Simple Summary
Neuroblastoma is a deadly childhood cancer that is more aggressive when the MYCN gene is turned on. Directly targeting MYCN has been very difficult. Overactive MYCN changes the way that cancer cells use nutrients, and teams of scientists have been working to understand how these changes may be used to fight neuroblastomas. This review outlines a number of the metabolic changes that are affected by MYCN amplification, including glycolysis, amino acid dependencies, polyamines, and aberrant glycosylation, and attempts to provide clinically relevant insight into their interconnectedness. Moreover, we highlight glycosylation as an emerging area of study in the context of neuroblastoma and summarize current efforts towards understanding how changes in carbohydrate display may affect neuroblastoma presentation and treatment.
Abstract
Neuroblastoma is a devastating pediatric solid tumor that, despite significant recent advances, still accounts for nearly 15% of all childhood cancer deaths. Patients are risk stratified based on a number of features, including amplification of the MYCN oncogene, yet targeting MYCN itself has been unsuccessful to date. The complex interplay between this oncogene and its many metabolic targets has proven challenging and is only beginning to be understood in the context of pediatric tumors. It is increasingly recognized, however, that MYCN-driven metabolic rewiring and concomitant increases in biosynthetic precursors has the potential to drive many aspects of tumor development. Furthermore, emerging research suggests that improving overall therapeutic outcomes for neuroblastoma patients may well require individual metabolic profiling, allowing personalized simultaneous targeting of multiple metabolic nodes. In this review, we outline clinically relevant research involving MYCN-driven metabolic derangements, including increased glucose uptake, polyamine synthesis, glycosylation, and others, and attempt to summarize the influence of MYCN on important metabolic genes and druggable protein targets. We spotlight emerging research in glycosylation and its modulation as an often overlooked but increasingly promising therapeutic area. It is our hope that this document will provide utility for both clinicians and scientists seeking to understand how the MYCN oncogene and metabolism are critically intertwined.
Keywords:
neuroblastoma; metabolism; MYCN; glycosylation; glycolysis; fatty acids; polyamines; sialic acid; fucosylation 1. Introduction
Neuroblastomas (NBs) arise from the sympathetic chain and are the most common extracranial solid tumor in children (~700 cases per year) [1]. Neuroblastoma is a histologically and clinically heterogeneous disease with disease progression ranging from spontaneous metastatic regression to overwhelming disease progression despite aggressive treatment with induction chemotherapy, surgery, radiation therapy, and immunologic therapy [2]. Children are risk stratified based upon age, extent of spread, histologic features, chromosomal aberrations, and MYCN-amplification [1,2]. MYCN-amplified (MYCN-amp) NBs account for 40% of children with high-risk disease characterized by extra copies of the MYCN gene detected by fluorescent in situ hybridization [3]. MYCN is a member of the MYC oncogenic transcription factor family (along with C-MYC and L-MYC) known to support cancer development and progression by driving proliferation, blocking differentiation, promoting genomic instability, inducing angiogenesis, and facilitating immune evasion [4,5]. MYCN-driven metabolic rewiring contributes to these cancer hallmarks by increasing biosynthetic precursors necessary to sustain proliferation and replicative immortality, altering redox balance to both resist cell death and drive genomic instability, and altering the tumor microenvironment to facilitate immune evasion [5].
Owing to its ubiquitous oncogenic functions across a wide array of cancers, C-MYC is a more common focus of cancer metabolism studies [4,5,6,7]. N-MYC is less ubiquitously expressed and commonly constrained to expression within tissues of neuroectodermal-derived many cancers, including NB, medulloblastoma, retinoblastoma, and small cell lung cancers [8]. C-MYC and N-MYC have numerous overlapping roles in their metabolic functions both driving glycolysis, amino acid dependence, context-dependent oxidative phosphorylation, ribosomal biogenesis, and fatty acid uptake (Figure 1) [4,5]. Metabolic intermediates (such as acetyl-CoA, alpha ketoglutarate, NAD+, etc.) can have drastic implications for how epigenetic machinery affects cellular states and plasticity, which is increasingly recognized to facilitate disease progression and therapeutic resistance [9,10]. Similarly, MYC-driven metabolic shifts have been shown to alter how post-translational modifications, including glycosylation, may be highjacked in cancer development and progression [11,12,13]. Glycosylation aberrancies contribute significantly to the heterogeneity of proteins and lipids, altering how these macromolecules are distributed, degraded, activated, and ultimately contribute to the interactome of cancer cells with their local tumor microenvironment [14,15]. These common threads demonstrate how oncogenic MYC signaling can contribute to sweeping metabolic changes that contribute to cancer cell progression and therapeutic failure.
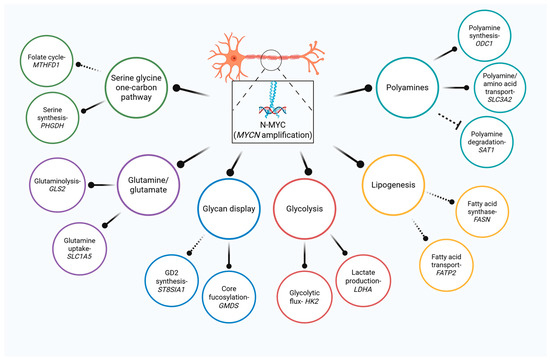
Figure 1.
Multi-nodal control of metabolism by N-MYC in NB. MYCN is a master regulator of central metabolism that drives glucose, amino acid, fatty acid, and polyamine metabolism. Complete nodes lines represent direct regulation while indirect regulation is illustrated by dashed connectors.
Herein, we seek to provide a central, but not all-encompassing, resource for both clinicians and scientists to understand how MYCN-driven metabolic perturbations have been shown to contribute to NB tumor formation, progression, and treatment failure. We attempt to summarize the key MYCN-driven perturbations within Table 1. The majority of these studies are limited to in vitro conditions where cancer cells are frequently cultured under artificial conditions that may not represent the true biology observed within our patients. Increasing utilization of advanced metabolomic techniques, including stable-isotope tracing to resolve metabolite flux and spatial metabolomics, in humans and human tissues samples, is likely to be a fruitful route toward developing new treatment strategies for this metabolically-active and hard-to-treat childhood cancer [16,17].

Table 1.
Summary of MYCN-influenced metabolic mediators in NB.
2. Oxidative Phosphorylation and Glycolysis
While oxidative phosphorylation produces ATP much more efficiently, cancer cells often rewire their metabolism towards glycolysis, even in the presence of oxygen, a phenomenon known as the Warburg effect [35,36]. This metabolic shift promotes increased glucose uptake, which ultimately supports macromolecule synthesis necessary for tumor progression. The Warburg effect allows the formation of key glycolytic intermediates, including glucose 6-phosphate, 3-phosphoglycerate, and pyruvate, supporting downstream N-linked glycosylation, lipid formation, and the production of glutathione, among others [5,36,37]. The central role of preferential glycolysis in many cancers is widely recognized; however, the regulatory landscape and potential therapeutic interventions remain poorly understood and under-investigated, particularly in pediatric cancers, underscoring a critical need for further investigation [37].
Thirty years ago, PET imaging demonstrated that NBs display increased glucose uptake relative to normal tissues [38]. More recently, intraoperative 13C glucose tracing in a small cohort of tumors revealed elevated lactate labeling in NBs compared to other tumor types, suggesting increased glycolytic activity in NBs relative to other pediatric tumors. Notably, only one of the NBs in this cohort was MYCN-amp [39]. Myc activation is known to increase glucose uptake, drive mitochondrial biogenesis, and initiate metabolic programs that use both oxidative phosphorylation and glycolysis [6,7,40]. In vitro work using MYCN ON/OFF NB cells has also demonstrated that MYCN activation in cells under stress can shift ATP production to mitochondrial oxidation of fatty acids, while aerobic glycolysis was also increased under stress but independent of MYCN status. Accordingly, limiting fatty acid oxidation decreased NB cell viability and tumor burden in mice [41]. Overall, however, the role of oxidative phosphorylation in MYCN-amp NB and its specific targeting remains poorly understood.
Hexokinase 2 (HK2) is the enzyme that catalyzes the first committed step of glycolysis by phosphorylating glucose to yield glucose-6-phosphate. HK2 is upregulated by MYCN induction in NB cells, along with a shift toward use of glycolysis and concomitant sensitization to 2-deoxyglucose [18]. MYCN-amp cells and primary tumors have also been demonstrated to express hypoxia inducible factor 1α (HIF1α), which cooperates with N-Myc to regulate both HK2 and LDHA [20]. HK2 is tumor-promoting in many cancers and is significantly upregulated in MYCN-amp primary tumors, making it an attractive target for inhibition [20,37]. 3-bromopyruvate, an inhibitor of HK2, has demonstrated synergism with rapamycin, an mTOR inhibitor in vitro [19]. Interestingly, “escape” of glycolytic dependency depends on mTORC1 expression, and some PI3K/mTOR inhibitors were also found to destabilize MYCN in vitro and in mouse models [42,43]. Together, these data demonstrate that a sustained effect when targeting glycolysis may only be accomplished by considering the many metabolic and survival nodes that these enzymes and intermediates can affect.
Increased expression of lactate dehydrogenase A (LDHA), which catalyzes the conversion of pyruvate to lactate, is associated with poor patient outcomes [44,45]. Results are mixed though regarding its overall role in MYCN-amp tumors. LDHA was shown to be required for normoxic MYCN-amp NB proliferation and tumorigenesis [20]; however, a study using patient data, CRISPR/Cas9 LDHA knockdown, and TH-MYCN mice indicated that LDHA expression was independent of MYCN and was actually dispensable for aerobic glycolysis, suggesting important alternative roles for the enzyme [44]. Several small-molecule LDHA inhibitors have been described, including FX11, which disrupts aerobic glycolysis, reduces cell viability and proliferation, and induces apoptosis in MYCN-amp NB cell lines, and Oxamate, a competitive inhibitor that can be delivered to tumors via targeted liposomes. However, preclinical studies remain limited, and the precise relationship among MYCN, LDHA, and the Warburg effect in NB is still unclear [21,45].
Recent work has emerged indicating that the combination of a mitochondrial uncoupler along with retinoic acid, a common NB treatment, would reverse the Warburg effect and promote more efficient NB differentiation than retinoic acid alone by activating mitochondrial respiration [46]. While overall successful, in vivo application produced only partial differentiation, likely owing to tumor-wide heterogeneity [46]. Indeed, work in lung cancer models has highlighted metabolic heterogeneity and subtype-specific metabolic alterations, suggesting that patients with many tumor types may benefit from metabolic studies to better understand the state of their tumor as well as its response to treatment [47,48].
3. MYCN-Regulated Amino Acid Dependencies
Deregulated MYC favors not only increased glucose uptake and energy and macromolecule generation, but also an increased dependency on certain amino acids to use as building blocks as well as replenish key intermediates in the tricarboxylic acid cycle [5,49]. Glutamine, serine, and cysteine act as central metabolic mediators, supplying carbon and nitrogen for nucleotide, protein, and lipid synthesis, and have been among the most extensively studied amino acids linked to MYCN-driven NB.
Glutamine is a conditionally essential amino acid that plays diverse roles in cell metabolism and growth, spanning from energy generation to end-product macromolecule synthesis [49,50]. N-Myc activates expression of the glutamine transporter SLC1A5/ASCT2 as well as mitochondrial glutaminase 2 (GLS2), which catalyzes the formation of glutamate from glutamine, is elevated in MYCN-amp tumors, and correlates with poor survival [22,23]. Glutamate can be converted to α-ketoglutarate and further used in the citric acid cycle or used for biosynthesis of glutathione, an important antioxidant. Glutathione biosynthesis was demonstrated in TH-MYCN++ mice as upregulated to promote cell survival during very early tumorigenesis [51]. This study further suggested that coupling existing chemotherapies with glutathione biosynthesis inhibition may potentiate the therapy. GLS2 and SLC1A5 inhibition or glutamine deprivation have been shown to trigger significant inhibition of aerobic glycolysis and cell death [22,23,52]. However, MYCN-amp NB cells have also been shown to synthesize glutamine de novo and survive glutamine starvation in the presence of glucose, hinting at the extreme metabolic adaptability of these cells [41]. High SLC1A5 expression has also recently reported to be correlated with immune cell infiltration and demonstrated that the SLC1A5 inhibitor V-9032 may also regulated ST8SIA1 expression within NB cells [53]. This study invites further inquiry into determining how glutamine uptake may be implicated into NB immune evasion within preclinical models.
N-Myc activates ATF4 mRNA, thus stabilizing N-Myc and allowing formation of a positive feedback loop necessary for transcriptional activation of the serine–glycine–one-carbon (SGOC) pathway [24]. This is a highly interconnected pathway, and for a review that includes its roles in cancer, we recommend reading Reina-Campos et al. [54]. At the heart of SGOC, glucose is used to generate serine and glycine. These two then contribute one-carbon units to the folate cycle, feeding into multiple other points that sustain homeostasis. Serine can also contribute to purine biosynthesis. Amplification of MYCN creates a SGOC gene signature consisting of PHGDH, PSAT1, MTHFD1L, MTHFD1, MTHFD2, and SHMT2, all of which are elevated in MYCN-amp cases compared to either low-risk or high-risk tumors without amplification [24]. Pharmacological inhibition of phosphoglycerate dehydrogenase (PHGDH), one of the enzymes necessary for the conversion of glucose to serine, was shown to trigger metabolic stress and G1 arrest specifically in MYCN-amp NB cells while non-amp cells were unaffected [24]. In vitro and in vivo trials demonstrate increased susceptibility of MYCN-amplified NBs to NCT-503, a small molecule inhibitor of PHGDH, suggesting that MYCN-amplified NBs are preferentially sensitive to the SGOC therapeutic strategies [24].
MYCN-amp cells also have an elevated requirement for folate, and the combination of MYCN inhibition and either silencing of methylenetetrahydrafolate dehydrogenase 1 (MTHFD1) or methotrexate treatment of MYCN-amp cells alters redox homeostasis, causes apoptosis in vitro, and significantly slows xenograft growth [25,26]. Knockdown or pharmacological inhibition of two MYCN-controlled enzymes involved in purine biosynthesis also demonstrated robust effects on MYCN-amp cell viability in vitro, suggesting that targeting SGOC may be a potent strategy that limits availability of multiple metabolites [55].
Given the fact that targeting MYCN alone has proven unsatisfying, the crosstalk of MYCN-driven amino acid dependencies with other biological processes may prove extremely useful. For example, depletion of cysteine, necessary for glutathione synthesis, in MYCN-amp cells was shown to trigger ferroptosis resulting from accumulated reactive oxygen species [56]. A feed-forward loop also exists between MYCN and the amino acid transporters SLC7A5 and SLC43A1, and specific inhibition of these transporters inhibits both glutamine and glucose metabolism while also inhibiting N-Myc synthesis [57]. Overall, however, very few therapies targeting amino acid dependencies have been successfully developed, and to the best of our knowledge, none have been clinically implemented in NB.
A major challenge to the translational integration of targeting amino acid dependencies is that much of this foundational work derives from studies in two-dimensional cultured cell systems, where nutrient availability, oxygen tension, and immune interactions differ dramatically from the tumor microenvironment in vivo and in situ. For instance, the composition of amino acids and redox buffers in standard culture media (e.g., high cystine, glutamine, and glucose) does not accurately reflect the nutrient gradients, stromal interactions, or hypoxic conditions present within bulky NBs [58]. As a result, metabolic dependencies observed in vitro may be exaggerated, or alternatively masked, compared to what occurs in patients, highlighting the need to develop physiologically relevant NB culture systems and highlighting the importance of validating cell culture within disease relevant models in vivo.
4. Polyamine Synthesis and Uptake
Polyamines are small organic compounds with at least two amino groups. They are essential for numerous processes in cell growth and differentiation and, as such, their synthesis is typically upregulated in fast-growing tumors such as NB [27,59]. Multiple enzymes involved in polyamine synthesis, including rate-limiting ornithine decarboxylase (ODC1), are dysregulated in MYCN-amp NB and, furthermore, high-risk tumors without MYCN amplification also feature high levels of ODC1, suggesting that targeting this enzyme may provide broad benefit in NB treatment [60,61].
MYCN amplification promotes increased polyamine synthesis by directly targeting ODC1 transcription as well as by repressing antizyme breakdown of ODC and polyamine catabolism [27,44]. Moreover, ODC1 has been shown in a subset of tumors to be co-amplified along with MYCN [27]. Tumor cells can also take up polyamines from the microenvironment via SLC3A2, a direct N-Myc target, and can compensate for single-agent inhibition of polyamine synthesis by upregulating a second transporter, ATP13A3 [28,62]. Both transporters have been shown to be druggable with the small molecule polyamine transport inhibitor AMXT 1501 [62,63].
Difluoromethylornithine (DFMO) was approved by the US FDA in late 2023 to target polyamine synthesis via ODC1 inhibition in high-risk post-immunotherapy patients in remission (NCT02395666). This maintenance therapy has been shown to increase both event-free and overall survival in high-risk MYCN-amp and non-amp cases [64,65]. DFMO is undergoing numerous clinical trials for additional indications and drug combinations, including AMX 1501, standard of care chemotherapies, and dinutuximab. Combining DFMO with celecoxib, which upregulates spermine/spermidine acetyltransferase (SAT1), thus regulating polyamine cellular export and depleting polyamines, is also under investigation [28,61,66]. DFMO treatment with restricted dietary arginine intake may also increase the benefit of DFMO treatment by limiting ornithine generation and thus tumor uptake, highlighting the role that careful examination of exogenous sources of important intermediates can play in cancer treatment [67]. DFMO also represses Lin28, an RNA binding protein that promotes stemness, by enhancing synthesis of let7, a tumor suppressor microRNA that is repressed by N-Myc [68]. This inhibitory axis regulates glycolytic metabolism, discussed earlier in this review, thus suggesting that DFMO treatment could have patient benefits on multiple fronts [69].
5. Fatty Acid Dependencies
Tumors reactivate de novo fatty acid synthesis in order to support rapid cell division and overall growth, which requires increased availability of lipids to form membranes, perform signaling functions, and serve as energy sources [70]. Multiple studies have shown a concerted role for c-Myc in fatty acid metabolism and abundance, but a role for MYCN and its amplification is generally less clear [29].
Fatty acids serve as the basis for more complex lipids. De novo fatty acid synthesis requires acetyl-CoA carboxylase and fatty acid synthase (FASN), a direct target of N-Myc, to produce palmitate, which is then modified further to produce other fatty acids [30,70]. MYCN additionally promotes de novo fatty acid synthesis through glucose-sensing transcription factor MondoA and lipogenic transcription factor SREBP1 [71,72]. MondoA loss significantly abrogates glutamine-derived lipid synthesis, and N-Myc overexpressing cells are particularly sensitive to in vitro FASN inhibition when MondoA is knocked down [71]. FASN inhibition was shown to reduce NB xenograft growth and increase differentiation through activation of ERK signaling with concomitant reductions in both c-Myc and N-Myc; however, those benefits were found to be independent of MYCN amplification [29].
Fatty acids can also be taken up by cells from their environment through specific transporters [70]. Moreover, NB cells use exogenous fatty acids to evade FASN inhibition [31]. MYCN promotes fatty acids uptake by NB cells through upregulation of the fatty acid transporter FATP2, encoded by SLC7A2, and pharmacological inhibition of this uptake in an animal model significantly impacts tumor growth and survival by targeting FATP2 overexpressing cells [31]. Interestingly, FATP2 has also been implicated in tumor neutrophil-derived myeloid suppressor cell activity, indicating that targeting lipid mediator formation may produce significant results in the tumor microenvironment [73].
In vitro work has demonstrated MYCN-amp NB cells to be highly dependent on fatty acid β-oxidation for growth and survival; however, rigorous validation in pre-clinical mouse models remains limited [41,74]. Several points of the β-oxidation pathway have emerged as promising therapeutic vulnerabilities in other cancers, suggesting that targeting lipid metabolism may hold untapped potential in NB as well [75]. At the same time, inhibition of fatty acid synthesis represents a particularly compelling strategy within the context of MYCN-driven metabolic reprogramming, as it may simultaneously restrict energy production and membrane biosynthesis. Despite this promise, the current pharmacological toolkit remains underdeveloped. Expanding this toolkit will require both adaptation of existing metabolic inhibitors from other cancer settings and development of NB-tailored approaches guided by in vivo validation. However, major challenges remain, including limited specificity, systemic toxicity given the importance of lipid metabolism in normal tissues, and the intrinsic redundancy of metabolic networks that allows NB cells to adaptively rewire their fuel utilization and bypass single-pathway inhibition [76,77]. Overcoming these barriers will necessitate rational combination strategies, improved drug design, and integration of metabolic biomarkers into translational studies.
6. Glycosylation
Glycosylation, a post-translational modification involving the addition of a carbohydrate to a protein or lipid, and its dysregulation have gained recognition as major players in cancer and metastasis more recently than some other metabolic derangements. Aerobic glycolysis, and in turn increased pools of fructose 6-phosphate, has the potential to fuel hexosamine biosynthesis. The final product, UDP-GlcNAc, serves as the base for elaboration of both N- and O-glycans; however, limited experiments have suggested that it is tightly regulated [14,78]. Membrane-bound as well as secreted proteins may be glycosylated with a wide variety of carbohydrate conformations, and the inherent heterogeneity of the resultant products has slowed their understanding compared to the templated processes involved in protein production. Significant alterations in surface glycan conformation, increased fucosylation or sialyation, as well as changes in glycan secretion, have been identified in numerous cancers including NB, with connections to MYCN beginning to be identified [11,15,79,80]. For example, Bley et al. recently analyzed mRNA sequencing results from MYCN-amp and non-amp NBs and identified over 30 differentially expressed glycosyltransferase genes between the two [81]. Moreover, Zhu et al. showed increased core fucosylation within neuroblastic regions of MYCN-amp tumors using matrix-assisted laser desorption ionization mass spectrometry imaging (MALDI-MSI) and subsequently identified increased expression of a key enzyme responsible for de novo GDP-fucose synthesis as a result of MYCN amplification [11].
N-glycosylation, the addition of an oligosaccharide to an asparagine residue, is a complex process beginning with a dolichol-phosphate oligosaccharide precursor and dependent on a large family of coordinated enzymes in the endoplasmic reticulum and Golgi [14]. A study comparing only two NB cell lines, one MYCN-amplified and one not, found that MYCN-amplified cells tended to display larger glycans, with a preference for sialic acid modifications [82]. Interestingly, a study of ganglioneuroblastoma and NB patients compared with matched controls found increased branched, sialylated N-linked glycans in the serum of NB patients, suggesting utility for serum glycan profiling; however, the study did not stratify by MYCN status [80].
O-glycosylation, which involves adding an oligosaccharide to a serine or threonine residue, is crucial for forming H antigen precursor of blood group-related antigens, some of which participate in selectin binding and, ultimately, tumor cell extravasation and metastasis [14,83]. Compared to some adult tumors, little is known about how aberrant O-glycosylation might affect NB progression or prognosis. In vitro, expression of Lewis glycan family members, and glycosyl and fucosyltransferases involved in their construction, tends to be higher in MYCN-amp cell lines than in non-amp lines [83], suggesting a role for these structures in more aggressive tumor presentations. However, a prior report using immunohistochemistry to identify blood group-related antigens on tumors of epithelial, neuroectoderm, and mesodermal origin found no expression of these antigens when analyzing a small number on frozen NBs and very limited work has been done since [84].
Core β1,3 galactosyltransferase (C1GALT1) catalyzes formation of the core 1 structure in GalNAc-type O-glycosylation, necessary for more complex core 2 and extended structures [85]. Its expression is increased in numerous adult cancers and correlates with poor outcomes therein. However, C1GALT1 is positively associated with survival in NB cases, even those with MYCN amplification, and loss of this enzyme promotes malignant behaviors in NB cells in vitro and in vivo [86]. This enzyme participates in GalNAc O-glycosylation of TrkA, a receptor tyrosine kinase necessary for sympathetic nervous differentiation, and TrkA activation suppresses MYCN expression [32,86,87]. When compared to the numerous tumors in which C1GALT1 seems to be tumor-promoting, these data suggest a unique role for O-glycosylation in NB that should be further explored.
6.1. Fucosylation
L-fucose is found on N- and O-linked glycans glycolipids, and directly conjugated to proteins via O-fucosylation of serine residues. It plays important roles in processes such as cell motility and angiogenesis. Dysregulated fucosylation, a known entity in neoplasms as diverse as glioblastoma, hepatocellular carcinoma, melanoma, and breast cancer, has been implicated in aggressive tumor growth and is used as a biomarker in some cases [88,89]. Fucose may be derived from exogenous sources of fucose, mannose, or glucose, or it may be taken in by recycling existing glycoconjugates. Regardless of source, it undergoes a multi-step reaction to form GDP-fucose prior to glycan addition. The addition of GDP-fucose to glycan structures relies on a set of fucosyltransferases (FUTs) and transporters thought to discriminate the heritage of their individual substrates [90]. While α-1,2/3/4 fucose linkages may be formed by multiple FUTs, core fucosylation, an α-1,6 linkage to the innermost asparagine-linked GlcNAC, is accomplished solely by FUT8 [14].
Relatively little is known about the role of fucosylation in NB. Zhu et al. demonstrated a pathogenic role for MYCN-enhanced overexpression of GDP-mannose 4,6 dehydratase (GMDS), the first committed enzyme step in de novo GDP-fucose synthesis, and found significantly increased core fucosylation in MYCN-amp neuroblastic tumor regions in situ [11]. This suggests a significant role for FUT8 or perhaps the enzymes that precede it in GDP-fucose biosynthesis, such as GMDS, in NBs. Indeed, FUT8 expression is negatively associated with overall survival in NB; however, this enzyme was not associated with disease staging or MYCN status [11]. Taken together, these findings highlight core fucosylation as a critical but understudied modification in NB, with FUT8 and upstream enzymes such as GMDS emerging as potential drivers of tumor aggressiveness. Defining the cell surface and secreted proteins that depend on this modification for their oncogenic potential represents a rational next step in advancing this area of investigation.
6.2. Sialylation
Sialic acids are highly abundant cell surface sugars that provide a negatively charged cap on glycans. A wide variety of structures exists, with the most common form found in humans designated Neu5Ac [14]. Oligosialic acids, up to three residues, are commonly found on gangliosides, while polysialic acid, a polymer of sometimes more than 100 residues in a chain formation, is less common but may be found on some structures important to NB, including neural cell adhesion molecule (NCAM) [14]. Polysialylated NCAM may facilitate NB cell migration [91], and serum levels have been suggested to indicate undifferentiated or high-stage disease [92]; however, a study using paraffin-embedded NB samples found no correlation of polysialylated NCAM with MYCN status [93].
One area of NB research and treatment in which sialic acid has gained significant favor is the disialoganglioside GD2, which is at present the only approved immunotherapy target for NB patients (dinutuximab). The structure, synthesis, and biology of GD2 are well reviewed in reference [94]. Compared to normal tissues, NBs express high levels of gangliosides, which are compound lipids that contain a carbohydrate moiety, an alcohol called sphingosine, a fatty acid, and at least one sialic acid [95]. In general, ganglioside expression in nervous tissues progresses from simple types such as GD2 toward more complex species from embryonic development through maturity [96]. This pattern becomes dysregulated in MYCN-amp NB, where the oncogene drives an adrenergic regulatory program, thus promoting persistence of the immature neuroblast state and high GD2 levels [33,34,97,98]. NBs may be very plastic and able to reversibly transition from one state to another in response to environmental factors [99,100]. In addition to being linked to chemotherapy resistance, this plasticity has been reported to influence GD2 surface display in commonly used cell lines, with a more mesenchymal state leading to lower GD2 expression by throttling GD3 synthase (ST8SIA1) expression [101,102].
GD2 has multiple functions in NB, including adhesion to extracellular matrix proteins and providing an anti-phagocytic signal by binding phagocyte Siglec 7 [103,104]. It is also shed by undifferentiated NB cells, and plasma GD2 concentrations are thought to be indicative of active disease [105]. A recent study proposing use of circulating GD2 as a biomarker found high serum concentrations in children with high-risk, MYCN-amp, or high-stage disease, suggesting that monitoring circulating GD2 could have utility as a prognostic indicator and treatment response [79]. GD2 expression on tumors may be somewhat heterogeneous, with infrequent tumors initially presenting as partially or fully GD2 negative. Loss as a result of treatment is relatively rare but should be considered in cases of relapse with previous anti-GD2 immunotherapy [106,107,108]. Taken together, the aforementioned studies indicate clinical utility for individual ganglioside profiling alongside MYCN status prior to initiating anti-GD2 therapy.
6.3. Modulation of Carbohydrate Display
The utility and relatively straightforward nature of carbohydrate modification in cancer treatment is only beginning to be realized. Fucose and sialic acid biosynthesis, as well as some fucosyl and sialyltransferases, can be inhibited by treatment with unnatural monosaccharide derivatives including Fucotrim I, 2-fluorofucose, FNANA, P-SiaFNEtoc, and others [109,110]. Zhu et al. showed that oral 2-fluorofucose treatment of immune-deficient mice after tumor initiation with MYCN-amp BE(2)-C cells significantly slowed tumor growth, thus indicating that fucosylation blockade may have considerable benefit in these aggressive tumors [11]. More work remains to be done in this area, however, including significant study into how the tumor’s immune microenvironment is altered with reduced fucosylation. Exogenous methods to enhance ST8SIA1 and GD2 expression are also an active area of investigation. The combination of peracetylated sialic acid and vorinostat, an HDAC inhibitor, effectively boosted GD2 expression in MYCN-amp cells, suggesting utility for this treatment combination for in vivo investigations [111]. Inhibition of MYCN- driven histone methyltransferase EZH2 has also been shown to upregulate GD2 in a mouse mesenchymal NB xenograft and, moreover, EZH2 and HDAC inhibition were synergistic in vitro, promoting expression of tumor suppressor genes in MYCN-amp cells, thus demonstrating that multiple routes to therapeutic benefit exist in this realm [102,112].
7. Conclusions
There is now abundant evidence from both in vitro models and in vivo preclinical systems that MYCN serves as a master regulator of metabolic transformation in NB. Its control over glycolysis, glutaminolysis, and fatty acid metabolism positions it as a central orchestrator of the biosynthetic and energetic programs that sustain tumor growth [113]. Traditional in vitro models, while indispensable, are often limited by supraphysiologic glucose and glutamine concentrations and non-physiologic oxygen content, conditions that may distort metabolic dependencies relative to the native tumor microenvironment [114]. However, the expanding toolkit of both spatial and stable isotope-resolved metabolomics (SIRM) now enables these regulatory networks to be mapped within the authentic context of human NB tissue and patient-derived samples, offering an opportunity to connect molecular rewiring, genetic features, and clinical behavior [16,17]. The capacity to measure direct metabolite flux within pediatric cancers was recently reported by Johnston et al. [39]. In addition, our group recently leveraged MALDI-MSI to profile the N-linked glycome of human NBs [11]. The spatial-preserving nature of this analysis noted marked N-linked glycan heterogeneity throughout the human tumor samples, emphasizing limitations to traditional metabolomic analysis of bulk homogenates. Together, these advances underscore the critical importance of studying neuroblastoma metabolism within the intact human tumor context, where spatial heterogeneity and physiologic conditions can be faithfully captured to reveal clinically actionable vulnerabilities.
Emerging evidence increasingly suggests that MYCN’s oncogenic consequences extend beyond core canonical metabolism into glycosylation, a complex post-translational signaling axis. Glycan remodeling represents a critical interface between metabolic rewiring and cell–cell communication, with profound implications for immune recognition, invasion, and therapy resistance. The hexosamine biosynthetic pathway (HBP) exemplifies this integration, serving as a central metabolic shunt supplied by glycolytic, glutamine, and fatty acid-derived intermediates to generate UDP-GlcNAc for N- and O-linked glycosylation. Further details of this integration of the HBP as a central hub is provided in a separate review by Akella et al. [115]. Despite differential end-product abundance manifest by cell surface glycan expression and upstream substrate sources that are MYCN-dependent, this dimension of MYCN biology remains understudied. Given the role of glycosylation in facilitating cell surface and paracrine interactions, our group views glycosylation as an integrated output of MYCN-driven transformation that warrants systematic exploration. Together, these converging areas of investigation position MYCN at the nexus of metabolic and post-translational control, reinforcing its role as a central driver of NB biology and a compelling therapeutic target.
Importantly, metabolic and glycosylation-dependent vulnerabilities already intersect with therapies that have transformed the clinical management of high-risk NB. Aberrant sialylation is exploited in anti-GD2–based immunotherapy, an FDA-approved antibody strategy that improves survival in children with high-risk disease. Similarly, the polyamine axis is another MYCN-coupled metabolic output that is being leveraged in the clinic using the irreversible ODC1 inhibitor eflornithine (DFMO) to reduce relapse risk following anti-GD2 therapy. These precedents validate the principle that MYCN-programmed metabolism is both mechanistically central and therapeutically actionable. A summary of pathway-specific therapeutic interventions is included in Table 2.

Table 2.
Pathway-specific metabolic vulnerabilities and therapeutic interventions in neuroblastoma.
NB metabolism is dynamic and heterogeneous. Systematic implementation of approaches such as spatial metabolomics and SIRM to map nutrient flux, quantify pathway end-products, and define regional heterogeneity in situ will enable investigators to move beyond reliance on expression-based surrogates to capture the functional metabolic state of high-risk NBs. These advanced metabolomic strategies may also define the clinical contexts in which known metabolic aberrancies, such as sialylation and polyamine biosynthesis, might be most effectively leveraged, while uncovering new metabolic nodes of oncogenic potential within this devastating and metabolically driven childhood cancer.
Author Contributions
Writing—original draft preparation, M.G.P., L.T.B. and M.D.B.; writing—review and editing, M.G.P. and E.J.R.; supervision, E.J.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was generously supported by the University of Kentucky Center for Cancer and Metabolism, funded through the NIH/NIGMS COBRE (P20 GM121327) and the Dick Vitale Pediatric Cancer Research Fund (V2023-026) to E.J.R.
Acknowledgments
Figure summary was created with BioRender.com under a publication license.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| NB | neuroblastoma |
| MYCN-amp | MYCN-amplified |
| LDHA | lactate dehydrogenase A |
| HK2 | hexokinase 2 |
| GLS2 | mitochondrial glutaminase |
| PHGDH | phosphoglycerate dehydrogenase |
| ODC | ornithine decarboxylase |
| SAT1 | spermine/spermidine acetyltransferase |
| FASN | fatty acid synthase |
| GMDS | GDP mannose 4,6-dehydratase |
| FUT8 | Fucosyltransferase 8 |
| HDAC | histone deacetylase |
| MALDI-MSI | matrix-assisted laser desorption ionization mass spectrometry imaging |
| SIRM | stable isotope-resolved metabolomics |
References
- DuBois, S.G.; Moreno, L.; Anderson, J.; Asgharzadeh, S.; Bagatell, R.; Beck-Popovic, M.; Belle, J.; Berlanga, P.; Bird, N.J.; Chesler, L.; et al. Accelerating Drug Development for Neuroblastoma: Consensus Statement from the Third Neuroblastoma Drug Development Strategy Forum. Pediatr. Blood Cancer 2025, 72, e31831. [Google Scholar] [CrossRef]
- DuBois, S.G.; Macy, M.E.; Henderson, T.O. High-Risk and Relapsed Neuroblastoma: Toward More Cures and Better Outcomes. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 768–780. [Google Scholar] [CrossRef]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Primers 2016, 2, 16078. [Google Scholar] [CrossRef]
- Stine, Z.E.; Walton, Z.E.; Altman, B.J.; Hsieh, A.L.; Dang, C.V. MYC, Metabolism, and Cancer. Cancer Discov. 2015, 5, 1024–1039. [Google Scholar] [CrossRef]
- Dang, C.V. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a014217. [Google Scholar] [CrossRef]
- Osthus, R.C.; Shim, H.; Kim, S.; Li, Q.; Reddy, R.; Mukherjee, M.; Xu, Y.; Wonsey, D.; Lee, L.A.; Dang, C.V. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 2000, 275, 21797–21800. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Y.; Zeller, K.I.; Potter, J.J.; Wonsey, D.R.; O’Donnell, K.A.; Kim, J.W.; Yustein, J.T.; Lee, L.A.; Dang, C.V. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol. Cell. Biol. 2005, 25, 6225–6234. [Google Scholar] [CrossRef]
- Rickman, D.S.; Schulte, J.H.; Eilers, M. The Expanding World of N-MYC-Driven Tumors. Cancer Discov. 2018, 8, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ma, R.; Wu, Y.; Zhai, Y.; Li, S. Reciprocal Regulation of Metabolic Reprogramming and Epigenetic Modifications in Cancer. Front. Genet. 2018, 9, 394. [Google Scholar] [CrossRef] [PubMed]
- Crispo, F.; Condelli, V.; Lepore, S.; Notarangelo, T.; Sgambato, A.; Esposito, F.; Maddalena, F.; Landriscina, M. Metabolic Dysregulations and Epigenetics: A Bidirectional Interplay that Drives Tumor Progression. Cells 2019, 8, 798. [Google Scholar] [CrossRef]
- Zhu, B.; Pitts, M.G.; Buoncristiani, M.D.; Bryant, L.T.; Lopez-Nunez, O.; Gurria, J.P.; Shedlock, C.; Ribas, R.; Keohane, S.; Liu, J.; et al. GDP-mannose 4,6-dehydratase is a key driver of MYCN-amplified neuroblastoma core fucosylation and tumorigenesis. Oncogene 2025, 44, 1272–1283. [Google Scholar] [CrossRef]
- Tian, L.; Li, H.; Zhao, P.; Liu, Y.; Lu, Y.; Zhong, R.; Jin, Y.; Tan, T.; Cheng, Y. C-Myc-induced hypersialylation of small cell lung cancer facilitates pro-tumoral phenotypes of macrophages. iScience 2023, 26, 107771. [Google Scholar] [CrossRef]
- Smith, B.A.H.; Deutzmann, A.; Correa, K.M.; Delaveris, C.S.; Dhanasekaran, R.; Dove, C.G.; Sullivan, D.K.; Wisnovsky, S.; Stark, J.C.; Pluvinage, J.V.; et al. MYC-driven synthesis of Siglec ligands is a glycoimmune checkpoint. Proc. Natl. Acad. Sci. USA 2023, 120, e2215376120. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Mohnen, D.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. (Eds.) Essentials of Glycobiology, 4th ed.; Cold Spring Harbor Laboratory Press: Woodbury, NY, USA, 2022. [Google Scholar]
- Lin, Y.; Lubman, D.M. The role of N-glycosylation in cancer. Acta Pharm. Sin. B 2024, 14, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- Stanback, A.E.; Conroy, L.R.; Young, L.E.A.; Hawkinson, T.R.; Markussen, K.H.; Clarke, H.A.; Allison, D.B.; Sun, R.C. Regional N-glycan and lipid analysis from tissues using MALDI-mass spectrometry imaging. STAR Protoc. 2021, 2, 100304. [Google Scholar] [CrossRef]
- Lane, A.N.; Higashi, R.M.; Fan, T.W. NMR and MS-based Stable Isotope-Resolved Metabolomics and Applications in Cancer Metabolism. Trends Analyt. Chem. 2019, 120, 115322. [Google Scholar] [CrossRef] [PubMed]
- Tjaden, B.; Baum, K.; Marquardt, V.; Simon, M.; Trajkovic-Arsic, M.; Kouril, T.; Siebers, B.; Lisec, J.; Siveke, J.T.; Schulte, J.H.; et al. N-Myc-induced metabolic rewiring creates novel therapeutic vulnerabilities in neuroblastoma. Sci. Rep. 2020, 10, 7157. [Google Scholar] [CrossRef]
- Levy, A.G.; Zage, P.E.; Akers, L.J.; Ghisoli, M.L.; Chen, Z.; Fang, W.; Kannan, S.; Graham, T.; Zeng, L.; Franklin, A.R.; et al. The combination of the novel glycolysis inhibitor 3-BrOP and rapamycin is effective against neuroblastoma. Investig. New Drugs 2012, 30, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Qing, G.; Skuli, N.; Mayes, P.A.; Pawel, B.; Martinez, D.; Maris, J.M.; Simon, M.C. Combinatorial regulation of neuroblastoma tumor progression by N-Myc and hypoxia inducible factor HIF-1alpha. Cancer Res. 2010, 70, 10351–10361. [Google Scholar] [CrossRef]
- Rellinger, E.J.; Craig, B.T.; Alvarez, A.L.; Dusek, H.L.; Kim, K.W.; Qiao, J.; Chung, D.H. FX11 inhibits aerobic glycolysis and growth of neuroblastoma cells. Surgery 2017, 161, 747–752. [Google Scholar] [CrossRef]
- Xiao, D.; Ren, P.; Su, H.; Yue, M.; Xiu, R.; Hu, Y.; Liu, H.; Qing, G. Myc promotes glutaminolysis in human neuroblastoma through direct activation of glutaminase 2. Oncotarget 2015, 6, 40655–40666. [Google Scholar] [CrossRef]
- Ren, P.; Yue, M.; Xiao, D.; Xiu, R.; Gan, L.; Liu, H.; Qing, G. ATF4 and N-Myc coordinate glutamine metabolism in MYCN-amplified neuroblastoma cells through ASCT2 activation. J. Pathol. 2015, 235, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Ye, B.; Ding, J.; Yu, Y.; Alptekin, A.; Thangaraju, M.; Prasad, P.D.; Ding, Z.C.; Park, E.J.; Choi, J.H.; et al. Metabolic Reprogramming by MYCN Confers Dependence on the Serine-Glycine-One-Carbon Biosynthetic Pathway. Cancer Res. 2019, 79, 3837–3850. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Li, M.; Wang, Y.; Zhang, Y.; Que, Y.; Lu, S.; Wang, J.; Zhu, J.; Huang, J.; Zhen, Z.; et al. MTHFD1 regulates the NADPH redox homeostasis in MYCN-amplified neuroblastoma. Cell Death Dis. 2024, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.T.; Flemming, C.L.; Gherardi, S.; Perini, G.; Oberthuer, A.; Fischer, M.; Juraeva, D.; Brors, B.; Xue, C.; Norris, M.D.; et al. MYCN amplification confers enhanced folate dependence and methotrexate sensitivity in neuroblastoma. Oncotarget 2015, 6, 15510–15523. [Google Scholar] [CrossRef] [PubMed]
- Hogarty, M.D.; Norris, M.D.; Davis, K.; Liu, X.; Evageliou, N.F.; Hayes, C.S.; Pawel, B.; Guo, R.; Zhao, H.; Sekyere, E.; et al. ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res. 2008, 68, 9735–9745. [Google Scholar] [CrossRef]
- Gamble, L.D.; Purgato, S.; Murray, J.; Xiao, L.; Yu, D.M.T.; Hanssen, K.M.; Giorgi, F.M.; Carter, D.R.; Gifford, A.J.; Valli, E.; et al. Inhibition of polyamine synthesis and uptake reduces tumor progression and prolongs survival in mouse models of neuroblastoma. Sci. Transl. Med. 2019, 11, eaau1099. [Google Scholar] [CrossRef]
- Ruiz-Perez, M.V.; Sainero-Alcolado, L.; Oliynyk, G.; Matuschek, I.; Balboni, N.; Ubhayasekera, S.; Snaebjornsson, M.T.; Makowski, K.; Aaltonen, K.; Bexell, D.; et al. Inhibition of fatty acid synthesis induces differentiation and reduces tumor burden in childhood neuroblastoma. iScience 2021, 24, 102128. [Google Scholar] [CrossRef]
- Moreno-Smith, M.; Milazzo, G.; Tao, L.; Fekry, B.; Zhu, B.; Mohammad, M.A.; Di Giacomo, S.; Borkar, R.; Reddy, K.R.K.; Capasso, M.; et al. Restoration of the molecular clock is tumor suppressive in neuroblastoma. Nat. Commun. 2021, 12, 4006. [Google Scholar] [CrossRef]
- Tao, L.; Mohammad, M.A.; Milazzo, G.; Moreno-Smith, M.; Patel, T.D.; Zorman, B.; Badachhape, A.; Hernandez, B.E.; Wolf, A.B.; Zeng, Z.; et al. MYCN-driven fatty acid uptake is a metabolic vulnerability in neuroblastoma. Nat. Commun. 2022, 13, 3728. [Google Scholar] [CrossRef]
- Nakagawara, A.; Arima, M.; Azar, C.G.; Scavarda, N.J.; Brodeur, G.M. Inverse relationship between trk expression and N-myc amplification in human neuroblastomas. Cancer Res. 1992, 52, 1364–1368. [Google Scholar] [PubMed]
- Zeid, R.; Lawlor, M.A.; Poon, E.; Reyes, J.M.; Fulciniti, M.; Lopez, M.A.; Scott, T.G.; Nabet, B.; Erb, M.A.; Winter, G.E.; et al. Enhancer invasion shapes MYCN-dependent transcriptional amplification in neuroblastoma. Nat. Genet. 2018, 50, 515–523. [Google Scholar] [CrossRef]
- Zimmerman, M.W.; Durbin, A.D.; He, S.; Oppel, F.; Shi, H.; Tao, T.; Li, Z.; Berezovskaya, A.; Liu, Y.; Zhang, J.; et al. Retinoic acid rewires the adrenergic core regulatory circuitry of childhood neuroblastoma. Sci. Adv. 2021, 7, eabe0834. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. We need to talk about the Warburg effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef]
- Tang, Q.; Wu, S.; Zhao, B.; Li, Z.; Zhou, Q.; Yu, Y.; Yang, X.; Wang, R.; Wang, X.; Wu, W.; et al. Reprogramming of glucose metabolism: The hallmark of malignant transformation and target for advanced diagnostics and treatments. Biomed. Pharmacother. 2024, 178, 117257. [Google Scholar] [CrossRef]
- Shulkin, B.L.; Mitchell, D.S.; Ungar, D.R.; Prakash, D.; Dole, M.G.; Castle, V.P.; Hernandez, R.J.; Koeppe, R.A.; Hutchinson, R.J. Neoplasms in a pediatric population: 2-[F-18]-fluoro-2-deoxy-D-glucose PET studies. Radiology 1995, 194, 495–500. [Google Scholar] [CrossRef]
- Johnston, K.; Pachnis, P.; Tasdogan, A.; Faubert, B.; Zacharias, L.G.; Vu, H.S.; Rodgers-Augustyniak, L.; Johnson, A.; Huang, F.; Ricciardo, S.; et al. Isotope tracing reveals glycolysis and oxidative metabolism in childhood tumors of multiple histologies. Med 2021, 2, 395–410. [Google Scholar] [CrossRef]
- Morrish, F.; Neretti, N.; Sedivy, J.M.; Hockenbery, D.M. The oncogene c-Myc coordinates regulation of metabolic networks to enable rapid cell cycle entry. Cell Cycle 2008, 7, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Oliynyk, G.; Ruiz-Perez, M.V.; Sainero-Alcolado, L.; Dzieran, J.; Zirath, H.; Gallart-Ayala, H.; Wheelock, C.E.; Johansson, H.J.; Nilsson, R.; Lehtio, J.; et al. MYCN-enhanced Oxidative and Glycolytic Metabolism Reveals Vulnerabilities for Targeting Neuroblastoma. iScience 2019, 21, 188–204. [Google Scholar] [CrossRef]
- Pusapati, R.V.; Daemen, A.; Wilson, C.; Sandoval, W.; Gao, M.; Haley, B.; Baudy, A.R.; Hatzivassiliou, G.; Evangelista, M.; Settleman, J. mTORC1-Dependent Metabolic Reprogramming Underlies Escape from Glycolysis Addiction in Cancer Cells. Cancer Cell 2016, 29, 548–562. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, L.; Clarke, P.A.; Barker, K.; Chanthery, Y.; Gustafson, C.W.; Tucker, E.; Renshaw, J.; Raynaud, F.; Li, X.; Burke, R.; et al. Inhibition of mTOR-kinase destabilizes MYCN and is a potential therapy for MYCN-dependent tumors. Oncotarget 2016, 7, 57525–57544. [Google Scholar] [CrossRef] [PubMed]
- Dorneburg, C.; Fischer, M.; Barth, T.F.E.; Mueller-Klieser, W.; Hero, B.; Gecht, J.; Carter, D.R.; de Preter, K.; Mayer, B.; Christner, L.; et al. LDHA in Neuroblastoma Is Associated with Poor Outcome and Its Depletion Decreases Neuroblastoma Growth Independent of Aerobic Glycolysis. Clin. Cancer Res. 2018, 24, 5772–5783. [Google Scholar] [CrossRef] [PubMed]
- Panosyan, W.S.; Panosyan, D.E.; Koster, J.; Panosyan, E.H. Anti-GD2 immunoliposomes loaded with oxamate for neuroblastoma. Pediatr. Res. 2023, 94, 458–461. [Google Scholar] [CrossRef]
- Jiang, H.; Tiche, S.J.; He, C.J.; Liu, J.; Bian, F.; Jedoui, M.; Forgo, B.; Islam, M.T.; Zhao, M.; Emengo, P.; et al. Restoring mitochondrial quantity and quality to reverse the Warburg effect and drive neuroblastoma differentiation. Proc. Natl. Acad. Sci. USA 2025, 122, e2502483122. [Google Scholar] [CrossRef]
- Chen, P.H.; Cai, L.; Huffman, K.; Yang, C.; Kim, J.; Faubert, B.; Boroughs, L.; Ko, B.; Sudderth, J.; McMillan, E.A.; et al. Metabolic Diversity in Human Non-Small Cell Lung Cancer Cells. Mol. Cell 2019, 76, 838–851.e835. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Shackelford, D.B. Metabolic Phenotypes, Dependencies, and Adaptation in Lung Cancer. Cold Spring Harb. Perspect. Med. 2021, 11, a037838. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef]
- Carter, D.R.; Sutton, S.K.; Pajic, M.; Murray, J.; Sekyere, E.O.; Fletcher, J.; Beckers, A.; De Preter, K.; Speleman, F.; George, R.E.; et al. Glutathione biosynthesis is upregulated at the initiation of MYCN-driven neuroblastoma tumorigenesis. Mol. Oncol. 2016, 10, 866–878. [Google Scholar] [CrossRef]
- Qing, G.; Li, B.; Vu, A.; Skuli, N.; Walton, Z.E.; Liu, X.; Mayes, P.A.; Wise, D.R.; Thompson, C.B.; Maris, J.M.; et al. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell 2012, 22, 631–644. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, M.; Dong, X.; Yang, Y.; Qin, X.; Zhou, X.; Fu, Y.; Wang, Y.; Wang, J.; Zhang, D. Predictive role of SLC1A5 in neuroblastoma prognosis and immunotherapy. BMC Cancer 2025, 25, 161. [Google Scholar] [CrossRef]
- Reina-Campos, M.; Diaz-Meco, M.T.; Moscat, J. The complexity of the serine glycine one-carbon pathway in cancer. J. Cell Biol. 2020, 219, e201907022. [Google Scholar] [CrossRef]
- Cheung, C.H.Y.; Hsu, C.L.; Tsuei, C.Y.; Kuo, T.T.; Huang, C.T.; Hsu, W.M.; Chung, Y.H.; Wu, H.Y.; Hsu, C.C.; Huang, H.C.; et al. Combinatorial targeting of MTHFD2 and PAICS in purine synthesis as a novel therapeutic strategy. Cell Death Dis. 2019, 10, 786. [Google Scholar] [CrossRef]
- Alborzinia, H.; Florez, A.F.; Kreth, S.; Bruckner, L.M.; Yildiz, U.; Gartlgruber, M.; Odoni, D.I.; Poschet, G.; Garbowicz, K.; Shao, C.; et al. MYCN mediates cysteine addiction and sensitizes neuroblastoma to ferroptosis. Nat. Cancer 2022, 3, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Jiang, J.; Gao, P.; Liu, H.; Qing, G. Oncogenic MYC Activates a Feedforward Regulatory Loop Promoting Essential Amino Acid Metabolism and Tumorigenesis. Cell Rep. 2017, 21, 3819–3832. [Google Scholar] [CrossRef] [PubMed]
- Vande Voorde, J.; Ackermann, T.; Pfetzer, N.; Sumpton, D.; Mackay, G.; Kalna, G.; Nixon, C.; Blyth, K.; Gottlieb, E.; Tardito, S. Improving the metabolic fidelity of cancer models with a physiological cell culture medium. Sci. Adv. 2019, 5, eaau7314. [Google Scholar] [CrossRef] [PubMed]
- Pegg, A.E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988, 48, 759–774. [Google Scholar]
- Rounbehler, R.J.; Li, W.; Hall, M.A.; Yang, C.; Fallahi, M.; Cleveland, J.L. Targeting ornithine decarboxylase impairs development of MYCN-amplified neuroblastoma. Cancer Res. 2009, 69, 547–553. [Google Scholar] [CrossRef]
- Evageliou, N.F.; Haber, M.; Vu, A.; Laetsch, T.W.; Murray, J.; Gamble, L.D.; Cheng, N.C.; Liu, K.; Reese, M.; Corrigan, K.A.; et al. Polyamine Antagonist Therapies Inhibit Neuroblastoma Initiation and Progression. Clin. Cancer Res. 2016, 22, 4391–4404. [Google Scholar] [CrossRef]
- Azfar, M.; Gao, W.; Van den Haute, C.; Xiao, L.; Karsa, M.; Pandher, R.; Karsa, A.; Spurling, D.; Ronca, E.; Bongers, A.; et al. The polyamine transporter ATP13A3 mediates difluoromethylornithine-induced polyamine uptake in neuroblastoma. Mol. Oncol. 2025, 19, 913–936. [Google Scholar] [CrossRef]
- Samal, K.; Zhao, P.; Kendzicky, A.; Yco, L.P.; McClung, H.; Gerner, E.; Burns, M.; Bachmann, A.S.; Sholler, G. AMXT-1501, a novel polyamine transport inhibitor, synergizes with DFMO in inhibiting neuroblastoma cell proliferation by targeting both ornithine decarboxylase and polyamine transport. Int. J. Cancer 2013, 133, 1323–1333. [Google Scholar] [CrossRef]
- Oesterheld, J.; Ferguson, W.; Kraveka, J.M.; Bergendahl, G.; Clinch, T.; Lorenzi, E.; Berry, D.; Wada, R.K.; Isakoff, M.S.; Eslin, D.E.; et al. Eflornithine as Postimmunotherapy Maintenance in High-Risk Neuroblastoma: Externally Controlled, Propensity Score-Matched Survival Outcome Comparisons. J. Clin. Oncol. 2024, 42, 90–102. [Google Scholar] [CrossRef]
- Sholler, G.L.S.; Ferguson, W.; Bergendahl, G.; Bond, J.P.; Neville, K.; Eslin, D.; Brown, V.; Roberts, W.; Wada, R.K.; Oesterheld, J.; et al. Maintenance DFMO Increases Survival in High Risk Neuroblastoma. Sci. Rep. 2018, 8, 14445. [Google Scholar] [CrossRef]
- Hogarty, M.D.; Ziegler, D.S.; Franson, A.; Chi, Y.Y.; Tsao-Wei, D.; Liu, K.; Vemu, R.; Gerner, E.W.; Bruckheimer, E.; Shamirian, A.; et al. Phase 1 study of high-dose DFMO, celecoxib, cyclophosphamide and topotecan for patients with relapsed neuroblastoma: A New Approaches to Neuroblastoma Therapy trial. Br. J. Cancer 2024, 130, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Cherkaoui, S.; Yang, L.; McBride, M.; Turn, C.S.; Lu, W.; Eigenmann, C.; Allen, G.E.; Panasenko, O.O.; Zhang, L.; Vu, A.; et al. Reprogramming neuroblastoma by diet-enhanced polyamine depletion. bioRxiv 2024, 2024.2001.2007.573662. [Google Scholar] [CrossRef] [PubMed]
- Lozier, A.M.; Rich, M.E.; Grawe, A.P.; Peck, A.S.; Zhao, P.; Chang, A.T.; Bond, J.P.; Sholler, G.S. Targeting ornithine decarboxylase reverses the LIN28/Let-7 axis and inhibits glycolytic metabolism in neuroblastoma. Oncotarget 2015, 6, 196–206. [Google Scholar] [CrossRef]
- Zhu, H.; Shyh-Chang, N.; Segre, A.V.; Shinoda, G.; Shah, S.P.; Einhorn, W.S.; Takeuchi, A.; Engreitz, J.M.; Hagan, J.P.; Kharas, M.G.; et al. The Lin28/let-7 axis regulates glucose metabolism. Cell 2011, 147, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Snaebjornsson, M.T.; Janaki-Raman, S.; Schulze, A. Greasing the Wheels of the Cancer Machine: The Role of Lipid Metabolism in Cancer. Cell Metab. 2020, 31, 62–76. [Google Scholar] [CrossRef]
- Carroll, P.A.; Diolaiti, D.; McFerrin, L.; Gu, H.; Djukovic, D.; Du, J.; Cheng, P.F.; Anderson, S.; Ulrich, M.; Hurley, J.B.; et al. Deregulated Myc requires MondoA/Mlx for metabolic reprogramming and tumorigenesis. Cancer Cell 2015, 27, 271–285. [Google Scholar] [CrossRef]
- Gouw, A.M.; Margulis, K.; Liu, N.S.; Raman, S.J.; Mancuso, A.; Toal, G.G.; Tong, L.; Mosley, A.; Hsieh, A.L.; Sullivan, D.K.; et al. The MYC Oncogene Cooperates with Sterol-Regulated Element-Binding Protein to Regulate Lipogenesis Essential for Neoplastic Growth. Cell Metab. 2019, 30, 556–572.e5. [Google Scholar] [CrossRef]
- Veglia, F.; Tyurin, V.A.; Blasi, M.; De Leo, A.; Kossenkov, A.V.; Donthireddy, L.; To, T.K.J.; Schug, Z.; Basu, S.; Wang, F.; et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature 2019, 569, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Zirath, H.; Frenzel, A.; Oliynyk, G.; Segerstrom, L.; Westermark, U.K.; Larsson, K.; Munksgaard Persson, M.; Hultenby, K.; Lehtio, J.; Einvik, C.; et al. MYC inhibition induces metabolic changes leading to accumulation of lipid droplets in tumor cells. Proc. Natl. Acad. Sci. USA 2013, 110, 10258–10263. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Temkin, S.M.; Hawkridge, A.M.; Guo, C.; Wang, W.; Wang, X.Y.; Fang, X. Fatty acid oxidation: An emerging facet of metabolic transformation in cancer. Cancer Lett. 2018, 435, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, S.; Zhang, P.; Zheng, S.; Li, X.; Li, J.; Pei, H. Emerging roles for fatty acid oxidation in cancer. Genes Dis. 2025, 12, 101491. [Google Scholar] [CrossRef]
- Batchuluun, B.; Pinkosky, S.L.; Steinberg, G.R. Lipogenesis inhibitors: Therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 2022, 21, 283–305. [Google Scholar] [CrossRef]
- Paneque, A.; Fortus, H.; Zheng, J.; Werlen, G.; Jacinto, E. The Hexosamine Biosynthesis Pathway: Regulation and Function. Genes 2023, 14, 933. [Google Scholar] [CrossRef]
- Balis, F.M.; Busch, C.M.; Desai, A.V.; Hibbitts, E.; Naranjo, A.; Bagatell, R.; Irwin, M.; Fox, E. The ganglioside G(D2) as a circulating tumor biomarker for neuroblastoma. Pediatr. Blood Cancer 2020, 67, e28031. [Google Scholar] [CrossRef]
- Qin, W.; Pei, H.; Li, X.; Li, J.; Yao, X.; Zhang, R. Serum Protein N-Glycosylation Signatures of Neuroblastoma. Front. Oncol. 2021, 11, 603417. [Google Scholar] [CrossRef]
- Bley, I.A.; Behrens, S.; Spohn, M.; Muller, I.; Schattling, B. Genetic Risk Profiling Reveals Altered Glycosyltransferase Expression as a Predictor for Patient Outcome in Neuroblastoma. J. Clin. Med. 2025, 14, 527. [Google Scholar] [CrossRef]
- Hu, Y.; Mayampurath, A.; Khan, S.; Cohen, J.K.; Mechref, Y.; Volchenboum, S.L. N-linked glycan profiling in neuroblastoma cell lines. J. Proteome Res. 2015, 14, 2074–2081. [Google Scholar] [CrossRef]
- Cuello, H.A.; Segatori, V.I.; Alberto, M.; Gulino, C.A.; Aschero, R.; Camarero, S.; Mutti, L.G.; Madauss, K.; Alonso, D.F.; Lubieniecki, F.; et al. Aberrant O-glycosylation modulates aggressiveness in neuroblastoma. Oncotarget 2018, 9, 34176–34188. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, H.S.; Cordon-Cardo, C.; Reuter, V.E.; Singhal, A.K.; Lloyd, K.O.; Livingston, P.O. Selection of tumor antigens as targets for immune attack using immunohistochemistry: II. Blood group-related antigens. Int. J. Cancer 1997, 73, 50–56. [Google Scholar] [CrossRef]
- Tran, D.T.; Ten Hagen, K.G. Mucin-type O-glycosylation during development. J. Biol. Chem. 2013, 288, 6921–6929. [Google Scholar] [CrossRef]
- Lin, N.Y.; Chen, S.T.; Chang, H.L.; Lu, M.Y.; Yang, Y.L.; Chou, S.W.; Lin, D.T.; Lin, K.H.; Jou, S.T.; Hsu, W.M.; et al. C1GALT1 expression predicts a favorable prognosis and suppresses malignant phenotypes via TrkA signaling in neuroblastoma. Oncogenesis 2022, 11, 8. [Google Scholar] [CrossRef]
- Higashi, M.; Sakai, K.; Fumino, S.; Aoi, S.; Furukawa, T.; Tajiri, T. The roles played by the MYCN, Trk, and ALK genes in neuroblastoma and neural development. Surg. Today 2019, 49, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Shan, M.; Yang, D.; Dou, H.; Zhang, L. Fucosylation in cancer biology and its clinical applications. Prog. Mol. Biol. Transl. Sci. 2019, 162, 93–119. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, E.; Liu, Q.; Burton, C.; Mockabee-Macias, A.; Lester, D.K.; Lau, E. L-fucose, a sugary regulator of antitumor immunity and immunotherapies. Mol. Carcinog. 2022, 61, 439–453. [Google Scholar] [CrossRef]
- Sosicka, P.; Ng, B.G.; Pepi, L.E.; Shajahan, A.; Wong, M.; Scott, D.A.; Matsumoto, K.; Xia, Z.J.; Lebrilla, C.B.; Haltiwanger, R.S.; et al. Origin of cytoplasmic GDP-fucose determines its contribution to glycosylation reactions. J. Cell Biol. 2022, 221, e202205038. [Google Scholar] [CrossRef]
- Seifert, A.; Glanz, D.; Glaubitz, N.; Horstkorte, R.; Bork, K. Polysialylation of the neural cell adhesion molecule: Interfering with polysialylation and migration in neuroblastoma cells. Arch. Biochem. Biophys. 2012, 524, 56–63. [Google Scholar] [CrossRef]
- Gluer, S.; Zense, M.; Radtke, E.; von Schweinitz, D. Polysialylated neural cell adhesion molecule in childhood ganglioneuroma and neuroblastoma of different histological grade and clinical stage. Langenbecks Arch. Surg. 1998, 383, 340–344. [Google Scholar] [CrossRef]
- Korja, M.; Jokilammi, A.; Salmi, T.T.; Kalimo, H.; Pelliniemi, T.T.; Isola, J.; Rantala, I.; Haapasalo, H.; Finne, J. Absence of polysialylated NCAM is an unfavorable prognostic phenotype for advanced stage neuroblastoma. BMC Cancer 2009, 9, 57. [Google Scholar] [CrossRef]
- Machy, P.; Mortier, E.; Birkle, S. Biology of GD2 ganglioside: Implications for cancer immunotherapy. Front. Pharmacol. 2023, 14, 1249929. [Google Scholar] [CrossRef]
- Groux-Degroote, S.; Guerardel, Y.; Delannoy, P. Gangliosides: Structures, Biosynthesis, Analysis, and Roles in Cancer. ChemBioChem 2017, 18, 1146–1154. [Google Scholar] [CrossRef]
- Ngamukote, S.; Yanagisawa, M.; Ariga, T.; Ando, S.; Yu, R.K. Developmental changes of glycosphingolipids and expression of glycogenes in mouse brains. J. Neurochem. 2007, 103, 2327–2341. [Google Scholar] [CrossRef] [PubMed]
- Paret, C.; Ustjanzew, A.; Ersali, S.; Seidmann, L.; Jennemann, R.; Ziegler, N.; Malki, K.E.; Russo, A.; Wingerter, A.; Ortmuller, F.; et al. GD2 Expression in Medulloblastoma and Neuroblastoma for Personalized Immunotherapy: A Matter of Subtype. Cancers 2022, 14, 6051. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Schwartz, E.; Seeger, R.; Ladisch, S. Expression of GD2 ganglioside by untreated primary human neuroblastomas. Cancer Res. 1986, 46, 440–443. [Google Scholar] [PubMed]
- van Groningen, T.; Koster, J.; Valentijn, L.J.; Zwijnenburg, D.A.; Akogul, N.; Hasselt, N.E.; Broekmans, M.; Haneveld, F.; Nowakowska, N.E.; Bras, J.; et al. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat. Genet. 2017, 49, 1261–1266. [Google Scholar] [CrossRef]
- Thirant, C.; Peltier, A.; Durand, S.; Kramdi, A.; Louis-Brennetot, C.; Pierre-Eugene, C.; Gautier, M.; Costa, A.; Grelier, A.; Zaidi, S.; et al. Reversible transitions between noradrenergic and mesenchymal tumor identities define cell plasticity in neuroblastoma. Nat. Commun. 2023, 14, 2575. [Google Scholar] [CrossRef]
- Avitabile, M.; Bonfiglio, F.; Aievola, V.; Cantalupo, S.; Maiorino, T.; Lasorsa, V.A.; Domenicotti, C.; Marengo, B.; Zbynek, H.; Vojtech, A.; et al. Single-cell transcriptomics of neuroblastoma identifies chemoresistance-associated genes and pathways. Comput. Struct. Biotechnol. J. 2022, 20, 4437–4445. [Google Scholar] [CrossRef]
- Mabe, N.W.; Huang, M.; Dalton, G.N.; Alexe, G.; Schaefer, D.A.; Geraghty, A.C.; Robichaud, A.L.; Conway, A.S.; Khalid, D.; Mader, M.M.; et al. Transition to a mesenchymal state in neuroblastoma confers resistance to anti-GD2 antibody via reduced expression of ST8SIA1. Nat. Cancer 2022, 3, 976–993. [Google Scholar] [CrossRef]
- Theruvath, J.; Menard, M.; Smith, B.A.H.; Linde, M.H.; Coles, G.L.; Dalton, G.N.; Wu, W.; Kiru, L.; Delaidelli, A.; Sotillo, E.; et al. Anti-GD2 synergizes with CD47 blockade to mediate tumor eradication. Nat. Med. 2022, 28, 333–344. [Google Scholar] [CrossRef]
- Cheresh, D.A.; Pierschbacher, M.D.; Herzig, M.A.; Mujoo, K. Disialogangliosides GD2 and GD3 are involved in the attachment of human melanoma and neuroblastoma cells to extracellular matrix proteins. J. Cell Biol. 1986, 102, 688–696. [Google Scholar] [CrossRef]
- Ladisch, S.; Wu, Z.L.; Feig, S.; Ulsh, L.; Schwartz, E.; Floutsis, G.; Wiley, F.; Lenarsky, C.; Seeger, R. Shedding of GD2 ganglioside by human neuroblastoma. Int. J. Cancer 1987, 39, 73–76. [Google Scholar] [CrossRef]
- Terzic, T.; Cordeau, M.; Herblot, S.; Teira, P.; Cournoyer, S.; Beaunoyer, M.; Peuchmaur, M.; Duval, M.; Sartelet, H. Expression of Disialoganglioside (GD2) in Neuroblastic Tumors: A Prognostic Value for Patients Treated with Anti-GD2 Immunotherapy. Pediatr. Dev. Pathol. 2018, 21, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Schumacher-Kuckelkorn, R.; Hero, B.; Ernestus, K.; Berthold, F. Lacking immunocytological GD2 expression in neuroblastoma: Report of 3 cases. Pediatr. Blood Cancer 2005, 45, 195–201. [Google Scholar] [CrossRef]
- Schumacher-Kuckelkorn, R.; Volland, R.; Gradehandt, A.; Hero, B.; Simon, T.; Berthold, F. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence. Pediatr. Blood Cancer 2017, 64, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Heise, T.; Pijnenborg, J.F.A.; Bull, C.; van Hilten, N.; Kers-Rebel, E.D.; Balneger, N.; Elferink, H.; Adema, G.J.; Boltje, T.J. Potent Metabolic Sialylation Inhibitors Based on C-5-Modified Fluorinated Sialic Acids. J. Med. Chem. 2019, 62, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Rossing, E.; Pijnenborg, J.F.A.; Boltje, T.J. Chemical tools to track and perturb the expression of sialic acid and fucose monosaccharides. Chem. Commun. 2022, 58, 12139–12150. [Google Scholar] [CrossRef]
- van den Bijgaart, R.J.E.; Kroesen, M.; Wassink, M.; Brok, I.C.; Kers-Rebel, E.D.; Boon, L.; Heise, T.; van Scherpenzeel, M.; Lefeber, D.J.; Boltje, T.J.; et al. Combined sialic acid and histone deacetylase (HDAC) inhibitor treatment up-regulates the neuroblastoma antigen GD2. J. Biol. Chem. 2019, 294, 4437–4449. [Google Scholar] [CrossRef]
- Chen, L.; Alexe, G.; Dharia, N.V.; Ross, L.; Iniguez, A.B.; Conway, A.S.; Wang, E.J.; Veschi, V.; Lam, N.; Qi, J.; et al. CRISPR-Cas9 screen reveals a MYCN-amplified neuroblastoma dependency on EZH2. J. Clin. Investig. 2018, 128, 446–462. [Google Scholar] [CrossRef] [PubMed]
- Bansal, M.; Gupta, A.; Ding, H.F. MYCN and Metabolic Reprogramming in Neuroblastoma. Cancers 2022, 14, 4113. [Google Scholar] [CrossRef] [PubMed]
- Cantor, J.R.; Sabatini, D.M. Cancer cell metabolism: One hallmark, many faces. Cancer Discov. 2012, 2, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Akella, N.M.; Ciraku, L.; Reginato, M.J. Fueling the fire: Emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 2019, 17, 52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).