Phase II Study of Nanoliposomal Irinotecan (Nal-IRI) with 5-Fluorouracil and Leucovorin in Refractory Advanced High-Grade Neuroendocrine Cancer of Gastroenteropancreatic (GEP) or Unknown Origin
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Study Design/Treatment
2.3. Safety Monitoring
2.4. Efficacy
2.5. Quality of Life (QoL)
2.6. Statistics
3. Results
3.1. Patient Characteristics
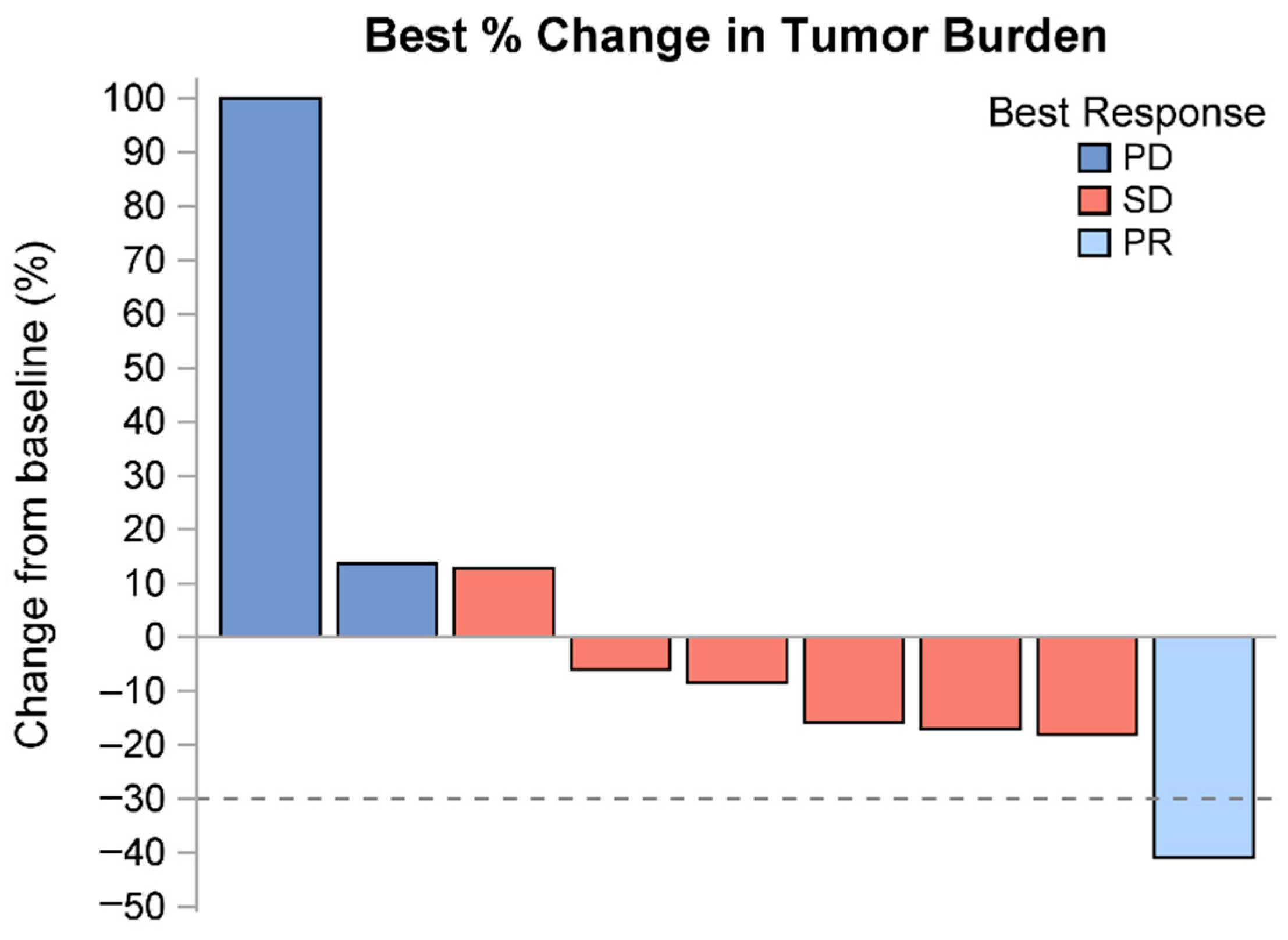
3.2. Treatment Response
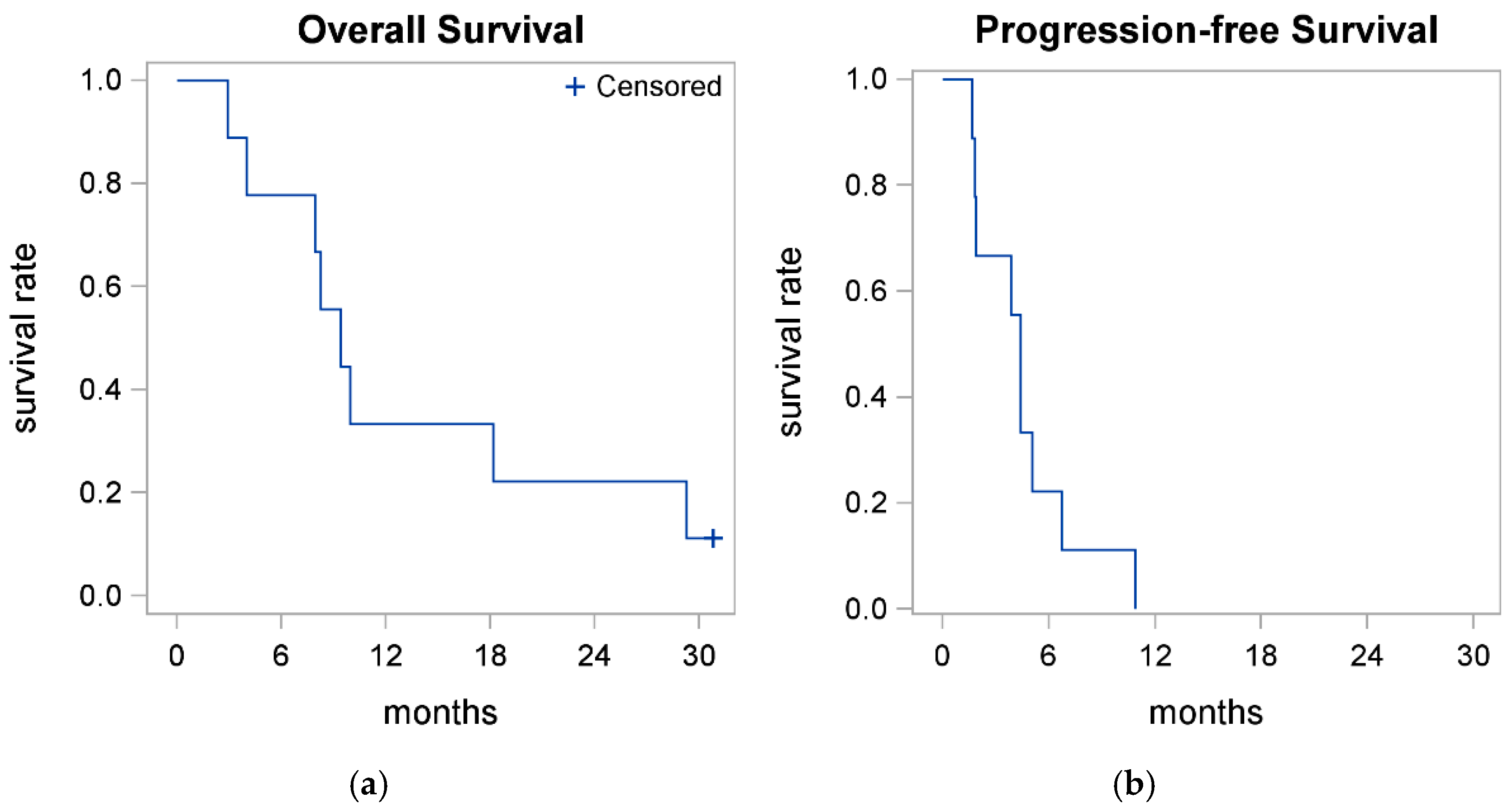
3.3. Treatment Efficacy-Survival
3.4. Exploratory Mutational Analysis
3.5. Safety
3.6. Quality of Life
4. Discussion
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rossi, R.E.; Massironi, S. The Increasing Incidence of Neuroendocrine Neoplasms Worldwide: Current Knowledge and Open Issues. J. Clin. Med. 2022, 11, 3794. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dasari, A. Epidemiology, Incidence, and Prevalence of Neuroendocrine Neoplasms: Are There Global Differences? Curr. Oncol. Rep. 2021, 23, 43. [Google Scholar] [CrossRef]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients with Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Mollazadegan, K.; Welin, S.; Crona, J. Systemic Treatment of Gastroenteropancreatic Neuroendocrine Carcinoma. Curr. Treat. Options Oncol. 2021, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Taherifard, E.; Bakhtiar, M.; Mahnoor, M.; Ahmed, R.; Cavalcante, L.; Zhang, J.; Saeed, A. Efficacy and safety of temozolomide-based regimens in advanced pancreatic neuroendocrine tumors: A systematic review and meta-analysis. BMC Cancer 2024, 24, 192. [Google Scholar] [CrossRef] [PubMed]
- Sorbye, H.; Welin, S.; Langer, S.W.; Vestermark, L.W.; Holt, N.; Osterlund, P.; Dueland, S.; Hofsli, E.; Guren, M.G.; Ohrling, K.; et al. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): The NORDIC NEC study. Ann. Oncol. 2012, 24, 152–160. [Google Scholar] [CrossRef]
- Eads, J.R.; Catalano, P.J.; Fisher, G.A.; Rubin, D.; Iagaru, A.; Klimstra, D.S.; Konda, B.; Kwong, M.S.; Chan, J.A.; De Jesus-Acosta, A.; et al. Randomized phase II study of platinum and etoposide (EP) versus temozolomide and capecitabine (CAPTEM) in patients (pts) with advanced G3 non-small cell gastroenteropancreatic neuroendocrine neoplasms (GEPNENs): ECOG-ACRIN EA2142. J. Clin. Oncol. 2022, 40, 4020. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Zhen, D.B.; Mayerson, E.; Chiorean, E.G.; Burgess, E.F.; Swisher, E.M.; Gay, C.M.; Byers, L.A.; Wistuba, I.I.; Mahdi, H.; Das, S.; et al. SWOG S2012: Randomized phase II/III trial of first line platinum/etoposide (P/E) with or without atezolizumab (NSC#783608) in patients (pts) with poorly differentiated extrapulmonary small cell neuroendocrine carcinomas (NEC). J. Clin. Oncol. 2022, 40, TPS4179. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Spigel, D.R.; Chen, Y.; Jove, M.; Juan-Vidal, O.; Rich, P.; Hayes, T.; Calderón, V.G.; Caro, R.B.; Navarro, A.; et al. RESILIENT part 1: A phase 2 dose-exploration and dose-expansion study of second-line liposomal irinotecan in adults with small cell lung cancer. Cancer 2022, 128, 1801–1811. [Google Scholar] [CrossRef]
- Morizane, C.; Machida, N.; Honma, Y.; Okusaka, T.; Boku, N.; Kato, K.; Nomura, S.; Hiraoka, N.; Sekine, S.; Taniguchi, H.; et al. Etoposide and Cisplatin vs. Irinotecan and Cisplatin for Neuroendocrine Carcinoma of the Digestive System. JAMA Oncol. 2022, 8, 1447–1455. [Google Scholar] [CrossRef]
- Hoskins, J.M.; Goldberg, R.M.; Qu, P.; Ibrahim, J.G.; McLeod, H.L. UGT1A1*28 Genotype and Irinotecan-Induced Neutropenia: Dose Matters. JNCI J. Natl. Cancer Inst. 2007, 99, 1290–1295. [Google Scholar] [CrossRef]
- Hulshof, E.C.; de With, M.; de Man, F.M.; Creemers, G.-J.; Deiman, B.A.; Swen, J.J.; Houterman, S.; Koolen, S.L.; Bins, S.; Thijs, A.M.; et al. UGT1A1 genotype-guided dosing of irinotecan: A prospective safety and cost analysis in poor metaboliser patients. Eur. J. Cancer 2022, 162, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Milano, G.; Innocenti, F.; Minami, H. Liposomal irinotecan (Onivyde): Exemplifying the benefits of nanotherapeutic drugs. Cancer Sci. 2022, 113, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Adiwijaya, B.; Lang, I.; Csõszi, T.; Cubillo, A.; Chen, J.; Wong, M.; Park, J.; Kim, J.; Rau, K.; Melichar, B.; et al. Population Pharmacokinetics of Liposomal Irinotecan in Patients with Cancer. Clin. Pharmacol. Ther. 2017, 102, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Khan, S.; Wang, Y.F.; Kamath, N.; Sarkar, A.K.; Ahmad, A.; Sheikh, S.; Ali, S.; Carbonaro, D.; Zhang, A.; et al. Preclinical Safety, Pharmacokinetics and Antitumor Efficacy Profile of Liposome-entrapped SN-38 Formulation. Anticancer. Res. 2005, 25, 331–341. [Google Scholar] [PubMed]
- Wang-Gillam, A.; Li, C.-P.; Bodoky, G.; Dean, A.; Shan, Y.-S.; Jameson, G.; Macarulla, T.; Lee, K.-H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): A global, randomised, open-label, phase 3 trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Hentic, O.; Hammel, P.; Couvelard, A.; Rebours, V.; Zappa, M.; Palazzo, M.; Maire, F.; Goujon, G.; Gillet, A.; Lévy, P.; et al. FOLFIRI regimen: An effective second-line chemotherapy after failure of etoposide–platinum combination in patients with neuroendocrine carcinomas grade 3. Endocr. -Relat. Cancer 2012, 19, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://qol.eortc.org/questionnaires/core/eortc-qlq-c30/ (accessed on 11 July 2024).
- McNamara, M.G.; Swain, J.; Craig, Z.; Sharma, R.; Faluyi, O.O.; Morgan, C.; Wall, L.R.; Chau, I.; Reed, N.; Sarker, D.; et al. NET-02: A multicenter, randomized, phase II trial of liposomal irinotecan (nal-IRI) and 5-fluorouracil (5-FU)/folinic acid or docetaxel as second-line therapy in patients (pts) with progressive poorly differentiated extra-pulmonary neuroen-docrine carcinoma (PD-EP-NEC). J. Clin. Oncol. 2022, 40 (Suppl. 16). [Google Scholar]
- McNamara, M.G.; Swain, J.; Craig, Z.; Sharma, R.; Faluyi, O.O.; Wadsley, J.; Morgan, C.; Wall, L.R.; Chau, I.; Reed, N.; et al. NET-02 final results: A randomised, phase II trial of liposomal irinotecan (nal-IRI)/5-fluorouracil (5-FU)/folinic acid or docetaxel as second-line (2L) therapy in patients (pts) with progressive poorly differentiated extrapulmonary neuroendocrine car-cinoma (PD-EP-NEC). J. Clin. Oncol. 2023, 41 (Suppl. 4). [Google Scholar]
- Kawato, Y.; Aonuma, M.; Hirota, Y.; Kuga, H.; Sato, K. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991, 51, 4187–4191. [Google Scholar] [PubMed]
- Kaneda, N.; Nagata, H.; Furuta, T.; Yokokura, T. Metabolism and pharmacokinetics of the camptothecin analogue CPT-11 in the mouse. Cancer Res. 1990, 506, 1715–1720. [Google Scholar]
- Kalra, A.V.; Kim, J.; Klinz, S.G.; Paz, N.; Cain, J.; Drummond, D.C.; Nielsen, U.B.; Fitzgerald, J.B. Preclinical Activity of Nanoliposomal Irinotecan Is Governed by Tumor Deposition and Intratumor Prodrug Conversion. Cancer Res. 2014, 74, 7003–7013. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.T.; Macarulla, T.; Blanc, J.F.; Mirakhur, B.; de Jong, F.A.; Belanger, B.; Bekaii-Saab, T.; Siveke, J.T. Early dose reduction/delay and the efficacy of liposomal irinotecan with fluorouracil and leucovorin in metastatic pancreatic ductal adeno-carcinoma (mPDAC): A post hoc analysis of NAPOLI-1. Pancreatology 2021, 21, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Nizamova, A.; Gimalova, G.; Abdullin, Z.; Khusnutdinova, E. The analysis of CHEK2 gene mutation in small cell lung cancer. In Proceedings of the ERS International Congress 2021 abstracts; 2021; p. PA662. [Google Scholar]
- Khanna, S.; Dahshan, D.; Fankhanel, E.; Griswold, D.; Goebel, L.J. Rectal Neuroendocrine Tumor in a Patient with CHEK2 Mutation. Marshall J. Med. 2020, 6, 9. [Google Scholar] [CrossRef]
- Kamgar, M.; Mehdi, M.; Thapa, B.; Szabo, A.; Ahmed, G.; Shreenivas, A.; Thomas, J.P.; Sriram, D.; Evans, D.B.; Tsai, S.; et al. Atm or Chek2 Alterations as Potential Biomarkers of Improved Outcomes with Irinotecan: Real-World Analysis of Advanced Pancreatic Ductal Adenocarcinoma. J. Clin. Oncol. 2023. [Google Scholar]
- Chen, L.; Liu, M.; Zhang, Y.; Guo, Y.; Chen, M.-H.; Chen, J. Genetic Characteristics of Colorectal Neuroendocrine Carcinoma: More Similar to Colorectal Adenocarcinoma. Clin. Color. Cancer 2020, 20, 177–185. [Google Scholar] [CrossRef]

| Non-Evaluable | Evaluable | Overall | ||
|---|---|---|---|---|
| Overall | N | 2 (18.2) | 9 (81.8) | 11 (100%) |
| Age | Mean/Std/N | 71.5/0.5/2 | 64.8/11.6/9 | 66.0/10.7/11 |
| Median/Min/Max | 71.5/71.2/71.8 | 66.1/50.0/87.8 | 66.7/50.0/87.8 | |
| Sex | Male | 1 (50.0%) | 6 (66.7%) | 7 (63.6%) |
| Female | 1 (50.0%) | 3 (33.3%) | 4 (36.4%) | |
| Race | White | 1 (50.0%) | 8 (88.9%) | 9 (81.8%) |
| Asian | 1 (50.0%) | 1 (9.1%) | ||
| Not Reported | 1 (11.1%) | 1 (9.1%) | ||
| Ethnicity | Non-Hispanic | 2 (100.0%) | 7 (77.8%) | 9 (81.8%) |
| Not Reported | 2 (22.2%) | 2 (18.2%) | ||
| BMI | Mean/Std/N | 22.5/2.4/2 | 28.2/5.9/9 | 27.1/5.8/11 |
| Median/Min/Max | 22.5/20.7/24.2 | 26.1/22.1/37.6 | 25.0/20.7/37.6 | |
| Diabetes | No | 2 (100.0%) | 9 (100.0%) | 11 (100.0%) |
| Dyspnea | No | 2 (100.0%) | 9 (100.0%) | 11 (100.0%) |
| HTN | No | 2 (100.0%) | 6 (66.7%) | 8 (72.7%) |
| Yes | 3 (33.3%) | 3 (27.3%) | ||
| Cancer History | No | 1 (50.0%) | 8 (88.9%) | 9 (81.8%) |
| Yes | 1 (50.0%) | 1 (11.1%) | 2 (18.2%) | |
| Weight Loss | No | 2 (100.0%) | 9 (100.0%) | 11 (100.0%) |
| ECOG | 0 | 5 (55.6%) | 5 (45.5%) | |
| 1 | 2 (100.0%) | 4 (44.4%) | 6 (54.5%) | |
| Disease Site | Esophagus | 2 (22.2%) | 2 (18.2%) | |
| Ampulla | 2 (22.2%) | 2 (18.2%) | ||
| Pancreas | 1 (11.1%) | 1 (9.1%) | ||
| GI Tract | 1 (11.1%) | 1 (9.1%) | ||
| Colon | 1 (11.1%) | 1 (9.1%) | ||
| Rectum | 1 (11.1%) | 1 (9.1%) | ||
| Cecum | 1 (11.1%) | 1 (9.1%) | ||
| Cervix | 1 (50.0%) | 1 (9.1%) | ||
| Not Reported | 1 (50.0%) | 1 (9.1%) | ||
| Stage | IV | 2 (100.0%) | 9 (100.0%) | 11 (100.0%) |
| Grade | Grade 3 | 2 (100.0%) | 8 (88.9%) | 10 (90.9%) |
| Grade X | 1 (11.1%) | 1 (9.1%) | ||
| Tumor Size (mm) | Mean/Std/N | 146.5/136.5/2 | 68.6/31.8/8 | 84.2/62.7/10 |
| Median/Min/Max | 146.5/50.0/243.0 | 77.0/10.0/110.0 | 77.0/10.0/243.0 | |
| Ki67 (%) | Mean/Std/N | 95.0/7.1/2 | 82.5/16.7/8 | 85.0/15.8/10 |
| Median/Min/Max | 95.0/90.0/100.0 | 90.0/50.0/95.0 | 90.0/50.0/100.0 | |
| UGT1A1-28 Allele | Negative | 1 (50.0%) | 4 (44.4%) | 5 (45.5%) |
| Heterozygous | 1 (50.0%) | 4 (44.4%) | 5 (45.5%) | |
| Not Reported | 1 (11.1%) | 1 (9.1%) | ||
| Mets: Bone | No | 2 (100.0%) | 8 (88.9%) | 10 (90.9%) |
| Yes | 1 (11.1%) | 1 (9.1%) | ||
| Mets: Brain | No | 2 (100.0%) | 9 (100.0%) | 11 (100.0%) |
| Mets: Liver | No | 1 (50.0%) | 2 (22.2%) | 3 (27.3%) |
| Yes | 1 (50.0%) | 7 (77.8%) | 8 (72.7%) | |
| Mets: LN | No | 1 (50.0%) | 8 (88.9%) | 9 (81.8%) |
| Yes | 1 (50.0%) | 1 (11.1%) | 2 (18.2%) | |
| Mets: Lung | No | 2 (100.0%) | 9 (100.0%) | 11 (100.0%) |
| # Prior Chemo Regimens | 1 | 2 (100.0%) | 5 (62.5%) | 7 (70.0%) |
| 2 | 3 (37.5%) | 3 (30.0%) | ||
| Prior Chemo | Yes | 2 (100.0%) | 9 (100.0%) | 11 (100.0%) |
| Prior Radiation | No | 2 (100.0%) | 8 (88.9%) | 10 (90.9%) |
| Yes | 1 (11.1%) | 1 (9.1%) | ||
| Prior Surgery | No | 2 (100.0%) | 9 (100.0%) | 11 (100.0%) |
| TP53 Mutation | No | 1 (11.1%) | 1 (9.1%) | |
| Yes | 2 (100.0%) | 8 (88.9%) | 10 (90.9%) | |
| CHEK2 Mutation | No | 2 (100.0%) | 1 (11.1%) | 3 (27.3%) |
| Yes | 8 (88.9%) | 8 (72.7%) | ||
| APC Mutation | No | 2 (100.0%) | 6 (66.7%) | 8 (72.7%) |
| Yes | 3 (33.3%) | 3 (27.3%) | ||
| NF1 Mutation | No | 1 (50.0%) | 8 (88.9%) | 9 (81.8%) |
| Yes | 1 (50.0%) | 1 (11.1%) | 2 (18.2%) | |
| TP53 Expression | Mean/Std/N | 86.0/9.7/2 | 18.6/34.0/9 | 30.9/40.9/11 |
| Median/Min/Max | 86.0/79.2/92.9 | 0.7/0.0/88.9 | 1.6/0.0/92.9 | |
| CHEK2 Expression | Mean/Std/N | 0.0/0.0/2 | 6.1/16.4/9 | 5.0/14.8/11 |
| Median/Min/Max | 0.0/0.0/0.0 | 0.4/0.0/49.7 | 0.3/0.0/49.7 | |
| NF1 Expression | Mean/Std/N | 24.2/34.3/2 | 0.0/0.0/9 | 4.4/14.6/11 |
| Median/Min/Max | 24.2/0.0/48.5 | 0.0/0.0/0.0 | 0.0/0.0/48.5 | |
| Non-Evaluable | Evaluable | Overall | ||
|---|---|---|---|---|
| Overall | N | 2 (18.2) | 9 (81.8) | 11 (100%) |
| Best Response | PR | 1 (11.1%) | 1 (9.1%) | |
| SD | 6 (66.7%) | 6 (54.5%) | ||
| PD | 1 (50.0%) | 2 (22.2%) | 3 (27.3%) | |
| Not Evaluable | 1 (50.0%) | 1 (9.1%) | ||
| Maximum Change in Tumor Burden (%) | Mean/Std/N | 2.2/40.2/9 | 2.2/40.2/9 | |
| Median/Min/Max | −8.4/−40.8/100.0 | −8.4/−40.8/100.0 | ||
| Reason Off Treatment | Progression | 1 (50.0%) | 8 (88.9%) | 9 (81.8%) |
| Death | 1 (50.0%) | 1 (9.1%) | ||
| Hospice | 1 (11.1%) | 1 (9.1%) | ||
| Reason Off Study | On Study | 1 (11.1%) | 1 (9.1%) | |
| Death | 1 (50.0%) | 8 (88.9%) | 9 (81.8%) | |
| Hospice | 1 (50.0%) | 1 (9.1%) | ||
| Dose Reductions | No | 2 (100.0%) | 4 (37.5%) | 6 (54.5%) |
| Yes | 5 (62.5%) | 5 (45.5%) | ||
| Dose Interruptions | No | 1 (50.0%) | 4 (37.5%) | 5 (45.5%) |
| Yes | 1 (50.0%) | 5 (62.5%) | 6 (54.5%) | |
| Subsequent Chemotherapy | No | 2 (100.0%) | 4 (37.5%) | 6 (54.5%) |
| Yes | 5 (62.5%) | 5 (45.5%) | ||
| Subsequent RT | No | 2 (100.0%) | 7 (77.8%) | 9 (81.8%) |
| Yes | 2 (22.2%) | 2 (18.2%) | ||
| 6-Month Rate | 12-Month Rate | Median | Median Follow-Up | |
|---|---|---|---|---|
| OS (Evaluable) | 0.78 (0.36, 0.94) | 0.33 (0.08, 0.62) | 9.4 (2.9, 29.3) | 30.8 |
| PFS (Evaluable) | 0.22 (0.03, 0.51) | 0.00 (0.01, 0.39) | 4.4 (1.7, 6.7) | - |
| Grade 3+ | Any AE | |
|---|---|---|
| Diarrhea | 2 (18.2%) | 5 (45.5%) |
| Nausea | 0 (0.0%) | 5 (45.5%) |
| Vomiting | 0 (0.0%) | 5 (45.5%) |
| Fatigue | 1 (9.1%) | 5 (45.5%) |
| Neutropenia | 2 (18.2%) | 3 (27.3%) |
| Dysgeusia | 0 (0.0%) | 2 (18.2%) |
| Sepsis | 2 (18.2%) | 2 (18.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, S.; Pattnaik, H.; Sonti, S.; Ramesh, M.; Jain, P.; Ramirez, R.A.; Fountzilas, C.; Vadehra, D.; Attwood, K.; Iyer, R. Phase II Study of Nanoliposomal Irinotecan (Nal-IRI) with 5-Fluorouracil and Leucovorin in Refractory Advanced High-Grade Neuroendocrine Cancer of Gastroenteropancreatic (GEP) or Unknown Origin. Cancers 2025, 17, 224. https://doi.org/10.3390/cancers17020224
Mukherjee S, Pattnaik H, Sonti S, Ramesh M, Jain P, Ramirez RA, Fountzilas C, Vadehra D, Attwood K, Iyer R. Phase II Study of Nanoliposomal Irinotecan (Nal-IRI) with 5-Fluorouracil and Leucovorin in Refractory Advanced High-Grade Neuroendocrine Cancer of Gastroenteropancreatic (GEP) or Unknown Origin. Cancers. 2025; 17(2):224. https://doi.org/10.3390/cancers17020224
Chicago/Turabian StyleMukherjee, Sarbajit, Harsha Pattnaik, Sahithi Sonti, Mrinalini Ramesh, Prantesh Jain, Robert A. Ramirez, Christos Fountzilas, Deepak Vadehra, Kristopher Attwood, and Renuka Iyer. 2025. "Phase II Study of Nanoliposomal Irinotecan (Nal-IRI) with 5-Fluorouracil and Leucovorin in Refractory Advanced High-Grade Neuroendocrine Cancer of Gastroenteropancreatic (GEP) or Unknown Origin" Cancers 17, no. 2: 224. https://doi.org/10.3390/cancers17020224
APA StyleMukherjee, S., Pattnaik, H., Sonti, S., Ramesh, M., Jain, P., Ramirez, R. A., Fountzilas, C., Vadehra, D., Attwood, K., & Iyer, R. (2025). Phase II Study of Nanoliposomal Irinotecan (Nal-IRI) with 5-Fluorouracil and Leucovorin in Refractory Advanced High-Grade Neuroendocrine Cancer of Gastroenteropancreatic (GEP) or Unknown Origin. Cancers, 17(2), 224. https://doi.org/10.3390/cancers17020224








