Anal Cancer Screening: 10-Year Experience of a Specialized Outpatient Clinic
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Patients
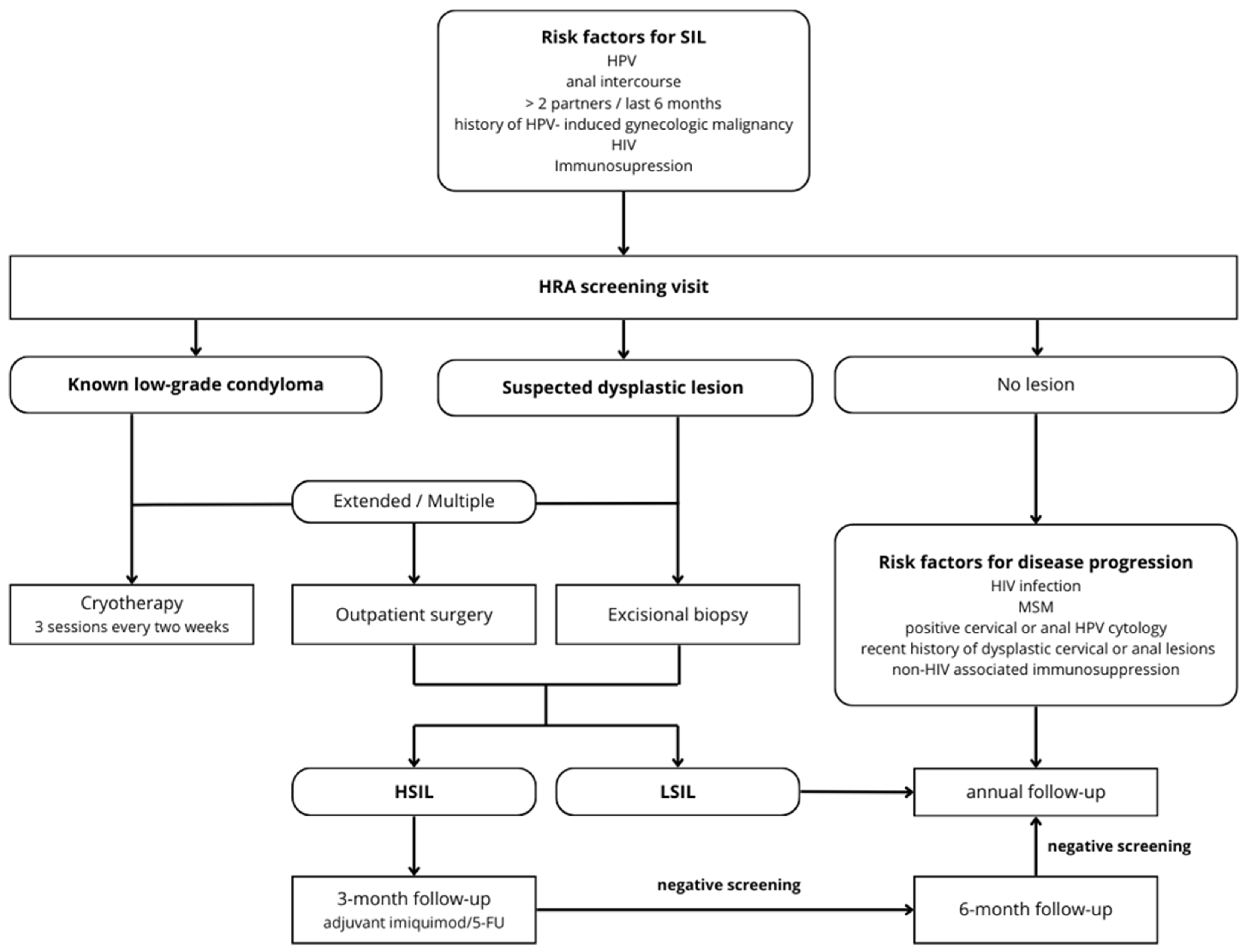
2.3. Screening and Treatment Protocols
2.4. Data Collection and Management
2.5. Statistical Analysis
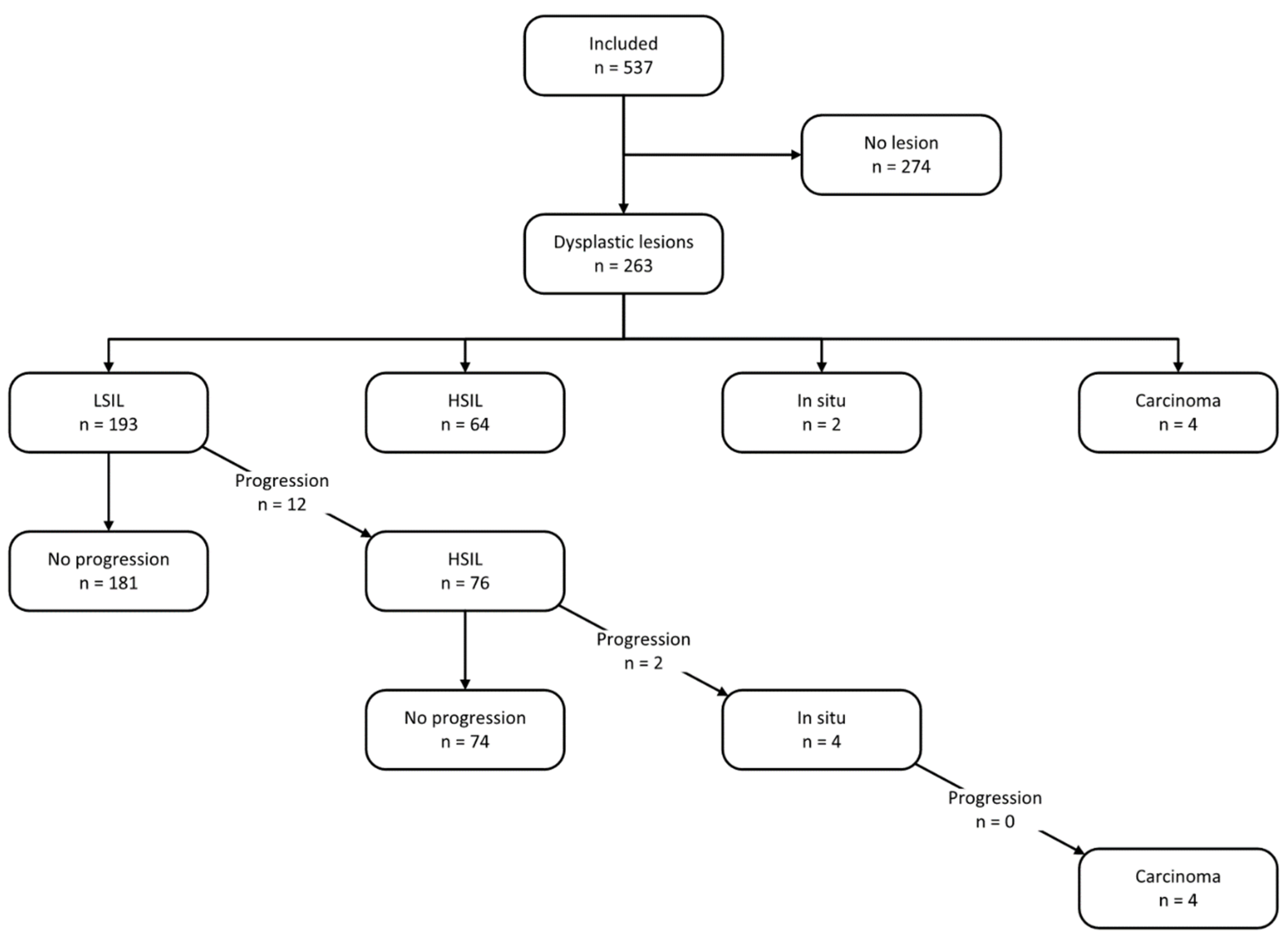
3. Results
3.1. Referrals and Demographics
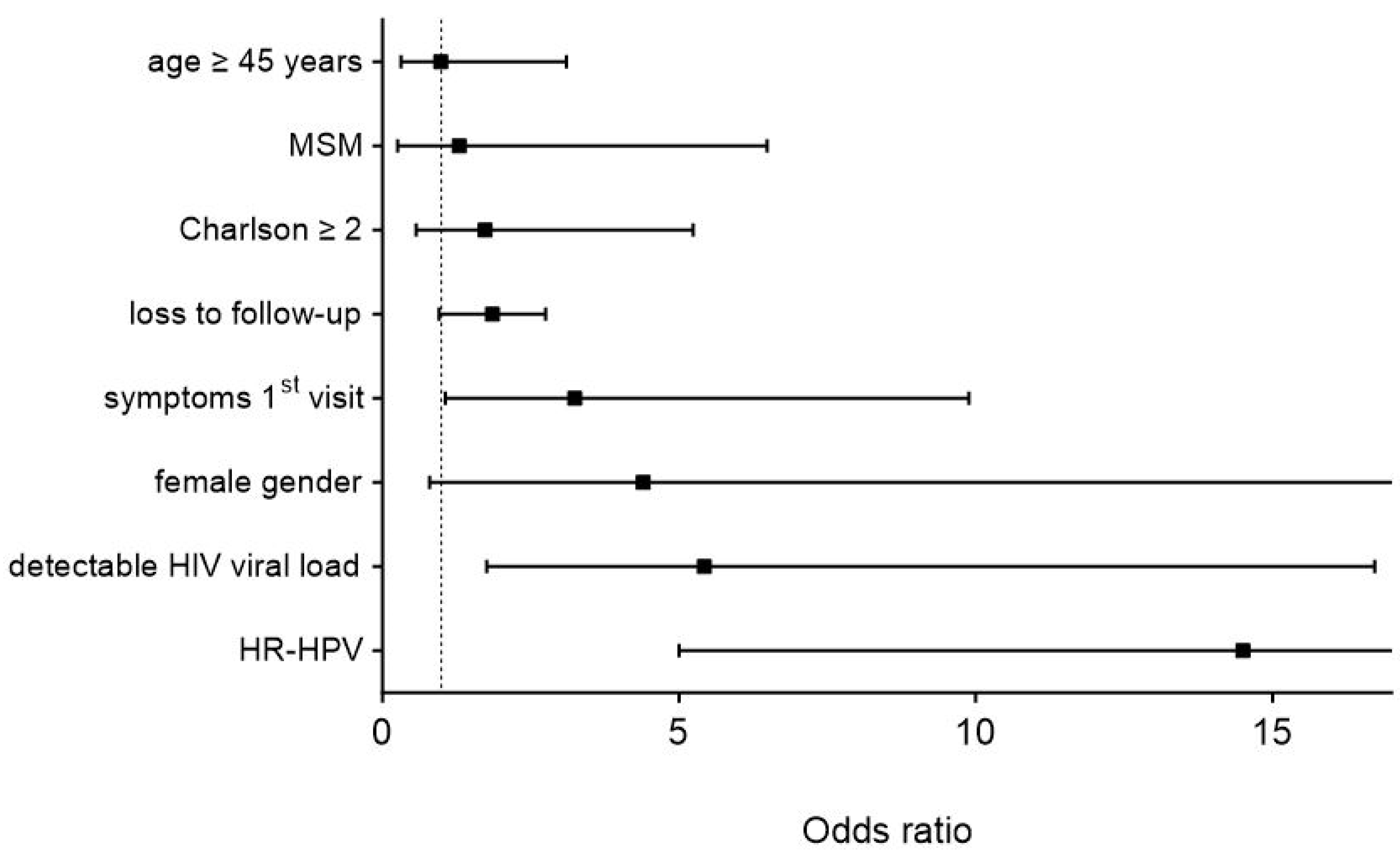
3.2. Risk Factors for Higher-Stage Lesions
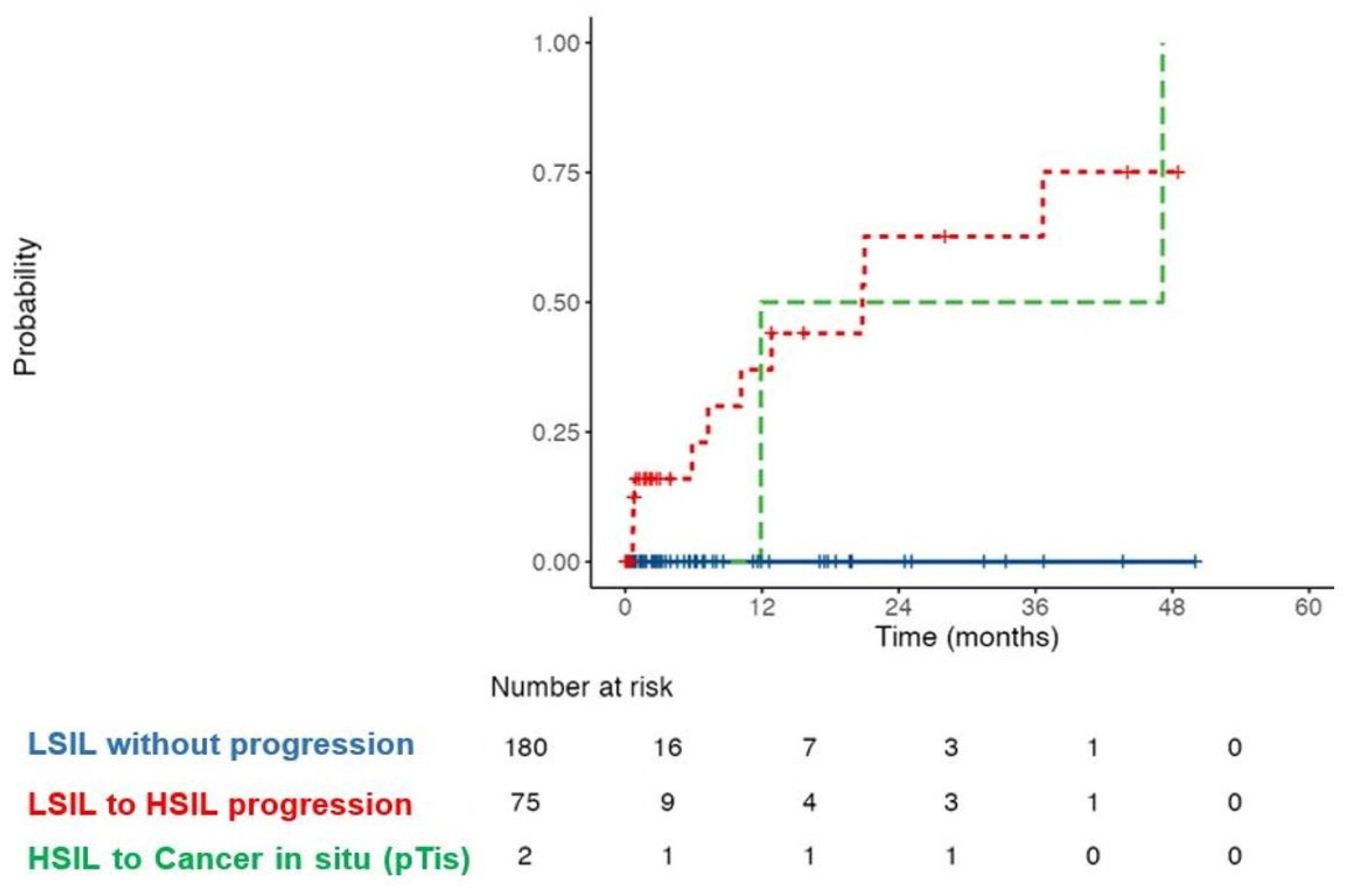
3.3. Treatment and Follow-Up
3.4. Algorithm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rao, S.; Guren, M.G.; Khan, K.; Brown, G.; Renehan, A.G.; Steigen, S.E.; Deutsch, E.; Martinelli, E.; Arnold, D. Anal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 1087–1100. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, A.A.; Suk, R.; Shiels, M.S.; Damgacioglu, H.; Lin, Y.Y.; Stier, E.A.; Nyitray, A.G.; Chiao, E.Y.; Nemutlu, G.S.; Chhatwal, J.; et al. Incidence Trends and Burden of Human Papillomavirus-Associated Cancers Among Women in the United States, 2001–2017. J. Natl. Cancer Inst. 2021, 113, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Gaba, Q.; Atun, R. Cancer incidence and survival for 11 cancers in the Commonwealth: A simulation-based modelling study. Lancet Oncol. 2024, 25, 1127–1134. [Google Scholar] [CrossRef]
- Sung, H.; Jiang, C.; Bandi, P.; Minihan, A.; Fidler-Benaoudia, M.; Islami, F.; Siegel, R.L.; Jemal, A. Differences in cancer rates among adults born between 1920 and 1990 in the USA: An analysis of population-based cancer registry data. Lancet Public Health 2024, 9, e583–e593. [Google Scholar] [CrossRef] [PubMed]
- Islami, F.; Ferlay, J.; Lortet-Tieulent, J.; Bray, F.; Jemal, A. International trends in anal cancer incidence rates. Int. J. Epidemiol. 2017, 46, 924–938. [Google Scholar] [CrossRef] [PubMed]
- Lozar, T.; Carchman, E. Pathophysiology of Anal Cancer. Surg. Oncol. Clin. 2025, 34, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Mignozzi, S.; Santucci, C.; Malvezzi, M.; Levi, F.; La Vecchia, C.; Negri, E. Global trends in anal cancer incidence and mortality. Eur. J. Cancer Prev. 2024, 33, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Shing, J.Z.; Engels, E.A.; Austin, A.A.; Clarke, M.A.; Hayes, J.H.; Kreimer, A.R.; Monterosso, A.; Horner, M.J.; Pawlish, K.S.; Luo, Q.; et al. Survival by sex and HIV status in patients with anal cancer in the USA between 2001 and 2019: A retrospective cohort study. Lancet HIV 2024, 11, e31–e41. [Google Scholar] [CrossRef] [PubMed]
- Scholefield, J.H.; Hickson, W.G.; Smith, J.H.; Rogers, K.; Sharp, F. Anal intraepithelial neoplasia: Part of a multifocal disease process. Lancet 1992, 340, 1271–1273. [Google Scholar] [CrossRef]
- Clavero, O.; McCloskey, J.; Molina, V.M.; Quiros, B.; Bravo, I.G.; de Sanjose, S.; Bosch, F.X.; Pimenoff, V.N. Squamous intraepithelial lesions of the anal squamocolumnar junction: Histopathological classification and HPV genotyping. Papillomavirus Res. 2017, 3, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. 2006, 110, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Aden, D.; Zaheer, S.; Khan, S.; Jairajpuri, Z.S.; Jetley, S. Navigating the landscape of HPV-associated cancers: From epidemiology to prevention. Pathol. Res. Pract. 2024, 263, 155574. [Google Scholar] [CrossRef] [PubMed]
- English, K.J. Anal carcinoma—Exploring the epidemiology, risk factors, pathophysiology, diagnosis, and treatment. World J. Exp. Med. 2024, 14, 98525. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.M.; Georges, D.; Shiels, M.S.; Engels, E.A.; Albuquerque, A.; Poynten, I.M.; de Pokomandy, A.; Easson, A.M.; Stier, E.A. A meta-analysis of anal cancer incidence by risk group: Toward a unified anal cancer risk scale. Int. J. Cancer 2021, 148, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Gaisa, M.M.; D’Souza, G.; Xia, N.; Giuliano, A.R.; Hawes, S.E.; Gao, L.; Cheng, S.H.; Dona, M.G.; Goldstone, S.E.; et al. Epidemiology of anal human papillomavirus infection and high-grade squamous intraepithelial lesions in 29 900 men according to HIV status, sexuality, and age: A collaborative pooled analysis of 64 studies. Lancet HIV 2021, 8, e531–e543. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.B.; Gaertner, W.B.; Glasgow, S.C.; Herzig, D.O.; Feingold, D.; Steele, S.R. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for Anal Squamous Cell Cancers (Revised 2018). Dis. Colon Rectum 2018, 61, 755–774. [Google Scholar] [CrossRef] [PubMed]
- Espirito Santo, I.; Duvoisin Cordoba, C.; Hubner, M.; Faes, S.; Hahnloser, D.; Grass, F. Anal cancer screening. A decade’s experience of a consultation in a tertiary centre. Rev. Med. Suisse 2023, 19, 1186–1190. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother. Psychosom. 2022, 91, 8–35. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Vargas, E.A.; Middleton, R.H. Modeling the three stages in HIV infection. J. Theor. Biol. 2013, 320, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.M.; Jay, N.; Cranston, R.D.; Darragh, T.M.; Holly, E.A.; Welton, M.L.; Palefsky, J.M. Progression of anal high-grade squamous intraepithelial lesions to invasive anal cancer among HIV-infected men who have sex with men. Int. J. Cancer 2014, 134, 1147–1155. [Google Scholar] [CrossRef]
- Lam, J.M.; Hoch, J.S.; Tinmouth, J.; Sano, M.; Raboud, J.; Salit, I.E. Cost-effectiveness of screening for anal precancers in HIV-positive men. AIDS 2011, 25, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Chilou, C.; Espirito Santo, I.; Faes, S.; St-Amour, P.; Jacot-Guillarmod, M.; Pache, B.; Hubner, M.; Hahnloser, D.; Grass, F. Concomitant Cervical and Anal Screening for Human Papilloma Virus (HPV): Worth the Effort or a Waste of Time? Cancers 2024, 16, 3534. [Google Scholar] [CrossRef] [PubMed]
- Gaisa, M.M.; Sigel, K.M.; Deshmukh, A.A.; Lenskaya, V.; Chan, C.A.; Silvera, R.; Winters, J.; Liu, Y. Comparing Anal Cancer Screening Algorithms Using Cytology and Human Papillomavirus DNA Testing in 3 High-Risk Populations. J. Infect. Dis. 2021, 224, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Wentzensen, N. Strategies for screening and early detection of anal cancers: A narrative and systematic review and meta-analysis of cytology, HPV testing, and other biomarkers. Cancer Cytopathol. 2018, 126, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Darragh, T.M.; Winkler, B. Anal cancer and cervical cancer screening: Key differences. Cancer Cytopathol. 2011, 119, 5–19. [Google Scholar] [CrossRef]
- Leeds, I.L.; Fang, S.H. Anal cancer and intraepithelial neoplasia screening: A review. World J. Gastrointest. Surg. 2016, 8, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Fracella, M.; Oliveto, G.; Roberto, P.; Cinti, L.; Gentile, M.; Coratti, E.; D’Ettorre, G.; Cavallari, E.N.; Romano, F.; Santinelli, L.; et al. The Epidemiology of Anal Human Papillomavirus (HPV) in HIV-Positive and HIV-Negative Women and Men: A Ten-Year Retrospective Observational Study in Rome (Italy). Pathogens 2024, 13, 163. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Deshmukh, A.A.; Suk, R.; Roberts, J.; Gilson, R.; Jay, N.; Stier, E.A.; Wentzensen, N. A systematic review and meta-analysis of cytology and HPV-related biomarkers for anal cancer screening among different risk groups. Int. J. Cancer 2022, 151, 1889–1901. [Google Scholar] [CrossRef] [PubMed]
- Ditescu, D.; Istrate-Ofiteru, A.M.; Rosu, G.C.; Iovan, L.; Liliac, I.M.; Zorila, G.L.; Balasoiu, M.; Cercelaru, L. Clinical and pathological aspects of condyloma acuminatum—Review of literature and case presentation. Rom. J. Morphol. Embryol. 2021, 62, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Squeo, G.C.; Geba, M.C.; Kane, W.J.; Thomas, T.A.; Newberry, Y.; Wang, X.Q.; Hedrick, T.L.; Friel, C.M.; Hoang, S.C. Impact of a High-Resolution Anoscopy Clinic on Management of Anal Dysplasia in Women Living With HIV. Am. Surg. 2023, 89, 4689–4695. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.; Flowers, L.; Mehta, C.C.; Chandler, R.; Knott, R.; McDonnell Holstad, M.; Watkins Bruner, D. Follow-Up to High-Resolution Anoscopy After Abnormal Anal Cytology in People Living with HIV. AIDS Patient Care STDS 2022, 36, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Moeckli, B.; Canner, J.; Najafian, A.; Carbunaru, S.; Cowell, N.; Atallah, C.; Paredes, E.; Chudnovets, A.; Fang, S.H. High-resolution anoscopy, is there a benefit in proceeding directly to the operating room? Tech. Coloproctol. 2021, 25, 461–466. [Google Scholar] [CrossRef]
- Stier, E.A.; Clarke, M.A.; Deshmukh, A.A.; Wentzensen, N.; Liu, Y.; Poynten, I.M.; Cavallari, E.N.; Fink, V.; Barroso, L.F.; Clifford, G.M.; et al. International Anal Neoplasia Society’s consensus guidelines for anal cancer screening. Int. J. Cancer 2024, 154, 1694–1702. [Google Scholar] [CrossRef]
- Achhra, A.C.; Chan, E.; Applebaum, S.; Guerrero, M.; Hao, R.; Pantel, H.; Virata, M.; Fikrig, M.; Barakat, L. Five-year evaluation of Anal Cancer Screening Program in Men Who Have Sex With Men with HIV at Two Academic Center Clinics. Clin. Infect. Dis. 2024, ciae541. [Google Scholar] [CrossRef] [PubMed]
- Pineda, C.E.; Berry, J.M.; Jay, N.; Palefsky, J.M.; Welton, M.L. High-resolution anoscopy targeted surgical destruction of anal high-grade squamous intraepithelial lesions: A ten-year experience. Dis. Colon Rectum 2008, 51, 829–837. [Google Scholar] [CrossRef]
- Machalek, D.A.; Poynten, M.; Jin, F.; Fairley, C.K.; Farnsworth, A.; Garland, S.M.; Hillman, R.J.; Petoumenos, K.; Roberts, J.; Tabrizi, S.N.; et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: A systematic review and meta-analysis. Lancet Oncol. 2012, 13, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Mallari, A.O.; Schwartz, T.M.; Luque, A.E.; Polashenski, P.S.; Rauh, S.M.; Corales, R.B. Anal cancer screening in HIV-infected patients: Is it time to screen them all? Dis. Colon Rectum 2012, 55, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Hillman, R.J.; Gunathilake, M.P.; Jin, F.; Tong, W.; Field, A.; Carr, A. Ability to detect high-grade squamous anal intraepithelial lesions at high resolution anoscopy improves over time. Sex. Health 2016, 13, 177–181. [Google Scholar] [CrossRef]
- van Heukelom, M.L.S.; Marra, E.; Cairo, I.; Van Eeden, A.; van der Loeff, M.F.S.; De Vries, H.J.; Prins, J.M. Detection Rate of High-Grade Squamous Intraepithelial Lesions as a Quality Assurance Metric for High-Resolution Anoscopy in HIV-Positive Men. Dis. Colon Rectum 2018, 61, 780–786. [Google Scholar] [CrossRef]
- Hillman, R.J.; Cuming, T.; Darragh, T.; Nathan, M.; Berry-Lawthorn, M.; Goldstone, S.; Law, C.; Palefsky, J.; Barroso, L.F.; Stier, E.A.; et al. 2016 IANS International Guidelines for Practice Standards in the Detection of Anal Cancer Precursors. J. Low. Genit. Tract Dis. 2016, 20, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wiley, D.; Barrett, B.W.; Hsu, H.; Palella, F.J.; Kwait, J.; Martinson, J.; D’Souza, G. Comparison of anal pre-cancer screening strategies among men who have sex with men. Int. J. STD AIDS 2023, 34, 87–97. [Google Scholar] [CrossRef]
- Chiao, E.Y.; Giordano, T.P.; Palefsky, J.M.; Tyring, S.; Serag, H.E. Screening HIV-infected individuals for anal cancer precursor lesions: A systematic review. Clin. Infect.Dis. 2006, 43, 223. [Google Scholar] [CrossRef] [PubMed]
- Natale, A.; Brunetti, T.; Orioni, G.; Gaspari, V. Screening of Anal HPV Precancerous Lesions: A Review after Last Recommendations. J. Clin. Med. 2024, 13, 5246. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Ploner, A.; Elfstrom, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundstrom, K.; Dillner, J.; Sparen, P. HPV Vaccination and the Risk of Invasive Cervical Cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Petrosky, E.; Bocchini, J.A., Jr.; Hariri, S.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E. Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 300–304. [Google Scholar] [PubMed]
- Utami, T.W.; Andrijono, A.; Putra, A.; Indarti, J.; Fleuren, G.; Jordanova, E.; Humairah, I.; Utomo, A. Possible different genotypes for human papillomavirus vaccination in lower middle-income countries towards cervical cancer elimination in 2030: A cross-sectional study. Clin. Exp. Vaccine Res. 2022, 11, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Office Fédéral de la Santé Publique (OFSP). Recommandation pour la vaccination contre les cancers HPV; Office fédéral de la Santé Publique OFSP: Berne, Switzerland, 2019. [Google Scholar]
- King, E.M.; Gilson, R.; Beddows, S.; Soldan, K.; Panwar, K.; Young, C.; Prah, P.; Jit, M.; Edmunds, W.J.; Sonnenberg, P. Human papillomavirus DNA in men who have sex with men: Type-specific prevalence, risk factors and implications for vaccination strategies. Br. J. Cancer 2015, 112, 1585–1593. [Google Scholar] [CrossRef]
- Stankiewicz Karita, H.C.; Hauge, K.; Magaret, A.; Mao, C.; Schouten, J.; Grieco, V.; Xi, L.F.; Galloway, D.A.; Madeleine, M.M.; Wald, A. Effect of Human Papillomavirus Vaccine to Interrupt Recurrence of Vulvar and Anal Neoplasia (VIVA): A Trial Protocol. JAMA Netw. Open 2019, 2, e190819. [Google Scholar] [CrossRef]

| Item | All Patients (n = 537) | LSIL (n = 181) | ≥HSIL (n = 82) | No SIL (n = 274) | p-Value |
|---|---|---|---|---|---|
| Age (median, IQR) | 47 (38–57) | 44 (36–53) | 53 (41–64) | 47 (39–57) | <0.001 |
| Male gender (%) | 377 (70.2%) | 139 (77.8%) | 46 (58.2%) | 194 (68.8%) | 0.001 |
| MSM (% of men) | 265 (70.3%) | 90 (64.7%) | 28 (60.1%) | 147 (75.8%) | 0.01 |
| HPV related symptoms (%) | 114 (21.2%) | 59 (32.6%) | 25 (30.5%) | 30 (10.9%) | <0.001 |
| Immunosuppression | |||||
| HIV-related (%) | 280 (52.1%) | 70 (39.7%) | 32 (40.5%) | 178 (63.1%) | <0.001 |
| Non-HIV-related (%) | 18 (3.4%) | 6 (3.4%) | (6.3%) | 7 (2.5%) | 0.05 |
| Detectable HIV viral load (%) | 40 (7.4%) | 9 (5.0%) | 10 (12.2%) | 21 (7.7%) | 0.03 |
| AIDS (A3, B3, C3) | 90 (16.8%) | 26 (14.4%) | 16 (19.5%) | 48 (17.5%) | 0.52 |
| Charlson Comorbidity Index ≥ 2 | 183 (34.1%) | 53 (29.3%) | 37 (45.1%) | 93 (33.9%) | 0.05 |
| Condyloma | |||||
| Endoanal (%) | 193 (35.9%) | 105 (58.0%) | 59 (72.0%) | 29 (10.6%) | <0.001 |
| Perianal (%) | 217 (40.4%) | 124 (68.5%) | 48 (58.5%) | 45 (16.4%) | <0.001 |
| Prior treatment | |||||
| Yes (%) | 206 (38.4%) | 80 (40.2%) | 43 (52.4%) | 83 (30.3%) | <0.001 |
| Surgical excision (%) | 128 (28.9%) | 41 (22.7%) | 29 (35.4%) | 58 (21.2%) | 0.02 |
| Cryotherapy (%) | 41 (7.63%) | 22 (12.2%) | 5 (6.1%) | 14 (5.1%) | 0.02 |
| Laser (%) | 25 (4.65%) | 11 (6.1%) | 4 (4.9%) | 10 (3.6%) | 0.49 |
| Local therapy (%) | 82 (15.3%) | 40 (22.1%) | 8 (9.8%) | 34 (12.4%) | 0.01 |
| Prior HPV genotyping | |||||
| HR-HPV (%) | 64 (11.9%) | 15 (8.3%) | 15 (18.3%) | 34 (12.4%) | <0.001 |
| HPV-16 | 27 (5.0%) | 5 (2.8%) | 13 (15.9%) | 9 (3.3%) | |
| HPV-18 | 7 (1.3%) | 1 (0.6%) | 2 (2.4%) | 4 (1.5%) | |
| Other HR types | 48 (8.9%) | 11 (6.1%) | 10 (2.2%) | 27 (9.9%) | |
| LR-HPV (%) | 36 (6.7%) | 10 (5.5%) | 8 (9.8%) | 18 (6.6%) | 0.44 |
| HPV-6 | 14 (2.6%) | 7 (3.9%) | 3 (3.7%) | 4 (1.6%) | |
| HPV-11 | 6 (1.1%) | 2 (1.1%) | 2 (2.4%) | 2 (0.7%) | |
| Other LR types | 24 (4.5%) | 4 (2.2%) | 5 (6.1%) | 15 (5.5%) | |
| HPV genotyping | |||||
| HR-HPV (%) | 111 (20.7%) | 57 (31.5%) | 47 (57.3%) | 7 (2.6%) | <0.001 |
| HPV-16 | 38 (7.1%) | 11 (6.1%) | 27 (32.9%) | 0 (0.0%) | |
| HPV-18 | 18 (3.4%) | 6 (3.3%) | 11 (13.4%) | 1 (0.4%) | |
| Other HR types | 86 (16.0%) | 48 (26.5%) | 31 (37.8%) | 7 (2.6%) | |
| LR-HPV (%) | 169 (31.5%) | 123 (68.0%) | 31 (37.8%) | 15 (5.5%) | <0.001 |
| HPV-6 | 107 (19.9%) | 81 (44.8%) | 14 (17.1%) | 12 (4.4%) | |
| HPV-11 | 42 (7.8%) | 29 (16.0%) | 11 (13.4%) | 2 (0.7%) | |
| Other LR types | 57 (10.6%) | 37 (20.4%) | 17 (20.7%) | 3 (1.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espirito Santo, I.; Kefleyesus, A.; Chilou, C.; Faes, S.; Clerc, D.; Hübner, M.; Hahnloser, D.; Grass, F. Anal Cancer Screening: 10-Year Experience of a Specialized Outpatient Clinic. Cancers 2025, 17, 193. https://doi.org/10.3390/cancers17020193
Espirito Santo I, Kefleyesus A, Chilou C, Faes S, Clerc D, Hübner M, Hahnloser D, Grass F. Anal Cancer Screening: 10-Year Experience of a Specialized Outpatient Clinic. Cancers. 2025; 17(2):193. https://doi.org/10.3390/cancers17020193
Chicago/Turabian StyleEspirito Santo, Iolanda, Amaniel Kefleyesus, Camille Chilou, Seraina Faes, Daniel Clerc, Martin Hübner, Dieter Hahnloser, and Fabian Grass. 2025. "Anal Cancer Screening: 10-Year Experience of a Specialized Outpatient Clinic" Cancers 17, no. 2: 193. https://doi.org/10.3390/cancers17020193
APA StyleEspirito Santo, I., Kefleyesus, A., Chilou, C., Faes, S., Clerc, D., Hübner, M., Hahnloser, D., & Grass, F. (2025). Anal Cancer Screening: 10-Year Experience of a Specialized Outpatient Clinic. Cancers, 17(2), 193. https://doi.org/10.3390/cancers17020193







