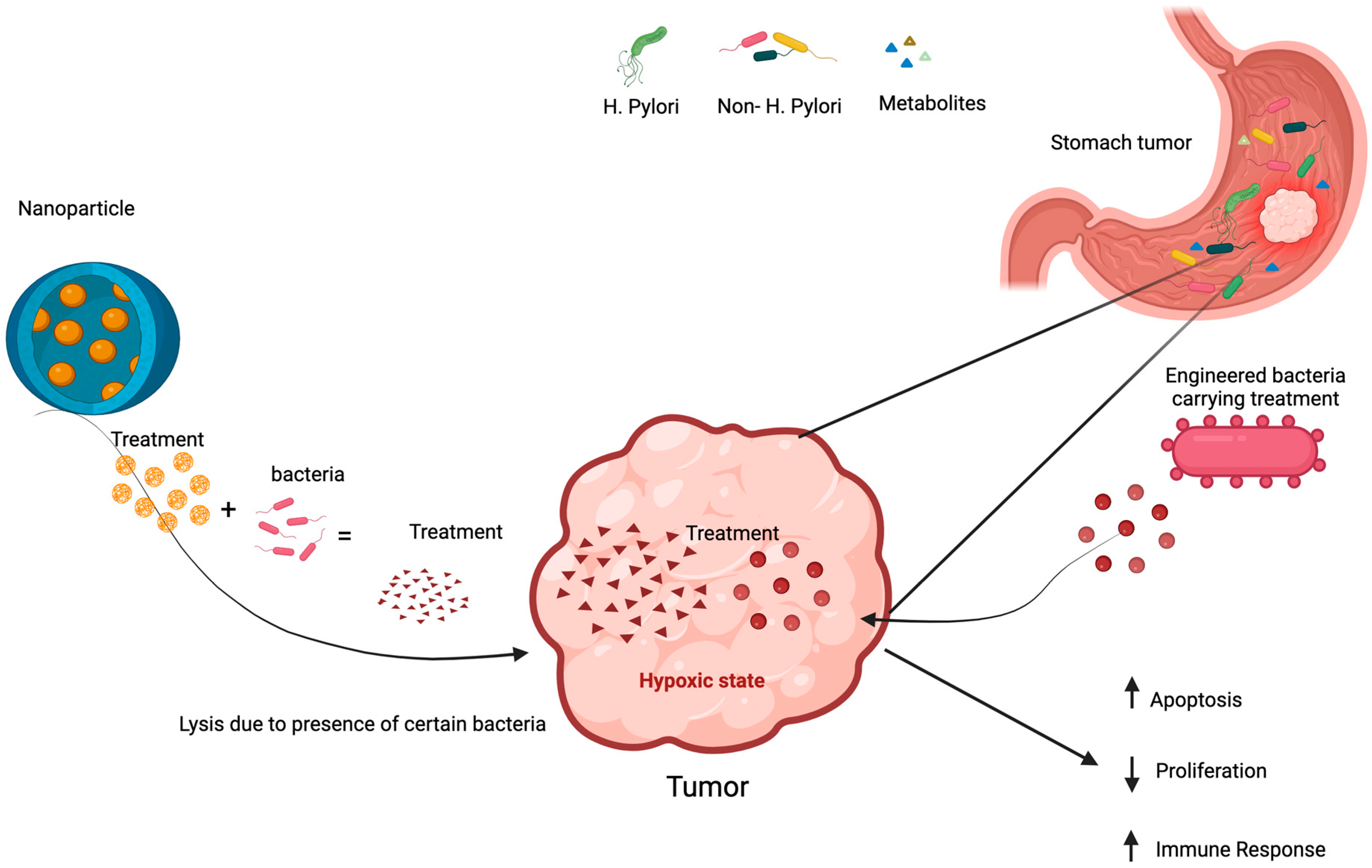
7.4. Engineered Microbes in Cancer Treatment
7.5. Nanoparticles Technology in Microbiome Manipulation
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, e00036-17. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Doré, J.; Schiffrin, E.J. The inflammatory status of old age can be nurtured from the intestinal environment. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J.; Plummer, N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Zitvogel, L.; Galluzzi, L.; Viaud, S.; Vétizou, M.; Daillère, R.; Merad, M.; Kroemer, G. Cancer and the gut microbiota: An unexpected link. Sci. Transl. Med. 2015, 7, 271ps1. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.V.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef] [PubMed]
- Belkaid, Y.; Hand, T.W. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar]
- Macpherson, A.J.; McCoy, K.D.; Johansen, F.-E.; Brandtzaeg, P. The immune geography of IgA induction and function. Mucosal Immunol. 2008, 1, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Hapfelmeier, S.; Lawson, M.A.E.; Slack, E.; Kirundi, J.K.; Stoel, M.; Heikenwalder, M.; Cahenzli, J.; Velykoredko, Y.; Balmer, M.L.; Endt, K.; et al. Reversible Microbial Colonization of Germ-Free Mice Reveals the Dynamics of IgA Immune Responses. Science 2010, 328, 1705–1709. [Google Scholar] [CrossRef]
- Park, J.-H.; Kotani, T.; Konno, T.; Setiawan, J.; Kitamura, Y.; Imada, S.; Usui, Y.; Hatano, N.; Shinohara, M.; Saito, Y.; et al. Promotion of intestinal epithelial cell turnover by commensal bacteria: Role of short-chain fatty acids. PLoS ONE 2016, 11, e0156334. [Google Scholar] [CrossRef] [PubMed]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic Bacteria Direct Expression of an Intestinal Bactericidal Lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Śliżewska, W. The Role of Probiotics in Cancer Prevention. Cancers 2020, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Cocozza, E.; Cemali, Ö.; Bayazıt, A.D.; Nanì, M.F.; Cerqua, I.; Morgillo, F.; Saygılı, S.K.; Canani, R.B.; Amero, P.; et al. Understanding the role of the gut microbiome in gastrointestinal cancer: A review. Front. Pharmacol. 2023, 14, 1130562. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Fulbright, L.E.; Ellermann, M.; Arthur, J.C. The microbiome and the hallmarks of cancer. PLoS Pathog. 2017, 13, e1006480. [Google Scholar] [CrossRef]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatumin colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Ivleva, E.A.; Grivennikov, S.I. Microbiota-driven mechanisms at different stages of cancer development. Neoplasia 2022, 32, 100829. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos-Manzano, C.; Puschhof, J.; Rosendahl Huber, A.; Van Hoeck, A.; Wood, H.M.; Nomburg, J.; Gurjao, C.; Manders, F.; Dalmasso, G.; Stege, P.B.; et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature 2020, 580, 269–273. [Google Scholar] [CrossRef]
- Kadosh, E.; Snir-Alkalay, I.; Venkatachalam, A.; May, S.; Lasry, A.; Elyada, E.; Zinger, A.; Shaham, M.; Vaalani, G.; Mernberger, M.; et al. The gut microbiome switches mutant p53 from tumour-suppressive to oncogenic. Nature 2020, 586, 133–138. [Google Scholar] [CrossRef]
- de Almeida, C.V.; Taddei, A.; Amedei, A. The controversial role of Enterococcus faecalis in colorectal cancer. Ther. Adv. Gastroenterol. 2018, 11, 1756284818783606. [Google Scholar] [CrossRef] [PubMed]
- Yangyanqiu, W.; Jian, C.; Yuqing, Y.; Zhanbo, Q.; Shuwen, H. Gut microbes involvement in gastrointestinal cancers through redox regulation. Gut Pathog. 2023, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, E.; Zhang, Y.; Zhang, L.; Montiel, M.; Zoltan, M.; Dong, W.; Quesada, P.; Sahin, I.; Chandra, V.; Lucas, A.S.; et al. Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 2019, 178, 795–806.e12. [Google Scholar] [CrossRef]
- Cheng, W.Y.; Wu, C.-Y.; Yu, J. The role of gut microbiota in cancer treatment: Friend or foe? Gut 2020, 69, 1867–1876. [Google Scholar] [CrossRef]
- Yang, W.; Yu, T.; Huang, X.; Bilotta, A.J.; Xu, L.; Lu, Y.; Sun, J.; Pan, F.; Zhou, J.; Zhang, W.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef] [PubMed]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Gou, H.; Zeng, R.; Lau, H.C.H.; Yu, J. Gut microbial metabolites: Shaping future diagnosis and treatment against gastrointestinal cancer. Pharmacol. Res. 2024, 208, 107373. [Google Scholar] [CrossRef] [PubMed]
- Ocvirk, S.; O’keefe, S.J. Dietary fat, bile acid metabolism and colorectal cancer. Semin. Cancer Biol. 2020, 73, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Bazzichetto, C.; Milella, M.; Zampiva, I.; Simionato, F.; Amoreo, C.A.; Buglioni, S.; Pacelli, C.; Le Pera, L.; Colombo, T.; Bria, E.; et al. Interleukin-8 in Colorectal Cancer: A Systematic Review and Meta-Analysis of Its Potential Role as a Prognostic Biomarker. Biomedicines 2022, 10, 2631. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Lian, S.; Ung, T.T.; Xia, Y.; Han, J.Y.; Jung, Y.D. Lithocholic Acid Stimulates IL-8 Expression in Human Colorectal Cancer Cells Via Activation of Erk1/2 MAPK and Suppression of STAT3 Activity. J. Cell Biochem. 2017, 118, 2958–2967. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.M.; Howarth, G.S.; Butler, R.N. Short-chain fatty acid modulation of apoptosis in the kato III human gastric carcinoma cell line. Cancer Biol. Ther. 2007, 6, 1051–1057. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, B.; Zheng, Q.; Li, H.; Meng, X.; Zhou, F.; Zhang, L. A Review of Gut Microbiota-Derived Metabolites in Tumor Progression and Cancer Therapy. Adv. Sci. 2023, 10, 2207366. [Google Scholar] [CrossRef]
- Encarnação, J.C.; Pires, A.S.; Amaral, R.A.; Gonçalves, T.J.; Laranjo, M.; Casalta-Lopes, J.E.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Abrantes, A.M.; Botelho, M.F. Butyrate, a dietary fiber derivative that improves irinotecan effect in colon cancer cells. J. Nutr. Biochem. 2018, 56, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Popov, J.; Caputi, V.; Nandeesha, N.; Rodriguez, D.A.; Pai, N. Microbiota-Immune Interactions in Ulcerative Colitis and Colitis Associated Cancer and Emerging Microbiota-Based Therapies. Int. J. Mol. Sci. 2021, 22, 11365. [Google Scholar] [CrossRef] [PubMed]
- Behary, J.; Amorim, N.; Jiang, X.-T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The Microbiome and Butyrate Regulate Energy Metabolism and Autophagy in the Mammalian Colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Obiso, R.J.; Lyerly, D.M.; Van Tassell, R.L.; Wilkins, T.D. Proteolytic activity of the Bacteroides fragilis enterotoxin causes fluid secretion and intestinal damage in vivo. Infect. Immun. 1995, 63, 3820–3826. [Google Scholar] [CrossRef] [PubMed]
- Zamani, S.; Taslimi, R.; Sarabi, A.; Jasemi, S.; Sechi, L.A.; Feizabadi, M.M. Enterotoxigenic Bacteroides fragilis: A Possible Etiological Candidate for Bacterially-Induced Colorectal Precancerous and Cancerous Lesions. Front. Cell. Infect. Microbiol. 2020, 9, 449. [Google Scholar] [CrossRef]
- Haghi, F.; Goli, E.; Mirzaei, B.; Zeighami, H. The association between fecal enterotoxigenic B. fragilis with colorectal cancer. BMC Cancer 2019, 19, 879. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Powell, J.; Mathioudakis, N.; Kane, S.; Fernandez, E.; Sears, C.L. Bacteroides fragilis Enterotoxin Induces Intestinal Epithelial Cell Secretion of Interleukin-8 through Mitogen-Activated Protein Kinases and a Tyrosine Kinase-Regulated Nuclear Factor-κB Pathway. Infect. Immun. 2004, 72, 5832–5839. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.M.; Yang, Y.S.; Peng, L.H. Microbiota in the stomach: New insights. J. Dig. Dis. 2014, 15, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Reyes, V.E. Helicobacter pylori and Its Role in Gastric Cancer. Microorganisms 2023, 11, 1312. [Google Scholar] [CrossRef] [PubMed]
- Polk, D.B.; Peek, R.M., Jr. Helicobacter pylori: Gastric cancer and beyond. Nat. Rev. Cancer 2010, 10, 403–414. [Google Scholar] [CrossRef]
- Sharafutdinov, I.; Tegtmeyer, N.; Linz, B.; Rohde, M.; Vieth, M.; Tay, A.C.-Y.; Lamichhane, B.; Tuan, V.P.; Fauzia, K.A.; Sticht, H.; et al. A single-nucleotide polymorphism in Helicobacter pylori promotes gastric cancer development. Cell Host Microbe 2023, 31, 1345–1358.e6. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Choi, H.; Leung, K.; Jiang, F.; Graham, D.Y.; Leung, W.K. Global prevalence of Helicobacter pylori infection between 1980 and 2022: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 8, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Zilberstein, B.; Quintanilha, A.G.; Santos, M.A.A.; Pajecki, D.; De Moura, E.G.; Alves, P.R.A.; Filho, F.M.; De Souza, J.A.U.; Gama-Rodrigues, J. Digestive tract microbiota in healthy volunteers. Clin. Sci. 2007, 62, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Wong, G.L.-H.; To, K.-F.; Wong, V.W.-S.; Lai, L.H.; Chow, D.K.-L.; Lau, J.Y.-W.; Sung, J.J.-Y.; Ding, C. Bacterial Microbiota Profiling in Gastritis without Helicobacter pylori Infection or Non-Steroidal Anti-Inflammatory Drug Use. PLoS ONE 2009, 4, e7985. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Contreras, A.; Goldfarb, K.C.; Godoy-Vitorino, F.; Karaoz, U.; Contreras, M.; Blaser, M.J.; Brodie, E.L.; Dominguez-Bello, M.G. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J. 2010, 5, 574–579. [Google Scholar] [CrossRef]
- Han, K.J.; Lee, N.-K.; Park, H.; Paik, H.-D. Anticancer and Anti-Inflammatory Activity of Probiotic Lactococcus lactis NK34. J. Microbiol. Biotechnol. 2015, 25, 1697–1701. [Google Scholar] [CrossRef]
- Li, Z.-P.; Liu, J.-X.; Lu, L.-L.; Wang, L.-L.; Xu, L.; Guo, Z.-H.; Dong, Q.-J. Overgrowth of Lactobacillus in gastric cancer. World J. Gastrointest. Oncol. 2021, 13, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, S.J.; Cole, J.A. Nitrite Accumulatin during Anaerobic Nitrate Reduction by Binary Suspensions of Bacteria Isolated from the Achlorhydric Stomach. Microbiology 1987, 133, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Vinasco, K.; Mitchell, H.M.; Kaakoush, N.O.; Castaño-Rodríguez, N. Microbial carcinogenesis: Lactic acid bacteria in gastric cancer. Biochim. Biophys. Acta (BBA) Rev. Cancer 2019, 1872, 188309. [Google Scholar] [CrossRef] [PubMed]
- Rosier, B.T.; Moya-Gonzalvez, E.M.; Corell-Escuin, P.; Mira, A. Isolation and Characterization of Nitrate-Reducing Bacteria as Potential Probiotics for Oral and Systemic Health. Front. Microbiol. 2020, 11, 555465. [Google Scholar] [CrossRef] [PubMed]
- González-Soltero, R.; Bailén, M.; de Lucas, B.; Ramírez-Goercke, M.I.; Pareja-Galeano, H.; Larrosa, M. Role of Oral and Gut Microbiota in Dietary Nitrate Metabolism and Its Impact on Sports Performance. Nutrients 2020, 12, 3611. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, X.; Zeng, R.; Wu, Q.; Sun, H.; Wu, W.; Zhang, X.; Sun, G.; Yan, B.; Wu, L.; et al. Changes of the Gastric Mucosal Microbiome Associated with Histological Stages of Gastric Carcinogenesis. Front. Microbiol. 2020, 11, 997. [Google Scholar] [CrossRef]
- Hernández-Ramírez, R.U.; Galván-Portillo, M.V.; Ward, M.H.; Agudo, A.; González, C.A.; Oñate-Ocaña, L.F.; Herrera-Goepfert, R.; Palma-Coca, O.; López-Carrillo, L. Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City. Int. J. Cancer 2009, 125, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Lara-Tejero, M.; Galán, J.E. A Bacterial Toxin That Controls Cell Cycle Progression as a Deoxyribonuclease I-Like Protein. Science 2000, 290, 354–357. [Google Scholar] [CrossRef]
- He, Z.; Tian, W.; Wei, Q.; Xu, J. Involvement of Fusobacterium nucleatum in malignancies except for colorectal cancer: A literature review. Front. Immunol. 2022, 13, 968649. [Google Scholar] [CrossRef] [PubMed]
- Gantuya, B.; El Serag, H.B.; Matsumoto, T.; Ajami, N.J.; Uchida, T.; Oyuntsetseg, K.; Bolor, D.; Yamaoka, Y. Gastric mucosal microbiota in a Mongolian population with gastric cancer and precursor conditions. Aliment. Pharmacol. Ther. 2020, 51, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Liou, J.-M.; Lee, Y.-C.; Hong, T.-C.; El-Omar, E.M.; Wu, M.-S. The interplay between Helicobacter pylori and gastrointestinal microbiota. Gut Microbes 2021, 13, 1909459. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, R.; Chen, S.; Sun, B.; Zhang, K. Analysis of gastric microbiome reveals three distinctive microbial communities associated with the occurrence of gastric cancer. BMC Microbiol. 2022, 22, 184. [Google Scholar] [CrossRef]
- Sarhadi, V.; Mathew, B.; Kokkola, A.; Karla, T.; Tikkanen, M.; Rautelin, H.; Lahti, L.; Puolakkainen, P.; Knuutila, S. Gut microbiota of patients with different subtypes of gastric cancer and gastrointestinal stromal tumors. Gut Pathog. 2021, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Ravegnini, G.; Fosso, B.; Di Saverio, V.; Sammarini, G.; Zanotti, F.; Rossi, G.; Ricci, M.; D’amico, F.; Valori, G.; Ioli, A.; et al. Gastric Adenocarcinomas and Signet-Ring Cell Carcinoma: Unraveling Gastric Cancer Complexity through Microbiome Analysis—Deepening Heterogeneity for a Personalized Therapy. Int. J. Mol. Sci. 2020, 21, 9735. [Google Scholar] [CrossRef]
- Winter, S.E.; Winter, M.G.; Xavier, M.N.; Thiennimitr, P.; Poon, V.; Keestra, A.M.; Laughlin, R.C.; Gomez, G.; Wu, J.; Lawhon, S.D.; et al. Host-Derived Nitrate Boosts Growth of E. coli in the Inflamed Gut. Science 2013, 339, 708–711. [Google Scholar] [CrossRef]
- Castaño-Rodríguez, N.; Goh, K.-L.; Fock, K.M.; Mitchell, H.M.; Kaakoush, N.O. Dysbiosis of the microbiome in gastric carcinogenesis. Sci. Rep. 2017, 7, 15957. [Google Scholar] [CrossRef] [PubMed]
- Strickertsson, J.A.B.; Desler, C.; Martin-Bertelsen, T.; Machado, A.M.D.; Wadstrøm, T.; Winther, O.; Rasmussen, L.J.; Friis-Hansen, L. Enterococcus faecalis Infection Causes Inflammation, Intracellular Oxphos-Independent ROS Production, and DNA Damage in Human Gastric Cancer Cells. PLoS ONE 2013, 8, e63147. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Dzink-Fox, J.; Feng, Y.; Muthupalani, S.; Mannion, A.J.; Sheh, A.; Whary, M.T.; Holcombe, H.R.; Piazuelo, B.M.; Bravo, L.E.; et al. Gastric Non-Helicobacter pylori Urease-Positive Staphylococcus epidermidis and Streptococcus salivarius Isolated from Humans Have Contrasting Effects on H. pylori-Associated Gastric Pathology and Host Immune Responses in a Murine Model of Gastric Cancer. mSphere 2022, 7, e0077221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Shi, D.; Li, M.; Li, Y.; Wang, X.; Li, W. The relationship between gastric microbiota and gastric disease. Scand. J. Gastroenterol. 2019, 54, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Delgado, S.; Cabrera-Rubio, R.; Mira, A.; Suárez, A.; Mayo, B. Microbiological Survey of the Human Gastric Ecosystem Using Culturing and Pyrosequencing Methods. Microb. Ecol. 2013, 65, 763–772. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, C.; Xu, S.; Xiang, C.; Wang, R.; Yang, D.; Lu, B.; Shi, L.; Tong, R.; Teng, Y.; et al. Fecal Microbiome Alteration May Be a Potential Marker for Gastric Cancer. Dis. Markers 2020, 2020, 3461315. [Google Scholar] [CrossRef]
- Aviles-Jimenez, F.; Vazquez-Jimenez, F.; Medrano-Guzman, R.; Mantilla, A.; Torres, J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Sci. Rep. 2014, 4, 4202. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.J.Y.; Coker, O.O.; Chu, E.; Szeto, C.H.; Luk, S.T.Y.; Lau, H.C.H.; Yu, J. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut 2020, 69, 1572–1581. [Google Scholar] [CrossRef]
- Hsieh, Y.-Y.; Tung, S.-Y.; Pan, H.-Y.; Yen, C.-W.; Xu, H.-W.; Lin, Y.-J.; Deng, Y.-F.; Hsu, W.-T.; Wu, C.-S.; Li, C. Increased Abundance of Clostridium and Fusobacterium in Gastric Microbiota of Patients with Gastric Cancer in Taiwan. Sci. Rep. 2018, 8, 158. [Google Scholar] [CrossRef]
- Long, X.; Wong, C.C.; Tong, L.; Chu, E.S.H.; Szeto, C.H.; Go, M.Y.Y.; Coker, O.O.; Chan, A.W.H.; Chan, F.K.L.; Sung, J.J.Y.; et al. Peptostreptococcus anaerobius promotes colorectal carcinogenesis and modulates tumour immunity. Nat. Microbiol. 2019, 4, 2319–2330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, X.; Zhou, Y.; Fu, K.; Lau, H.C.-H.; Chun, T.W.-Y.; Cheung, A.H.-K.; Coker, O.O.; Wei, H.; Wu, W.K.-K.; et al. Parvimonas micra promotes colorectal tumorigenesis and is associated with prognosis of colorectal cancer patients. Oncogene 2022, 41, 4200–4210. [Google Scholar] [CrossRef]
- Eribe, E.R.K.; Olsen, I. Leptotrichia species in human infections II. J. Oral Microbiol. 2017, 9, 1368848. [Google Scholar] [CrossRef]
- Boehm, E.T.; Thon, C.; Kupcinskas, J.; Steponaitiene, R.; Skieceviciene, J.; Canbay, A.; Malfertheiner, P.; Link, A. Fusobacterium nucleatum is associated with worse prognosis in Lauren’s diffuse type gastric cancer patients. Sci. Rep. 2020, 10, 16240. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, J.; Xin, Y.; Geng, C.; Tian, Z.; Yu, X.; Dong, Q. Bacterial overgrowth and diversification of microbiota in gastric cancer. Eur. J. Gastroenterol. Hepatol. 2016, 28, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Sgamato, C.; Rocco, A.; Compare, D.; Priadko, K.; Romano, M.; Nardone, G. Exploring the Link between Helicobacter pylori, Gastric Microbiota and Gastric Cancer. Antibiotics 2024, 13, 484. [Google Scholar] [CrossRef]
- Maddern, A.S.; Coller, J.K.; Bowen, J.M.; Gibson, R.J. The Association between the Gut Microbiome and Development and Progression of Cancer Treatment Adverse Effects. Cancers 2023, 15, 4301. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, L.; Leng, X.X.; Xie, Y.L.; Kang, Z.R.; Zhao, L.C.; Song, L.H.; Zhou, C.B.; Fang, J.Y. Fusobacterium nucleatum induces chemoresistance in colorectal cancer by inhibiting pyroptosis via the Hippo pathway. Gut Microbes 2024, 16, 2333790. [Google Scholar] [CrossRef] [PubMed]
- Csendes, D.; Gutlapalli, S.D.; Prakash, K.; Swarnakari, K.M.; Bai, M.; Manoharan, M.P.; Raja, R.; Jamil, A.; Desai, A.; Desai, D.M.; et al. Gastrointestinal Microbiota and Breast Cancer Chemotherapy Interactions: A Systematic Review. Cureus 2022, 14, e31648. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; He, J.; Li, H.; You, J.; Qin, H. Alterations in intestinal microbiota of colorectal cancer patients receiving radical surgery combined with adjuvant CapeOx therapy. Sci. China Life Sci. 2019, 62, 1178–1193. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, S.; Li, H.; Yang, F.; Mushtaq, N.; Ullah, S.; Shi, Y.; An, C.; Xu, J. The influence of gut microbiota dysbiosis to the efficacy of 5-Fluorouracil treatment on colorectal cancer. Biomed. Pharmacother. 2018, 108, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Iida, N.; Dzutsev, A.; Stewart, C.A.; Smith, L.; Bouladoux, N.; Weingarten, R.A.; Molina, D.A.; Salcedo, R.; Back, T.; Cramer, S.; et al. Commensal Bacteria Control Cancer Response to Therapy by Modulating the Tumor Microenvironment. Science 2019, 342, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, Y.; Weng, W.; Guo, B.; Cai, G.; Ma, Y.; Cai, S. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 2019, 38, 14. [Google Scholar] [CrossRef]
- Yu, T.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef]
- Chang, C.-W.; Lee, H.-C.; Li, L.-H.; Chiau, J.-S.C.; Wang, T.-E.; Chuang, W.-H.; Chen, M.-J.; Wang, H.-Y.; Shih, S.-C.; Liu, C.-Y.; et al. Fecal Microbiota Transplantation Prevents Intestinal Injury, Upregulation of Toll-Like Receptors, and 5-Fluorouracil/Oxaliplatin-Induced Toxicity in Colorectal Cancer. Int. J. Mol. Sci. 2020, 21, 386. [Google Scholar] [CrossRef] [PubMed]
- Pflug, N.; Kluth, S.; Vehreschild, J.J.; Bahlo, J.; Tacke, D.; Biehl, L.; Eichhorst, B.; Fischer, K.; Cramer, P.; Fink, A.-M.; et al. Efficacy of antineoplastic treatment is associated with the use of antibiotics that modulate intestinal microbiota. OncoImmunology 2016, 5, e1150399. [Google Scholar] [CrossRef] [PubMed]
- Dyawanapelly, S.; Kumar, A.; Chourasia, M.K. Lessons Learned from Gemcitabine: Impact of Therapeutic Carrier Systems and Gemcitabine’s Drug Conjugates on Cancer Therapy. Crit. Rev. Ther. Drug Carr. Syst. 2017, 34, 63–96. [Google Scholar] [CrossRef] [PubMed]
- Geller, L.T.; Barzily-Rokni, M.; Danino, T.; Jonas, O.H.; Shental, N.; Nejman, D.; Gavert, N.; Zwang, Y.; Cooper, Z.A.; Shee, K.; et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 2017, 357, 1156–1160. [Google Scholar] [CrossRef] [PubMed]
- Voorde, J.V.; Sabuncuoğlu, S.; Noppen, S.; Hofer, A.; Ranjbarian, F.; Fieuws, S.; Balzarini, J.; Liekens, S. Nucleoside-catabolizing Enzymes in Mycoplasma-infected Tumor Cell Cultures Compromise the Cytostatic Activity of the Anticancer Drug Gemcitabine. J. Biol. Chem. 2014, 289, 13054–13065. [Google Scholar] [CrossRef]
- Bawaneh, A.; Wilson, A.S.; Levi, N.; Howard-McNatt, M.M.; Chiba, A.; Soto-Pantoja, D.R.; Cook, K.L. Intestinal Microbiota Influence Doxorubicin Responsiveness in Triple-Negative Breast Cancer. Cancers 2022, 14, 4849. [Google Scholar] [CrossRef]
- Sougiannis, A.; VanderVeen, B.; Enos, R.; Velazquez, K.; Bader, J.; Carson, M.; Chatzistamou, I.; Walla, M.; Pena, M.; Kubinak, J.; et al. Impact of 5 fluorouracil chemotherapy on gut inflammation, functional parameters, and gut microbiota. Brain Behav. Immun. 2019, 80, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Lalla, R.V.; Bowen, J.; Barasch, A.; Elting, L.; Epstein, J.; Keefe, D.M.; McGuire, D.B.; Migliorati, C.; Nicolatou-Galitis, O.; Peterson, D.E.; et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 2014, 120, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Kee, B.K.; Morris, J.S.; Slack, R.S.; Crocenzi, T.; Wong, L.; Esparaz, B.; Overman, M.; Glover, K.; Jones, D.; Wen, S.; et al. A phase II, randomized, double blind trial of calcium aluminosilicate clay versus placebo for the prevention of diarrhea in patients with metastatic colorectal cancer treated with irinotecan. Support. Care Cancer 2014, 23, 661–670. [Google Scholar] [CrossRef]
- Tang, L.; Gong, L.; Zhou, G.; Liu, L.; Zhang, D.; Tang, J.; Zheng, J. Design of low temperature-responsive hydrogels used as a temperature indicator. Polymer 2019, 173, 182–189. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Yao, N.; Wei, K.-K.; Jiang, L.; Hanif, S.; Wang, Z.-X.; Pei, C.-X. The efficacy and safety of probiotics for prevention of chemoradiotherapy-induced diarrhea in people with abdominal and pelvic cancer: A systematic review and meta-analysis. Eur. J. Clin. Nutr. 2016, 70, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Lu, C.; Gao, F.; Qian, Z.; Yin, Y.; Kan, S.; Chen, D. Selenium-enriched Bifidobacterium longum DD98 attenuates irinotecan-induced intestinal and hepatic toxicity in vitro and in vivo. Biomed. Pharmacother. 2021, 143, 112192. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, A.S.; Singh, N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology 2012, 291, 1–9. [Google Scholar] [CrossRef]
- Cuozzo, M.; Castelli, V.; Avagliano, C.; Cimini, A.; D’angelo, M.; Cristiano, C.; Russo, R. Effects of Chronic Oral Probiotic Treatment in Paclitaxel-Induced Neuropathic Pain. Biomedicines 2021, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-J.; An, J.; Ha, E.-M. Lactobacillus plantarum-derived metabolites sensitize the tumor-suppressive effects of butyrate by regulating the functional expression of SMCT1 in 5-FU-resistant colorectal cancer cells. J. Microbiol. 2021, 60, 100–117. [Google Scholar] [CrossRef]
- Teng, H.; Wang, Y.; Sui, X.; Fan, J.; Li, S.; Lei, X.; Shi, C.; Sun, W.; Song, M.; Wang, H.; et al. Gut microbiota-mediated nucleotide synthesis attenuates the response to neoadjuvant chemoradiotherapy in rectal cancer. Cancer Cell 2022, 41, 124–138.e6. [Google Scholar] [CrossRef] [PubMed]
- Dalal, N.; Jalandra, R.; Bayal, N.; Yadav, A.K.; Harshulika, N.; Sharma, M.; Makharia, G.K.; Kumar, P.; Singh, R.; Solanki, P.R.; et al. Gut microbiota-derived metabolites in CRC progression and causation. J. Cancer Res. Clin. Oncol. 2021, 147, 3141–3155. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Beckermann, K.E.; Johnson, D.B.; Das, S. Combining anti-cytotoxic T-lymphocyte antigen 4 (CTLA-4) and -programmed cell death protein 1 (PD-1) agents for cancer immunotherapy. Expert Opin. Biol. Ther. 2021, 21, 1623–1634. [Google Scholar] [CrossRef]
- Ahmed, J.; Kumar, A.; Parikh, K.; Anwar, A.; Knoll, B.M.; Puccio, C.; Chun, H.; Fanucchi, M.; Lim, S.H. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. OncoImmunology 2018, 7, e1507670. [Google Scholar] [CrossRef]
- Wu, M.; Huang, Q.; Xie, Y.; Wu, X.; Ma, H.; Zhang, Y.; Xia, Y. Improvement of the anticancer efficacy of PD-1/PD-L1 blockade via combination therapy and PD-L1 regulation. J. Hematol. Oncol. 2022, 15, 24. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Liu, T.; Xing, J.; Zhang, H.; Wang, D.; Tang, D. Bidirectional effects of intestinal microbiota and antibiotics: A new strategy for colorectal cancer treatment and prevention. J. Cancer Res. Clin. Oncol. 2022, 148, 2387–2404. [Google Scholar] [CrossRef]
- Deng, Y.; Hou, X.; Wang, H.; Du, H.; Liu, Y. Influence of Gut Microbiota-Mediated Immune Regulation on Response to Chemotherapy. Pharmaceuticals 2024, 17, 604. [Google Scholar] [CrossRef]
- Inamura, K. Roles of microbiota in response to cancer immunotherapy. Semin. Cancer Biol. 2020, 65, 164–175. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Yoon, Y.; Kim, G.; Jeon, B.-N.; Fang, S.; Park, H. Bifidobacterium Strain-Specific Enhances the Efficacy of Cancer Therapeutics in Tumor-Bearing Mice. Cancers 2021, 13, 957. [Google Scholar] [CrossRef]
- Kang, X.; Lau, H.C.-H.; Yu, J. Modulating gut microbiome in cancer immunotherapy: Harnessing microbes to enhance treatment efficacy. Cell Rep. Med. 2024, 5, 101478. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Cheng, S.; Kou, Y.; Wang, Z.; Jin, R.; Hu, H.; Zhang, X.; Gong, J.-F.; Li, J.; Lu, M.; et al. The Gut Microbiome Is Associated with Clinical Response to Anti–PD-1/PD-L1 Immunotherapy in Gastrointestinal Cancer. Cancer Immunol. Res. 2020, 8, 1251–1261. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Wang, D.; Long, J.; Yang, X.; Lin, J.; Song, Y.; Xie, F.; Xun, Z.; Wang, Y.; Wang, Y.; et al. Gut microbiome is associated with the clinical response to anti-PD-1 based immunotherapy in hepatobiliary cancers. J. Immunother. Cancer 2021, 9, e003334. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zheng, X.; Pan, T.; Yang, X.; Chen, X.; Zhang, B.; Peng, L.; Xie, C. Dynamic microbiome and metabolome analyses reveal the interaction between gut microbiota and anti-PD-1 based immunotherapy in hepatocellular carcinoma. Int. J. Cancer. 2022, 151, 1321–1334. [Google Scholar] [CrossRef] [PubMed]
- Ponziani, F.R.; De Luca, A.; Picca, A.; Marzetti, E.; Petito, V.; Del Chierico, F.; Reddel, S.; Sterbini, F.P.; Sanguinetti, M.; Putignani, L.; et al. Gut Dysbiosis and Fecal Calprotectin Predict Response to Immune Checkpoint Inhibitors in Patients with Hepatocellular Carcinoma. Hepatol. Commun. 2022, 6, 1492–1501. [Google Scholar] [CrossRef]
- Chung, M.-W.; Kim, M.-J.; Won, E.J.; Lee, Y.J.; Yun, Y.-W.; Cho, S.B.; Joo, Y.-E.; Hwang, J.-E.; Bae, W.K.; Chung, I.-J.; et al. Gut microbiome composition can predict the response to nivolumab in advanced hepatocellular carcinoma patients. World J. Gastroenterol. 2021, 27, 7340–7349. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, L.K.; Sharma, M.; Chandel, D.; Shukla, G. Prophylactic intervention of probiotics (L. acidophilus, L. rhamnosus GG) and celecoxib modulate Bax-mediated apoptosis in 1,2-dimethylhydrazine-induced experimental colon carcinogenesis. BMC Cancer 2018, 18, 1111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, J.; Xia, Q. Role of gut microbiome in cancer immunotherapy: From predictive biomarker to therapeutic target. Exp. Hematol. Oncol. 2023, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Dai, A.; Ghilardi, G.; Amelsberg, K.V.; Devlin, S.M.; Pajarillo, R.; Slingerland, J.B.; Beghi, S.; Herrera, P.S.; Giardina, P.; et al. Gut microbiome correlates of response and toxicity following anti-CD19 CAR T cell therapy. Nat. Med. 2022, 28, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Hu, M.; Sun, T.; Li, J.; Zhou, Y.; Yan, Y.; Xuan, B.; Wang, J.; Xiong, H.; Ji, L.; et al. Multi-kingdom gut microbiota analyses define bacterial-fungal interplay and microbial markers of pan-cancer immunotherapy across cohorts. Cell Host Microbe 2023, 31, 1930–1943.e4. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, D.; Yang, Y.; Jiang, X.; Zhang, J.; Zeng, X.; Wu, Z.; Sun, Y.; Guo, Y. Effect of Lactobacillus acidophilus CICC 6074 S-Layer Protein on Colon Cancer HT-29 Cell Proliferation and Apoptosis. J. Agric. Food Chem. 2020, 68, 2639–2647. [Google Scholar] [CrossRef]
- Peiffer, L.B.; White, J.R.; Jones, C.B.; Slottke, R.E.; Ernst, S.E.; Moran, A.E.; Graff, J.N.; Sfanos, K.S. Composition of gastrointestinal microbiota in association with treatment response in individuals with metastatic castrate resistant prostate cancer progressing on enzalutamide and initiating treatment with anti-PD-1 (pembrolizumab). Neoplasia 2022, 32, 100822. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Sun, Z.; Cao, Y.; Mu, Z.; Ji, X. Commensal microbiota contributes to predicting the response to immune checkpoint inhibitors in non-small-cell lung cancer patients. Cancer Sci. 2021, 112, 3005–3017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ferrero, M.; Dong, N.; D’auria, G.; Reyes-Prieto, M.; Herreros-Pomares, A.; Calabuig-Fariñas, S.; Duréndez, E.; Aparisi, F.; Blasco, A.; et al. Analysis of the Gut Microbiota: An Emerging Source of Biomarkers for Immune Checkpoint Blockade Therapy in Non-Small Cell Lung Cancer. Cancers 2021, 13, 2514. [Google Scholar] [CrossRef]
- Newsome, R.C.; Gharaibeh, R.Z.; Pierce, C.M.; da Silva, W.V.; Paul, S.; Hogue, S.R.; Yu, Q.; Antonia, S.; Conejo-Garcia, J.R.; Robinson, L.A.; et al. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. 2022, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.-F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef]
- Jiang, S.; Redelman-Sidi, G. BCG in Bladder Cancer Immunotherapy. Cancers 2022, 14, 3073. [Google Scholar] [CrossRef]
- De Lucia, M.; Cotugno, G.; Bignone, V.; Garzia, I.; Nocchi, L.; Langone, F.; Petrovic, B.; Sasso, E.; Pepe, S.; Froechlich, G.; et al. Retargeted and Multi-cytokine-Armed Herpes Virus Is a Potent Cancer Endovaccine for Local and Systemic Anti-tumor Treatment. Mol. Ther. Oncolytics 2020, 19, 253–264. [Google Scholar] [CrossRef]
- Wei, X.; Du, M.; Chen, Z.; Yuan, Z. Recent Advances in Bacteria-Based Cancer Treatment. Cancers 2022, 14, 4945. [Google Scholar] [CrossRef]
- Yadav, R.P.; Huo, C.; Budhathoki, R.; Budthapa, P.; Bhattarai, B.R.; Rana, M.; Kim, K.H.; Parajuli, N. Antibacterial, Antifungal, and Cytotoxic Effects of Endophytic Streptomyces Species Isolated from the Himalayan Regions of Nepal and Their Metabolite Study. Biomedicines 2024, 12, 2192. [Google Scholar] [CrossRef]
- Monu, M.; Sehrawat, K.D.; Singh, A.; Chaudhary, G.; Bamal, D.; Sehrawat, A.R. An overview on the therapeutic potential and anticancer mechanism of Monacolin K/Lovastatin. Pharmacol. Res. Mod. Chin. Med. 2022, 5, 100187. [Google Scholar] [CrossRef]
- Thomas, C.J.; Delgado, K.; Sawant, K.; Roy, J.; Gupta, U.; Song, C.S.; Poojary, R.; de Figueiredo, P.; Song, J. Harnessing Bacterial Agents to Modulate the Tumor Microenvironment and Enhance Cancer Immunotherapy. Cancers 2024, 16, 3810. [Google Scholar] [CrossRef]
- Lin, D.; Shen, Y.; Liang, T. Oncolytic virotherapy: Basic principles, recent advances and future directions. Signal Transduct. Target. Ther. 2023, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhu, X.; Wang, Y.; Lu, S. The gut microbiota improves the efficacy of immune-checkpoint inhibitor immunotherapy against tumors: From association to cause and effect. Cancer Lett. 2024, 598, 217123. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, D.C. Facing a new challenge. Chin. Med. J. 2019, 132, 1135–1138. [Google Scholar] [CrossRef]
- Wardill, H.R.; van der Aa, S.A.; Ferreira, A.R.d.S.; Havinga, R.; Tissing, W.J.; Harmsen, H.J. Antibiotic-induced disruption of the microbiome exacerbates chemotherapy-induced diarrhoea and can be mitigated with autologous faecal microbiota transplantation. Eur. J. Cancer 2021, 153, 27–39. [Google Scholar] [CrossRef]
- Janney, A.; Powrie, F.; Mann, E.H. Host–microbiota maladaptation in colorectal cancer. Nature 2020, 585, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; Rinaldi, V.; Hassan, C.; Diana, F.; Winn, S.; Castagna, G.; Attili, A.F. Ascorbic acid and intestinal metaplasia in the stomach: A prospective, randomized study. Aliment. Pharmacol. Ther. 2000, 14, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.D.; Meng, X.J.; Shi, Y.; Hu, Y.B.; Zhu, S.S.; Wang, C.W. Interventional study of high dose folic acid in gastric carcinogenesis in beagles. Gut 2002, 50, 61–64. [Google Scholar] [CrossRef]
- Gail, M.H.; Brown, L.M.; You, W.-C. Re: Chemoprevention of Gastric Dysplasia: Randomized Trial of Antioxidant Supplements and Anti-Helicobacter pylori Therapy. JNCI J. Natl. Cancer Inst. 2001, 93, 559. [Google Scholar] [CrossRef] [PubMed]
- You, W.-C.; Brown, L.M.; Zhang, L.; Li, J.-Y.; Jin, M.-L.; Chang, Y.-S.; Ma, J.-L.; Pan, K.-F.; Liu, W.-D.; Hu, Y.; et al. Randomized Double-Blind Factorial Trial of Three Treatments To Reduce the Prevalence of Precancerous Gastric Lesions. JNCI J. Natl. Cancer Inst. 2006, 98, 974–983. [Google Scholar] [CrossRef]
- Stavrou, G. Gut microbiome, surgical complications and probiotics. Ann. Gastroenterol. 2016, 30, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Doublier, S.; Cirrincione, S.; Scardaci, R.; Botta, C.; Lamberti, C.; Di Giuseppe, F.; Angelucci, S.; Rantsiou, K.; Cocolin, L.; Pessione, E. Putative probiotics decrease cell viability and enhance chemotherapy effectiveness in human cancer cells: Role of butyrate and secreted proteins. Microbiol. Res. 2022, 260, 127012. [Google Scholar] [CrossRef]
- Lee, K.A.; Shaw, H.M.; Bataille, V.; Nathan, P.; Spector, T.D. Role of the gut microbiome for cancer patients receiving immunotherapy: Dietary and treatment implications. Eur. J. Cancer 2020, 138, 149–155. [Google Scholar] [CrossRef]
- Yao, M.; Xie, J.; Du, H.; McClements, D.J.; Xiao, H.; Li, L. Progress in microencapsulation of probiotics: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 857–874. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Motoori, M.; Yano, M.; Miyata, H.; Sugimura, K.; Saito, T.; Omori, T.; Fujiwara, Y.; Miyoshi, N.; Akita, H.; Gotoh, K.; et al. Randomized study of the effect of synbiotics during neoadjuvant chemotherapy on adverse events in esophageal cancer patients. Clin. Nutr. 2015, 36, 93–99. [Google Scholar] [CrossRef]
- Hansen, S.L.; Purup, S.; Christensen, L.P. Bioactivity of falcarinol and the influenceof processing and storage on its content in carrots (Daucus carota L.). J. Sci. Food Agric. 2003, 83, 1010–1017. [Google Scholar] [CrossRef]
- Zaini, R.G.; Brandt, K.; Clench, M.R.; Le Maitre, C.L. Effects of Bioactive Compounds from Carrots (Daucus carota L.), Polyacetylenes, Beta-Carotene and Lutein on Human Lymphoid Leukaemia Cells. Anti-Cancer Agents Med. Chem. 2012, 12, 640–652. [Google Scholar] [CrossRef] [PubMed]
- Tarifa, M.C.; Piqueras, C.M.; Genovese, D.B.; Brugnoni, L.I. Microencapsulation of Lactobacillus casei and Lactobacillus rhamnosus in pectin and pectin-inulin microgel particles: Effect on bacterial survival under storage conditions. Int. J. Biol. Macromol. 2021, 179, 457–465. [Google Scholar] [CrossRef]
- Fukaya, M.; Yokoyama, Y.; Usui, H.; Fujieda, H.; Sakatoku, Y.; Takahashi, T.; Miyata, K.; Niikura, M.; Sugimoto, T.; Asahara, T.; et al. Impact of synbiotics treatment on bacteremia induced during neoadjuvant chemotherapy for esophageal cancer: A randomised controlled trial. Clin. Nutr. 2021, 40, 5781–5791. [Google Scholar] [CrossRef] [PubMed]
- Zaharuddin, L.; Mokhtar, N.M.; Nawawi, K.N.M.; Ali, R.A.R. A randomized double-blind placebo-controlled trial of probiotics in post-surgical colorectal cancer. BMC Gastroenterol. 2019, 19, 131. [Google Scholar] [CrossRef]
- Mantzourani, I.; Chondrou, P.; Bontsidis, C.; Karolidou, K.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Galanis, A.; Plessas, S. Assessment of the probiotic potential of lactic acid bacteria isolated from kefir grains: Evaluation of adhesion and antiproliferative properties in in vitro experimental systems. Ann. Microbiol. 2019, 69, 751–763. [Google Scholar] [CrossRef]
- Dubey, V.; Ghosh, A.R.; Bishayee, K.; Khuda-Bukhsh, A.R. Appraisal of the anti-cancer potential of probiotic Pediococcus pentosaceus GS4 against colon cancer: In vitro and in vivo approaches. J. Funct. Foods 2016, 23, 66–79. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Chu, S.-H.; Jeon, J.Y.; Lee, M.-K.; Park, J.-H.; Lee, D.-C.; Lee, J.-W.; Kim, N.-K. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: A double-blind, randomized, placebo-controlled trial. Dig. Liver Dis. 2014, 46, 1126–1132. [Google Scholar] [CrossRef]
- Al-Nabulsi, A.A.; Jaradat, Z.W.; Al Qudsi, F.R.; Elsalem, L.; Osaili, T.M.; Olaimat, A.N.; Esposito, G.; Liu, S.-Q.; Ayyash, M.M. Characterization and bioactive properties of exopolysaccharides produced by Streptococcus thermophilus and Lactobacillus bulgaricus isolated from labaneh. LWT 2022, 167, 113817. [Google Scholar] [CrossRef]
- Lee, H.A.; Kim, H.; Lee, K.-W.; Park, K.-Y. Dead Nano-Sized Lactobacillus plantarum Inhibits Azoxymethane/Dextran Sulfate Sodium-Induced Colon Cancer in Balb/c Mice. J. Med. Food 2015, 18, 1400–1405. [Google Scholar] [CrossRef] [PubMed]
- McNabney, S.M.; Henagan, T.M. Short Chain Fatty Acids in the Colon and Peripheral Tissues: A Focus on Butyrate, Colon Cancer, Obesity and Insulin Resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef]
- Song, M.W.; Chung, Y.; Kim, K.-T.; Hong, W.S.; Chang, H.J.; Paik, H.-D. Probiotic characteristics of Lactobacillus brevis B13-2 isolated from kimchi and investigation of antioxidant and immune-modulating abilities of its heat-killed cells. LWT 2020, 128, 109452. [Google Scholar] [CrossRef]
- Ardestani, S.K.; Tafvizi, F.; Ebrahimi, M.T. Heat-killed probiotic bacteria induce apoptosis of HT-29 human colon adenocarcinoma cell line via the regulation of Bax/Bcl2 and caspases pathway. Hum. Exp. Toxicol. 2019, 38, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Keller, D.S.; Windsor, A.; Cohen, R.; Chand, M. Colorectal cancer in inflammatory bowel disease: Review of the evidence. Tech. Coloproctol. 2019, 23, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Tiptiri-Kourpeti, A.; Spyridopoulou, K.; Santarmaki, V.; Aindelis, G.; Tompoulidou, E.; Lamprianidou, E.E.; Saxami, G.; Ypsilantis, P.; Lampri, E.S.; Simopoulos, C.; et al. Lactobacillus casei Exerts Anti-Proliferative Effects Accompanied by Apoptotic Cell Death and Up-Regulation of TRAIL in Colon Carcinoma Cells. PLoS ONE 2016, 11, e0147960. [Google Scholar] [CrossRef]
- Yu, H.; Li, X.X.; Han, X.; Chen, B.X.; Zhang, X.H.; Gao, S.; Xu, D.-Q.; Wang, Y.; Wang, Y.; Gao, Z.-K.; et al. Fecal microbiota transplantation inhibits colorectal cancer progression: Reversing intestinal microbial dysbiosis to enhance anti-cancer immune responses. Front. Microbiol. 2023, 14, 1126808. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, T.; Wang, Y.; Gao, Y.; Kong, Y.; Liu, Z.; Deng, X. A randomised trial of probiotics to reduce severity of physiological and microbial disorders induced by partial gastrectomy for patients with gastric cancer. J. Cancer 2019, 10, 568–576. [Google Scholar] [CrossRef]
- Nekouian, R.; Rasouli, B.S.; Ghadimi-Darsajini, A.; Iragian, G.-R. In vitro activity of probiotic Lactobacillus reuteri against gastric cancer progression by downregulation of urokinase plasminogen activator/urokinase plasminogen activator receptor gene expression. J. Cancer Res. Ther. 2017, 13, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Concina, F.; Bertuccio, P.; Fiori, F.; Parpinel, M.; Garavello, W.; Crispo, A.; Libra, M.; Negri, E.; Serraino, D.; et al. Prebiotics and the Risk of Upper Digestive Tract and Stomach Cancers: The PrebiotiCa Study. J. Acad. Nutr. Diet. 2023, 123, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Besselink, M.G.H.; van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witteman, B.J.M.; et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Munakata, S.; Arakawa, C.; Kohira, R.; Fujita, Y.; Fuchigami, T.; Mugishima, H. A case of D-lactic acid encephalopathy associated with use of probiotics. Brain Dev. 2009, 32, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Salminen, M.K.; Rautelin, H.; Tynkkynen, S.; Poussa, T.; Saxelin, M.; Valtonen, V.; Järvinen, A. Lactobacillus Bacteremia, Clinical Significance, and Patient Outcome, with Special Focus on Probiotic, L. rhamnosus GG. Clin. Infect. Dis. 2004, 38, 62–69. [Google Scholar] [CrossRef]
- Rubin, I.M.C.; Stevnsborg, L.; Mollerup, S.; Petersen, A.M.; Pinholt, M. Bacteraemia caused by Lactobacillus rhamnosus given as a probiotic in a patient with a central venous catheter: A WGS case report. Infect. Prev. Pr. 2022, 4, 100200. [Google Scholar] [CrossRef]
- Salminen, M.K.; Tynkkynen, S.; Rautelin, H.; Saxelin, M.; Vaara, M.; Ruutu, P.; Sarna, S.; Valtonen, V.; Järvinen, A. Lactobacillus Bacteremia during a Rapid Increase in Probiotic Use of Lactobacillus rhamnosus GG in Finland. Clin. Infect. Dis. 2002, 35, 1155–1160. [Google Scholar] [CrossRef]
- Mikucka, A.; Deptuła, A.; Bogiel, T.; Chmielarczyk, A.; Nurczyńska, E.; Gospodarek-Komkowska, E. Bacteraemia Caused by Probiotic Strains of Lacticaseibacillus rhamnosus—Case Studies Highlighting the Need for Careful Thought before Using Microbes for Health Benefits. Pathogens 2022, 11, 977. [Google Scholar] [CrossRef] [PubMed]
- Kochan, P.; Chmielarczyk, A.; Szymaniak, L.; Brykczynski, M.; Galant, K.; Zych, A.; Pakosz, K.; Giedrys-Kalemba, S.; Lenouvel, E.; Heczko, P.B. Lactobacillus rhamnosus administration causes sepsis in a cardiosurgical patient—Is the time right to revise probiotic safety guidelines? Clin. Microbiol. Infect. 2011, 17, 1589–1592. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Su, J.; Liu, C.; Mai, Z.; Yin, S.; Yang, C.; Fu, L. Human microbiomes in cancer development and therapy. Medcomm 2023, 4, e221. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Feng, Y.; Li, L.; Kong, F.; Gao, J.; Kong, X. Engineered bacterial outer membrane vesicles: A versatile bacteria-based weapon against gastrointestinal tumors. Theranostics 2024, 14, 761–787. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; He, J.; Cheng, H.; Zhu, Z.; Xu, H. Enhanced therapeutic effect of an antiangiogenesis peptide on lung cancer in vivo combined with salmonella VNP20009 carrying a Sox2 shRNA construct. J. Exp. Clin. Cancer Res. 2016, 35, 107. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, S.; Esmaeili, A.; Omidi, Y. Anti-cancer properties of Escherichia coli Nissle 1917 against HT-29 colon cancer cells through regulation of Bax/Bcl-xL and AKT/ PTEN signaling pathways. Iran J. Basic Med. Sci. 2020, 23, 886–893. [Google Scholar]
- Li, R.; Helbig, L.; Fu, J.; Bian, X.; Herrmann, J.; Baumann, M.; Stewart, A.F.; Müller, R.; Li, A.; Zips, D.; et al. Expressing cytotoxic compounds in Escherichia coli Nissle 1917 for tumor-targeting therapy. Res. Microbiol. 2019, 170, 74–79. [Google Scholar] [CrossRef]
- Wang, H.; Bastian, S.E.P.; Lawrence, A.; Howarth, G.S. Factors Derived from Escherichia coli Nissle 1917, Grown in Different Growth Media, Enhance Cell Death in a Model of 5-Fluorouracil-Induced Caco-2 Intestinal Epithelial Cell Damage. Nutr. Cancer 2015, 67, 316–326. [Google Scholar] [CrossRef]
- Ho, C.L.; Tan, H.Q.; Chua, K.J.; Kang, A.; Lim, K.H.; Ling, K.L.; Yew, W.S.; Lee, Y.S.; Thiery, J.P.; Chang, M.W. Engineered commensal microbes for diet-mediated colorectal-cancer chemoprevention. Nat. Biomed. Eng. 2018, 2, 27–37. [Google Scholar] [CrossRef]
- Gurbatri, C.R.; Radford, G.A.; Vrbanac, L.; Im, J.; Thomas, E.M.; Coker, C.; Taylor, S.R.; Jang, Y.; Sivan, A.; Rhee, K.; et al. Engineering tumor-colonizing E. coli Nissle 1917 for detection and treatment of colorectal neoplasia. Nat. Commun. 2024, 15, 646. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-J.; Hong, Y.-H. In situ delivery of biobutyrate by probiotic Escherichia coli for cancer therapy. Sci. Rep. 2021, 11, 18172. [Google Scholar] [CrossRef] [PubMed]
- Fluckiger, A.; Daillère, R.; Sassi, M.; Sixt, B.S.; Liu, P.; Loos, F.; Richard, C.; Rabu, C.; Alou, M.T.; Goubet, A.-G.; et al. Cross-reactivity between tumor MHC class I–restricted antigens and an enterococcal bacteriophage. Science 2020, 369, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Bronte, V.; Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nat. Rev. Immunol. 2005, 5, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Canale, F.P.; Basso, C.; Antonini, G.; Perotti, M.; Li, N.; Sokolovska, A.; Neumann, J.; James, M.J.; Geiger, S.; Jin, W.; et al. Metabolic modulation of tumours with engineered bacteria for immunotherapy. Nature 2021, 598, 662–666. [Google Scholar] [CrossRef]
- Shimizu, T.; Ohtani, K.; Hirakawa, H.; Ohshima, K.; Yamashita, A.; Shiba, T.; Ogasawara, N.; Hattori, M.; Kuhara, S.; Hayashi, H. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. USA 2002, 99, 996–1001. [Google Scholar] [CrossRef]
- Pahle, J.; Menzel, L.; Niesler, N.; Kobelt, D.; Aumann, J.; Rivera, M.; Walther, W. Rapid eradication of colon carcinoma by Clostridium perfringens Enterotoxin suicidal gene therapy. BMC Cancer 2017, 17, 129. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.-B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Wang, K.; Oppong-Gyebi, A.; Hu, J. Application of Nanotechnology in Cancer Diagnosis and Therapy—A Mini-Review. Int. J. Med. Sci. 2020, 17, 2964–2973. [Google Scholar] [CrossRef]
- Song, W.; Tiruthani, K.; Wang, Y.; Shen, L.; Hu, M.; Dorosheva, O.; Qiu, K.; Kinghorn, K.A.; Liu, R.; Huang, L. Trapping of Lipopolysaccharide to Promote Immunotherapy against Colorectal Cancer and Attenuate Liver Metastasis. Adv. Mater. 2018, 30, e1805007. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.-H.; Bao, Y.; Du, X.-J.; Tan, Z.-B.; Jiang, Q.; Wang, H.-X.; Zhu, Y.-H.; Wang, J. Differential Anticancer Drug Delivery with a Nanogel Sensitive to Bacteria-Accumulated Tumor Artificial Environment. ACS Nano 2013, 7, 10636–10645. [Google Scholar] [CrossRef]
| Cancer-Promoting Microbe (Genus Level) | Phylum | Ref. | Pathogenesis/Mechanism Linked to Gastric Cancer | Ref. |
|---|---|---|---|---|
| Helicobacter pylori | Proteobacteria | [57,61,62,63] |
| [45] |
| Escherichia/Shigella | Proteobacteria | [61,64,65] | Produce N-nitroso toxins generate DNA double-strand breaks within the host’s epithelial cells, thus promoting a transient cell cycle arrest, allowing for genomic mutations to arise, and finally leading to tumor formation. | [66] |
| Campylobacter | Proteobacteria | [63,67] | Produce N-nitroso toxins. | [56] |
| Enterococcus | Firmicutes | [61,64] | Produce reactive oxygen species (ROS) and reduced expression of the MMR gene involved in DNA repair. | [68] |
| Staphylococcus | Firmicutes | [69] | Produce N-nitroso toxins. | [70] |
| Streptococcus | Firmicutes | [48,57,63,71,72,73] | Produce N-nitroso toxins. Produce Short-chain fatty acids (SCFAs)—Acetic acid. | [63] |
| Lactobacillus | Firmicutes | [57,61,67,72,73] | Produce N-nitroso compounds. Produce Short-chain fatty acids (SCFAs). | [52,70] |
| Megasphaera | Firmicutes | [72,74] | --- | |
| Veillonella | Firmicutes | [48,57,63,67,71,72] | Produce N-nitroso toxins. | [56] |
| Clostridium | Firmicutes | [75] | Produce N-nitroso toxins. | [75] |
| Peptostreptococcus | Firmicutes | [63] | Induces the activation of the PI3K–Akt pathway, leading to increased cell proliferation and (NF-κB) activation, and triggers a pro-inflammatory response. | [76] |
| Lactococcus | Firmicutes | [54] | Lactic acid-producing bacteria produce reactive oxygen species (ROS), which induce DNA damage, reduce nitrate to nitrite, drive the activation of oncogenes, enhance angiogenesis, and inhibit apoptosis. | [54] |
| Parvimonas | Firmicutes | [63] | Enhanced secretion of inflammatory cytokines l-23 and Il-17. | [77] |
| Bulleidia | Firmicutes | [73] | --- | |
| Haemophilus | Pseudomonadota | [48,67,71,73] | Produce N-nitroso toxins. | [56] |
| Pasturellaceae | Pseudomonadota | [48,71] | Produce N-nitroso toxins. | [56] |
| Neisseria | Pseudomonadota | [63,73] | Produce N-nitroso toxins. | [56] |
| Leptotrichia | Fusobacteriota | [67] | Produce endotoxins which activate pro-inflammatory interleukins (IL)-1β, IL-6, IL-8, and IL-10. | [78] |
| Fusobacterium | Fusobacteriota | [57,65,67,72] | FadA adhesin, which binds to E-cadherin, activates β-catenin signaling and accordingly, various inflammatory pathways. | [60,79] |
| Actinomyces | Actinobacteria | [74] | Produce N-nitroso toxins. | [56] |
| Rothia | Actinobacteria | [48,71] | Produce N-nitroso toxins. | [55] |
| Bacteroides | Bacteroidetes | [65,74] | --- | [80] |
| Prevotella | Bacteroidetes | [61,72,73] | Produce N-nitroso toxins. Produce Short-chain fatty acids (SCFAs)—Acetic acid. | [56] |
| Nitrospirae | Nitrospirota | [81] | Produce N-nitroso toxins. | [56] |
| Dialister | Bacillota | [82] | --- | |
| Granulicatella | Bacillota | [82] | --- | |
| Herbaspirillum | Pseudomonadota | [82] | --- | |
| Comamonas | Pseudomonadota | [82] | --- | |
| Chryseobacterium | Bacteroidota | [82] | --- |
| NCT Number | Study Type | Types of Cancer | Purpose | Patients (n) (Adult) | Intervention Model Description | Study Status |
|---|---|---|---|---|---|---|
| NCT06088940 | An interventional double-blinded, placebo-controlled, randomized study; parallel assignment | Cancer | To compare the effects of probiotics versus placebo on gut bacteria and their correlation with gastrointestinal and psychosocial functions. | 66 | Survivors of cancer will receive one probiotic (strains of Lactobacillus and Bifidobacterium) and one placebo (maltodextrin) capsule every day for 12 weeks. The effect of probiotics on diarrhea/gas/bloating/anxiety/fatigue symptoms and cognitive function will be observed. | Not yet recruiting |
| NCT06022822 | Interventional, randomized placebo-controlled trial | Prostate cancer | This phase 2 randomized control trial assesses the effect of Uro-A supplementation compared to placebo in men with biopsy-confirmed prostate cancer undergoing radical prostatectomy progressive disease. | 90 | The primary endpoint will be analyzed using a linear regression model. | Recruiting or phase 2 |
| NCT05349227 | Crossover assignment in an open-label, interventional randomized research | Breast cancer, lung cancer, stomach cancer, ovarian cancer, and lung cancer | To assess the gut microbiota in fecal samples at the beginning of the trial and six months after enrollment, as well as to track changes in depression, cognitive performance or impairment, and sleep-related impairment. | 625 | Individuals will be assigned to groups that will be monitored by wrist-worn devices and receive either six months of digital coaching right after or six months of monitoring and digital health coaching thereafter. | Recruiting |
| NCT04700527 | A Randomized Controlled Study | Cancer | To assess and compare gastrointestinal toxicity from radiation therapy between subjects who receive therapeutic short-chain fatty acid and those who receive placebo, identifying a safe, low-cost therapeutic to reduce gastrointestinal toxicity from therapeutic or environmental radiation. | 122 | GI toxicities (PRO-CTCAE v5 for patients and CTCAE v5 for physicians) will be recorded and compared between the 2 groups to identify any differences. | Recruiting/phase 2 |
| NCT06039644 | Parallel Assignment, Randomized Controlled Trial | Breast Cancer | To explore after consumption of probiotics of lactobacillus composite strain powder sachets for 6 months in BC chemotherapy, and whether it assists patients in alleviating the side effects of chemotherapy. | 100 | The questionnaire will be finished to record within 24 weeks the side effects, including nausea, vomiting, diarrhea, stomatitis, peripheral neuropathy, skin rashes, and hand-food syndrome before and after the treatment. | Recruiting |
| NCT04775355 | An observational prospective study | Prostate cancer | To examine the gut microbial population after radiation and androgen deprivation therapy and identify alterations linked to post-treatment toxicity. | 30 | Questionnaires will be filled out by participants prior to, during, and following radiation treatment. | Recruiting |
| NCT05112614 | An observational prospective study | Cancer | This study examines how gut microbiome can affect cancer therapy in patients with cancer undergoing cancer therapy or stem cell transplantation. Information from this study may help medical professionals improve the way cancer treatment condition is delivered and increase its efficacy and success. | 5000 | Will be performed using the Shogun pipeline for metagenomics data followed by analysis using QIIME 2.0. | Recruiting |
| Gut Microbiome | Experimental Model | Chemotherapy Regimen | Types of Cancer | Effects | Mechanism | Ref. | |
|---|---|---|---|---|---|---|---|
| Efficacy | Toxicity | ||||||
| Fusobacterium nucleatum | Mice | Oxaliplatin and 5-fluorouracil | Colorectal cancer | ↓ | - | Inducing autophagy activation to promote chemoresistance in a TLR4/MYD88-dependent manner; upregulating BIRC3 that directly inhibited caspase cascade | [90] |
| Mycoplasma hyorhinis | Mice | Gemcitabine | Breast cancer | ↓ | - | Metabolizing GEM into inactive form by deamination | [95] |
| Akkermansia muciniphila Lactobacillus | Mice | Doxorubicin | Breast cancer | ↑ | - | - | [96] |
| Bifidobacterium longum | Mice | Irinotecan | Irinotecan-induced diarrhea | - | ↓ | Decreasing the pro-inflammatory cytokines IL-1b and IL-18 | [102] |
| Probiotic Mixture Slab51® | Mice Stool sample | Paclitaxel | Paclitaxel-induced neuropathy | - | ↓ | Modulating the serum pro-inflammatory cytokine concentration | [104] |
| Lactobacillus plantarum | Bacterial Cell culture | Colorectal cancer | ↑ | - | Secreting metabolites to increase the expression of the butyrate transporter | [105] | |
| Bacteroides vulgatus | Mice | 5-fluorouracil | Colorectal cancer | ↑ | - | Decreasing the abundance of F. nucleatum, and more efficient capacity of DNA repair | [106] |
| Cancer Type | Immunotherapy | Microbes Enriched in Responders | Sequencing Methods | Patients (n) | Ref. |
|---|---|---|---|---|---|
| Gastrointestinal | anti-PD-1/PD-L1 | Prevotella/Bacteroides ratio, Prevotellaceae, Ruminococcaceae, Lachnospiraceae, Eubacterium, Lactobacillus, and Streptococcus | 16S rRNA (V3-V4) and metagenomics | 74 adults | [118] |
| Hepatobiliary | anti-PD-1 | Lachnospiraceae bacterium GAM79, Alistipes sp. Marseille-P5997, Ruminococcus calidus, and Erysipelotichaceae bacterium GAM147 | metagenomic | 65 adults | [119] |
| HCC | anti-PD-1 | Faecalibacterium, Blautia, Lachnospiracea incertae Sedis, Megamonas, Ruminococcus, Coprococcus, Dorea, and Haemophilus | 16S rRNA (V3-V4) | 35 adults | [120] |
| HCC | nivoluma | Akkermansia | 16S rDNA (V3–V4) | 11 | [121,122] |
| HCC | camrelizumab | Akkermansia muciniphila and Ruminococcaceae spp. | 16S rDNA (V3–V4) | 11 | [121,122] |
| Thoracic carcinoma | anti-PD-1 | Akkermansiaceae, Enterococcaceae, Enterobacteriaceae, Carnobacteriaceae, and Clostridiales Family XI | 16S sRNA (V4) | 42 | [123,124] |
| B cell lymphoma | anti-CD19 CAR-T | Bacteroides, Ruminococcus, Eubacterium, and Akkerman | metagenomic | 172 | [125] |
| Pan-cancer | immune checkpoint inhibitors | Trichophyton benhamiae, Cryptococcus amylolentus, Suillus clintonianus, Pseudogymnoascus sp. 05NY08, Schizosaccharomyces octosporus, Podospora anserina, and Verticillium longisporum | metagenomic | 862 | [126,127] |
| Prostate Cancer | pembrolizumab | Streptococcus | 16S rRNA and qPCR | 23 | [128] |
| NSCLC | anti-PD-1/PD-L1 | Desulfovibrio, Bifidobacterium, Anaerostipes, Faecalibacterium, and Alistipes | 16S rRNA (V3-V4) | 75 | [129] |
| NSCLC | immune checkpoint inhibitors | Phascolarctobacterium | 16S rRNA (V3-V4) | 69 | [130,131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Matouq, J.; Al-Ghafli, H.; Alibrahim, N.N.; Alsaffar, N.; Radwan, Z.; Ali, M.D. Unveiling the Interplay Between the Human Microbiome and Gastric Cancer: A Review of the Complex Relationships and Therapeutic Avenues. Cancers 2025, 17, 226. https://doi.org/10.3390/cancers17020226
Al-Matouq J, Al-Ghafli H, Alibrahim NN, Alsaffar N, Radwan Z, Ali MD. Unveiling the Interplay Between the Human Microbiome and Gastric Cancer: A Review of the Complex Relationships and Therapeutic Avenues. Cancers. 2025; 17(2):226. https://doi.org/10.3390/cancers17020226
Chicago/Turabian StyleAl-Matouq, Jenan, Hawra Al-Ghafli, Noura N. Alibrahim, Nida Alsaffar, Zaheda Radwan, and Mohammad Daud Ali. 2025. "Unveiling the Interplay Between the Human Microbiome and Gastric Cancer: A Review of the Complex Relationships and Therapeutic Avenues" Cancers 17, no. 2: 226. https://doi.org/10.3390/cancers17020226
APA StyleAl-Matouq, J., Al-Ghafli, H., Alibrahim, N. N., Alsaffar, N., Radwan, Z., & Ali, M. D. (2025). Unveiling the Interplay Between the Human Microbiome and Gastric Cancer: A Review of the Complex Relationships and Therapeutic Avenues. Cancers, 17(2), 226. https://doi.org/10.3390/cancers17020226






