Dose-Escalated SBRT for Borderline and Locally Advanced Pancreatic Cancer: Resectability Rate and Pathological Results of a Multicenter Prospective Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study End Points
2.3. Statistical Analysis
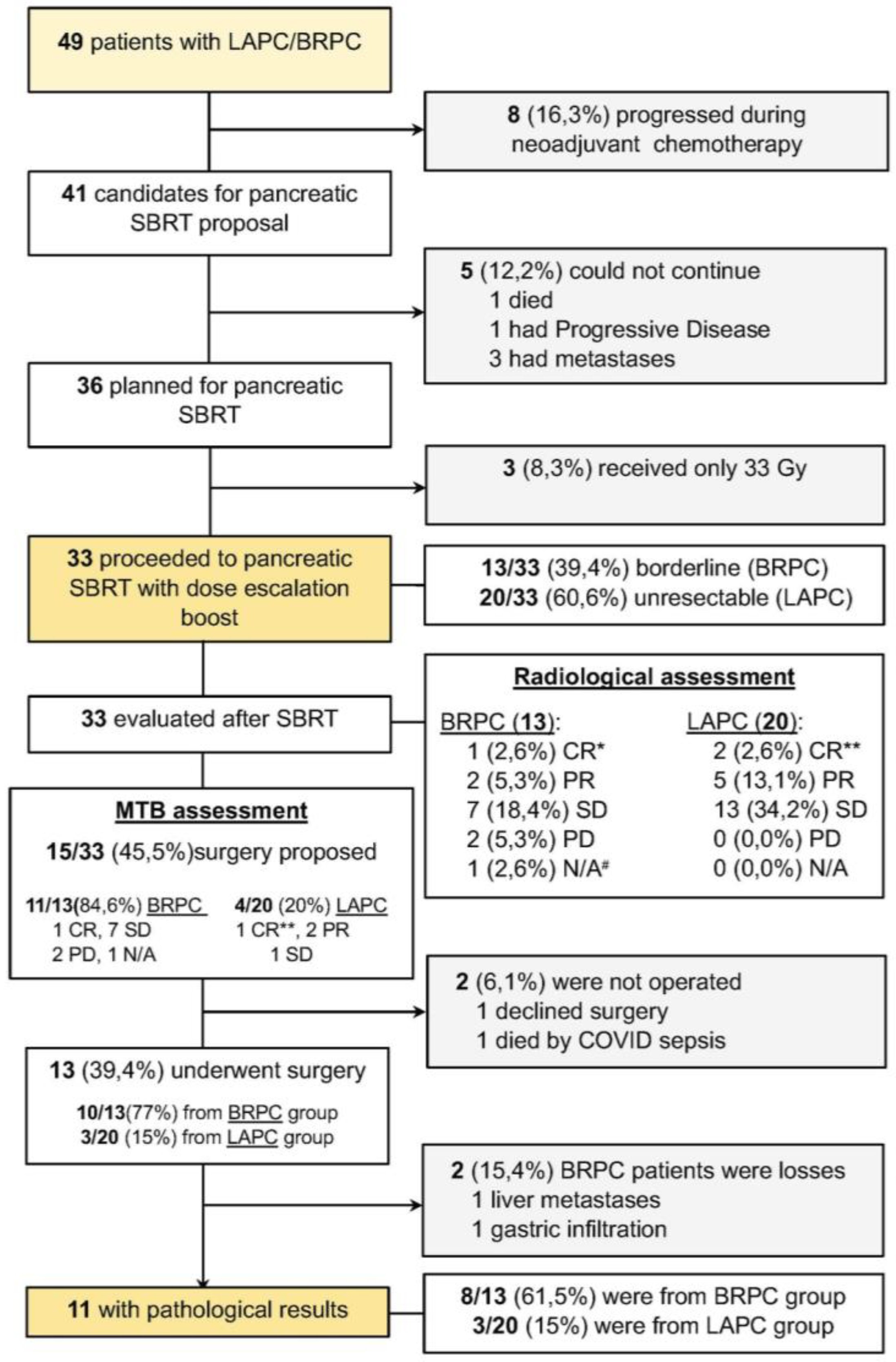
3. Results
4. Discussion
4.1. Feasibility and Safety of Dose-Escalated SBRT
4.2. Comparisons with the Existing Literature
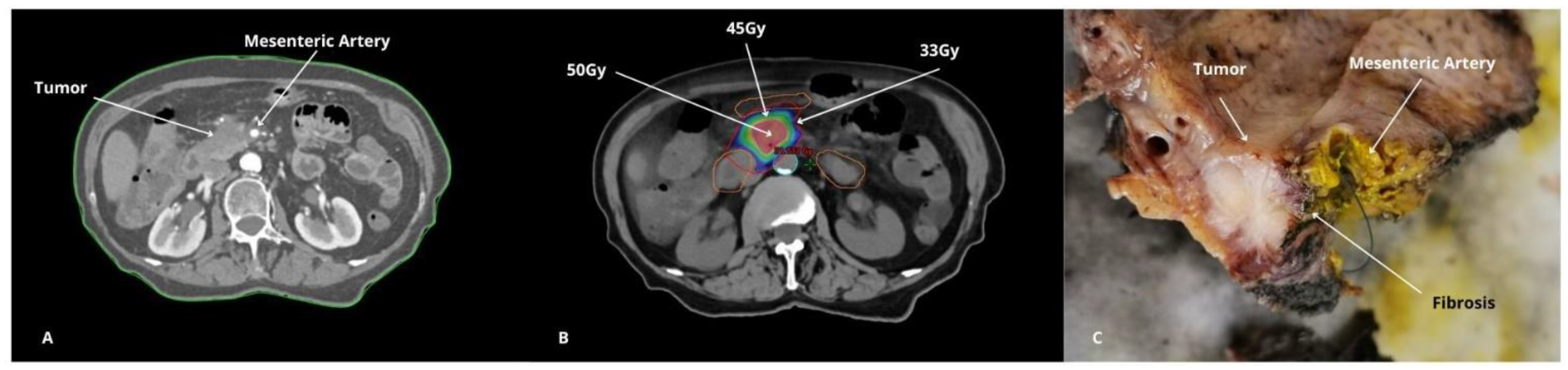
4.3. Technological Advances in Radiotherapy Delivery
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Overall survival | OS |
| Chemotherapy | ChT |
| Radiotherapy | RT |
| Chemoradiotherapy | CRT |
| Borderline resectable pancreatic cancer | BRPC |
| Locally advanced pancreatic cancer | LAPC |
| Local control | LC |
| Local progression | LP |
| Stereotactic body radiotherapy | SBRT |
| Conventional fractionated radiotherapy | CFRT |
| Biological effective dose | BED |
| Organs at risk | OAR |
| Planned treatment volume | PTV |
| Standard linear accelerator | LINAC |
| Deep inspiration breath holding | DIBH |
| Computer tomography | CT |
| Magnetic resonance | MR |
| 4D computed tomography | 4D/CT |
| Gross tumor volume | GTV |
| Simultaneous integrated boost | SIB |
| Magnetic resonance-guided radiation therapy | MRgRT |
| Gy | Gray |
| Tumor Response Scoring | TRS |
| Stereotactic ablative radiotherapy | SABR |
| The Society of Abdominal Radiology/American Pancreatic Association | SAR/APA |
| Multidisciplinary tumor board | MTB |
| Classification system of the College of American Pathologists | CAP |
| Freedom from local progression | FFLP |
| Cancer-specific survival | CSS |
| Disease-free survival | DFS |
| Freedom from distant failure | FFDF |
| Median follow-up | mfup |
| Follow-up | FUP |
References
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’reilly, E.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Callery, M.P.; Chang, K.J.; Fishman, E.K.; Talamonti, M.S.; Traverso, L.W.; Linehan, D.C. Pretreatment Assessment of Resectable and Borderline Resectable Pancreatic Cancer: Expert Consensus Statement. Ann. Surg. Oncol. 2009, 16, 1727–1733. [Google Scholar] [CrossRef]
- Vauthey, J.-N.; Dixon, E. AHPBA/SSO/SSAT Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer: Rationale and Overview of the Conference. Ann. Surg. Oncol. 2009, 16, 1725–1726. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, M.; Gogineni, E.; Herman, J.; Saif, M.W. New Potential Options for SBRT in Pancreatic Cancer. Cancer Med. J. 2021, 4, 41–50. [Google Scholar]
- Blair, A.B.; Rosati, L.M.; Rezaee, N.; Gemenetzis, G.; Zheng, L.; Hruban, R.H.; Cameron, J.L.; Weiss, M.J.; Wolfgang, C.L.; Herman, J.M.; et al. Postoperative complications after resection of borderline resectable and locally advanced pancreatic cancer: The impact of neoadjuvant chemotherapy with conventional radiation or stereotactic body radiation therapy. Surgery 2018, 163, 1090–1096. [Google Scholar] [CrossRef]
- Burkoň, P.; Trna, J.; Slávik, M.; Němeček, R.; Kazda, T.; Pospíšil, P.; Dastych, M.; Eid, M.; Novotný, I.; Procházka, T.; et al. Stereotactic Body Radiotherapy (SBRT) of Pancreatic Cancer—A Critical Review and Practical Consideration. Biomedicines 2022, 10, 2480. [Google Scholar] [CrossRef] [PubMed]
- Suker, M.; Nuyttens, J.J.; Eskens, F.A.; Haberkorn, B.C.; Coene, P.-P.L.; van der Harst, E.; Bonsing, B.A.; Vahrmeijer, A.L.; Mieog, J.D.; Swijnenburg, R.J.; et al. Efficacy and feasibility of stereotactic radiotherapy after folfirinox in patients with locally advanced pancreatic cancer (LAPC-1 trial). eClinicalMedicine 2019, 17, 100200. [Google Scholar] [CrossRef]
- Chen, Y.; Sun, X.J.; Jiang, T.H.; Mao, A.W. Combined radiochemotherapy in patients with locally advanced pancreatic cancer: A meta-analysis. World J. Gastroenterol. 2013, 19, 7461–7471. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Oh, D.Y.; Kim, K.; Chie, E.K.; Kim, T.Y.; Lee, K.H.; Han, S.W.; Im, S.A.; Kim, T.Y.; Ha, S.W.; et al. Concurrent chemoradiotherapy versus chemotherapy alone for unresectable locally advanced pancreatic cancer: A retrospective cohort study. Cancer Res. Treat. 2016, 48, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Fietkau, R.; Ghadimi, M.; Grützmann, R.; Wittel, U.A.; Jacobasch, L.; Uhl, W.; Jacobasch, L.; Uhl, W.; Croner, R.S.; Bechstein, W.O.; et al. Randomized phase III trial of induction chemotherapy followed by chemoradiotherapy or chemotherapy alone for nonresec-table locally advanced pancreatic cancer: First results of the CONKO-007 trial. J. Clin. Oncol. 2022, 40 (Suppl. S16), 4008. [Google Scholar] [CrossRef]
- Comito, T.; Massaro, M.; Teriaca, M.A.; Franzese, C.; Franceschini, D.; Navarria, P.; Clerici, E.; Di Cristina, L.; Bertolini, A.; Tomatis, S.; et al. Can STEreotactic Body Radiation Therapy (SBRT) Improve the Prognosis of Unresectable Locally Advanced Pancreatic Cancer? Long-Term Clinical Outcomes, Toxicity and Prognostic Factors on 142 Patients (STEP Study). Curr. Oncol. 2023, 30, 7073–7088. [Google Scholar] [CrossRef]
- Li, D.; Morris, J.S.; Liu, J.; Hassan, M.M.; Day, R.S.; Bondy, M.L.; Abbruzzese, J.L. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 2009, 301, 2553–2562. [Google Scholar] [CrossRef] [PubMed]
- A Shouman, M.; Fuchs, F.; Walter, F.; Corradini, S.; Westphalen, C.B.; Vornhülz, M.; Beyer, G.; Andrade, D.; Belka, C.; Niyazi, M.; et al. Stereotactic body radiotherapy for pancreatic cancer—A systematic review of prospective data. Clin. Transl. Radiat. Oncol. 2024, 45, 100738. [Google Scholar] [CrossRef] [PubMed]
- Rosati, L.M.; Herman, J.M. Role of Stereotactic Body Radiotherapy in the Treatment of Elderly and Poor Performance Status Patients With Pancreatic Cancer. J. Oncol. Pract. 2017, 13, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Arcelli, A.; Guido, A.; Buwenge, M.; Simoni, N.; Mazzarotto, R.; Macchia, G.; Deodato, F.; Cilla, S.; Bonomo, P.; Scotti, V.; et al. Higher Biologically Effective Dose Predicts Survival in SBRT of Pancreatic Cancer: A Multicentric Analysis (PAULA-1). Anticancer. Res. 2019, 40, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Mazzarotto, R.; Simoni, N.; Guariglia, S.; Rossi, G.; Micera, R.; De Robertis, R.; Pierelli, A.; Zivelonghi, E.; Malleo, G.; Paiella, S.; et al. Dosimetric Feasibility Study of Dose Escalated Stereotactic Body Radiation Therapy (SBRT) in Locally Advanced Pancreatic Cancer (LAPC) Patients: It Is Time to Raise the Bar. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Liu, J.; Lee, P.; McGee, H.M.; Chung, V.; Melstrom, L.; Singh, G.; Raoof, M.; Amini, A.; Chen, Y.-J.; Williams, T.M. Advances in Radiation Oncology for Pancreatic Cancer: An Updated Review. Cancers 2022, 14, 5725. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Balter, J.M.; Normolle, D.; Adusumilli, S.; Cao, Y.; Chenevert, T.L.; Ben-Josef, E. Characterization of Pancreatic Tumor Motion Using Cine MRI: Surrogates for Tumor Position Should Be Used With Caution. Int. J. Radiat. Oncol. 2009, 74, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Kaučić, H.; Kosmina, D.; Schwarz, D.; Mack, A.; Šobat, H.; Čehobašić, A.; Leipold, V.; Andrašek, I.; Avdičević, A.; Mlinarić, M. Stereotactic Stereotactic Ablative Radiotherapy Using CALYPSO® Extracranial Tracking for Intrafractional Tumor Motion Manage-ment—A New Potential Local Treatment for Unresectable Locally Advanced Pancreatic Cancer? Results from a Retrospective Study. Cancers 2022, 14, 2688. [Google Scholar] [CrossRef]
- Kishi, T.; Matsuo, Y.; Nakamura, A.; Nakamoto, Y.; Itasaka, S.; Mizowaki, T.; Togashi, K.; Hiraoka, M. Comparative evaluation of respiratory-gated and ungated FDG-PET for target volume definition in radiotherapy treatment planning for pancreatic cancer. Radiother. Oncol. 2016, 120, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Xiong, W.; Li, X.; Reyngold, M.; Gewanter, R.M.; Cuaron, J.J.; Yorke, E.D.; Li, T. Intrafraction tumor motion during deep inspi-ration breath hold pancreatic cancer treatment. J. Appl. Clin. Med. Phys. 2019, 20, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Brunner, T.B.; Nestle, U.; Adebahr, S.; Gkika, E.; Wiehle, R.; Baltas, D.; Grosu, A.L. Simultaneous integrated protection: A new concept for high-precision radiation therapy. Strahlenther. Onkol. 2016, 192, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Gkika, E.; Kostyszyn, D.; Fechter, T.; Moustakis, C.; Ernst, F.; Boda-Heggemann, J.; Sarria, G.; Dieckmann, K.; Dobiasch, S.; Duma, M.N.; et al. Interobserver agreement on definition of the target volume in stereotactic radiotherapy for pancreatic adenocarcinoma using different imaging modalities. Strahlenther. Onkol. 2023, 199, 973–981. [Google Scholar] [CrossRef]
- Koay, E.J.; Hanania, A.N.; Hall, W.A.; Taniguchi, C.M.; Rebueno, N.; Myrehaug, S.; Aitken, K.L.; Dawson, L.A.; Crane, C.H.; Herman, J.M.; et al. Dose-Escalated Radiation Therapy for Pancreatic Cancer: A Simultaneous Integrated Boost Approach. Pract. Radiat. Oncol. 2020, 10, e495–e507. [Google Scholar] [CrossRef]
- Yang, W.; Reznik, R.; Fraass, B.A.; Nissen, N.; Hendifar, A.; Wachsman, A.; Sandler, H.; Tuli, R. Dosimetric evaluation of simultaneous integrated boost during stereotactic body radiation therapy for pancreatic cancer. Med. Dosim. 2014, 40, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Rudra, S.; Jiang, N.; Rosenberg, S.A.; Olsen, J.R.; Roach, M.C.; Wan, L.; Portelance, L.; Mellon, E.A.; Bruynzeel, A.; Lagerwaard, F.; et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pan-creatic cancer. Cancer Med. 2019, 8, 2123–2132. [Google Scholar]
- Hassanzadeh, C.; Rudra, S.; Bommireddy, A.; Hawkins, W.G.; Wang-Gillam, A.; Fields, R.C.; Cai, B.; Park, J.; Green, O.; Roach, M.; et al. Ablative Five-Fraction Stereotactic Body Radiation Therapy for Inoperable Pancreatic Cancer Using Online MR-Guided Adaptation. Adv. Radiat. Oncol. 2020, 6, 100506. [Google Scholar] [CrossRef]
- Massaccesi, M.; Cusumano, D.; Boldrini, L.; Dinapoli, N.; Fionda, B.; Teodoli, S.; Azario, L.; Mattiucci, G.C.; Balducci, M.; Cellini, F.; et al. A new frontier of image guidance: Organs at risk avoidance with MRI-guided respiratory-gated intensity modulated radiotherapy: Technical note and report of a case. J. Appl. Clin. Med. Phys. 2019, 20, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Chuong, M.D.; Bryant, J.; Mittauer, K.E.; Hall, M.; Kotecha, R.; Alvarez, D.; Romaguera, T.; Rubens, M.; Adamson, S.; Godley, A.; et al. Ablative 5-Fraction Stereotactic Magnetic Resonance–Guided Radiation Therapy With On-Table Adaptive Replanning and Elective Nodal Irradiation for Inoperable Pancreas Cancer. Pract. Radiat. Oncol. 2021, 11, 134–147. [Google Scholar] [CrossRef]
- Parikh, P.J.; Lee, P.; Low, D.A.; Kim, J.; Mittauer, K.E.; Bassetti, M.F.; Glide-Hurst, C.K.; Raldow, A.C.; Yang, Y.; Portelance, L.; et al. A MultiInstitutional Phase 2 Trial of Ablative 5-Fraction Stereotactic Magnetic Resonance–Guided On-Table Adaptive Radiation Therapy for Borderline Resectable and Locally Ad-vanced Pancreatic Cancer. Int. J. Radiat. Oncol. 2023, 117, 799–808. [Google Scholar]
- Ladbury, C.; Amini, A.; Schwer, A.; Liu, A.; Williams, T.; Lee, P. Clinical Applications of Magnetic Resonance-Guided Radi-otherapy: A Narrative Review. Cancers 2023, 15, 2916. [Google Scholar] [CrossRef] [PubMed]
- Shaib, W.L.; Ip, A.; Cardona, K.; Alese, O.B.; Maithel, S.K.; Kooby, D.; Landry, J.; El-Rayes, B.F. Contemporary Management of Borderline Resectable and Locally Advanced Unresectable Pancreatic Cancer. Oncol. 2016, 21, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Stoop, T.F.; Oba, A.; Wu, Y.H.A.; Beaty, L.E.; Colborn, K.L.; Janssen, B.V.; Al-Musawi, M.H.; Franco, S.R.; Sugawara, T.; Franklin, O.; et al. Pathological Complete Response in Patients With Resected Pancreatic Adenocarcinoma After Preoperative Chemotherapy. JAMA Netw. Open 2024, 7, e2417625. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.M.; Lu, D.J.; Nguyen, A.T.; Hendifar, A.E.; Nissen, N.N.; Gong, J.; Osipov, A.; Gangi, A.; Attiyeh, M.A.; Atkins, K.M.; et al. Patterns of Failure in Patients With Borderline Resectable/Locally Advanced Pancreatic Cancer After Preoperative Chemo-therapy and Stereotactic Body Radiation Therapy. Adv. Radiat. Oncol. 2024, 9, 101471. [Google Scholar] [CrossRef]
- Tozzi, A.; Comito, T.; Alongi, F.; Navarria, P.; Iftode, C.; Mancosu, P.; Reggiori, G.; Clerici, E.; Rimassa, L.; Zerbi, A.; et al. SBRT in unresectable advanced pancreatic cancer: Preliminary results of a mono-institutional experience. Radiat. Oncol. 2013, 8, 148. [Google Scholar] [CrossRef]
- Waheed, A.; Murland, S.; Yip, E.; Heikal, A.; Ghosh, S.; Abraham, A.; Paulson, K.; Tankel, K.; Usmani, N.; Severin, D.; et al. Sharing Mono-Institutional Experience of Treating Pancreatic Cancer with Stereotactic Body Radiation Therapy (SBRT). Curr. Oncol. 2024, 31, 5974–5986. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Heestand, G.M.; Chang, D.T.; Pollom, E.L. Neoadjuvant treatment strategies for resectable pancreas cancer: A propen-sity-matched analysis of the National Cancer Database. Radiother. Oncol. 2020, 143, 101–107. [Google Scholar] [CrossRef]
- Hill, C.S.; Rosati, L.M.; Hu, C.; Fu, W.; Sehgal, S.; Hacker-Prietz, A.; Wolfgang, C.L.; Weiss, M.J.; Burkhart, R.A.; Hruban, R.H.; et al. Neoadjuvant Stereotactic Body Radiotherapy After Upfront Chemotherapy Improves Pathologic Outcomes Compared With Chemotherapy Alone for Patients With Borderline Resectable or Locally Advanced Pancreatic Adenocarcinoma Without Increasing Perioperative Toxicity. Ann. Surg. Oncol. 2022, 29, 2456–2468. [Google Scholar] [CrossRef]
- Bouchart, C.; Navez, J.; Borbath, I.; Geboes, K.; Vandamme, T.; Closset, J.; Moretti, L.; Demetter, P.; Paesmans, M.; Van Laethem, J.-L. Preoperative treatment with mFOLFIRINOX or Gemcitabine/Nab-paclitaxel +/− isotoxic high-dose stereotactic body Radiation Therapy (iHD-SBRT) for borderline resectable pancreatic adenocarcinoma (the STEREOPAC trial): Study protocol for a randomised comparative multicenter phase II trial. BMC Cancer 2023, 23, 1–13. [Google Scholar] [CrossRef]
- Salas, B.; Ferrera-Alayón, L.; Espinosa-López, A.; Vera-Rosas, A.; Salcedo, E.; Kannemann, A.; Alayon, A.; Chicas-Sett, R.; Lloret, M.; Lara, P. Dose-escalated SBRT for borderline and locally advanced pancreatic cancer. Feasibility, safety and preliminary clinical results of a multicenter study. Clin. Transl. Radiat. Oncol. 2024, 45, 100753. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.L.; Trotti, A. AJCC Cancer Staging Handbook from the AJCC Cancer Staging Manual; Springer: New York, NY, USA, 2010. [Google Scholar]
- Lee, E.S.; Lee, J.M. Imaging diagnosis of pancreatic cancer: A state-of-the-art review. World J. Gastroenterol. 2014, 20, 7864–7877. [Google Scholar] [CrossRef]
- Brunner, T.B.; Haustermans, K.; Huguet, F.; Morganti, A.G.; Mukherjee, S.; Belka, C.; Krempien, R.; Hawkins, M.A.; Valentini, V.; Roeder, F. ESTRO ACROP guidelines for target volume definition in pancreatic cancer. Radiother. Oncol. 2020, 154, 60–69. [Google Scholar] [CrossRef]
- Keall, P.J.; Mageras, G.S.; Balter, J.M.; Emery, R.S.; Forster, K.M.; Jiang, S.B.; Kapatoes, J.M.; Low, D.A.; Murphy, M.J.; Murray, B.R.; et al. The management of respiratory motion in radiation oncology report of AAPM Task Group 76a). Med. Phys. 2006, 33, 3874–3900. [Google Scholar] [CrossRef] [PubMed]
- Be, K.H.; Khor, R.; Joon, D.L.; Starvaggi, B.; Chao, M.; Ng, S.P.; Ng, M.; Pu, L.Z.C.T.; Efthymiou, M.; Vaughan, R.; et al. Long-term clinical outcomes of lipiodol marking using standard gastroscopy for image-guided radiotherapy of upper gastrointestinal cancers. World J. Gastroenterol. 2021, 27, 7387–7401. [Google Scholar] [CrossRef]
- Lyu, Y.; Ye, S.; Wang, B. Comparison of metal versus plastic stent for preoperative biliary drainage in patients with pancreatic cancer undergoing neoadjuvant therapy: A meta-analysis and systematic review. BMC Gastroenterol. 2023, 23, 1–8. [Google Scholar] [CrossRef]
- Timmerman, R. A Story of Hypofractionation and the Table on the Wall. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Al-Hawary, M.M.; Francis, I.R.; Chari, S.T.; Fishman, E.K.; Hough, D.M.; Lu, D.S.; Macari, M.; Megibow, A.J.; Miller, F.H.; Mortele, K.J.; et al. Pancreatic Ductal Adenocarcinoma Radiology Reporting Template: Consensus Statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014, 270, 248–260. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma (Version 3.2024). Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf (accessed on 15 November 2024).
- Burgart, L.J.; Chopp, W.V.; Jain, D. Protocol for the Examination of Specimens from Patients with Carcinoma of the Exocrine Pancreas. College of American Pathologists. (Version 4.2.0.1) June 2021. Available online: https://documents.cap.org/protocols/Panc.Endo_4.1.0.0.REL_CAPCP.pdf (accessed on 15 November 2024).
- Washington, K.B.J.; Berlin, J.; Branton, P.; Burgart, L.J.; Carter, D.K.; Compton, C.C.; Fitzgibbons, P.; Frankel, W.; Jessup, J.; Kakar, S. Protocol for the Examination of Specimens from Patients with Carcinoma of the Exocrine Pancreas. College of American Pathologists. Available online: https://documents.cap.org/protocols/cp-pancreas-exocrine-2016-v3301.pdf (accessed on 15 November 2024).
| Characteristic | Number of Patients (%) |
|---|---|
| Follow-up study * | 23.60 ± 12.95 (6–71) |
| Age * | 61.70 ± 9.51 (37–82) |
| Sex Female Male | 19 (57.6%) 14 (42.4%) |
| Status Dead Alive | 32 (96.7%) 1 (3.3%) |
| Location of primary tumor Pancreatic head Body Uncinated process (UP) Overlapping head/UP Overlapping head/body Overlapping body/tail Tail | 15 (45.5%) 4 (12.1%) 4 (12.1%) 3 (9.1%) 3 (9.1%) 3 (9.1%) 1 (3.0%) |
| cT stage at diagnosis cT1 cT2 cT3 cT4 | 0 (0.0%) 6 (18.2%) 15 (45.5%) 12 (36.3%) |
| cN stage at diagnosis cN0 cN1 cN2 | 25 (75.8%) 6 (18.1%) 2 (6.1%) |
| cM stage at diagnosis cM0 cM1 | 33 (100%) 0 (0.0%) |
| Histology Adenocarcinoma | 33 (100%) |
| Duodenal infiltration No Dubious Yes | 31 (93.9%) 2 (6.1%) 0 (0.0%) |
| Characteristic | Number of Patients (%) |
|---|---|
| Radiological post-neoadjuvant resectability-Clinical borderline group (n = 13) | |
| Underwent surgery and was completed | 8 (61.5%) |
| Underwent surgery and was cancelled # | 2 (15.4%) |
| Died before surgery | 1 (7.7%) |
| No response or PD | 2 (15.4%) |
| Radiological post-neoadjuvant resectability-Clinical unresectable group (n = 20) | |
| Underwent surgery and was completed | 3 (15.0%) |
| Response but not underwent surgery | 5 (25.0%) |
| No response | 12 (60.0%) |
| Post-neoadjuvant surgery | |
| Yes | 13 (39.4%) |
| No | 20 (60.6%) |
| Possibility of pathological analysis in operated patients (n = 13) | |
| Borderline group | 10 (76.9%) |
| Yes | 8 (80.0%) |
| No # | 2 (20.0%) |
| Unresectable group | 3 (23.1%) |
| Yes | 3 (100.0%) |
| No | 0 (0.0%) |
| ypT stage * | |
| ypT1 | 2 (18.2%) |
| ypT2 | 6 (54.5%) |
| ypT3 | 2 (18.2%) |
| ypT4 | 0 (0.0%) |
| ypTx | 1 (9.1%) |
| ypN stage * | |
| ypN0 | 5 (45.4%) |
| ypN1 | 3 (27.3%) |
| ypN2 | 3 (27.3%) |
| Pathological response (TRS system) *† | |
| Score 0 | 0 (0.0%) |
| Score 1 | 3 (27.3%) |
| Score 2 | 6 (54.5%) |
| Score 3 | 2 (18.2%) |
| Resection margins * | |
| R0 surgery (tumour-free) | 8 (72.7%) |
| R1 surgery (microscopic disease) | 3 (27.3%) |
| R2 surgery (macroscopic disease) | 0 (0.0%) |
| Pretreatment | Total | p-Value | |||
|---|---|---|---|---|---|
| BRPC | LAPC | ||||
| RESECTABILITY | Non-resectable | 2(13.6%) | 16 (80%) | 18 (55.5%) | |
| Resectable | 11(86.4%) | 4 (20%) | 15 (45.5%) | p < 0.0001 | |
| 13(39.4%) | 20 (60.6%) | 33 (100.0%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salas-Salas, B.; Ferrera-Alayon, L.; Espinosa-Lopez, A.; Perez-Rodriguez, M.L.; Afonso, A.A.; Vera-Rosas, A.; Garcia-Plaza, G.; Chicas-Sett, R.; Martinez-Martin, M.S.; Salcedo, E.; et al. Dose-Escalated SBRT for Borderline and Locally Advanced Pancreatic Cancer: Resectability Rate and Pathological Results of a Multicenter Prospective Study. Cancers 2025, 17, 191. https://doi.org/10.3390/cancers17020191
Salas-Salas B, Ferrera-Alayon L, Espinosa-Lopez A, Perez-Rodriguez ML, Afonso AA, Vera-Rosas A, Garcia-Plaza G, Chicas-Sett R, Martinez-Martin MS, Salcedo E, et al. Dose-Escalated SBRT for Borderline and Locally Advanced Pancreatic Cancer: Resectability Rate and Pathological Results of a Multicenter Prospective Study. Cancers. 2025; 17(2):191. https://doi.org/10.3390/cancers17020191
Chicago/Turabian StyleSalas-Salas, Barbara, Laura Ferrera-Alayon, Alberto Espinosa-Lopez, Maria Luisa Perez-Rodriguez, Antonio Alayón Afonso, Andres Vera-Rosas, Gabriel Garcia-Plaza, Rodolfo Chicas-Sett, Maria Soledad Martinez-Martin, Elisa Salcedo, and et al. 2025. "Dose-Escalated SBRT for Borderline and Locally Advanced Pancreatic Cancer: Resectability Rate and Pathological Results of a Multicenter Prospective Study" Cancers 17, no. 2: 191. https://doi.org/10.3390/cancers17020191
APA StyleSalas-Salas, B., Ferrera-Alayon, L., Espinosa-Lopez, A., Perez-Rodriguez, M. L., Afonso, A. A., Vera-Rosas, A., Garcia-Plaza, G., Chicas-Sett, R., Martinez-Martin, M. S., Salcedo, E., Kannemann, A., Lloret-Saez-Bravo, M., & Lara, P. C. (2025). Dose-Escalated SBRT for Borderline and Locally Advanced Pancreatic Cancer: Resectability Rate and Pathological Results of a Multicenter Prospective Study. Cancers, 17(2), 191. https://doi.org/10.3390/cancers17020191





