Racial and Geographic Disparities in Colorectal Cancer Incidence and Associated County-Level Risk Factors in Mississippi, 2003–2020: An Ecological Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Area, and CRC Data Sources
2.2. Predictor Variables
2.3. Descriptive Statistical Analysis
2.4. Spatial Cluster Analysis
2.5. Regression Analysis
2.6. Cartographic Display
3. Results
3.1. Descriptive Statistics
3.2. Spatial Clustering Results
3.3. Regression Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Virostko, J.; Capasso, A.; Yankeelov, T.E.; Goodgame, B. Recent trends in the age at diagnosis of colorectal cancer in the US National Cancer Data Base, 2004–2015. Cancer 2019, 125, 3828–3835. [Google Scholar] [CrossRef] [PubMed]
- Ansa, B.E.; Coughlin, S.S.; Alema-Mensah, E.; Smith, S.A. Evaluation of colorectal cancer incidence trends in the United States (2000–2014). J. Clin. Med. 2018, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Irby, K.; Anderson, W.F.; Henson, D.E.; Devesa, S.S. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975–2002). Cancer Epidemiol. Biomark. Prev. 2006, 15, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Crawford, N.D.; Jones, C.P.; Richardson, L.C. Understanding Racial and Ethnic Disparities in Colorectal Cancer Screening. Ethn. Dis. 2010, 20, 359–365. [Google Scholar] [PubMed]
- CDC. U.S. Cancer Statistics Data Visualizations Tool, Based on 2020 Submission Data (1999–2018). 2020 Released in June 2021. Available online: www.cdc.gov/cancer/dataviz (accessed on 20 May 2024).
- Mississippi State Department of Health. Colorectal Cancer in Mississippi 2015–2019. Available online: https://msdh.ms.gov/page/resources/8121.pdf (accessed on 4 January 2025).
- National Cancer Institute. State Cancer Profiles. Available online: https://www.statecancerprofiles.cancer.gov/quick-profiles/index.php?statename=Mississippi (accessed on 4 January 2025).
- Centers for Disease Control and Prevention. Cancer Data and Statistics. Available online: https://www.cdc.gov/cancer/data/index.html (accessed on 4 January 2025).
- National Cancer Institute. GIS Portal for Cancer Research—NCI Cancer Atlas. Available online: https://gis.cancer.gov/canceratlas/ (accessed on 4 January 2025).
- Hinshaw, T.; Lea, S.; Arcury, J.; Parikh, A.A.; Snyder, R.A. Racial and geographic disparities in stage-specific incidence and mortality in the colorectal cancer hotspot region of eastern North Carolina, 2008–2016. Cancer Causes Control 2021, 32, 271–278. [Google Scholar] [CrossRef]
- U.S. Census Bureau, Department of Commerce. Mississippi, County Health Rankings & Rodamaps. Population Health Institute, University of Wisconsin 2024. Available online: https://www.countyhealthrankings.org/health-data/mississippi?year=2020 (accessed on 2 February 2024).
- U.S. Census Bureau, Department of Commerce. TIGER/Line Shapefile, 2016, State, Mississippi, Current County Subdivision State-Based [Shapefile Zip File]. 2016. Available online: https://catalog.data.gov/dataset/tiger-line-shapefile-2016-state-mississippi-current-county-subdivision-state-based (accessed on 2 January 2024).
- MedCalc. Comparison of Two Rates. 2020. Available online: https://www.medcalc.org/calc/rate_comparison.php (accessed on 1 February 2024).
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Getis, A.; Ord, J.K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 1992, 24, 189–206. [Google Scholar] [CrossRef]
- Kutner, M.H.; Nachtsheim, C.J.; Neter, J.; Li, W. Applied Linear Statistical Models; McGraw-Hill: New York, NY, USA, 2005. [Google Scholar]
- Li, Z.; Oshan, T.; Fotheringham, S.; Kang, W.; Wolf, L.; Yu, H.; Sachdeva, M.; Bardin, S. MGWR 2.2 User Manual; Spatial Analysis Research Center (SPARC) Arizona State University: Tempe, AZ, USA, 2019; p. 38. [Google Scholar]
- Anselin, L.; Syabri, I.; Kho, Y. GeoDa: An introduction to spatial data analysis. In Handbook of Applied Spatial Analysis: Software Tools, Methods and Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 73–89. [Google Scholar]
- Jenks, G.F. The data model concept in statistical mapping. Int. Yearb. Cartogr. 1967, 7, 186–190. [Google Scholar]
- CDC. United States Cancer Statistics (USCS). Colorectal Cancer Incidence, United States—2003–2019. 2023. Available online: https://www.cdc.gov/colorectal-cancer/statistics/index.html (accessed on 19 May 2024).
- Dolan, L.; Smith, K.S.; Marlin, M.B.; Bell, L.N.; Blythe, E.; Greene, M.W.; Frugé, A.D. Food security, obesity, and meat-derived carcinogen exposure in US adults. Food Chem. Toxicol. 2021, 155, 112412. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Laure Preterre, A.; Iqbal, K.; Bechthold, A.; De Henauw, S.; Michels, N.; Devleesschauwer, B. Food groups and risk of colorectal cancer. Int. J. Cancer 2018, 142, 1748–1758. [Google Scholar] [CrossRef]
- Chen, K.Y.; Blackford, A.L.; Hussaini, S.Q. County-level food insecurity to predict cancer incidence and mortality in the United States, 2015–2020. J. Clin. Oncol. 2023, 41, 10539. [Google Scholar] [CrossRef]
- Gowda, C.; Hadley, C.; Aiello, A.E. The association between food insecurity and inflammation in the US adult population. Am. J. Public Health 2012, 102, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Lokar, K.; Zagar, T.; Zadnik, V. Estimation of the ecological fallacy in the geographical analysis of the association of socio-economic deprivation and cancer incidence. Int. J. Environ. Res. Public Health 2019, 16, 296. [Google Scholar] [CrossRef] [PubMed]
- Meliker, J.R.; Sloan, C.D. Spatio-temporal epidemiology: Principles and opportunities. Spat. Spatio-Temporal Epidemiol. 2011, 2, 1–9. [Google Scholar] [CrossRef]
- Faruque, F.S.; Zhang, X.; Nichols, E.N.; Bradley, D.L.; Reeves-Darby, R.; Reeves-Darby, V.; Duhé, R.J. The impact of preventive screening resource distribution on geographic and population-based disparities in colorectal cancer in Mississippi. BMC Res. Notes 2015, 8, 423. [Google Scholar] [CrossRef][Green Version]
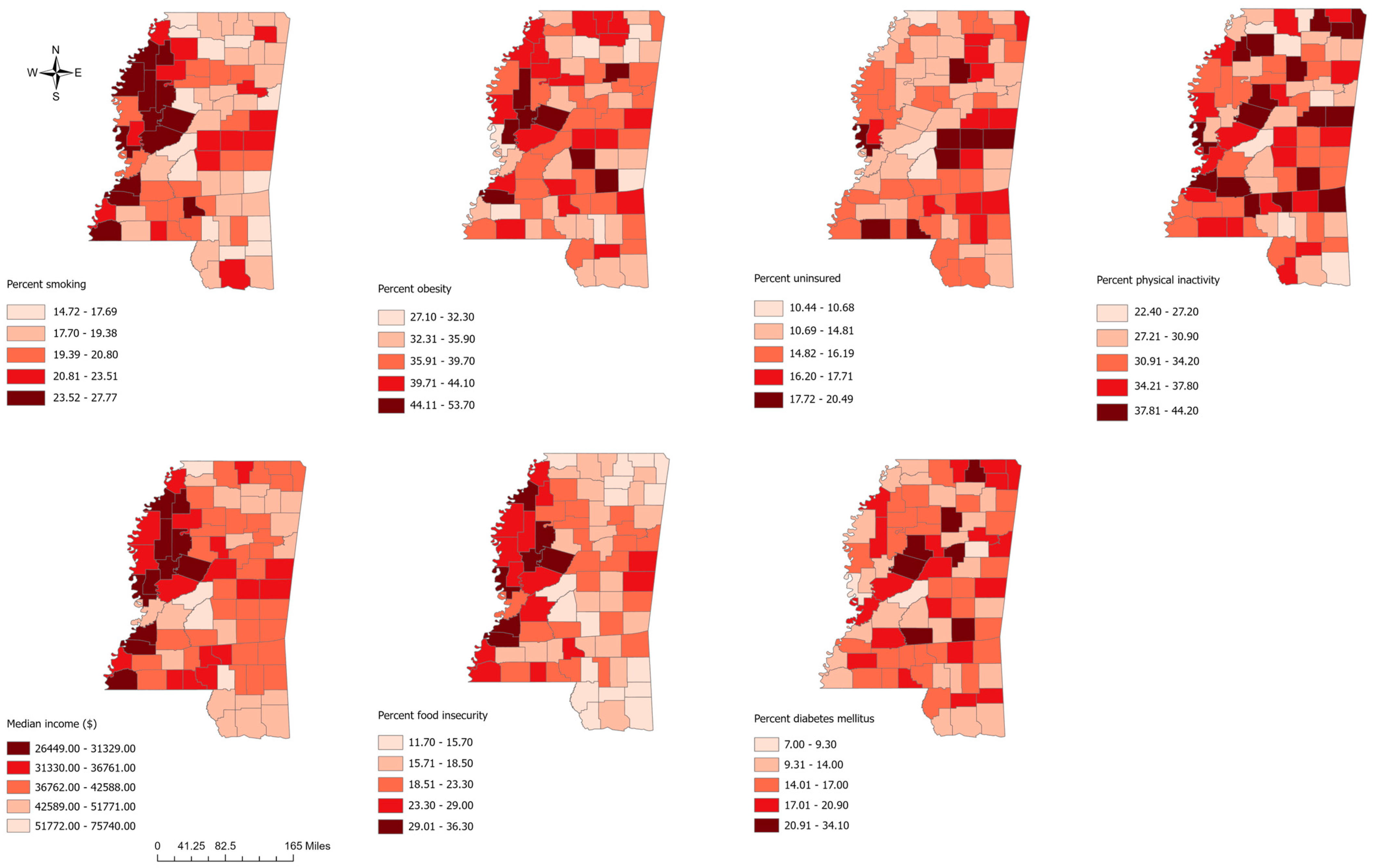
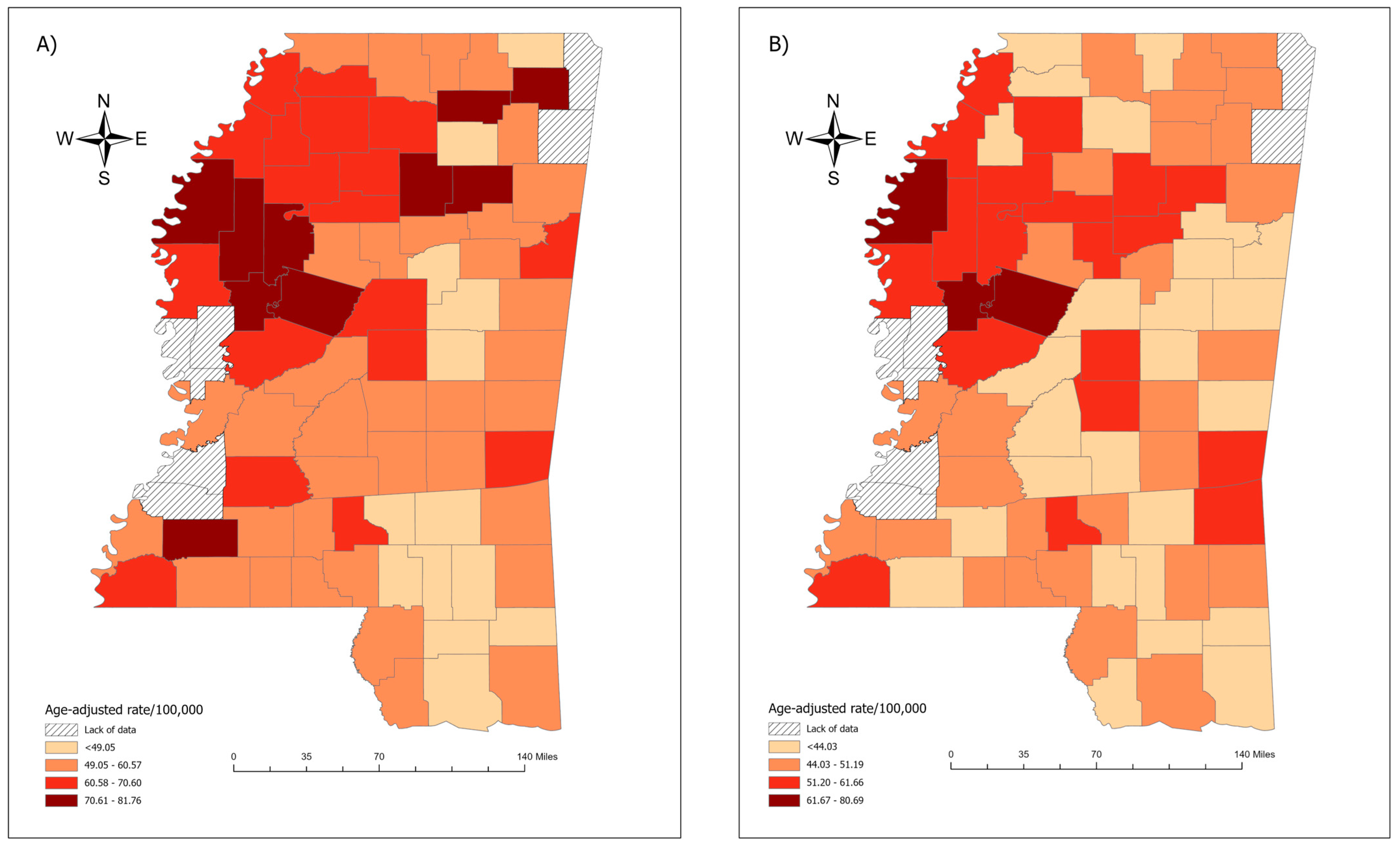
 .
.
 .
.
| Exposure Variable | Model 1 (Unadjusted) | Model 2 (Minimally Adjusted) | Model 3 (Fully Adjusted) |
|---|---|---|---|
| Multiple OLS regression | |||
| % Food Insecurity (β, 95% CI) | 0.36 (−0.075, 0.79) | 0.93 (0.25, 1.62) | 1.03 (0.26, 1.81) |
| AIC | 555.12 | 554.41 | 557.89 |
| Multivariable Logistic Regression | |||
| % Food Insecurity (OR, 95% CI) | 1.00 (0.91, 1.10) | 1.25 (1.02, 1.52) | 1.31 (1.05, 1.64) |
| AIC | 104.03 | 93.32 | 96.81 |
| Multivariable Ordinal Logistic Regression | |||
| % Food Insecurity (OR, 95% CI) | 1.07 (0.98, 1.16) | 1.19 (1.03, 1.36) | 1.22 (1.04, 1.42) |
| AIC | 216.35 | 216.57 | 217.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, S.; McKay, S.; Williams, J.L.; Meliker, J.R. Racial and Geographic Disparities in Colorectal Cancer Incidence and Associated County-Level Risk Factors in Mississippi, 2003–2020: An Ecological Study. Cancers 2025, 17, 192. https://doi.org/10.3390/cancers17020192
Sarkar S, McKay S, Williams JL, Meliker JR. Racial and Geographic Disparities in Colorectal Cancer Incidence and Associated County-Level Risk Factors in Mississippi, 2003–2020: An Ecological Study. Cancers. 2025; 17(2):192. https://doi.org/10.3390/cancers17020192
Chicago/Turabian StyleSarkar, Shamim, Sasha McKay, Jennie L. Williams, and Jaymie R. Meliker. 2025. "Racial and Geographic Disparities in Colorectal Cancer Incidence and Associated County-Level Risk Factors in Mississippi, 2003–2020: An Ecological Study" Cancers 17, no. 2: 192. https://doi.org/10.3390/cancers17020192
APA StyleSarkar, S., McKay, S., Williams, J. L., & Meliker, J. R. (2025). Racial and Geographic Disparities in Colorectal Cancer Incidence and Associated County-Level Risk Factors in Mississippi, 2003–2020: An Ecological Study. Cancers, 17(2), 192. https://doi.org/10.3390/cancers17020192






