The Neuroblastoma Microenvironment, Heterogeneity and Immunotherapeutic Approaches
Abstract
Simple Summary
Abstract
1. Introduction
2. Neuroblastoma Heterogeneity and Origin
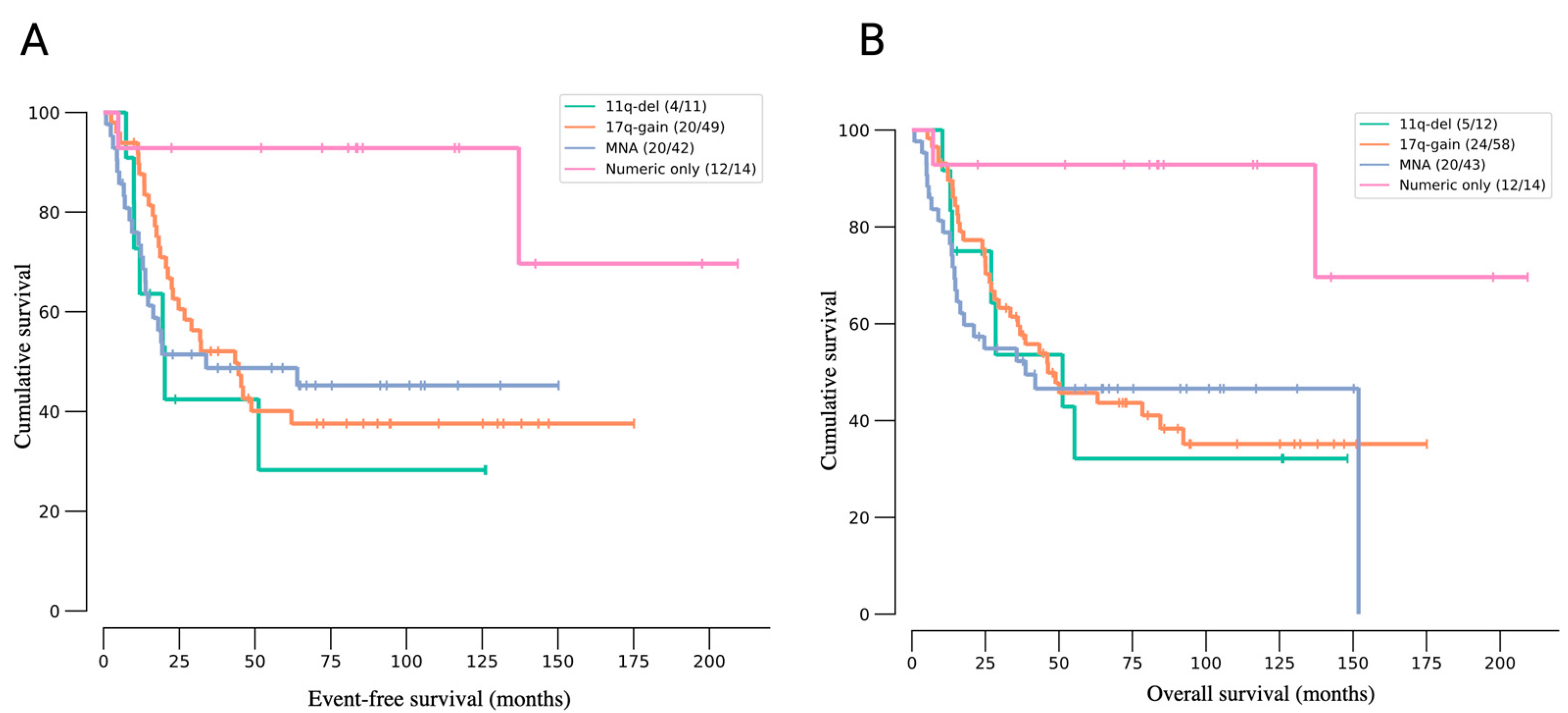
3. Molecular Landscape of Neuroblastoma
4. Current Treatments of Patients with Neuroblastoma
5. Emerging Therapies
6. The Immune Landscape of Neuroblastoma
7. Immunotherapeutic Approaches for Neuroblastoma
7.1. Neuroblastoma Vaccines
| NCT Identifier | Study Title | Status/Outcome |
|---|---|---|
| NCT06057948 | A Study of a Vaccine in Combination With Beta-glucan in People With Neuroblastoma | Recruiting |
| NCT00048386 | Neuroblastoma Vaccine for Treatment of High-Risk Neuroblastoma After Chemotherapy | Completed; no results posted |
| NCT01192555 | Allogeneic Tumor Cell Vaccination With Oral Metronomic Cytoxan in Patients With High-Risk Neuroblastoma | Active, not recruiting |
| NCT00405327 | A Pilot Study of Tumor Cell Vaccine for High-risk Solid Tumor Patients Following Stem Cell Transplantation | Completed; no results posted |
| NCT00911560 | Bivalent Vaccine With Escalating Doses of the Immunological Adjuvant OPT-821, in Combination With Oral β-glucan for High-Risk Neuroblastoma | Active, not recruiting |
| NCT04936529 | A Study of a Vaccine in Combination With β-glucan and GM-CSF in People With Neuroblastoma | Recruiting |
| NCT01241162 | Decitabine Followed by a Cancer Antigen Vaccine for Patients With Neuroblastoma and Sarcoma | Completed; vaccine tolerance and feasibility were indicated for patients with relapsed solid tumors [118] |
| NCT04239040 | GVAX Plus Checkpoint Blockade in Neuroblastoma | Recruiting |
| NCT00703222 | A Phase I/II Study Of Immunization With Lymphotactin And Interleukin 2 Gene Modified Neuroblastoma Tumor Cells (CHESAT) | Active, not recruiting |
| NCT01953900 | iC9-GD2-CAR-VZV-CTLs/Refractory or Metastatic GD2-positive Sarcoma and Neuroblastoma (VEGAS) | Active, not recruiting |
| NCT00101309 | Vaccine Therapy and Interleukin-2 in Treating Young Patients With Relapsed or Refractory Ewing’s Sarcoma or Neuroblastoma | Unknown |
| NCT04049864 | DNA Vaccination Against Neuroblastoma | Unknown |
| NCT02998983 | Racotumomab in Patients With High-risk Neuroblastoma | Completed; no results posted |
7.2. Monoclonal Antibodies
| NCT Identifier | Study Title | Status/Outcome |
|---|---|---|
| NCT04909515 | Naxitamab and Granulocyte–Macrophage Colony Stimulating Factor (GMCSF) and Isotretinoin for Consolidation of Patients With High-Risk Neuroblastoma in First Remission. | Withdrawn |
| NCT05489887 | Naxitamab Added to Induction for Newly Diagnosed High-Risk Neuroblastoma | Recruiting |
| NCT04560166 | Naxitamab and GM-CSF in Combination With IT in Patients With High-Risk Neuroblastoma | Terminated (due to business priorities) |
| NCT06013618 | Clinical Analysis of Naxitamab (hu3F8) in the Treatment of Pediatric High Risk or Refractory/Relapsed Neuroblastoma | Recruiting |
| NCT03363373 | Naxitamab for High-Risk Neuroblastoma Patients With Primary Refractory Disease or Incomplete Response to Salvage Treatment in Bone and/or Bone Marrow | Recruiting |
| NCT04501757 | Naxitamab and GM-CSF in People With Neuroblastoma | No longer available |
| NCT06047535 | Naxitamab and Granulocyte–Macrophage Colony Stimulating Factor (GM-CSF) Combined With Isotretinoin for Maintenance Treatment of Patients With High-Risk Neuroblastoma in First Complete Response. | Not yet recruiting |
| NCT02308527 | Activity Study of Bevacizumab With Temozolomide ± Irinotecan for Neuroblastoma in Children | Active, not recruiting |
| NCT02693171 | Post-Marketing Assessment of Immunogenicity and Safety of UnituxinÆ in High-Risk Neuroblastoma Patients | Terminated (sponsor decision) |
| NCT05272371 | Immunotherapy With Dinutuximab Beta in Combination With Chemotherapy for the Treatment of Patients With Primary Neuroblastoma Refractory to Standard Therapy and With Relapsed or Progressive Disease | Recruiting |
| NCT01701479 | Long-Term Continuous Infusion ch14.18/CHO Plus s.c. Aldesleukin (IL-2) | Unknown |
| NCT03794349 | Irinotecan Hydrochloride, Temozolomide, and Dinutuximab With or Without Eflornithine in Treating Patients With Relapsed or Refractory Neuroblastoma | Active, not recruiting |
| NCT02914405 | Phase I Study of 131-I mIBG Followed by Nivolumab and Dinutuximab Beta Antibodies in Children With Relapsed/Refractory Neuroblastoma | Recruiting |
| NCT02169609 | Safety Study of Dinutuximab Combined With Immunotherapy to Treat Neuroblastoma | Completed; no results posted |
| NCT01711554 | Lenalidomide and Dinutuximab With or Without Isotretinoin in Treating Younger Patients With Refractory or Recurrent Neuroblastoma | Active, not recruiting |
| NCT05400603 | Allogeneic Expanded Gamma Delta T-cells With GD2 Chemoimmunotherapy in Relapsed or Refractory Neuroblastoma | Recruiting |
| NCT02743429 | Phase II Study of Monoclonal Antibody ch14.18/CHO Continuous Infusion in Patients With Primary Refractory or Relapsed Neuroblastoma | Active, not recruiting |
| NCT05373901 | Evaluation of the Safety and Pharmacokinetics of Dinutuximab Beta as Maintenance Therapy in Chinese Patients With High-risk Neuroblastoma | Completed; no results posted |
| NCT02573896 | Immunotherapy of Relapsed Refractory Neuroblastoma With Expanded NK Cells | Active, not recruiting |
| NCT06172296 | Dinutuximab With Chemotherapy, Surgery, and Stem Cell Transplantation for the Treatment of Children With Newly Diagnosed High-Risk Neuroblastoma | Not yet recruiting |
| NCT01704716 | High-Risk Neuroblastoma Study 1.8 of SIOP-Europe (SIOPEN) | Recruiting |
| NCT01592045 | ch14.18 Pharmacokinetic Study in High-Risk Neuroblastoma | Completed; pharmacokinetics results can be accessed on clinicaltrials.org |
| NCT04211675 | NK Cells Infusions With Irinotecan, Temozolomide, and Dinutuximab | Recruiting |
| NCT03126916 | Testing the Addition of 131I-MIBG or Lorlatinib to Intensive Therapy in People With High-Risk Neuroblastoma (NBL) | Active, not recruiting |
| NCT02641782 | NB2013-HR German (GPOH)/Dutch (DCOG) Trial | Terminated |
| NCT02258815 | CH14.18 1021 Antibody and IL2 After Haplo SCT in Children With Relapsed Neuroblastoma | Completed; feasible treatment, with low chance of graft-versus-host disease [136] |
| NCT04253015 | A Post-Authorization Safety Study Patient Registry of Patients With Neuroblastoma Being Treated With Dinutuximab Beta | Recruiting |
| NCT01041638 | Monoclonal Antibody Ch14.18, Sargramostim, Aldesleukin, and Isotretinoin After Autologous Stem Cell Transplant in Treating Patients With Neuroblastoma | Completed; identified toxicities from treatment and potential biomarkers [137] |
| NCT04385277 | Treatment With Dinutuximab, Sargramostim (GM-CSF), and Isotretinoin in Combination With Irinotecan and Temozolomide After Intensive Therapy for People With High-Risk Neuroblastoma (NBL) | Active, not recruiting |
| NCT01767194 | Irinotecan Hydrochloride and Temozolomide With Temsirolimus or Dinutuximab in Treating Younger Patients With Refractory or Relapsed Neuroblastoma | Completed; patients with relapsed/refractory disease exhibited significant antitumor response [138] |
| NCT03786783 | Dinutuximab, Sargramostim, and Combination Chemotherapy in Treating Patients With Newly Diagnosed High-Risk Neuroblastoma | Active, not recruiting |
| NCT04751383 | Testing the Combination of Two Immunotherapy Drugs (Magrolimab and Dinutuximab) in Patients With Relapsed or Refractory Neuroblastoma or Relapsed Osteosarcoma | Suspended (unacceptable toxicity) |
| NCT03332667 | MIBG With Dinutuximab +/− Vorinostat | Active, not recruiting |
| NCT05421897 | Rapid Administration Pilot for Infusing Dinutuximab | Recruiting |
| NCT06071897 | Induction Chemoimmunotherapy for Patients With High-Risk Neuroblastoma | Recruiting |
| NCT04238819 | A Study of Abemaciclib (LY2835219) in Combination With Other Anti-Cancer Treatments in Children and Young Adult Participants With Solid Tumors, Including Neuroblastoma | Recruiting |
| NCT00030719 | Combination Chemotherapy With or Without Filgrastim Before Surgery, High-Dose Chemotherapy, and Radiation Therapy Followed by Isotretinoin With or Without Monoclonal Antibody in Treating Patients With Neuroblastoma | Unknown |
| NCT01857934 | Therapy for Children With Advanced Stage Neuroblastoma | Active, not recruiting |
| NCT05608148 | Clinical Trial of GAIA-102 for Refractory/Relapse Neuroblastomas or Malignant Pediatric Solid Tumors With Lung Metastases | Recruiting |
| NCT00026312 | Isotretinoin With or Without Dinutuximab, Aldesleukin, and Sargramostim Following Stem Cell Transplant in Treating Patients With Neuroblastoma | Active, not recruiting |
| NCT01704872 | ch14.18/CHO Bridging Study | Completed; similar side effects as observed in ch14.18/SP2/0 studies; treatment accepted for further evaluation [139] |
| NCT05754684 | Quadruple Immunotherapy for Neuroblastoma | Recruiting |
| NCT02559778 | Pediatric Precision Laboratory Advanced Neuroblastoma Therapy | Recruiting |
| NCT00005576 | Monoclonal Antibody Therapy With Sargramostim and Interleukin-2 in Treating Children With Neuroblastoma | Completed; no results posted |
| NCT04221035 | High-Risk Neuroblastoma Study 2 of SIOP-Europa-Neuroblastoma (SIOPEN) | Recruiting |
| NCT01701479 | Long-Term Continuous Infusion ch14.18/CHO Plus s.c. Aldesleukin (IL-2) | Unknown |
| NCT02914405 | Phase I Study of 131-I mIBG Followed by Nivolumab and Dinutuximab Beta Antibodies in Children With Relapsed/Refractory Neuroblastoma | Recruiting |
| NCT02743429 | Phase II Study of Monoclonal Antibody ch14.18/CHO Continuous Infusion in Patients With Primary Refractory or Relapsed Neuroblastoma | Active, not recruiting |
| NCT01704716 | High-Risk Neuroblastoma Study 1.8 of SIOP-Europe (SIOPEN) | Recruiting |
| NCT02258815 | CH14.18 1021 Antibody and IL2 After Haplo SCT in Children With Relapsed Neuroblastoma | Completed; the antibody dosing regimen was adequate and the lymphoid immune compartment exhibited strong performance [140] |
| NCT00743496 | A Phase I Trial Of The Humanized Anti-GD2 Antibody In Children And Adolescents With Neuroblastoma, Osteosarcoma, Ewing Sarcoma, and Melanoma | Completed; pre-existing antitherapeutic antibodies may be associated with increased antitumor effects [141] |
| NCT02130869 | A Pilot Study of Immunotherapy Including Haploidentical NK Cell Infusion Following CD133+ Positively Selected Autologous Hematopoietic Stem Cells in Children With High-Risk Solid Tumors or Lymphomas | Completed; no results posted |
| NCT02159443 | Pretreatment Anti-Therapeutic Antibodies (PATA) in Patients Treated With hu14.18K322A Antibody | Completed; no results posted |
| NCT01576692 | Combination Chemotherapy, Monoclonal Antibody, and Natural Killer Cells in Treating Young Patients With Recurrent or Refractory Neuroblastoma | Completed; combination therapy was tolerable, safe, and feasible for patients with relapsed/refractory neuroblastoma and exhibits promising antitumor effects [142] |
| NCT01857934 | Therapy for Children With Advanced Stage Neuroblastoma | Active, not recruiting |
| NCT00582608 | Tumor Detection Using Iodine-131-Labeled Monoclonal Antibody 8H9 | Terminated |
| NCT00089245 | Radiolabeled MAB Therapy in Patients With Refractory, Recurrent, or Advanced CNS or Leptomeningeal Cancer | Terminated |
7.3. Oncolytic Virotherapy
7.4. Adoptive Cell Therapy
| NCT Identifier | Study Title | Status/Outcome |
|---|---|---|
| NCT02761915 | A Phase I Trial of Anti-GD2 T-cells (1RG-CART) | Completed; CAR T-cell therapy appears safe, with no on-target off-tumor toxicity. However, two patients exhibited cytokine release syndrome [168]. |
| NCT02107963 | A Phase I Trial of T-cells Expressing an Anti-GD2 Chimeric Antigen Receptor in Children and Young Adults With GD2+ Solid Tumors | Completed; feasibility and safety of administration was identified [169] |
| NCT05650749 | GPC2 CAR T-cells for Relapsed or Refractory Neuroblastoma | Recruiting |
| NCT03721068 | Study of CAR T-cells Targeting the GD2 With IL-15+iCaspase9 for Relapsed/Refractory Neuroblastoma or Relapsed/Refractory Osteosarcoma | Recruiting |
| NCT01822652 | Third-Generation GD-2 Chimeric Antigen Receptor and iCaspase Suicide Safety Switch, Neuroblastoma, GRAIN | Active, not recruiting |
| NCT03373097 | Anti-GD2 CAR T-cells in Pediatric Patients Affected by High Risk and/or Relapsed/Refractory Neuroblastoma or Other GD2-positive Solid Tumors | Recruiting |
| NCT03294954 | GD2 Specific CAR and Interleukin-15 Expressing Autologous NKT Cells to Treat Children With Neuroblastoma | Recruiting |
| NCT04637503 | 4SCAR-T Therapy Targeting GD2, PSMA, and CD276 for Treating Neuroblastoma | Unknown |
| NCT01953900 | iC9-GD2-CAR-VZV-CTLs/Refractory or Metastatic GD2-positive Sarcoma and Neuroblastoma | Active, not recruiting |
| NCT02439788 | Third-Generation GD2-specific Chimeric Antigen Receptor Transduced Autologous Natural Killer T-cells for Neuroblastoma | Withdrawn |
| NCT04897321 | B7-H3-Specific Chimeric Antigen Receptor Autologous T-cell Therapy for Pediatric Patients With Solid Tumors (3CAR) | Recruiting |
| NCT02765243 | Anti-GD2 Fourth-Generation CAR T-cells Targeting Refractory and/or Recurrent Neuroblastoma | Completed; this therapy exhibited antitumor response without serious toxicities [170] |
| NCT04539366 | Testing a New Immune Cell Therapy, GD2-Targeted Modified T-cells (GD2CART), in Children, Adolescents, and Young Adults With Relapsed/Refractory Osteosarcoma and Neuroblastoma, the GD2-CAR PERSIST Trial | Suspended |
| NCT05990751 | Multi-modular Chimeric Antigen Receptor Targeting GD2 in Neuroblastoma | Not yet recruiting |
| NCT00085930 | Blood T-cells and EBV Specific CTLs Expressing GD-2 Specific Chimeric T-cell Receptors to Neuroblastoma Patients | Active, not recruiting |
| NCT02311621 | Engineered Neuroblastoma Cellular Immunotherapy (ENCIT)-01 | Active, not recruiting |
| NCT04864821 | Clinical Study of CD276 Targeted Autologous Chimeric Antigen Receptor T-cell Infusion in Patients With CD276-Positive Advanced Solid Tumor | Unknown |
| NCT02919046 | Study Evaluating Efficacy and Safety With CAR-T for Relapsed or Refractory Neuroblastoma in Children | Unknown |
| NCT03635632 | C7R-GD2.CAR T-cells for Patients With Relapsed or Refractory Neuroblastoma and Other GD2-Positive Cancers (GAIL-N) | Active, not recruiting |
| NCT04483778 | B7H3 CAR T-cell Immunotherapy for Recurrent/Refractory Solid Tumors in Children and Young Adults | Active, not recruiting |
| NCT05562024 | TAA06 Injection in the Treatment of Patients With B7-H3-Positive Relapsed/Refractory Neuroblastoma | Recruiting |
| NCT03618381 | EGFR806 CAR T-cell Immunotherapy for Recurrent/Refractory Solid Tumors in Children and Young Adults | Recruiting |
| NCT02457650 | T-cell Receptor-transduced T-cells Targeting NY-ESO-1 for Treatment of Patients With NY-ESO-1-Expressing Malignancies | Unknown |
| NCT05296564 | Anti-NY-ESO-1 TCR Gene Engineered Lymphocytes Given by Infusion to Patients With NY-ESO-1-Expressing Metastatic Cancers | Recruiting |
| NCT02508038 | Alpha/Beta CD19+ Depleted Haploidentical Transplantation + Zometa for Pediatric Hematologic Malignancies and Solid Tumors | Recruiting |
| NCT00085930 | Blood T-cells and EBV-Specific CTLs Expressing GD-2 Specific Chimeric T-cell Receptors to Neuroblastoma Patients | Active, not recruiting |
| NCT00874315 | Allogeneic Hematopoietic Stem Cell Transplantation for Relapsed or Refractory High-Risk NBL. | Withdrawn |
| NCT01807468 | Haploidentical Stem Cell Transplantation and NK Cell Therapy in Patients With High-risk Solid Tumors | Unknown |
| NCT04211675 | NK Cell Infusions With Irinotecan, Temozolomide, and Dinutuximab | Recruiting |
| NCT01287104 | A Phase I Study of NK Cell Infusion Following Allogeneic Peripheral Blood Stem Cell Transplantation From Related or Matched Unrelated Donors in Pediatric Patients With Solid Tumors and Leukemias | Completed; while killing efficacy and receptor expression activation was significant, five out of nine participants demonstrated severe graft-versus-host disease [171] |
| NCT00698009 | Haploidentical Natural Killer (NK) Cells in Patients With Relapsed or Refractory Neuroblastoma | Terminated |
| NCT02100891 | Phase 2 STIR Trial: Haploidentical Transplant and Donor Natural Killer Cells for Solid Tumors | Completed; this treatment was well-tolerated, and overall survival was improved [172] |
| NCT01156350 | Haploidentical Hematopoietic Stem Cell Transplantation Following Reduced-intensity Conditioning in Children With Neuroblastoma | Unknown |
| NCT03242603 | Immunotherapy of Neuroblastoma Patients Using a Combination of Anti-GD2 and NK Cells | Unknown |
| NCT02650648 | Humanized Anti-GD2 Antibody Hu3F8 and Allogeneic Natural Killer Cells for High-Risk Neuroblastoma | Active, not recruiting |
| NCT00788125 | Dasatinib, Ifosfamide, Carboplatin, and Etoposide in Treating Young Patients With Metastatic or Recurrent Malignant Solid Tumors | Terminated |
| NCT01386619 | NK DLI in Patients After Human Leukocyte Antigen (HLA)-Haploidentical Hematopoietic Stem Cell Transplantation (HSCT) | Completed; NKG2D-mediated cytotoxicity was repaired after haploidentical NK-DLI treatment [173] |
| NCT02573896 | Immunotherapy of Relapsed Refractory Neuroblastoma With Expanded NK Cells | Active, not recruiting |
| NCT05754684 | Quadruple Immunotherapy for Neuroblastoma | Recruiting |
| NCT00877110 | Anti-GD2 3F8 Antibody and Allogeneic Natural Killer Cells for High-Risk Neuroblastoma | Completed; observed antitumor effect after activation of NK cells. Follow up study: NCT02650648 [161] |
| NCT01857934 | Therapy for Children With Advanced Stage Neuroblastoma | Active, not recruiting |
| NCT01875601 | NK White Blood Cells and Interleukin in Children and Young Adults With Advanced Solid Tumors | Completed; feasibility, safety, and tolerance of this strategy were observed [174] |
| NCT03209869 | Treatment of Relapsed or Refractory Neuroblastoma and Osteosarcoma With Expanded Haploidentical NK Cells and Hu14.18-IL2 | Withdrawn |
| NCT02130869 | A Pilot Study of Immunotherapy Including Haploidentical NK Cell Infusion Following CD133+ Positively Selected Autologous Hematopoietic Stem Cells in Children With High-Risk Solid Tumors or Lymphomas | Completed; no results posted |
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chung, C.; Boterberg, T.; Lucas, J.; Panoff, J.; Valteau-Couanet, D.; Hero, B.; Bagatell, R.; Hill-Kayser, C.E. Neuroblastoma. Pediatr. Blood Cancer 2021, 68, e28473. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, J.I.; Dyberg, C.; Wickström, M. Neuroblastoma—A Neural Crest Derived Embryonal Malignancy. Front. Mol. Neurosci. 2019, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, K.I.; Treis, D.; Johnsen, J.I. Nuroblastoma Heterogeneity, Plasticity, and Emerging Therapies. Curr. Oncol. Rep. 2022, 24, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Eggert, A.; Caron, H. Neuroblastoma: Biology, Prognosis, and Treatment. Hematol. Oncol. Clin. N. Am. 2010, 24, 65–86. [Google Scholar] [CrossRef] [PubMed]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Qiu, B.; Matthay, K.K. Advancing Therapy for Neuroblastoma. Nat. Rev. Clin. Oncol. 2022, 19, 515–533. [Google Scholar] [CrossRef] [PubMed]
- Laug, W.E.; Siegel, S.E.; Shaw, K.N.; Landing, B.; Baptista, J.; Gutenstein, M. Initial Urinary Catecholamine Metabolite Concentrations and Prognosis in Neuroblastoma. Pediatrics 1978, 62, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Naseem, S.; Varma, N.; Das, R.; Ahluwalia, J.; Sachdeva, M.U.S.; Sharma, P.; Kumar, N.; Marwaha, R.K. Bone Marrow Involvement in Neuroblastoma: A Study of Hemato-Morphological Features. Indian J. Hematol. Blood Transfus. 2015, 31, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, A. Biological Categories of Neuroblastoma Based on the International Neuroblastoma Pathology Classification for Treatment Stratification. Pathol. Int. 2021, 71, 232–244. [Google Scholar] [CrossRef]
- Shimada, H.; Ambros, I.M.; Dehner, L.P.; Hata, J.; Joshi, V.V.; Roald, B.; Stram, D.O.; Gerbing, R.B.; Lukens, J.N.; Matthay, K.K.; et al. The International Neuroblastoma Pathology Classification (the Shimada System). Cancer 1999, 86, 364–372. [Google Scholar] [CrossRef]
- Monclair, T.; Brodeur, G.M.; Ambros, P.F.; Brisse, H.J.; Cecchetto, G.; Holmes, K.; Kaneko, M.; London, W.B.; Matthay, K.K.; Nuchtern, J.G.; et al. The International Neuroblastoma Risk Group (INRG) Staging System: An INRG Task Force Report. J. Clin. Oncol. 2009, 27, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Cohn, S.L.; Pearson, A.D.J.; London, W.B.; Monclair, T.; Ambros, P.F.; Brodeur, G.M.; Faldum, A.; Hero, B.; Iehara, T.; Machin, D.; et al. The International Neuroblastoma Risk Group (INRG) Classification System: An INRG Task Force Report. J. Clin. Oncol. 2009, 27, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, V.P.; Matthay, K.K. Neuroblastoma: Clinical and Biological Approach to Risk Stratification and Treatment. Cell Tissue Res. 2018, 372, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Gomez, R.L.; Ibragimova, S.; Ramachandran, R.; Philpott, A.; Ali, F.R. Tumoral Heterogeneity in Neuroblastoma. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188805. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Valind, A.; Holmquist Mengelbier, L.; Bredin, S.; Cornmark, L.; Jansson, C.; Wali, A.; Staaf, J.; Viklund, B.; Øra, I.; et al. Four Evolutionary Trajectories Underlie Genetic Intratumoral Variation in Childhood Cancer. Nat. Genet. 2018, 50, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.A.; Biedler, J.L.; Spengler, B.A. A Role for Distinct Cell Types in Determining Malignancy in Human Neuroblastoma Cell Lines and Tumors. Cancer Lett. 2003, 197, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Boeva, V.; Louis-Brennetot, C.; Peltier, A.; Durand, S.; Pierre-Eugène, C.; Raynal, V.; Etchevers, H.C.; Thomas, S.; Lermine, A.; Daudigeos-Dubus, E.; et al. Heterogeneity of Neuroblastoma Cell Identity Defined by Transcriptional Circuitries. Nat. Genet. 2017, 49, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Olsen, T.K.; Otte, J.; Mei, S.; Kameneva, P.; Björklund, Å.; Kryukov, E.; Hou, Z.; Johansson, A.; Sundström, E.; Martinsson, T.; et al. Malignant Schwann Cell Precursors Mediate Intratumoral Plasticity in Human Neuroblastoma; Cancer Biology: Palo Alto, CA, USA, 2020. [Google Scholar]
- van Groningen, T.; Koster, J.; Valentijn, L.J.; Zwijnenburg, D.A.; Akogul, N.; Hasselt, N.E.; Broekmans, M.; Haneveld, F.; Nowakowska, N.E.; Bras, J.; et al. Neuroblastoma Is Composed of Two Super-Enhancer-Associated Differentiation States. Nat. Genet. 2017, 49, 1261–1266. [Google Scholar] [CrossRef]
- Gartlgruber, M.; Sharma, A.K.; Quintero, A.; Dreidax, D.; Jansky, S.; Park, Y.-G.; Kreth, S.; Meder, J.; Doncevic, D.; Saary, P.; et al. Super Enhancers Define Regulatory Subtypes and Cell Identity in Neuroblastoma. Nat. Cancer 2021, 2, 114–128. [Google Scholar] [CrossRef]
- Anderson, D.J.; Carnahan, J.F.; Michelsohn, A.; Patterson, P.H. Antibody Markers Identify a Common Progenitor to Sympathetic Neurons and Chromaffin Cells in Vivo and Reveal the Timing of Commitment to Neuronal Differentiation in the Sympathoadrenal Lineage. J. Neurosci. 1991, 11, 3507–3519. [Google Scholar] [CrossRef]
- Kerosuo, L.; Neppala, P.; Hsin, J.; Mohlin, S.; Vieceli, F.M.; Török, Z.; Laine, A.; Westermarck, J.; Bronner, M.E. Enhanced Expression of MycN/CIP2A Drives Neural Crest toward a Neural Stem Cell-like Fate: Implications for Priming of Neuroblastoma. Proc. Natl. Acad. Sci. USA 2018, 115, E7351–E7360. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Yang, R.; Zhan, Y.; Lai, H.-D.; Ye, C.-J.; Yao, X.-Y.; Luo, W.-Q.; Cheng, X.-M.; Miao, J.-J.; Wang, J.-F.; et al. Single-Cell Characterization of Malignant Phenotypes and Developmental Trajectories of Adrenal Neuroblastoma. Cancer Cell 2020, 38, 716–733.e6. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.-K.V.; Dyer, M.A. Neuroblastoma: Developmental Biology, Cancer Genomics, and Immunotherapy. Nat. Rev. Cancer 2013, 13, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Vega-Lopez, G.A.; Cerrizuela, S.; Aybar, M.J. Trunk Neural Crest Cells: Formation, Migration and Beyond. Int. J. Dev. Biol. 2017, 61, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Jansky, S.; Sharma, A.K.; Körber, V.; Quintero, A.; Toprak, U.H.; Wecht, E.M.; Gartlgruber, M.; Greco, A.; Chomsky, E.; Grünewald, T.G.P.; et al. Single-Cell Transcriptomic Analyses Provide Insights into the Developmental Origins of Neuroblastoma. Nat. Genet. 2021, 53, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Furlan, A.; Dyachuk, V.; Kastriti, M.E.; Calvo-Enrique, L.; Abdo, H.; Hadjab, S.; Chontorotzea, T.; Akkuratova, N.; Usoskin, D.; Kamenev, D.; et al. Multipotent Peripheral Glial Cells Generate Neuroendocrine Cells of the Adrenal Medulla. Science 2017, 357, eaal3753. [Google Scholar] [CrossRef]
- Kastriti, M.E.; Faure, L.; Von Ahsen, D.; Bouderlique, T.G.; Boström, J.; Solovieva, T.; Jackson, C.; Bronner, M.; Meijer, D.; Hadjab, S.; et al. Schwann Cell Precursors Represent a Neural Crest-like State with Biased Multipotency. EMBO J. 2022, 41, e108780. [Google Scholar] [CrossRef]
- Blavier, L.; Yang, R.-M.; DeClerck, Y.A. The Tumor Microenvironment in Neuroblastoma: New Players, New Mechanisms of Interaction and New Perspectives. Cancers 2020, 12, 2912. [Google Scholar] [CrossRef] [PubMed]
- Braekeveldt, N.; von Stedingk, K.; Fransson, S.; Martinez-Monleon, A.; Lindgren, D.; Axelson, H.; Levander, F.; Willforss, J.; Hansson, K.; Øra, I.; et al. Patient-Derived Xenograft Models Reveal Intratumor Heterogeneity and Temporal Stability in Neuroblastoma. Cancer Res. 2018, 78, 5958–5969. [Google Scholar] [CrossRef]
- Verhoeven, B.M.; Mei, S.; Olsen, T.K.; Gustafsson, K.; Valind, A.; Lindström, A.; Gisselsson, D.; Fard, S.S.; Hagerling, C.; Kharchenko, P.V.; et al. The Immune Cell Atlas of Human Neuroblastoma. Cell Rep. Med. 2022, 3, 100657. [Google Scholar] [CrossRef]
- Nurmik, M.; Ullmann, P.; Rodriguez, F.; Haan, S.; Letellier, E. In Search of Definitions: Cancer-associated Fibroblasts and Their Markers. Int. J. Cancer 2020, 146, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Spaeth, E.L.; Dembinski, J.L.; Sasser, A.K.; Watson, K.; Klopp, A.; Hall, B.; Andreeff, M.; Marini, F. Mesenchymal Stem Cell Transition to Tumor-Associated Fibroblasts Contributes to Fibrovascular Network Expansion and Tumor Progression. PLoS ONE 2009, 4, e4992. [Google Scholar] [CrossRef] [PubMed]
- Neviani, P.; Wise, P.M.; Murtadha, M.; Liu, C.W.; Wu, C.-H.; Jong, A.Y.; Seeger, R.C.; Fabbri, M. Natural Killer-Derived Exosomal miR-186 Inhibits Neuroblastoma Growth and Immune Escape Mechanisms. Cancer Res. 2019, 79, 1151–1164. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.C.; Wan, Z.; Sheard, M.A.; Sun, J.; Jackson, J.R.; Malvar, J.; Xu, Y.; Wang, L.; Sposto, R.; Kim, E.S.; et al. TGFβR1 Blockade with Galunisertib (LY2157299) Enhances Anti-Neuroblastoma Activity of the Anti-GD2 Antibody Dinutuximab (Ch14.18) with Natural Killer Cells. Clin. Cancer Res. 2017, 23, 804–813. [Google Scholar] [CrossRef] [PubMed]
- Zeine, R.; Salwen, H.R.; Peddinti, R.; Tian, Y.; Guerrero, L.; Yang, Q.; Chlenski, A.; Cohn, S.L. Presence of Cancer-Associated Fibroblasts Inversely Correlates with Schwannian Stroma in Neuroblastoma Tumors. Mod. Pathol. 2009, 22, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; Nakata, R.; Sheard, M.A.; Shimada, H.; Buettner, R.; Groshen, S.G.; Ji, L.; Yu, H.; Jove, R.; Seeger, R.C.; et al. Critical Role of STAT3 in IL-6-Mediated Drug Resistance in Human Neuroblastoma. Cancer Res. 2013, 73, 3852–3864. [Google Scholar] [CrossRef] [PubMed]
- Borriello, L.; Nakata, R.; Sheard, M.A.; Fernandez, G.E.; Sposto, R.; Malvar, J.; Blavier, L.; Shimada, H.; Asgharzadeh, S.; Seeger, R.C.; et al. Cancer-Associated Fibroblasts Share Characteristics and Protumorigenic Activity with Mesenchymal Stromal Cells. Cancer Res. 2017, 77, 5142–5157. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.; Kock, A.; Idborg, H.; Arsenian Henriksson, M.; Martinsson, T.; Johnsen, J.I.; Korotkova, M.; Kogner, P.; Jakobsson, P.-J. COX/mPGES-1/PGE2 Pathway Depicts an Inflammatory-Dependent High-Risk Neuroblastoma Subset. Proc. Natl. Acad. Sci. USA 2015, 112, 8070–8075. [Google Scholar] [CrossRef]
- Kock, A.; Larsson, K.; Bergqvist, F.; Eissler, N.; Elfman, L.H.M.; Raouf, J.; Korotkova, M.; Johnsen, J.I.; Jakobsson, P.-J.; Kogner, P. Inhibition of Microsomal Prostaglandin E Synthase-1 in Cancer-Associated Fibroblasts Suppresses Neuroblastoma Tumor Growth. eBioMedicine 2018, 32, 84–92. [Google Scholar] [CrossRef]
- Quinn, C.H.; Beierle, A.M.; Beierle, E.A. Artificial Tumor Microenvironments in Neuroblastoma. Cancers 2021, 13, 1629. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Komorowski, M.; Tisonczyk, J.; Kolakowska, A.; Drozdz, R.; Kozbor, D. Modulation of the Tumor Microenvironment by CXCR4 Antagonist-Armed Viral Oncotherapy Enhances the Antitumor Efficacy of Dendritic Cell Vaccines against Neuroblastoma in Syngeneic Mice. Viruses 2018, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Di Matteo, S.; Avanzini, M.A.; Pelizzo, G.; Calcaterra, V.; Croce, S.; Spaggiari, G.M.; Theuer, C.; Zuccotti, G.; Moretta, L.; Pelosi, A.; et al. Neuroblastoma Tumor-Associated Mesenchymal Stromal Cells Regulate the Cytolytic Functions of NK Cells. Cancers 2022, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.; Morandi, F.; Cilli, M.; Daga, A.; Bocelli-Tyndall, C.; Gambini, C.; Pistoia, V.; Raffaghello, L. Close Interactions between Mesenchymal Stem Cells and Neuroblastoma Cell Lines Lead to Tumor Growth Inhibition. PLoS ONE 2012, 7, e48654. [Google Scholar] [CrossRef] [PubMed]
- Pajtler, K.W.; Mahlow, E.; Odersky, A.; Lindner, S.; Stephan, H.; Bendix, I.; Eggert, A.; Schramm, A.; Schulte, J.H. Neuroblastoma in Dialog with Its Stroma: NTRK1 Is a Regulator of Cellular Cross-Talk with Schwann Cells. Oncotarget 2014, 5, 11180–11192. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, L. HMGB1-Induced Autophagy in Schwann Cells Promotes Neuroblastoma Proliferation. Int. J. Clin. Exp. Pathol. 2015, 8, 504–510. [Google Scholar] [PubMed]
- Bown, N.; Cotterill, S.; Lastowska, M.; O’Neill, S.; Pearson, A.D.; Plantaz, D.; Meddeb, M.; Danglot, G.; Brinkschmidt, C.; Christiansen, H.; et al. Gain of Chromosome Arm 17q and Adverse Outcome in Patients with Neuroblastoma. N. Engl. J. Med. 1999, 340, 1954–1961. [Google Scholar] [CrossRef] [PubMed]
- Brady, S.W.; Liu, Y.; Ma, X.; Gout, A.M.; Hagiwara, K.; Zhou, X.; Wang, J.; Macias, M.; Chen, X.; Easton, J.; et al. Pan-Neuroblastoma Analysis Reveals Age- and Signature-Associated Driver Alterations. Nat. Commun. 2020, 11, 5183. [Google Scholar] [CrossRef] [PubMed]
- Janoueix-Lerosey, I.; Schleiermacher, G.; Michels, E.; Mosseri, V.; Ribeiro, A.; Lequin, D.; Vermeulen, J.; Couturier, J.; Peuchmaur, M.; Valent, A.; et al. Overall Genomic Pattern Is a Predictor of Outcome in Neuroblastoma. J. Clin. Oncol. 2009, 27, 1026–1033. [Google Scholar] [CrossRef]
- Maris, J.M. Recent Advances in Neuroblastoma. N. Engl. J. Med. 2010, 362, 2202–2211. [Google Scholar] [CrossRef]
- Ambros, P.F.; Ambros, I.M.; Brodeur, G.M.; Haber, M.; Khan, J.; Nakagawara, A.; Schleiermacher, G.; Speleman, F.; Spitz, R.; London, W.B.; et al. International Consensus for Neuroblastoma Molecular Diagnostics: Report from the International Neuroblastoma Risk Group (INRG) Biology Committee. Br. J. Cancer 2009, 100, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Meddeb, M.; Danglot, G.; Chudoba, I.; Vénuat, A.-M.; Bénard, J.; Avet-Loiseau, H.; Vasseur, B.; Le Paslier, D.; Terrier-Lacombe, M.-J.; Hartmann, O.; et al. Additional Copies of a 25 Mb Chromosomal Region Originating from 17q23.1-17qter Are Present in 90% of High-Grade Neuroblastomas. Genes Chromosomes Cancer 1996, 17, 156–165. [Google Scholar] [CrossRef]
- Milosevic, J.; Treis, D.; Fransson, S.; Gallo-Oller, G.; Sveinbjörnsson, B.; Eissler, N.; Tanino, K.; Sakaguchi, K.; Martinsson, T.; Wickström, M.; et al. PPM1D Is a Therapeutic Target in Childhood Neural Tumors. Cancers 2021, 13, 6042. [Google Scholar] [CrossRef]
- Mlakar, V.; Dupanloup, I.; Gonzales, F.; Papangelopoulou, D.; Ansari, M.; Gumy-Pause, F. 17q Gain in Neuroblastoma: A Review of Clinical and Biological Implications. Cancers 2024, 16, 338. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, S.; Misiak, D.; Bell, J.L.; Fuchs, T.; Lederer, M.I.; Bley, N.; Hämmerle, M.; Ghazy, E.; Sippl, W.; Schulte, J.H.; et al. IGF2BP1 Induces Neuroblastoma via a Druggable Feedforward Loop with MYCN Promoting 17q Oncogene Expression. Mol. Cancer 2023, 22, 88. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, J.J.; Domingo-Fernández, R.; Ebus, M.E.; Lindner, S.; Koster, J.; Drabek, K.; Mestdagh, P.; van Sluis, P.; Valentijn, L.J.; van Nes, J.; et al. LIN28B Induces Neuroblastoma and Enhances MYCN Levels via Let-7 Suppression. Nat. Genet. 2012, 44, 1199–1206. [Google Scholar] [CrossRef]
- Mossé, Y.P.; Laudenslager, M.; Longo, L.; Cole, K.A.; Wood, A.; Attiyeh, E.F.; Laquaglia, M.J.; Sennett, R.; Lynch, J.E.; Perri, P.; et al. Identification of ALK as a Major Familial Neuroblastoma Predisposition Gene. Nature 2008, 455, 930–935. [Google Scholar] [CrossRef]
- Huber, K.; Karch, N.; Ernsberger, U.; Goridis, C.; Unsicker, K. The Role of Phox2B in Chromaffin Cell Development. Dev. Biol. 2005, 279, 501–508. [Google Scholar] [CrossRef]
- Lerone, M.; Ognibene, M.; Pezzolo, A.; Martucciello, G.; Zara, F.; Morini, M.; Mazzocco, K. Molecular Genetics in Neuroblastoma Prognosis. Children 2021, 8, 456. [Google Scholar] [CrossRef]
- Dyberg, C.; Fransson, S.; Andonova, T.; Sveinbjörnsson, B.; Lännerholm-Palm, J.; Olsen, T.K.; Forsberg, D.; Herlenius, E.; Martinsson, T.; Brodin, B.; et al. Rho-Associated Kinase Is a Therapeutic Target in Neuroblastoma. Proc. Natl. Acad. Sci. USA 2017, 114, E6603–E6612. [Google Scholar] [CrossRef]
- Molenaar, J.J.; Ebus, M.E.; Koster, J.; van Sluis, P.; van Noesel, C.J.M.; Versteeg, R.; Caron, H.N. Cyclin D1 and CDK4 Activity Contribute to the Undifferentiated Phenotype in Neuroblastoma. Cancer Res. 2008, 68, 2599–2609. [Google Scholar] [CrossRef]
- Javanmardi, N.; Fransson, S.; Djos, A.; Umapathy, G.; Östensson, M.; Milosevic, J.; Borenäs, M.; Hallberg, B.; Kogner, P.; Martinsson, T.; et al. Analysis of ALK, MYCN, and the ALK Ligand ALKAL2 (FAM150B/AUGα) in Neuroblastoma Patient Samples with Chromosome Arm 2p Rearrangements. Genes Chromosomes Cancer 2020, 59, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, Y.; Liu, Y.; Alexandrov, L.B.; Edmonson, M.N.; Gawad, C.; Zhou, X.; Li, Y.; Rusch, M.C.; Easton, J.; et al. Pan-Cancer Genome and Transcriptome Analyses of 1699 Paediatric Leukaemias and Solid Tumours. Nature 2018, 555, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Valentijn, L.J.; Koster, J.; Zwijnenburg, D.A.; Hasselt, N.E.; van Sluis, P.; Volckmann, R.; van Noesel, M.M.; George, R.E.; Tytgat, G.A.M.; Molenaar, J.J.; et al. TERT Rearrangements Are Frequent in Neuroblastoma and Identify Aggressive Tumors. Nat. Genet. 2015, 47, 1411–1414. [Google Scholar] [CrossRef]
- Eleveld, T.F.; Oldridge, D.A.; Bernard, V.; Koster, J.; Colmet Daage, L.; Diskin, S.J.; Schild, L.; Bentahar, N.B.; Bellini, A.; Chicard, M.; et al. Relapsed Neuroblastomas Show Frequent RAS-MAPK Pathway Mutations. Nat. Genet. 2015, 47, 864–871. [Google Scholar] [CrossRef]
- Pugh, T.J.; Morozova, O.; Attiyeh, E.F.; Asgharzadeh, S.; Wei, J.S.; Auclair, D.; Carter, S.L.; Cibulskis, K.; Hanna, M.; Kiezun, A.; et al. The Genetic Landscape of High-Risk Neuroblastoma. Nat. Genet. 2013, 45, 279–284. [Google Scholar] [CrossRef]
- Matthay, K.K.; Maris, J.M.; Schleiermacher, G.; Nakagawara, A.; Mackall, C.L.; Diller, L.; Weiss, W.A. Neuroblastoma. Nat. Rev. Dis. Prim. 2016, 2, 16078. [Google Scholar] [CrossRef] [PubMed]
- Gautier, M.; Thirant, C.; Delattre, O.; Janoueix-Lerosey, I. Plasticity in Neuroblastoma Cell Identity Defines a Noradrenergic-to-Mesenchymal Transition (NMT). Cancers 2021, 13, 2904. [Google Scholar] [CrossRef]
- Iehara, T.; Hamazaki, M.; Tajiri, T.; Kawano, Y.; Kaneko, M.; Ikeda, H.; Hosoi, H.; Sugimoto, T.; Sawada, T.; Japanese Infantile Neuroblastoma Cooperative Study Group. Successful Treatment of Infants with Localized Neuroblastoma Based on Their MYCN Status. Int. J. Clin. Oncol. 2013, 18, 389–395. [Google Scholar] [CrossRef]
- Nuchtern, J.G.; London, W.B.; Barnewolt, C.E.; Naranjo, A.; McGrady, P.W.; Geiger, J.D.; Diller, L.; Schmidt, M.L.; Maris, J.M.; Cohn, S.L.; et al. A Prospective Study of Expectant Observation as Primary Therapy for Neuroblastoma in Young Infants: A Children’s Oncology Group Study. Ann. Surg. 2012, 256, 573–580. [Google Scholar] [CrossRef]
- Strother, D.R.; London, W.B.; Schmidt, M.L.; Brodeur, G.M.; Shimada, H.; Thorner, P.; Collins, M.H.; Tagge, E.; Adkins, S.; Reynolds, C.P.; et al. Outcome after Surgery Alone or with Restricted Use of Chemotherapy for Patients with Low-Risk Neuroblastoma: Results of Children’s Oncology Group Study P9641. J. Clin. Oncol. 2012, 30, 1842–1848. [Google Scholar] [CrossRef]
- Twist, C.J.; Schmidt, M.L.; Naranjo, A.; London, W.B.; Tenney, S.C.; Marachelian, A.; Shimada, H.; Collins, M.H.; Esiashvili, N.; Adkins, E.S.; et al. Maintaining Outstanding Outcomes Using Response- and Biology-Based Therapy for Intermediate-Risk Neuroblastoma: A Report from the Children’s Oncology Group Study ANBL0531. J. Clin. Oncol. 2019, 37, 3243–3255. [Google Scholar] [CrossRef] [PubMed]
- Kohler, J.A.; Rubie, H.; Castel, V.; Beiske, K.; Holmes, K.; Gambini, C.; Casale, F.; Munzer, C.; Erminio, G.; Parodi, S.; et al. Treatment of Children over the Age of One Year with Unresectable Localised Neuroblastoma without MYCN Amplification: Results of the SIOPEN. Study. Eur. J. Cancer 2013, 49, 3671–3679. [Google Scholar] [CrossRef] [PubMed]
- Rubie, H.; De Bernardi, B.; Gerrard, M.; Canete, A.; Ladenstein, R.; Couturier, J.; Ambros, P.; Munzer, C.; Pearson, A.D.J.; Garaventa, A.; et al. Excellent Outcome with Reduced Treatment in Infants with Nonmetastatic and Unresectable Neuroblastoma without MYCN Amplification: Results of the Prospective INES 99.1. J. Clin. Oncol. 2011, 29, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M.S.; Naranjo, A.; Zhang, F.F.; Cohn, S.L.; London, W.B.; Gastier-Foster, J.M.; Ramirez, N.C.; Pfau, R.; Reshmi, S.; Wagner, E.; et al. Revised Neuroblastoma Risk Classification System: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2021, 39, 3229–3241. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.D.J.; Pinkerton, C.R.; Lewis, I.J.; Imeson, J.; Ellershaw, C.; Machin, D.; European Neuroblastoma Study Group; Children’s Cancer and Leukaemia Group (CCLG formerly United Kingdom Children’s Cancer Study Group). High-Dose Rapid and Standard Induction Chemotherapy for Patients Aged over 1 Year with Stage 4 Neuroblastoma: A Randomised Trial. Lancet Oncol. 2008, 9, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Smith, V.; Foster, J. High-Risk Neuroblastoma Treatment Review. Children 2018, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Park, J.R.; Kreissman, S.G.; London, W.B.; Naranjo, A.; Cohn, S.L.; Hogarty, M.D.; Tenney, S.C.; Haas-Kogan, D.; Shaw, P.J.; Kraveka, J.M.; et al. Effect of Tandem Autologous Stem Cell Transplant vs. Single Transplant on Event-Free Survival in Patients with High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA 2019, 322, 746–755. [Google Scholar] [CrossRef]
- Fischer, J.; Pohl, A.; Volland, R.; Hero, B.; Dübbers, M.; Cernaianu, G.; Berthold, F.; von Schweinitz, D.; Simon, T. Complete Surgical Resection Improves Outcome in INRG High-Risk Patients with Localized Neuroblastoma Older than 18 Months. BMC Cancer 2017, 17, 520. [Google Scholar] [CrossRef]
- Gaze, M.N.; Boterberg, T.; Dieckmann, K.; Hörmann, M.; Gains, J.E.; Sullivan, K.P.; Ladenstein, R. Results of a Quality Assurance Review of External Beam Radiation Therapy in the International Society of Paediatric Oncology (Europe) Neuroblastoma Group’s High-Risk Neuroblastoma Trial: A SIOPEN Study. Int. J. Radiat Oncol. Biol. Phys. 2013, 85, 170–174. [Google Scholar] [CrossRef]
- Ladenstein, R.; Pötschger, U.; Pearson, A.D.J.; Brock, P.; Luksch, R.; Castel, V.; Yaniv, I.; Papadakis, V.; Laureys, G.; Malis, J.; et al. Busulfan and Melphalan versus Carboplatin, Etoposide, and Melphalan as High-Dose Chemotherapy for High-Risk Neuroblastoma (HR-NBL1/SIOPEN): An International, Randomised, Multi-Arm, Open-Label, Phase 3 Trial. Lancet Oncol. 2017, 18, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Mazloom, A.; Louis, C.U.; Nuchtern, J.; Kim, E.; Russell, H.; Allen-Rhoades, W.; Krance, R.; Paulino, A.C. Radiation Therapy to the Primary and Postinduction Chemotherapy MIBG-Avid Sites in High-Risk Neuroblastoma. Int. J. Radiat Oncol. Biol. Phys. 2014, 90, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.-K.V.; Cheung, I.Y.; Kushner, B.H.; Ostrovnaya, I.; Chamberlain, E.; Kramer, K.; Modak, S. Murine Anti-GD2 Monoclonal Antibody 3F8 Combined with Granulocyte-Macrophage Colony-Stimulating Factor and 13-Cis-Retinoic Acid in High-Risk Patients with Stage 4 Neuroblastoma in First Remission. J. Clin. Oncol. 2012, 30, 3264–3270. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.L.; Gilman, A.L.; Ozkaynak, M.F.; London, W.B.; Kreissman, S.G.; Chen, H.X.; Smith, M.; Anderson, B.; Villablanca, J.G.; Matthay, K.K.; et al. Anti-GD2 Antibody with GM-CSF, Interleukin-2, and Isotretinoin for Neuroblastoma. N. Engl. J. Med. 2010, 363, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- London, W.B.; Bagatell, R.; Weigel, B.J.; Fox, E.; Guo, D.; Van Ryn, C.; Naranjo, A.; Park, J.R. Historical Time to Disease Progression and Progression-Free Survival in Patients with Recurrent/Refractory Neuroblastoma Treated in the Modern Era on Children’s Oncology Group Early-Phase Trials. Cancer 2017, 123, 4914–4923. [Google Scholar] [CrossRef] [PubMed]
- Lodrini, M.; Wünschel, J.; Thole-Kliesch, T.M.; Grimaldi, M.; Sprüssel, A.; Linke, R.B.; Hollander, J.F.; Tiburtius, D.; Künkele, A.; Schulte, J.H.; et al. Circulating Cell-Free DNA Assessment in Biofluids from Children with Neuroblastoma Demonstrates Feasibility and Potential for Minimally Invasive Molecular Diagnostics. Cancers 2022, 14, 2080. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhou, S.; Qi, D.; Xiang, S.-H.; Wong, E.T.; Wang, X.; Fonkem, E.; Hsieh, T.; Yang, J.; Kirmani, B.; et al. Nucleolin Is a Functional Binding Protein for Salinomycin in Neuroblastoma Stem Cells. J. Am. Chem. Soc. 2019, 141, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Wang, W.; Liu, G.; Wang, X.; Xian, W.; McKeon, F.; Foster, J.; Zhou, J.; Zhang, R. Molecular Targeting Therapies for Neuroblastoma: Progress and Challenges. Med. Res. Rev. 2021, 41, 961–1021. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, D.; Montemurro, L.; Raieli, S.; Lampis, S.; Pession, A.; Hrelia, P.; Tonelli, R. MYCN Impact on High-Risk Neuroblastoma: From Diagnosis and Prognosis to Targeted Treatment. Cancers 2022, 14, 4421. [Google Scholar] [CrossRef]
- Dalianis, T.; Lukoseviciute, M.; Holzhauser, S.; Kostopoulou, O.N. New Approaches towards Targeted Therapy for Childhood Neuroblastoma. Anticancer Res. 2023, 43, 3829–3839. [Google Scholar] [CrossRef]
- Greengard, E.G. Molecularly Targeted Therapy for Neuroblastoma. Children 2018, 5, 142. [Google Scholar] [CrossRef]
- Johnsen, J.I.; Dyberg, C.; Fransson, S.; Wickström, M. Molecular Mechanisms and Therapeutic Targets in Neuroblastoma. Pharmacol. Res. 2018, 131, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, W.; Li, K. Applications and Prospects of Targeted Therapy for Neuroblastoma. World Jnl. Ped. Surg. 2020, 3, e000164. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, J.; Yang, J. Promising Molecular Targets and Novel Therapeutic Approaches in Neuroblastoma. Curr. Pharmacol. Rep. 2023, 9, 43–58. [Google Scholar] [CrossRef]
- Frosch, J.; Leontari, I.; Anderson, J. Combined Effects of Myeloid Cells in the Neuroblastoma Tumor Microenvironment. Cancers 2021, 13, 1743. [Google Scholar] [CrossRef] [PubMed]
- Iolascon, A.; Giordani, L.; Borriello, A.; Carbone, R.; Izzo, A.; Tonini, G.P.; Gambini, C.; Ragione, F.D. Reduced Expression of Transforming Growth Factor-Beta Receptor Type III in High Stage Neuroblastomas. Br. J. Cancer 2000, 82, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Mussai, F.; Egan, S.; Hunter, S.; Webber, H.; Fisher, J.; Wheat, R.; McConville, C.; Sbirkov, Y.; Wheeler, K.; Bendle, G.; et al. Neuroblastoma Arginase Activity Creates an Immunosuppressive Microenvironment That Impairs Autologous and Engineered Immunity. Cancer Res. 2015, 75, 3043–3053. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated Regulation of Myeloid Cells by Tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Stip, M.C.; Teeuwen, L.; Dierselhuis, M.P.; Leusen, J.H.W.; Krijgsman, D. Targeting the Myeloid Microenvironment in Neuroblastoma. J. Exp. Clin. Cancer Res. 2023, 42, 337. [Google Scholar] [CrossRef]
- Morandi, F.; Barco, S.; Stigliani, S.; Croce, M.; Persico, L.; Lagazio, C.; Scuderi, F.; Belli, M.L.; Montera, M.; Cangemi, G.; et al. Altered Erythropoiesis and Decreased Number of Erythrocytes in Children with Neuroblastoma. Oncotarget 2017, 8, 53194–53209. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, X.-Y.; Chen, K.; Qin, L.-J.; Wang, F.-H.; Miao, L.; Li, L.; Wang, H.-Y. Phosphoserine Phosphatase as an Indicator for Survival through Potentially Influencing the Infiltration Levels of Immune Cells in Neuroblastoma. Front. Cell Dev. Biol. 2022, 10, 873710. [Google Scholar] [CrossRef] [PubMed]
- Erbe, A.K.; Diccianni, M.B.; Mody, R.; Naranjo, A.; Zhang, F.F.; Birstler, J.; Kim, K.; Feils, A.S.; Hung, J.-T.; London, W.B.; et al. KIR/KIR-Ligand Genotypes and Clinical Outcomes Following Chemoimmunotherapy in Patients with Relapsed or Refractory Neuroblastoma: A Report from the Children’s Oncology Group. J. Immunother. Cancer 2023, 11, e006530. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, S.; Feng, J.; Zhao, X. Prognostic Value of Inflammation Biomarkers for Survival of Patients with Neuroblastoma. Cancer Manag. Res. 2020, 12, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-L.; Reynolds, C.P.; Seeger, R.C. Neutrophils Are Cytotoxic and Growth-Inhibiting for Neuroblastoma Cells with an Anti-GD2 Antibody but, without Cytotoxicity, Can Be Growth-Stimulating. Cancer Immunol. Immunother. 2000, 48, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Evers, M.; Stip, M.; Keller, K.; Willemen, H.; Nederend, M.; Jansen, M.; Chan, C.; Budding, K.; Nierkens, S.; Valerius, T.; et al. Anti-GD2 IgA Kills Tumors by Neutrophils without Antibody-Associated Pain in the Preclinical Treatment of High-Risk Neuroblastoma. J. Immunother. Cancer 2021, 9, e003163. [Google Scholar] [CrossRef] [PubMed]
- Zafari, R.; Razi, S.; Rezaei, N. The Role of Dendritic Cells in Neuroblastoma: Implications for Immunotherapy. Immunobiology 2022, 227, 152293. [Google Scholar] [CrossRef] [PubMed]
- Melaiu, O.; Chierici, M.; Lucarini, V.; Jurman, G.; Conti, L.A.; De Vito, R.; Boldrini, R.; Cifaldi, L.; Castellano, A.; Furlanello, C.; et al. Cellular and Gene Signatures of Tumor-Infiltrating Dendritic Cells and Natural-Killer Cells Predict Prognosis of Neuroblastoma. Nat. Commun. 2020, 11, 5992. [Google Scholar] [CrossRef] [PubMed]
- Asgharzadeh, S.; Salo, J.A.; Ji, L.; Oberthuer, A.; Fischer, M.; Berthold, F.; Hadjidaniel, M.; Liu, C.W.-Y.; Metelitsa, L.S.; Pique-Regi, R.; et al. Clinical Significance of Tumor-Associated Inflammatory Cells in Metastatic Neuroblastoma. J. Clin. Oncol. 2012, 30, 3525–3532. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, O.; Yoshida, M.; Koma, Y.; Yanai, T.; Hasegawa, D.; Kosaka, Y.; Nishimura, N.; Yokozaki, H. Collaboration of Cancer-Associated Fibroblasts and Tumour-Associated Macrophages for Neuroblastoma Development. J. Pathol. 2016, 240, 211–223. [Google Scholar] [CrossRef]
- Masih, K.E.; Wei, J.S.; Milewski, D.; Khan, J. Exploring and Targeting the Tumor Immune Microenvironment of Neuroblastoma. J. Cell Immunol. 2021, 3, 305–316. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer Vaccines as Promising Immuno-Therapeutics: Platforms and Current Progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef] [PubMed]
- Srivatsan, S.; Patel, J.M.; Bozeman, E.N.; Imasuen, I.E.; He, S.; Daniels, D.; Selvaraj, P. Allogeneic Tumor Cell Vaccines. Hum. Vaccines Immunother. 2014, 10, 52–63. [Google Scholar] [CrossRef]
- Dranoff, G.; Jaffee, E.; Lazenby, A.; Golumbek, P.; Levitsky, H.; Brose, K.; Jackson, V.; Hamada, H.; Pardoll, D.; Mulligan, R.C. Vaccination with Irradiated Tumor Cells Engineered to Secrete Murine Granulocyte-Macrophage Colony-Stimulating Factor Stimulates Potent, Specific, and Long-Lasting Anti-Tumor Immunity. Proc. Natl. Acad. Sci. USA 1993, 90, 3539–3543. [Google Scholar] [CrossRef]
- Pangilinan, C.R.; Lee, C.-H. Highlights of Immunomodulation in Salmonella-Based Cancer Therapy. Biomedicines 2021, 9, 1566. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Cheung, I.Y.; Modak, S.; Kramer, K.; Ragupathi, G.; Cheung, N.-K.V. Phase I trial of a bivalent gangliosides vaccine in combination with β-glucan. Clin. Cancer Res. 2014, 20, 1375–1382. [Google Scholar] [CrossRef]
- Liebscher, L.; Weißenborn, C.; Langwisch, S.; Gohlke, B.-O.; Preissner, R.; Rabinovich, G.A.; Christiansen, N.; Christiansen, H.; Zenclussen, A.C.; Fest, S. A Minigene DNA Vaccine Encoding Peptide Epitopes Derived from Galectin-1 Has Protective Antitumoral Effects in a Model of Neuroblastoma. Cancer Lett. 2021, 509, 105–114. [Google Scholar] [CrossRef]
- George, R.; Krishnadas, D.K.; Bai, F.; Diller, L.; Shusterman, S.; Sullivan, J.E.; Lucas, K.G. Phase 1 Trial of Decitabine and CT Antigen-Specific Vaccine in Relapsed Pediatric Solid Tumors. J. Clin. Oncol. 2014, 32, 10070. [Google Scholar] [CrossRef]
- Furman, W.L. Monoclonal Antibody Therapies for High Risk Neuroblastoma. Biologics 2021, 15, 205–219. [Google Scholar] [CrossRef]
- Zeng, Y.; Fest, S.; Kunert, R.; Katinger, H.; Pistoia, V.; Michon, J.; Lewis, G.; Ladenstein, R.; Lode, H.N. Anti-Neuroblastoma Effect of Ch14.18 Antibody Produced in CHO Cells Is Mediated by NK-Cells in Mice. Mol. Immunol. 2005, 42, 1311–1319. [Google Scholar] [CrossRef]
- Cheung, N.K.; Lazarus, H.; Miraldi, F.D.; Abramowsky, C.R.; Kallick, S.; Saarinen, U.M.; Spitzer, T.; Strandjord, S.E.; Coccia, P.F.; Berger, N.A. Ganglioside GD2 Specific Monoclonal Antibody 3F8: A Phase I Study in Patients with Neuroblastoma and Malignant Melanoma. J. Clin. Oncol. 1987, 5, 1430–1440. [Google Scholar] [CrossRef]
- Cheung, N.-K.V.; Modak, S. Oral (1→3),(1→4)-Beta-D-Glucan Synergizes with Antiganglioside GD2 Monoclonal Antibody 3F8 in the Therapy of Neuroblastoma. Clin. Cancer Res. 2002, 8, 1217–1223. [Google Scholar] [PubMed]
- Kushner, B.H.; Kramer, K.; Cheung, N.-K.V. Phase II Trial of the Anti-GD2 Monoclonal Antibody 3F8 and Granulocyte-Macrophage Colony-Stimulating Factor for Neuroblastoma. J. Clin. Oncol. 2001, 19, 4189–4194. [Google Scholar] [CrossRef]
- Cheung, N.-K.V.; Guo, H.; Hu, J.; Tassev, D.V.; Cheung, I.Y. Humanizing Murine IgG3 Anti-GD2 Antibody m3F8 Substantially Improves Antibody-Dependent Cell-Mediated Cytotoxicity While Retaining Targeting In Vivo. OncoImmunology 2012, 1, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Kushner, B.H.; Cheung, I.Y.; Modak, S.; Basu, E.M.; Roberts, S.S.; Cheung, N.-K. Humanized 3F8 Anti-GD2 Monoclonal Antibody Dosing with Granulocyte-Macrophage Colony-Stimulating Factor in Patients with Resistant Neuroblastoma: A Phase 1 Clinical Trial. JAMA Oncol. 2018, 4, 1729–1735. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.D.; Hank, J.A.; Reaman, G.H.; Frierdich, S.; Seeger, R.C.; Gan, J.; Anderson, P.M.; Ettinger, L.J.; Cairo, M.S.; Blazar, B.R.; et al. A Phase I/IB Trial of Murine Monoclonal Anti-GD2 Antibody 14.G2a plus Interleukin-2 in Children with Refractory Neuroblastoma. Cancer 1997, 80, 317–333. [Google Scholar] [CrossRef]
- Handgretinger, R.; Baader, P.; Dopfer, R.; Klingebiel, T.; Reuland, P.; Treuner, J.; Reisfeld, R.A.; Niethammer, D. A Phase I Study of Neuroblastoma with the Anti-Ganglioside GD2 Antibody 14.G2a. Cancer Immunol. Immunother. 1992, 35, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Gilman, A.L.; Ozkaynak, M.F.; Matthay, K.K.; Krailo, M.; Yu, A.L.; Gan, J.; Sternberg, A.; Hank, J.A.; Seeger, R.; Reaman, G.H.; et al. Phase I Study of Ch14.18 with Granulocyte-Macrophage Colony-Stimulating Factor and Interleukin-2 in Children with Neuroblastoma After Autologous Bone Marrow Transplantation or Stem-Cell Rescue: A Report From the Children’s Oncology Group. J. Clin. Oncol. 2009, 27, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Handgretinger, R.; Anderson, K.; Lang, P.; Dopfer, R.; Klingebiel, T.; Schrappe, M.; Reuland, P.; Gillies, S.D.; Reisfeld, R.A.; Niethammer, D. A Phase I Study of Human/Mouse Chimeric Antiganglioside GD2 Antibody Ch14.18 in Patients with Neuroblastoma. Eur. J. Cancer 1995, 31, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Anghelescu, D.L.; Goldberg, J.L.; Faughnan, L.G.; Wu, J.; Mao, S.; Furman, W.L.; Santana, V.M.; Navid, F. Comparison of Pain Outcomes between Two Anti-GD2 Antibodies in Patients with Neuroblastoma. Pediatr. Blood Cancer 2015, 62, 224–228. [Google Scholar] [CrossRef]
- Furman, W.L.; Federico, S.M.; McCarville, M.B.; Shulkin, B.L.; Davidoff, A.M.; Krasin, M.J.; Sahr, N.; Sykes, A.; Wu, J.; Brennan, R.C.; et al. A Phase II Trial of Hu14.18K322A in Combination with Induction Chemotherapy in Children with Newly Diagnosed High-Risk Neuroblastoma. Clin. Cancer Res. 2019, 25, 6320–6328. [Google Scholar] [CrossRef]
- Espinosa-Cotton, M.; Cheung, N.-K.V. Bispecific Antibodies for the Treatment of Neuroblastoma. Pharmacol. Ther. 2022, 237, 108241. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Dondero, A.; Augugliaro, R.; Cantoni, C.; Carnemolla, B.; Sementa, A.R.; Negri, F.; Conte, R.; Corrias, M.V.; Moretta, L.; et al. Identification of 4Ig-B7-H3 as a Neuroblastoma-Associated Molecule That Exerts a Protective Role from an NK Cell-Mediated Lysis. Proc. Natl. Acad. Sci. USA 2004, 101, 12640–12645. [Google Scholar] [CrossRef] [PubMed]
- Picarda, E.; Ohaegbulam, K.C.; Zang, X. Molecular Pathways: Targeting B7-H3 (CD276) for Human Cancer Immunotherapy. Clin. Cancer Res. 2016, 22, 3425–3431. [Google Scholar] [CrossRef] [PubMed]
- Yarmarkovich, M.; Marshall, Q.F.; Warrington, J.M.; Premaratne, R.; Farrel, A.; Groff, D.; Li, W.; di Marco, M.; Runbeck, E.; Truong, H.; et al. Targeting of Intracellular Oncoproteins with Peptide-Centric CARs. Nature 2023, 623, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Flaadt, T.; Ladenstein, R.L.; Ebinger, M.; Lode, H.N.; Arnardóttir, H.B.; Poetschger, U.; Schwinger, W.; Meisel, R.; Schuster, F.R.; Döring, M.; et al. Anti-GD2 Antibody Dinutuximab Beta and Low-Dose Interleukin 2 after Haploidentical Stem-Cell Transplantation in Patients with Relapsed Neuroblastoma: A Multicenter, Phase I/II Trial. J. Clin. Oncol. 2023, 41, 3135–3148. [Google Scholar] [CrossRef] [PubMed]
- Ozkaynak, M.F.; Gilman, A.L.; London, W.B.; Naranjo, A.; Diccianni, M.B.; Tenney, S.C.; Smith, M.; Messer, K.S.; Seeger, R.; Reynolds, C.P.; et al. A Comprehensive Safety Trial of Chimeric Antibody 14.18 with GM-CSF, IL-2, and Isotretinoin in High-Risk Neuroblastoma Patients Following Myeloablative Therapy: Children’s Oncology Group Study ANBL0931. Front. Immunol. 2018, 9, 1355. [Google Scholar] [CrossRef] [PubMed]
- Mody, R.; Yu, A.L.; Naranjo, A.; Zhang, F.F.; London, W.B.; Shulkin, B.L.; Parisi, M.T.; Servaes, S.-E.-N.; Diccianni, M.B.; Hank, J.A.; et al. Irinotecan, Temozolomide, and Dinutuximab with GM-CSF in Children with Refractory or Relapsed Neuroblastoma: A Report from the Children’s Oncology Group. J. Clin. Oncol. 2020, 38, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Ladenstein, R.; Weixler, S.; Baykan, B.; Bleeke, M.; Kunert, R.; Katinger, D.; Pribill, I.; Glander, P.; Bauer, S.; Pistoia, V.; et al. Ch14.18 Antibody Produced in CHO Cells in Relapsed or Refractory Stage 4 Neuroblastoma Patients: A SIOPEN Phase 1 Study. MAbs 2013, 5, 801–809. [Google Scholar] [CrossRef]
- Seitz, C.M.; Flaadt, T.; Mezger, M.; Lang, A.-M.; Michaelis, S.; Katz, M.; Syring, D.; Joechner, A.; Rabsteyn, A.; Siebert, N.; et al. Immunomonitoring of Stage IV Relapsed Neuroblastoma Patients Undergoing Haploidentical Hematopoietic Stem Cell Transplantation and Subsequent GD2 (Ch14.18/CHO) Antibody Treatment. Front. Immunol. 2021, 12, 690467. [Google Scholar] [CrossRef]
- Goldberg, J.L.; Navid, F.; Hank, J.A.; Erbe, A.K.; Santana, V.; Gan, J.; de Bie, F.; Javaid, A.M.; Hoefges, A.; Merdler, M.; et al. Pre-Existing Antitherapeutic Antibodies against the Fc Region of the Hu14.18K322A mAb Are Associated with Outcome in Patients with Relapsed Neuroblastoma. J. Immunother. Cancer 2020, 8, e000590. [Google Scholar] [CrossRef]
- Federico, S.M.; McCarville, M.B.; Shulkin, B.L.; Sondel, P.M.; Hank, J.A.; Hutson, P.; Meagher, M.; Shafer, A.; Ng, C.Y.; Leung, W.; et al. A Pilot Trial of Humanized Anti-GD2 Monoclonal Antibody (Hu14.18K322A) with Chemotherapy and Natural Killer Cells in Children with Recurrent/Refractory Neuroblastoma. Clin. Cancer Res. 2017, 23, 6441–6449. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-T.; Dai, S.-Y.; Zhan, Y.; Yang, R.; Chen, D.-Q.; Li, Y.; Zhou, E.-Q.; Dong, R. Progress of Oncolytic Virotherapy for Neuroblastoma. Front. Pediatr. 2022, 10, 1055729. [Google Scholar] [CrossRef]
- de Matos, A.L.; Franco, L.S.; McFadden, G. Oncolytic Viruses and the Immune System: The Dynamic Duo. Mol. Ther. Methods Clin. Dev. 2020, 17, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Cascallo, M.; Alonso, M.M.; Rojas, J.J.; Perez-Gimenez, A.; Fueyo, J.; Alemany, R. Systemic Toxicity–Efficacy Profile of ICOVIR-5, a Potent and Selective Oncolytic Adenovirus Based on the pRB Pathway. Mol. Ther. 2007, 15, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Komarova, S.; Kawakami, Y.; Stoff-Khalili, M.A.; Curiel, D.T.; Pereboeva, L. Mesenchymal Progenitor Cells as Cellular Vehicles for Delivery of Oncolytic Adenoviruses. Mol. Cancer Ther 2006, 5, 755–766. [Google Scholar] [CrossRef]
- Ruano, D.; López-Martín, J.A.; Moreno, L.; Lassaletta, Á.; Bautista, F.; Andión, M.; Hernández, C.; González-Murillo, Á.; Melen, G.; Alemany, R.; et al. First-in-Human, First-in-Child Trial of Autologous MSCs Carrying the Oncolytic Virus Icovir-5 in Patients with Advanced Tumors. Mol. Ther. 2020, 28, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, H.; Zhu, X.; Feng, D.; Zhang, D.; Zhuo, B.; Zheng, J. Oncolytic Adenovirus-Mediated Short Hairpin RNA Targeting MYCN Gene Induces Apoptosis by Upregulating RKIP in Neuroblastoma. Tumor Biol. 2015, 36, 6037–6043. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, B.; Zhang, H.; Zhu, X.; Feng, D.; Zhang, D.; Zhuo, B.; Li, L.; Zheng, J. Oncolytic Adenovirus Armed with shRNA Targeting MYCN Gene Inhibits Neuroblastoma Cell Proliferation and In Vivo Xenograft Tumor Growth. J. Cancer Res. Clin. Oncol. 2013, 139, 933–941. [Google Scholar] [CrossRef]
- Tanimoto, T.; Tazawa, H.; Ieda, T.; Nouso, H.; Tani, M.; Oyama, T.; Urata, Y.; Kagawa, S.; Noda, T.; Fujiwara, T. Elimination of MYCN-Amplified Neuroblastoma Cells by Telomerase-Targeted Oncolytic Virus via MYCN Suppression. Mol. Ther.—Oncolytics 2020, 18, 14–23. [Google Scholar] [CrossRef]
- Cripe, T.P.; Ngo, M.C.; Geller, J.I.; Louis, C.U.; Currier, M.A.; Racadio, J.M.; Towbin, A.J.; Rooney, C.M.; Pelusio, A.; Moon, A.; et al. Phase 1 Study of Intratumoral Pexa-Vec (JX-594), an Oncolytic and Immunotherapeutic Vaccinia Virus, in Pediatric Cancer Patients. Mol. Ther. 2015, 23, 602–608. [Google Scholar] [CrossRef]
- Ma, J.; Jin, C.; Čančer, M.; Wang, H.; Ramachandran, M.; Yu, D. Concurrent Expression of HP-NAP Enhances Antitumor Efficacy of Oncolytic Vaccinia Virus but Not for Semliki Forest Virus. Mol. Ther.—Oncolytics 2021, 21, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.J.; Ahern, C.; Weigel, B.J.; Poirier, J.T.; Rudin, C.M.; Chen, Y.; Cripe, T.P.; Bernhardt, M.B.; Blaney, S.M. Phase I Trial of Seneca Valley Virus (NTX-010) in Children with Relapsed/Refractory Solid Tumors: A Report of the Children’s Oncology Group. Pediatr. Blood Cancer 2015, 62, 743–750. [Google Scholar] [CrossRef]
- Zappa, E.; Vitali, A.; Anders, K.; Molenaar, J.J.; Wienke, J.; Künkele, A. Adoptive Cell Therapy in Paediatric Extracranial Solid Tumours: Current Approaches and Future Challenges. Eur. J. Cancer 2023, 194, 113347. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Guo, F.; Wang, Y.; Cui, J. NK Cell Therapy: A Rising Star in Cancer Treatment. Cancers 2021, 13, 4129. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.-T.T.; Lee, S.-H.; Kim, S.-K.; Cho, D. Expansion of NK Cells Using Genetically Engineered K562 Feeder Cells. Methods Mol. Biol. 2016, 1441, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Dondero, A.; Cilli, M.; Ognio, E.; Pezzolo, A.; De Giovanni, B.; Gambini, C.; Pistoia, V.; Moretta, L.; Moretta, A.; et al. Human NK Cell Infusions Prolong Survival of Metastatic Human Neuroblastoma-Bearing NOD/Scid Mice. Cancer Immunol. Immunother. 2007, 56, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, H.-W.; Sheard, M.A.; Sposto, R.; Somanchi, S.S.; Cooper, L.J.N.; Lee, D.A.; Seeger, R.C. Growth and Activation of Natural Killer Cells Ex Vivo from Children with Neuroblastoma for Adoptive Cell Therapy. Clin. Cancer Res. 2013, 19, 2132–2143. [Google Scholar] [CrossRef] [PubMed]
- Heinze, A.; Grebe, B.; Bremm, M.; Huenecke, S.; Munir, T.A.; Graafen, L.; Frueh, J.T.; Merker, M.; Rettinger, E.; Soerensen, J.; et al. The Synergistic Use of IL-15 and IL-21 for the Generation of NK Cells From CD3/CD19-Depleted Grafts Improves Their Ex Vivo Expansion and Cytotoxic Potential Against Neuroblastoma: Perspective for Optimized Immunotherapy Post Haploidentical Stem Cell Transplantation. Front. Immunol. 2019, 10, 2816. [Google Scholar] [CrossRef] [PubMed]
- Talleur, A.C.; Triplett, B.M.; Federico, S.; Mamcarz, E.; Janssen, W.; Wu, J.; Shook, D.; Leung, W.; Furman, W.L. Consolidation Therapy for Newly Diagnosed Pediatric Patients with High-Risk Neuroblastoma Using Busulfan/Melphalan, Autologous Hematopoietic Cell Transplantation, Anti-GD2 Antibody, Granulocyte-Macrophage Colony-Stimulating Factor, Interleukin-2, and Haploidentical Natural Killer Cells. Biol. Blood Marrow Transpl. 2017, 23, 1910–1917. [Google Scholar] [CrossRef]
- Modak, S.; Le Luduec, J.-B.; Cheung, I.Y.; Goldman, D.A.; Ostrovnaya, I.; Doubrovina, E.; Basu, E.; Kushner, B.H.; Kramer, K.; Roberts, S.S.; et al. Adoptive Immunotherapy with Haploidentical Natural Killer Cells and Anti-GD2 Monoclonal Antibody m3F8 for Resistant Neuroblastoma: Results of a Phase I Study. Oncoimmunology 2018, 7, e1461305. [Google Scholar] [CrossRef]
- Sterner, R.C.; Sterner, R.M. CAR-T Cell Therapy: Current Limitations and Potential Strategies. Blood Cancer J. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mehrabadi, A.Z.; Ranjbar, R.; Farzanehpour, M.; Shahriary, A.; Dorostkar, R.; Hamidinejad, M.A.; Ghaleh, H.E.G. Therapeutic Potential of CAR T Cell in Malignancies: A Scoping Review. Biomed. Pharmacother. 2022, 146, 112512. [Google Scholar] [CrossRef] [PubMed]
- Tokarew, N.; Ogonek, J.; Endres, S.; von Bergwelt-Baildon, M.; Kobold, S. Teaching an Old Dog New Tricks: Next-Generation CAR T Cells. Br. J. Cancer 2019, 120, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, P.; Huang, H. Engineering Better Chimeric Antigen Receptor T Cells. Exp. Hematol. Oncol. 2020, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Lukacs, J.D.; Johnston, B. The Current Landscape of NKT Cell Immunotherapy and the Hills Ahead. Cancers 2021, 13, 5174. [Google Scholar] [CrossRef] [PubMed]
- Heczey, A.; Xu, X.; Courtney, A.N.; Tian, G.; Barragan, G.A.; Guo, L.; Amador, C.M.; Ghatwai, N.; Rathi, P.; Wood, M.S.; et al. Anti-GD2 CAR-NKT Cells in Relapsed or Refractory Neuroblastoma: Updated Phase 1 Trial Interim Results. Nat. Med. 2023, 29, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Straathof, K.; Flutter, B.; Wallace, R.; Jain, N.; Loka, T.; Depani, S.; Wright, G.; Thomas, S.; Cheung, G.W.-K.; Gileadi, T.; et al. Antitumor Activity without On-Target off-Tumor Toxicity of GD2-Chimeric Antigen Receptor T Cells in Patients with Neuroblastoma. Sci. Transl. Med. 2020, 12, eabd6169. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowska, S.; Murty, T.; Alimadadi, A.; Contreras, C.F.; Duault, C.; Subrahmanyam, P.B.; Reynolds, W.; Gutierrez, N.A.; Baskar, R.; Wu, C.J.; et al. Immune Determinants of CAR-T Cell Expansion in Solid Tumor Patients Receiving GD2 CAR-T Cell Therapy. Cancer Cell 2024, 42, 35–51.e8. [Google Scholar] [CrossRef]
- Yu, L.; Huang, L.; Lin, D.; Lai, X.; Wu, L.; Liao, X.; Liu, J.; Zeng, Y.; Liang, L.; Zhang, G.; et al. GD2-Specific Chimeric Antigen Receptor-Modified T Cells for the Treatment of Refractory and/or Recurrent Neuroblastoma in Pediatric Patients. J. Cancer Res. Clin. Oncol. 2022, 148, 2643–2652. [Google Scholar] [CrossRef]
- Shah, N.N.; Baird, K.; Delbrook, C.P.; Fleisher, T.A.; Kohler, M.E.; Rampertaap, S.; Lemberg, K.; Hurley, C.K.; Kleiner, D.E.; Merchant, M.S.; et al. Acute GVHD in Patients Receiving IL-15/4-1BBL Activated NK Cells Following T-Cell-Depleted Stem Cell Transplantation. Blood 2015, 125, 784–792. [Google Scholar] [CrossRef]
- Thakar, M.S.; Browning, M.; Hari, P.; Charlson, J.A.; Margolis, D.A.; Logan, B.; Schloemer, N.; Kelly, M.E.; Newman, A.; Johnson, B.; et al. Phase II Trial Using Haploidentical Hematopoietic Cell Transplantation (HCT) Followed by Donor Natural Killer (NK) Cell Infusion and Sirolimus Maintenance for Patients with High-Risk Solid Tumors. J. Clin. Oncol. 2020, 38, e23551. [Google Scholar] [CrossRef]
- Stern, M.; Passweg, J.R.; Meyer-Monard, S.; Esser, R.; Tonn, T.; Soerensen, J.; Paulussen, M.; Gratwohl, A.; Klingebiel, T.; Bader, P.; et al. Pre-Emptive Immunotherapy with Purified Natural Killer Cells after Haploidentical SCT: A Prospective Phase II Study in Two Centers. Bone Marrow Transpl. 2013, 48, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Segal, J.; Kaczanowska, S.; Bernstein, D.; Zhang, N.; Dinh, A.; Somerville, R.; Highfill, S.; Stroncek, D.; Conlon, K.; Waldmann, T.; et al. 621 A Phase I Study of Autologous Activated NK Cells ± rhIL15 in Children and Young Adults with Refractory Solid Tumors. J. Immunother. Cancer 2023, 11, 708. [Google Scholar] [CrossRef]

| NCT Identifier | Study Title | Status/Outcome |
|---|---|---|
| NCT01953900 | iC9-GD2-CAR-VZV-CTLs/Refractory or Metastatic GD2-positive Sarcoma and Neuroblastoma | Active, not recruiting |
| NCT01048892 | Seneca Valley Virus-001 and Cyclophosphamide in Treating Young Patients With Relapsed or Refractory Neuroblastoma, Rhabdomyosarcoma, or Rare Tumors With Neuroendocrine Features | Completed; tolerance and feasibility of NTX-010 with or without CTX was observed [153] |
| NCT01460901 | Study of Donor-Derived, Multi-virus-specific, Cytotoxic T-Lymphocytes for Relapsed/Refractory Neuroblastoma | Completed; all three patients died of the disease and response was non-complete [154] |
| NCT00314925 | Safety Study of Seneca Valley Virus in Patients With Solid Tumors With Neuroendocrine Features | Unknown |
| NCT05593328 | Study of Onvansertib in Combination With FOLFIRI and Bevacizumab Versus FOLFIRI and Bevacizumab for Second-Line Treatment of Metastatic Colorectal Cancer in Participants With a Kirsten Rat Sarcoma Virus Gene (KRAS) or Neuroblastoma-RAS (NRAS) Mutation | Active, not recruiting |
| NCT01169584 | Safety Study of Recombinant Vaccinia Virus to Treat Refractory Solid Tumors in Pediatric Patients | Completed; evaluated safety of administration to children, with no serious adverse effects [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polychronopoulos, P.A.; Bedoya-Reina, O.C.; Johnsen, J.I. The Neuroblastoma Microenvironment, Heterogeneity and Immunotherapeutic Approaches. Cancers 2024, 16, 1863. https://doi.org/10.3390/cancers16101863
Polychronopoulos PA, Bedoya-Reina OC, Johnsen JI. The Neuroblastoma Microenvironment, Heterogeneity and Immunotherapeutic Approaches. Cancers. 2024; 16(10):1863. https://doi.org/10.3390/cancers16101863
Chicago/Turabian StylePolychronopoulos, Panagiotis Alkinoos, Oscar C. Bedoya-Reina, and John Inge Johnsen. 2024. "The Neuroblastoma Microenvironment, Heterogeneity and Immunotherapeutic Approaches" Cancers 16, no. 10: 1863. https://doi.org/10.3390/cancers16101863
APA StylePolychronopoulos, P. A., Bedoya-Reina, O. C., & Johnsen, J. I. (2024). The Neuroblastoma Microenvironment, Heterogeneity and Immunotherapeutic Approaches. Cancers, 16(10), 1863. https://doi.org/10.3390/cancers16101863






